Influence of Different Land-Use Types on Soil Arthropod Communities in an Urban Area: A Case Study from Rome (Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling Sites and Sampling Design
2.2. Soil Characterization and Landscape Characteristics
2.3. Microarthropod Collection and Identification
2.4. Soil Biology Quality Indices
2.5. Microarthropod Density and Diversity
2.6. Statistical Analyses
3. Results
3.1. Soil and Landscape Characteristics of Urban Green Spaces
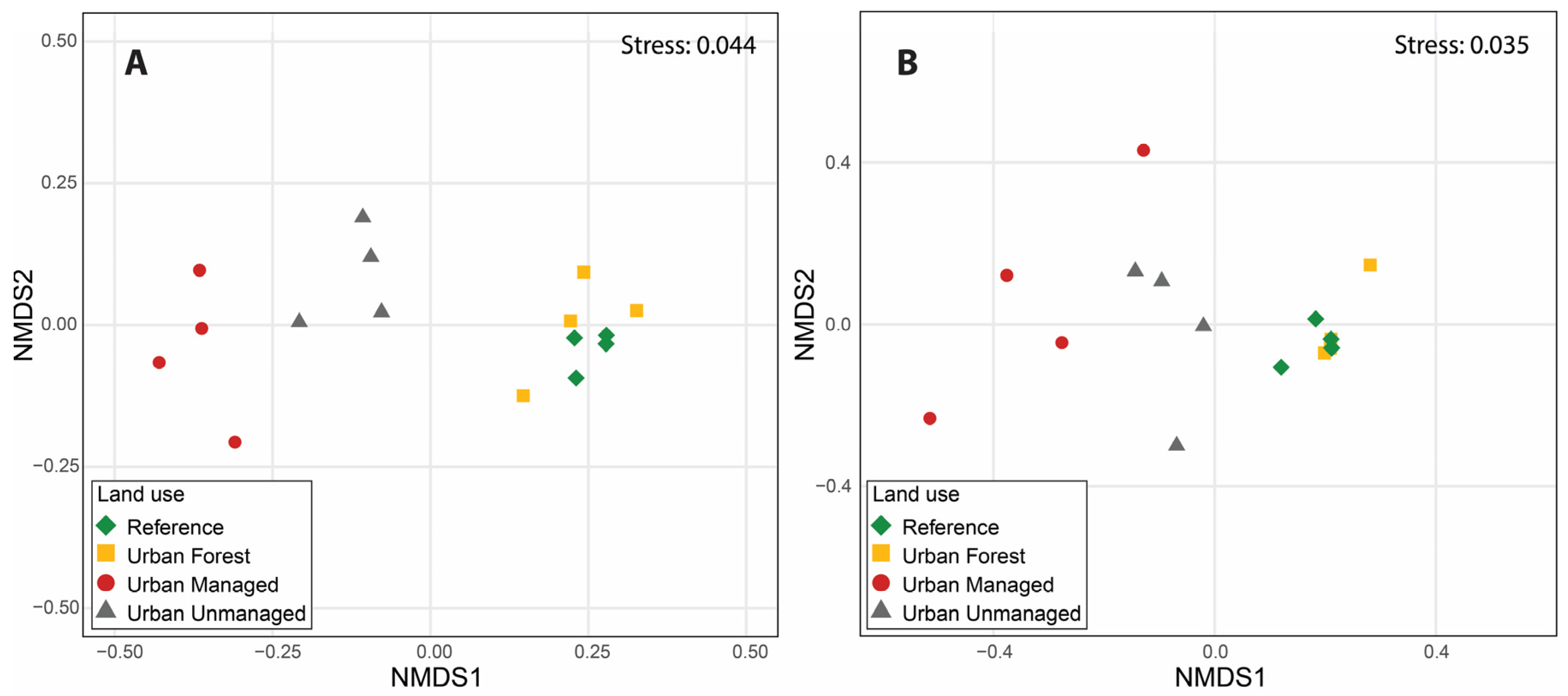
3.2. Soil Microarthropod Diversity and Biological Quality
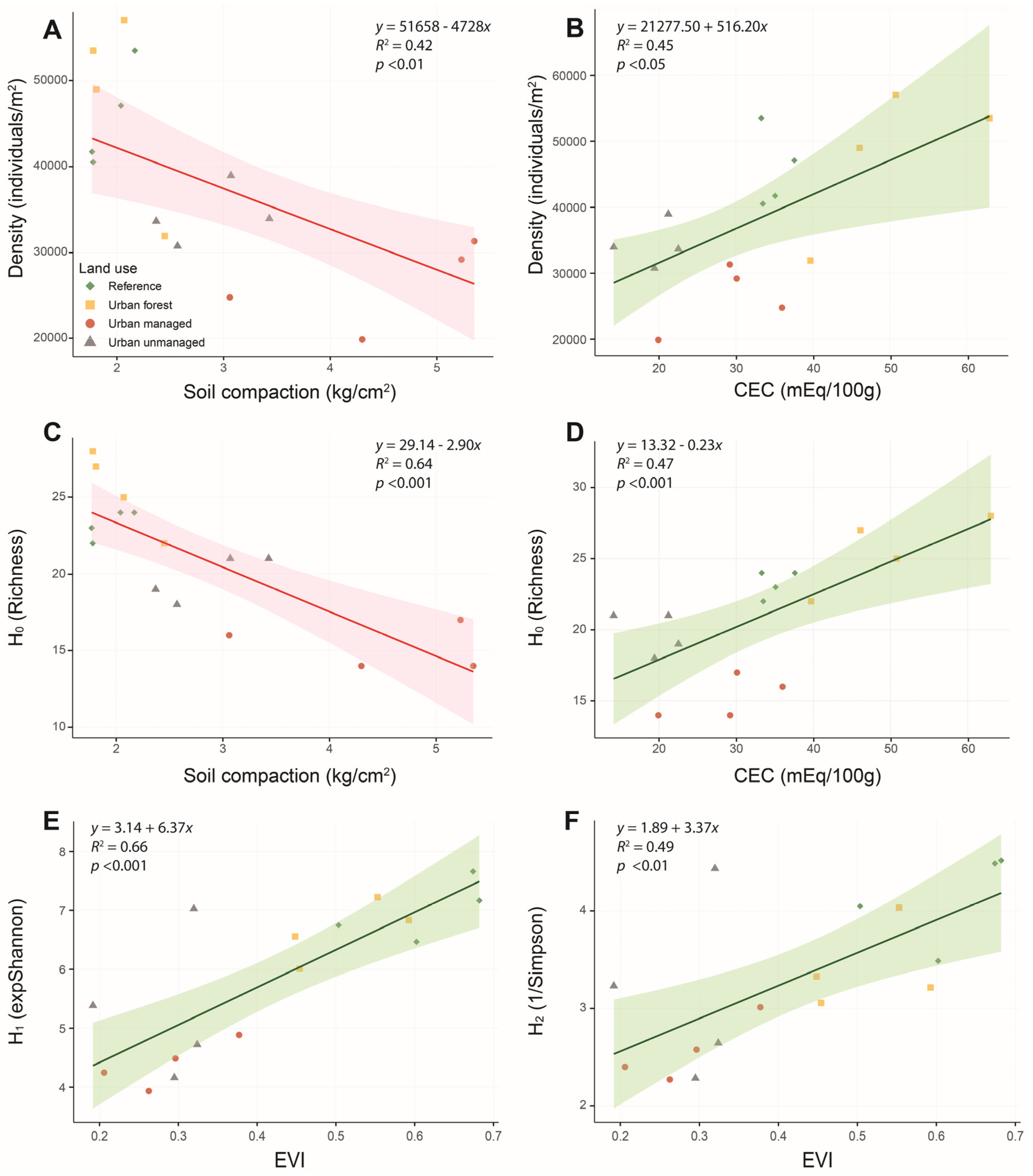
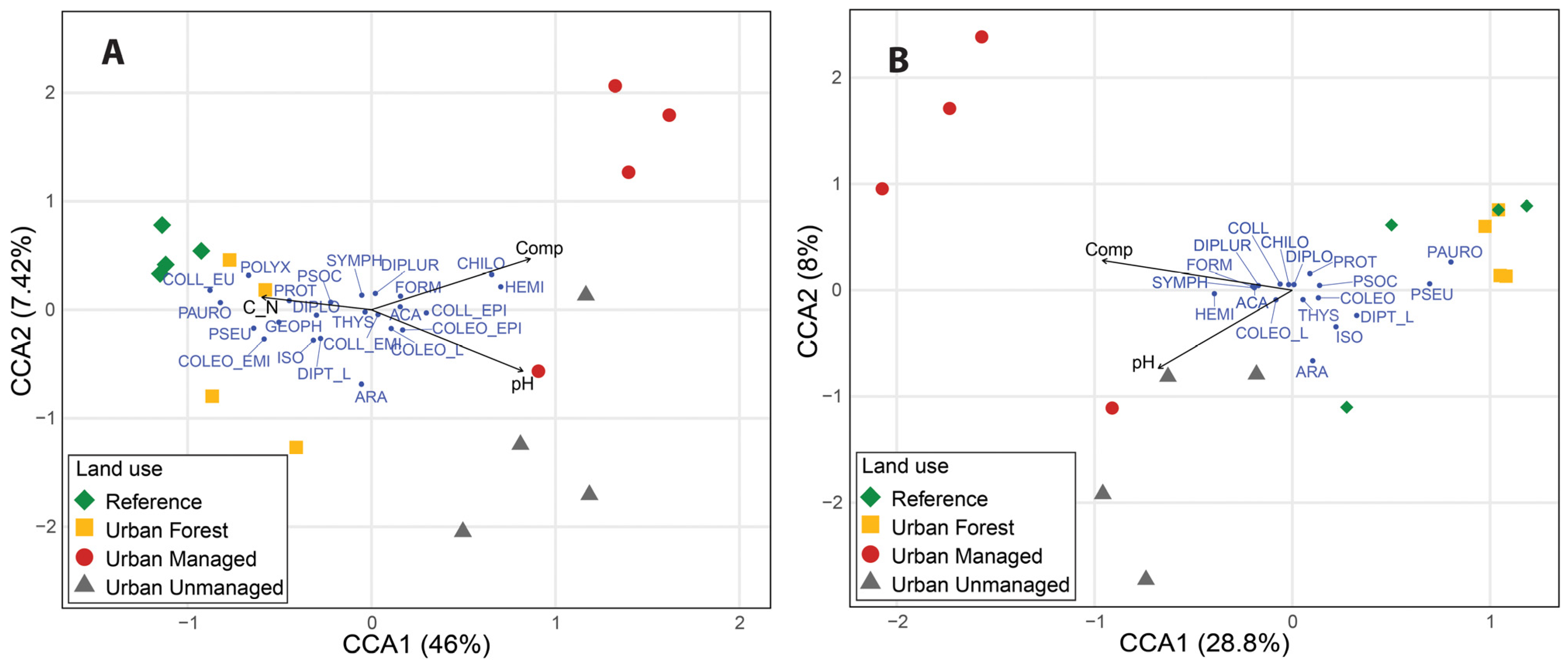
3.3. Environmental Factors and Soil Microarthropod Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolters, V. Biodiversity of soils animals and its function. Eur. J. Soil Biol. 2001, 37, 221–227. [Google Scholar]
- Decaëns, T.; Jiménez, J.J.; Gioia, C.; Measey, G.J.; Lavelle, P. The values of soil animals for conservation biology. Eur. J. Soil Biol. 2006, 42, 23–38. [Google Scholar] [CrossRef]
- Pulleman, M.; Creamer, R.; Hamer, U.; Helder, J.; Pelosi, C.; Peres, G.; Rutgers, M. Soil biodiversity, biological indicators and soil ecosystem services—An overview of European approaches. Curr. Opin. Environ. Sustain. 2012, 4, 529–538. [Google Scholar]
- Pavao-Zuckerman, M.A. Urbanization, soils, and ecosystem services. In Soil Ecology and Ecosystem Services; Wall, D.H., Bardgett, R.D., Behan-Pelletier, V., Herrick, J.E., Jones, T.H., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 270–278. [Google Scholar]
- Adhikari, K.; Hartemink, A.E. Linking soils to ecosystem services—A global review. Geoderma 2016, 262, 101–111. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Page-Dumroese, D.S.; Patel-Weynand, T.; Geiser, L.H. Forest and Rangeland Soils of the United States under Changing Conditions: A Comprehensive Science Synthesis; Springer Nature: New York, NY, USA, 2020; p. 289. [Google Scholar]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; De Goede, R.; Brussaard, L. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Caon, L.; Vargas, R. Threats to soils: Global trends and perspectives. In A Contribution from the Intergovernmental Technical Panel on Soils, Global Soil Partnership; Pierzynski, G., Ed.; Food and Agriculture: Rome, Italy, 2017. [Google Scholar]
- Szlavecz, K.; Yesilonis, I.D.; Pouyat, R.V. Soil as a foundation to urban biodiversity. In Urban Biodiversity: From Research to Practice; Ossola, A., Niemela, J., Eds.; Routledge: London, UK, 2017; pp. 18–36. [Google Scholar]
- Orgiazzi, A.; Panagos, P. Soil biodiversity and soil erosion: It is time to get married—Adding an earthworm factor to soil erosion modelling. Glob. Ecol. Biogeogr. 2018, 27, 1155–1167. [Google Scholar] [CrossRef]
- Huang, Y.; Yesilonis, I.; Szlavecz, K. Soil microarthropod communities of urban green spaces in Baltimore, Maryland, USA. Urban For. Urban Greening 2020, 53, 126676. [Google Scholar] [CrossRef]
- Trammell, T.L.; Schneid, B.P.; Carreiro, M.M. Forest soils adjacent to urban interstates: Soil physical and chemical properties, heavy metals, disturbance legacies, and relationships with woody vegetation. Urban Ecosyst. 2011, 14, 525–552. [Google Scholar] [CrossRef]
- Tenenbaum, D.E.; Band, L.E.; Kenworthy, S.T.; Tague, C.L. Analysis of soil moisture patterns in forested and suburban catchments in Baltimore, Maryland, using high-resolution photogrammetric and LIDAR digital elevation datasets. Hydrol. Process. 2006, 20, 219–240. [Google Scholar] [CrossRef]
- Zhu, W.X.; Hope, D.; Gries, C.; Grimm, N.B. Soil characteristics and the accumulation of inorganic nitrogen in an arid urban ecosystem. Ecosystems 2006, 9, 711–724. [Google Scholar] [CrossRef]
- Godefroid, S.; Koedam, N. The impact of forest paths upon adjacent vegetation: Effects of the path surfacing material on the species composition and soil compaction. Biol. Conserv. 2004, 119, 405–419. [Google Scholar] [CrossRef]
- Galli, L.; Bonacchi, A.; Capurro, M.; Conti, I.; Crovetto, F.; Ferrari, C.; Menta, C. Assessment of the impact of trampling on soil Arthropoda in a Mediterranean habitat. Acta Soc. Zool. Bohem. 2015, 79, 193–198. [Google Scholar]
- Rao, P.; Hutyra, L.R.; Raciti, S.M.; Templer, P.H. Atmospheric nitrogen inputs and losses along an urbanization gradient from Boston to Harvard Forest, MA. Biogeochemistry 2013, 121, 229–245. [Google Scholar] [CrossRef]
- Savva, Y.; Szlavecz, K.; Pouyat, R.V.; Groffman, P.M.; Heisler, G. Effects of land use and vegetation cover on soil temperature in an urban ecosystem. Soil Sci. Soc. Am. J. 2010, 74, 469–480. [Google Scholar] [CrossRef]
- Ossola, A.; Livesley, S.J. Drivers of soil heterogeneity in the urban landscape. In Urban Landscape Ecology: Science, Policy and Practice; Francis, R.A., Millington, J.D.A., Chadwick, M.A., Eds.; Routledge: London, UK, 2016; pp. 19–41. [Google Scholar]
- Shaw, R.K.; Isleib, J.T. The case of the New York City Soil Survey Program, United States. In IUSS Working Group SUITMA: Soils within Cities—Global Approaches to Their Sustainable Management—Composition, Properties, and Functions of Soils of the Urban Environment; Levin, M.J., Kim, K.H.J., Morel, J.L., Burghardt, W., Charzynski, P., Shaw, R.K., Eds.; Schweizerbart: Stuttgart, Germany, 2017; pp. 107–113. [Google Scholar]
- Pouyat, R.V.; Setälä, H.; Szlavecz, K.; Yesilonis, I.D.; Cilliers, S.; Hornung, E.; Yarwood, S.; Kotze, D.J.; Dombos, M.; McGuire, M.P.; et al. Introducing GLUSEEN: A new open access and experimental network in urban soil ecology. J. Urban Ecol. 2017, 3, jux002. [Google Scholar] [CrossRef][Green Version]
- Heidt, V.; Neef, M. Benefits of urban green space for improving urban climate. In Ecology, Planning, and Management of Urban Forests; Carreiro, M.M., Song, Y.C., Wu, J., Eds.; Springer: New York, NY, USA, 2008; pp. 97–106. [Google Scholar] [CrossRef]
- Paoletti, M.G.; Bressan, M.; Edwards, C.A. Soil invertebrates as bioindicators of human disturbance. Crit. Rev. Plant Sci. 1996, 15, 21–62. [Google Scholar] [CrossRef]
- Blair, J.M.; Bohlen, P.J.; Freckman, D.W. Soil invertebrates as indicators of soil quality. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; SSSA Special Publication 49, SSSA: Madison, WI, USA, 1997; pp. 429–437. [Google Scholar]
- Van Straalen, N.M. Evaluation of bioindicator systems derived from soil arthropod communities. Appl. Soil Ecol. 1998, 9, 429–437. [Google Scholar] [CrossRef]
- Paoletti, M.G.; Osler, G.H.R.; Kinnear, A.; Black, D.G.; Thomson, L.J.; Tsitsilas, A.; D’Inca, A. Detritivores as indicators of landscape stress and soil degradation. Aust. J. Exp. Agric. 2007, 47, 412. [Google Scholar] [CrossRef]
- Yan, S.; Singh, A.N.; Fu, S.; Liao, C.; Wang, S.; Li, Y.; Cui, Y.; Hu, L. A soil fauna index for assessing soil quality. Soil Biol. Biochem. 2012, 47, 158–165. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S. Biological indices for soil quality evaluation: Perspectives and limitations. Land Degrad. Dev. 2016, 27, 14–25. [Google Scholar] [CrossRef]
- Seastedt, T.R. The role of microarthropods in decomposition and mineralization processes. Annu. Rev. Entomol. 1984, 29, 25–46. [Google Scholar] [CrossRef]
- Coleman, D.C.; Callaham, M.A.; Crossley, D.A., Jr. Fundamentals of Soil Ecology, 3rd ed.; Academic Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- McIntyre, N.E.; Rango, J.; Fagan, W.F.; Faeth, S.H. Ground arthropod community structure in a heterogeneous urban environment. Landsc. Urban Plan. 2001, 52, 257–274. [Google Scholar] [CrossRef]
- Santorufo, L.; Van Gestel, C.A.; Rocco, A.; Maisto, G. Soil invertebrates as bioindicators of urban soil quality. Environ. Pollut. 2012, 161, 57–63. [Google Scholar] [CrossRef]
- Parisi, V. The biological soil quality, a method based on microarthropods. Acta Nat. L’Ateneo Parm. 2001, 37, 97–106. (In Italian) [Google Scholar]
- Parisi, V.; Menta, C.; Gardi, C.; Jacomini, C.; Mozzanica, E. Microarthropod communities as a tool to assess soil quality and biodiversity: A New Approach in Italy. Agric. Ecosyst. Environ. 2005, 105, 323–333. [Google Scholar] [CrossRef]
- Menta, C.; Conti, F.D.; Pinto, S. Microarthropods biodiversity in natural, seminatural and cultivated soils—QBS-ar approach. Appl. Soil Ecol. 2018, 123, 740–743. [Google Scholar] [CrossRef]
- Menta, C.; Conti, F.D.; Pinto, S.; Bodini, A. Soil Biological Quality index (QBS-ar): 15 years of application at global scale. Ecol. Indic. 2018, 85, 773–780. [Google Scholar] [CrossRef]
- Menta, C.; Bonati, B.; Staffilani, F.; Conti, F.D. Agriculture management and soil fauna monitoring: The case of Emilia-Romagna region (Italy). Agric. Res. Technol. 2017, 4, 1–3. [Google Scholar] [CrossRef]
- Mantoni, C.; Pellegrini, M.; Dapporto, L.; Del Gallo, M.; Pace, L.; Silveri, D.; Fattorini, S. Comparison of soil biology quality in organically and conventionally managed agro-ecosystems using microarthropods. Agriculture 2021, 11, 1022. [Google Scholar] [CrossRef]
- Gallese, F.; Gismero-Rodriguez, L.; Govednik, A.; Giagnoni, L.; Lumini, E.; Suhadolc, M.; Maienza, A. Soil microarthropods as tools for monitoring soil quality: The qbs-ar index in three european agroecosystems. Agriculture 2025, 15, 89. [Google Scholar] [CrossRef]
- Naglič, V.; Šibanc, N.; Grebenc, T.; Bertoncelj, I. Soil mesofauna diversity in agricultural systems of Slovenia using the QBS index and its modifications. Acta Biol. Slov. 2025, 68, 104–117. [Google Scholar] [CrossRef]
- Blasi, S.; Menta, C.; Balducci, L.; Conti, F.D.; Petrini, E.; Piovesan, G. Soil microarthropod communities from Mediterranean forest ecosystems in Central Italy under different disturbances. Environ. Monit. Assess. 2013, 187, 1637–1655. [Google Scholar] [CrossRef]
- Galli, L.; Capurro, M.; Menta, C.; Rellini, I. Is the QBS-ar index a good tool to detect the soil quality in Mediterranean areas? A cork tree Quercus suber L. (Fagaceae) wood as a case of study. Ital. J. Zool. 2014, 81, 126–135. [Google Scholar] [CrossRef]
- Menta, C.; Leoni, A.; Gardi, C.; Conti, F.D. Are grasslands important habitats for soil microarthropod conservation? Biodivers. Conserv. 2011, 20, 1073–1087. [Google Scholar] [CrossRef]
- Fusco, T.; Fortini, L.; Casale, F.; Jacomini, C.; Di Giulio, A. Assessing soil quality of Italian Western Alps protected areas by QBS-ar: Impact of management and habitat type on soil microarthropods. Environ. Monit. Assess. 2023, 195, 1287. [Google Scholar] [CrossRef]
- Çakır, M.; Akburak, S.; Makineci, E.; Bolat, F. Recovery of soil biological quality (QBS-ar) and soil microarthropod abundance following a prescribed fire in the Quercus frainetto forest. Appl. Soil Ecol. 2023, 184, 104768. [Google Scholar] [CrossRef]
- Madej, G.; Barkzyk, G.; Gdawiec, M. Evaluation of soil biological quality index (QBS-ar): Its sensitivity and usefulness in the post-mining chronosequence—Preliminary research. Pol. J. Environ. Stud. 2011, 20, 1367–1372. [Google Scholar]
- Menta, C.; Conti, F.D.; Pinto, S.; Leoni, A.; Lozano-Fondón, C. Monitoring soil restoration in an open-pit mine in northern Italy. Appl. Soil Ecol. 2014, 83, 22–29. [Google Scholar] [CrossRef]
- Maisto, G.; Santorufo, L.; Milano, V.; Arena, C. Relationships between Quercus ilex L. litter characteristics and soil microarthropod community in an urban environment at different climatic conditions. Appl. Soil Ecol. 2016, 99, 98–109. [Google Scholar] [CrossRef]
- Ungaro, F.; Maienza, A.; Ugolini, F.; Lanini, G.M.; Baronti, S.; Calzolari, C. Assessment of joint soil ecosystem services supply in urban green spaces: A case study in Northern Italy. Urban For. Urban Green. 2022, 67, 127455. [Google Scholar] [CrossRef]
- Tóth, Z.; Dombos, M.; Hornung, E. Urban soil quality deteriorates even with low heavy metal levels: An arthropod-based multi-indices approach. Ecol. Appl. 2023, 33, e2848. [Google Scholar] [CrossRef] [PubMed]
- Celesti-Grapow, L.; Fanelli, G. The vanishing landscape of the Campagna Romana. Landsc. Urban Plan. 1993, 24, 69–76. [Google Scholar] [CrossRef]
- Zapparoli, M. Urban development and insect biodiversity of the Rome area, Italy. Landsc. Urban Plan. 1997, 38, 77–86. [Google Scholar] [CrossRef]
- Di Pietro, S.; Mantoni, C.; Fattorini, S. Influence of urbanization on the avian species-area relationship: Insights from the breeding birds of Rome. Urban Ecosyst. 2021, 24, 779–788. [Google Scholar] [CrossRef]
- Vigna Taglianti, A. Storia dell’entomologia romana. In Atti XII Congresso Nazionale Italiano di Entomologia; Accademia Nazionale Italiana di Entomologia: Roma, Italy, 1980; Volume 1, pp. 5–66. [Google Scholar]
- Zapparoli, M. Aspetti del popolamento degli invertebrati. In L’ecosistema Roma, ambiente e territorio, conoscenze attuali e prospettive per il Duemila; Cignini, B., Massari, G., Pignatti, S., Eds.; Fratelli Palombi Editori: Roma, Italy, 1995; pp. 97–105. [Google Scholar]
- Fusco, T.; Fattorini, S.; Fortini, L.; Ruzzier, E.; Di Giulio, A. Ground spiders (Chelicerata, Araneae) of an urban green space: Intensive sampling in a protected area of Rome (Italy) reveals a high diversity and new records to the Italian territory. Biodivers. Data J. 2024, 12, e122896. [Google Scholar] [CrossRef]
- Trucchi, E.; Pitzalis, M.; Zapparoli, M.; Bologna, M. Short-term effects of canopy and surface fire on centipede (Chilopoda) communities in a semi-natural Mediterranean forest. Entomol. Fenn. 2009, 20, 129–138. [Google Scholar] [CrossRef][Green Version]
- Baini, F.; Pitzalis, M.; Taiti, S.; Vigna Taglianti, A.; Zapparoli, M.; Bologna, M.A. Effects of reforestation with Quercus species on selected arthropod assemblages (Isopoda Oniscidea, Chilopoda, Coleoptera Carabidae) in a Mediterranean area. Forest Ecol. Manag. 2012, 286, 183–191. [Google Scholar] [CrossRef]
- Baini, F.; Bologna, M.A.; Pitzalis, M.; Taiti, S.; Vigna Taglianti, A.; Zapparoli, M. Assessing patterns of co-occurrence and nestedness of arthropod assemblages in an artificial–natural Mediterranean forest mosaic (Isopoda Oniscidea, Coleoptera Carabidae). Rend. Lincei Sci. Fis. Nat. 2014, 25, 339–350. [Google Scholar] [CrossRef]
- Baini, F.; Zapparoli, M. Centipedes (Chilopoda) in urban forest habitats: The case of the metropolitan city of Rome area (Central Italy). Rend. Lincei Sci. Fis. Nat. 2022, 33, 591–601. [Google Scholar] [CrossRef]
- Pitzalis, M.; Fattorini, S.; Trucchi, E.; Bologna, M.A. Comparative analysis of species diversity of Isopoda Oniscidea and Collembola communities in burnt and unburnt habitats in central Italy. Ital. J. Zool. 2005, 72, 127–140. [Google Scholar] [CrossRef][Green Version]
- Fattorini, S.; Mantoni, C.; Bergamaschi, D.; Fortini, L.; Sánchez, F.J.; Di Biase, L.; Di Giulio, A. Activity density of carabid beetles along an urbanization gradient. Acta Zool. Acad. Sci. Hungaricae 2020, 66 (Suppl.), 21–36. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Mazziotta, A.; Piattella, E. Changes in food resources and conservation of scarab beetles: From sheep to dog dung in a green urban area of Rome (Coleoptera, Scarabaeoidea). Biol. Conserv. 2004, 123, 547–556. [Google Scholar] [CrossRef]
- Fattorini, S. Insect extinction by urbanization: A long-term study in Rome. Biol. Conserv. 2010, 144, 370–375. [Google Scholar] [CrossRef]
- Fattorini, S. Insect rarity, extinction and conservation in urban Rome (Italy): A 120-year-long study of tenebrionid beetles. Insect Conserv. Divers. 2011, 4, 307–315. [Google Scholar] [CrossRef]
- Fattorini, S. Species ecological preferences predict extinction risk in urban tenebrionid beetle guilds. Anim. Biol. 2012, 63, 93–106. [Google Scholar] [CrossRef]
- Fattorini, S. Faunistic knowledge and insect species loss in an urban area: The tenebrionid beetles of Rome. J. Insect Conserv. 2013, 17, 637–643. [Google Scholar] [CrossRef]
- Fattorini, S. Urban biodiversity hotspots are not related to the structure of green spaces: A case study of tenebrionid beetles from Rome, Italy. Urban Ecosyst. 2014, 17, 1033–1045. [Google Scholar] [CrossRef]
- Blasi, C.; Carranza, M.L.; Filesi, L.; Tilia, A.; Acosta, A. Relation between climate and vegetation along a Mediterranean-Temperate boundary in central Italy. Global Ecol. Biogeogr. 1999, 8, 17–27. [Google Scholar] [CrossRef]
- World Meteorological Organization. Available online: https://worldweather.wmo.int/en/dataguide.html (accessed on 12 November 2024).
- Pica, A.; Vergari, F.; Fredi, P.; Del Monte, M. The Aeterna Urbs geomorphological heritage (Rome, Italy). Geoheritage 2016, 8, 31–42. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Capotorti, G.; Del Vico, E.; Lattanzi, E.; Tilia, A.; Blasi, C. The vascular flora of Rome. Plant Biosyst. 2013, 147, 1059–1087. [Google Scholar] [CrossRef]
- D’Avino, L.; Gambelli, M.A.; Bigiotti, G.; Vitali, F.; Tondini, E.; L’Abate, G.; Jacomini, C.; Cassi, F.; Menta, C.; QBS-ar SISS Working Group. QBS-ar and QBS-ar_BF index toolbox for biodiversity assessment of microarthropods community in soil (3.0). Zenodo 2024. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Carter, M.R.; Angers, D.A.; Monreal, C.M.; Ellert, B.H. Towards a minimum data set to assess soil organic matter quality in agricultural soils. Can. J. Soil Sci. 1994, 74, 367–385. [Google Scholar] [CrossRef]
- Tóth, Z.; Hornung, E. Taxonomic and functional response of millipedes (Diplopoda) to urban soil disturbance in a metropolitan area. Insects 2019, 11, 25. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Yeates, G.W.; Anderson, J.M. Patterns and determinants of soil biological diversity. In Biological Diversity and Function in Soils; Bardgett, R.D., Usher, M., Hopkins, D., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 100–118. [Google Scholar] [CrossRef]
- Liker, A.; Papp, Z.; Bókony, V.; Lendvai, A.Z. Lean birds in the city: Body size and condition of house sparrows along the urbanization gradient. J. Anim. Ecol. 2008, 77, 789–795. [Google Scholar] [CrossRef]
- Didan, K. MODIS/Terra Vegetation Indices 16-Day L3 Global 250 m SIN Grid V061. NASA EOSDIS Land Process. DAAC 2021. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, S.; Zhang, L.; Liu, S. Remotely Sensed assessment of urbanization effects on vegetation phenology in China’s 32 major cities. Remote Sens. Environ. 2016, 176, 272–281. [Google Scholar] [CrossRef]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer: Berlin/Heidelberg, Germany, 2012; p. 426. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Petrén, H.; Köllner, T.G.; Junker, R.R. Quantifying chemodiversity considering biochemical and structural properties of compounds with the R package CHEMODIV. New Phytol. 2023, 237, 2478–2492. [Google Scholar]
- Fattorini, S. The role of vegetation in elevational diversity patterns of tenebrionid beetles in Central Italy. Diversity 2024, 16, 110. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chao, A. Distance-based functional diversity measures and their decomposition: A framework based on Hill numbers. PLoS ONE 2014, 9, e100014. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 23 March 2025).
- Alberdi, A.; Gilbert, M.T.P. hilldiv: An R package for the integral analysis of diversity based on Hill numbers. BioRxiv 2019. [Google Scholar] [CrossRef]
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. MASS: Support Functions and Datasets for Venables and Ripley’s MASS. R Package Version 7.3-51.5, 2019. Available online: https://cran.r-project.org/web/packages/MASS/ (accessed on 20 December 2024).
- Breiman, L. Random forests. Mach. Learn 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.46.0. 2022. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 20 December 2024).
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer-Verlag: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.6., 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 20 December 2024).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.3-5, 2016. Available online: https://cran.r-project.org/package=vegan (accessed on 20 December 2024).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2022; Available online: http://www.posit.co/ (accessed on 20 December 2024).
- Pouyat, R.V.; Groffman, P.M.; Yesilonis, I.; Hernandez, L. Soil carbon pools and fluxes in urban ecosystems. Environ. Pollut. 2002, 116, S107–S118. [Google Scholar] [CrossRef]
- Puskás, I.; Farsang, A. Diagnostic indicators for characterizing urban soils of Szeged, Hungary. Geoderma 2009, 148, 267–281. [Google Scholar] [CrossRef]
- Pickett, S.T.; Cadenasso, M.L.; Grove, J.M.; Boone, C.G.; Groffman, P.M.; Irwin, E.; Warren, P. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Manag. 2011, 92, 331–362. [Google Scholar] [CrossRef]
- Zhang, P.; Dong, Y.; Guo, Y.; Wang, C.; Wang, G.; Ma, Z.; Wang, W. Urban forest soil is becoming alkaline under rapid urbanization: A case study of Changchun, northeast China. Catena 2023, 224, 106993. [Google Scholar] [CrossRef]
- Roeland, S.; Moretti, M.; Amorim, J.H.; Branquinho, C.; Fares, S.; Morelli, F.; Niinemets, Ü.; Paoletti, E.; Pinho, P.; Sgrigna, G.; et al. Towards an integrative approach to evaluate the environmental ecosystem services provided by urban forest. J. For. Res. 2019, 30, 1981–1996. [Google Scholar] [CrossRef]
- Jim, C.Y. Urban soil characteristics and limitations for landscape planting in Hong Kong. Landsc. Urban Plan. 1998, 40, 235–249. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Szlavecz, K.; Yesilonis, I.D.; Groffman, P.M.; Schwarz, K. Chemical, physical, and biological characteristics of urban soils. Urban Ecosyst. Ecol. 2010, 55, 119–152. [Google Scholar] [CrossRef]
- Rota, E.; Caruso, T.; Migliorini, M.; Monaci, F.; Agamennone, V.; Biagini, G.; Bargagli, R. Diversity and abundance of soil arthropods in urban and suburban holm oak stands. Urban Ecosyst. 2015, 18, 715–728. [Google Scholar] [CrossRef]
- Malloch, B.; Tatsumi, S.; Seibold, S.; Cadotte, M.W.; MacIvor, J.S. Urbanization and plant invasion alter the structure of litter microarthropod communities. J. Anim. Ecol. 2020, 89, 2496–2507. [Google Scholar] [CrossRef]
- Henneron, L.; Aubert, M.; Archaux, F.; Bureau, F.; Dumas, Y.; Ningre, F.; Richter, C.; Balandier, P.; Chauvat, M. Forest plant community as a driver of soil biodiversity: Experimental evidence from collembolan assemblages through large-scale and long-term removal of oak canopy trees Quercus petraea. Oikos 2016, 126, 420–434. [Google Scholar] [CrossRef]
- Milano, V.; Cortet, J.; Baldantoni, D.; Bellino, A.; Dubs, F.; Nahmani, J.; Maisto, G. Collembolan biodiversity in Mediterranean urban parks: Impact of history, urbanization, management and soil characteristics. Appl. Soil Ecol. 2017, 119, 428–437. [Google Scholar] [CrossRef]
- David, J.F.; Ponge, J.F.; Arpin, P.; Vannier, G. Reactions of the macrofauna of a forest mull to experimental perturbations of litter supply. Oikos 1991, 61, 316–326. [Google Scholar] [CrossRef][Green Version]
- Uno, S.; Cotton, J.; Philpott, S.M. Diversity, abundance, and species composition of ants in urban green spaces. Urban Ecosyst. 2010, 13, 425–441. [Google Scholar] [CrossRef]
- Gardiner, M.M.; Burkman, C.E.; Prajzner, S.P. The value of urban vacant land to support arthropod biodiversity and ecosystem services. Environ. Entomol. 2013, 42, 1123–1136. [Google Scholar] [CrossRef]
- Burkman, C.E.; Gardiner, M.M. Spider assemblages within greenspaces of a deindustrialized urban landscape. Urban Ecosyst. 2015, 18, 793–818. [Google Scholar] [CrossRef]
- Ferreira, L.M.; De Souza, L.C.; Conti, D.D.M.; Capellani Quaresma, C.; Reis Tavares, A.; Gonçalves da Silva, K.; De Camargo, P.B. Soil biodiversity in urban forests as a consequence of litterfall management: Implications for São Paulo’s ecosystem services. Sustainability 2018, 10, 684. [Google Scholar] [CrossRef]
- Koricho, H.H.; Seboka, A.D.; Fufa, F.; Gebreyesus, T.; Song, S. Study on the ecosystem services of urban forests: Implications for climate change mitigation in the case of Adama City of Oromiya Regional State, Ethiopia. Urban Ecosyst. 2022, 25, 575–584. [Google Scholar] [CrossRef]
- Byrne, L.B.; Bruns, M.A. The effects of lawn management on soil microarthropods. J. Agric. Urban Entomol. 2004, 21, 150–156. [Google Scholar]
- Lemanski, K.; Scheu, S. The influence of fertilizer addition, cutting frequency, and herbicide application on soil organisms in grassland. Biol. Fertil. Soils 2015, 51, 197–205. [Google Scholar] [CrossRef]
- Proske, A.; Lokatis, S.; Rolff, J. Impact of mowing frequency on arthropod abundance and diversity in urban habitats: A meta-analysis. Urban For. Urban Green. 2022, 76, 127714. [Google Scholar] [CrossRef]
- Liu, R.; Meller, R.; Steinberger, Y. Changes in a soil microarthropod community in the vicinity of dominant tree species under trampling management at the Safari Zoological Center, Israel. Acarologia 2019, 59, 33–45. [Google Scholar] [CrossRef]
- Moriyama, M.; Numata, H. Urban soil compaction reduces cicada diversity. Zool. Lett. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Menta, C.; Remelli, S. Soil health and arthropods: From complex system to worthwhile investigation. Insects 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Gebrewahid, Y.; Teka, K.; Gebre-Egziabhier, T.B.; Tewolde-Berhan, S.; Birhane, E.; Eyasu, G.; Meresa, E. Dispersed trees on smallholder farms enhance soil fertility in semi-arid Ethiopia. Ecol. Process. 2019, 8, 1–8. [Google Scholar] [CrossRef]
- Christian, E.; Szeptycki, A. Distribution of Protura along an urban gradient in Vienna. Pedobiologia 2004, 48, 445–452. [Google Scholar] [CrossRef]
- Eitminaviciute, I. Microarthropod communities in anthropogenic urban soils. 1. Structure of microarthropod complexes in soils of roadside lawns. Entomol. Rev. 2006, 86, 128–135. [Google Scholar] [CrossRef]
- Magro, S.; Gutiérrez-López, M.; Casado, M.A.; Jiménez, M.D.; Trigo, D.; Mola, I.; Balaguer, L. Soil functionality at the roadside: Zooming in on a microarthropod community in an anthropogenic soil. Ecol. Eng. 2013, 60, 81–87. [Google Scholar] [CrossRef]
- Santorufo, L.; Van Gestel, C.A.; Maisto, G. Sampling season affects conclusions on soil arthropod community structure responses to metal pollution in Mediterranean urban soils. Geoderma 2014, 226, 47–53. [Google Scholar] [CrossRef]
- Maisto, G.; Milano, V.; Santorufo, L. Relationships among site characteristics, taxonomical structure, and functional trait distribution of arthropods in forest, urban, and agricultural soils of Southern Italy. Ecol. Res. 2017, 32, 511–521. [Google Scholar] [CrossRef]

| Environmental Variables | Urban Unmanaged (n = 4) | Urban Managed (n = 4) | Urban Forest (n = 4) | Reference (n = 4) |
|---|---|---|---|---|
| Soil pH | 7.95 ± 0.12 a | 7.34 ± 0.05 b | 6.95 ± 0.09 c | 6.61 ± 0.10 c |
| Conductivity (dS/m) | 1.32 ± 0.54 a | 1.48 ± 0.31 a | 1.01 ± 0.18 a | 1.30 ± 0.28 a |
| Organic matter (%) | 4.47 ± 0.99 a | 6.47 ± 0.65 a | 12.47 ± 0.98 b | 11.40 ± 0.73 b |
| SOC (%) | 2.60 ± 0.57 a | 3.75 ± 0.38 a | 7.23 ± 0.57 b | 6.45 ± 0.30 b |
| Total N (%) | 0.31 ± 0.08 a | 0.36 ± 0.03 a | 0.70 ± 0.13 b | 0.49 ± 0.05 ab |
| C/N ratio | 8.90 ± 1.44 a | 10.4 ± 0.40 ab | 10.91 ± 1.12 ab | 13.37 ± 1.01 b |
| P2O5 (mg/kg) | 103.70 ± 40.09 a | 154.8 ± 33.09 a | 151.42 ± 21.1 a | 54.60 ± 22.62 a |
| CaO (mg/kg) | 7903.50 ± 1016.57 a | 9232.25 ± 975.36 ab | 11,835.50 ± 763.9 b | 6861.00 ± 841.36 a |
| MgO (mg/kg) | 563.25 ± 62.81 a | 552.00 ± 70.58 a | 719.50 ± 109.33 a | 656.00 ± 92.68 a |
| Na (%) | 2.13 ± 0.21 a | 2.17 ± 0.23 a | 0.67 ± 0.15 b | 0.63 ± 0.09 b |
| CEC (mEq/100 g) | 19.30 ± 1.85 a | 28.80 ± 3.33 ac | 49.90 ± 4.92 b | 34.88 ± 0.99 c |
| Compaction (kg/cm2) | 2.86 ± 0.24 ab | 4.49 ± 0.53 a | 2.03 ± 0.16 bc | 1.94 ± 0.10 c |
| Moisture (%) | 20.40 ± 0.65 a | 21.55 ± 0.85 a | 22.83 ± 0.74 ab | 24.17 ± 0.10 b |
| Soil temperature (°C) | 21.04 ± 0.45 ab | 21.31 ± 0.54 a | 19.61 ± 0.19 b | 17.90 ± 0.35 c |
| Surface temperature (°C) | 22.83 ± 1.12 ab | 25.30 ± 0.87 a | 21.54 ± 0.33 b | 20.75 ± 0.43 b |
| UI | 1.83 ± 0.76 a | 1.80 ± 0.66 a | −1.41 ± 0.42 b | −2.22 ± 0.09 b |
| EVI | 0.28 ± 0.03 a | 0.29 ± 0.04 a | 0.51 ± 0.04 b | 0.62 ± 0.04 b |
| Biological Form | Label | EMI | Urban Unmanaged (n = 4) | Urban Managed (n = 4) | Urban Forest (n = 4) | Reference (n = 4) |
|---|---|---|---|---|---|---|
| Acari | ACA | 20 | 18,658.3 ± 2367.1 | 15,900 ± 1645.3 | 25,125 ± 3514.7 | 20,083.3 ± 1309.0 |
| Araneae | ARA | 1 | 41.7 ± 25.0 | 0 | 75 ± 43.8 | 33.3 ± 33.3 |
| Opiliones * | 10 * | 8.3 ± 8.3 | 0 | 0 | 0 | |
| Pseudoscorpiones | PSEU | 10 | 33.3 ± 33.3 | 0 | 100 ± 49.1 | 58.3 ± 21.0 |
| Palpigrada * | 20 * | 0 | 0 | 41.7 ± 41.7 | 0 | |
| Isopoda | ISO | 10 | 266.7 ± 52.7 | 8.3 ± 8.3 | 975.0 ± 107.5 | 558.3 ± 15.9 |
| Symphyla | SYMPH | 20 | 150.0 ± 61.6 | 216.7 ± 16.7 | 608.3 ± 169.1 | 558.3 ± 45.9 |
| Diplopoda | DIPLO | 10 | 258.3 ± 86.5 | 66.7 ± 27.2 | 1175.0 ± 127.2 | 925.0 ± 207.4 |
| Diplopoda Polyxenida | POLYX | 20 | 0 | 16.7 ± 16.7 | 200 ± 75.8 | 241.7 ± 25.0 |
| Chilopoda | CHILO | 10 | 50.0 ± 16.7 | 75.0 ± 43.8 | 25.0 ± 15.7 | 33.3 ± 33.3 |
| Chilopoda Geophilomorpha | GEOPH | 20 | 41.7 ± 41.7 | 16.7 ± 16.7 | 158.3 ± 53.4 | 191.7 ± 28.5 |
| Pauropoda | PAURO | 20 | 0 | 0 | 158.3 ± 39.4 | 158.3 ± 67.2 |
| Collembola epiedaphic | COLL_EPI | 1 | 391.7 ± 123.5 | 150.0 ± 95.7 | 225.0 ± 142.3 | 33.3 ± 33.3 |
| COLL_EPI | 2 | 1258.3± 622.2 | 1150.0 ± 183.3 | 658.3 ± 217.0 | 166.7 ± 88.2 | |
| COLL_EPI | 4 | 3716.7± 864.7 | 2525.0 ± 291.3 | 3516.7 ± 474.8 | 2616.7 ± 391.9 | |
| Collembola emiedaphic | COLL_EMIED | 6 | 3866.7 ± 524.2 | 2075.0 ± 276.3 | 4075.0 ± 512.9 | 2725.0 ± 653.8 |
| COLL_EMIED | 8 | 1058.3 ± 234.3 | 716.7 ± 161.9 | 2258.3 ± 612.3 | 1900.0 ± 407.8 | |
| COLL_EMIED | 10 | 2325.0 ± 330.1 | 1616.7 ± 427.2 | 2966.7 ± 345.1 | 6941.7 ± 783.4 | |
| Collembola euedaphic | COLL_EUED | 20 | 0 | 0 | 2350.0 ± 474.8 | 5400.0 ± 928.3 |
| Diplura | DIPLUR | 20 | 100.0 ± 33.3 | 108.3 ± 45.9 | 158.3 ± 41.7 | 283.3 ± 44.1 |
| Protura | PROT | 20 | 25.0 ± 15.9 | 25.0 ± 15.9 | 275.0 ± 98.5 | 275.0 ± 36.7 |
| Coleoptera epigeic | COLEO_EPI | 1 | 200.0 ± 60.9 | 58.3 ± 8.3 | 91.7 ± 47.9 | 58.3 ± 39.4 |
| COLEO_EPI | 5 | 41.7 ± 25.0 | 0 | 166.7 ± 36.0 | 58.3 ± 21.0 | |
| Coleoptera emiedaphic | COLEO_EMIED | 10 | 58.3 ± 58.3 | 0 | 25.0 ± 16.0 | 66.7 ± 23.6 |
| Coleoptera larvae | COLEO_L | 10 | 391.7 ± 96.6 | 166.7 ± 83.9 | 316.7 ± 61.6 | 300.0 ± 49.1 |
| Diptera * | 1 * | 16.7 ± 16.7 | 16.7 ± 16.7 | 75.0 ± 75 | 25.0 ± 25.0 | |
| Diptera larvae | DIPT_L | 10 | 216.7 ± 91.8 | 0 | 525.0 ± 153.0 | 375.0 ± 103.1 |
| Hymenoptera * | 1 * | 16.7 ± 16.7 | 0 | 33.3 ± 13.6 | 16.7 ± 9.6 | |
| Hymenoptera Formicidae | FORM | 5 | 866.7 ± 201.8 | 1041.7 ± 535.8 | 1116.7 ± 325.6 | 1241.7 ± 230.7 |
| Hymenoptera larvae * | 1 * | 41.7 ± 25 | 16.7 ± 16.7 | 0 | 0 | |
| Hemiptera | HEMI | 1 | 191.7 ± 87.5 | 250.0 ± 102.3 | 91.7 ± 43.8 | 50.0 ± 39.7 |
| Psocoptera | PSOC | 1 | 33.33 ± 33.3 | 58.3 ± 39.4 | 250.0 ± 115.9 | 208.3 ± 75.0 |
| Thysanoptera | THYS | 1 | 100.0 ± 43 | 66.7 ± 47.1 | 200.0 ± 49.1 | 175.0 ± 62.9 |
| Community metrics | ||||||
| Total density | 34,425.0 ± 1702.4 ab | 26,341.7 ± 2515.0 a | 48,016.7 ± 5591.8 b | 45,758.3 ± 2955.3 b | ||
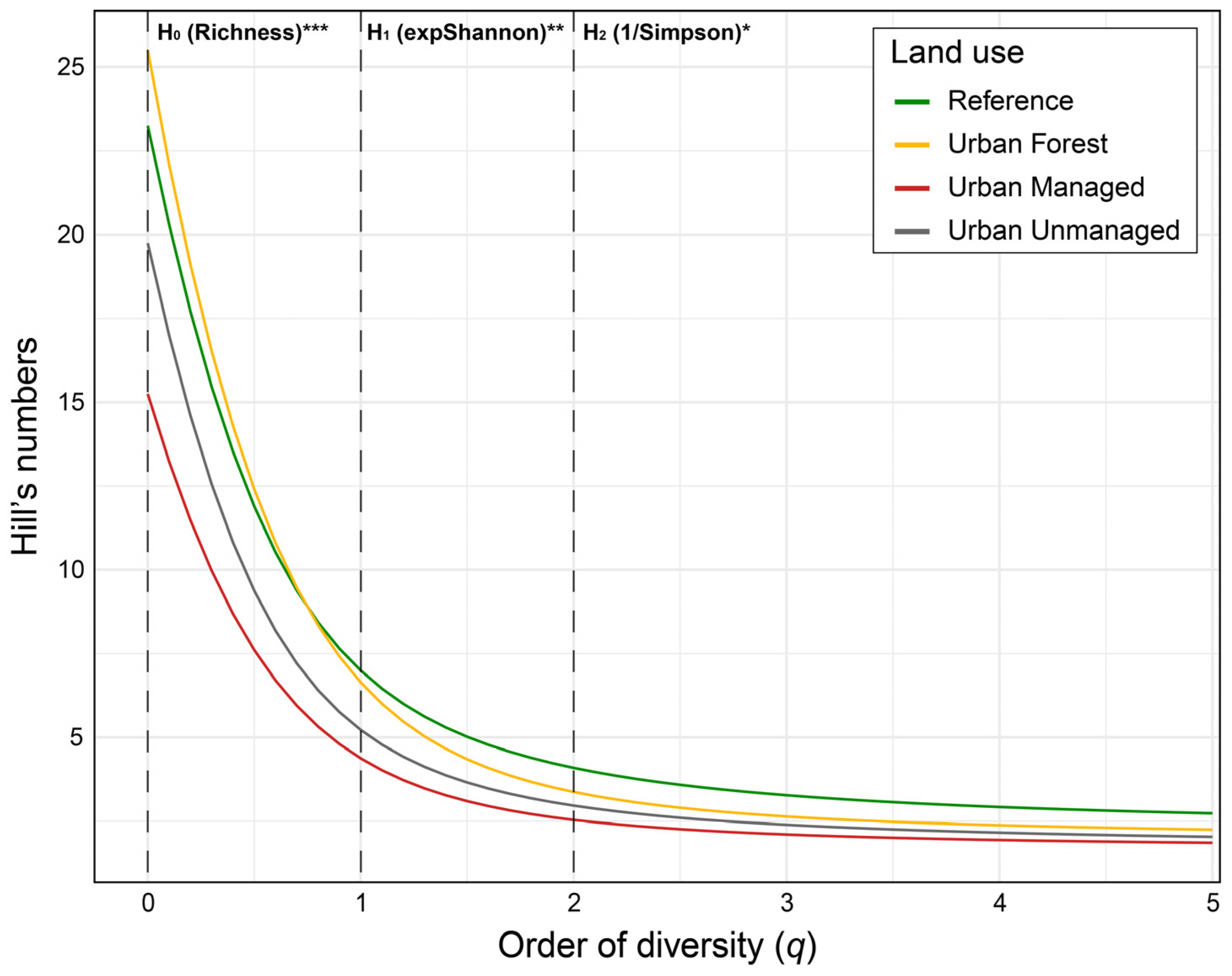
| H0: BFs richness (q = 0) | 19.75 ± 0.75 a | 15.25 ± 0.75 b | 25.5 ± 1.3 c | 23.25 ± 0.5 bc | ||
| H1: exp(Shannon) (q = 1) | 5.32 ± 0.62 ab | 4.39 ± 0.20 a | 6.66 ± 0.26 bc | 7.01 ± 0.26 c | ||
| H2: 1/Simpson (q = 2) | 3.15 ± 0.47 ab | 2.57 ± 0.16 a | 3.41 ± 0.22 ab | 4.14 ± 0.24 b | ||
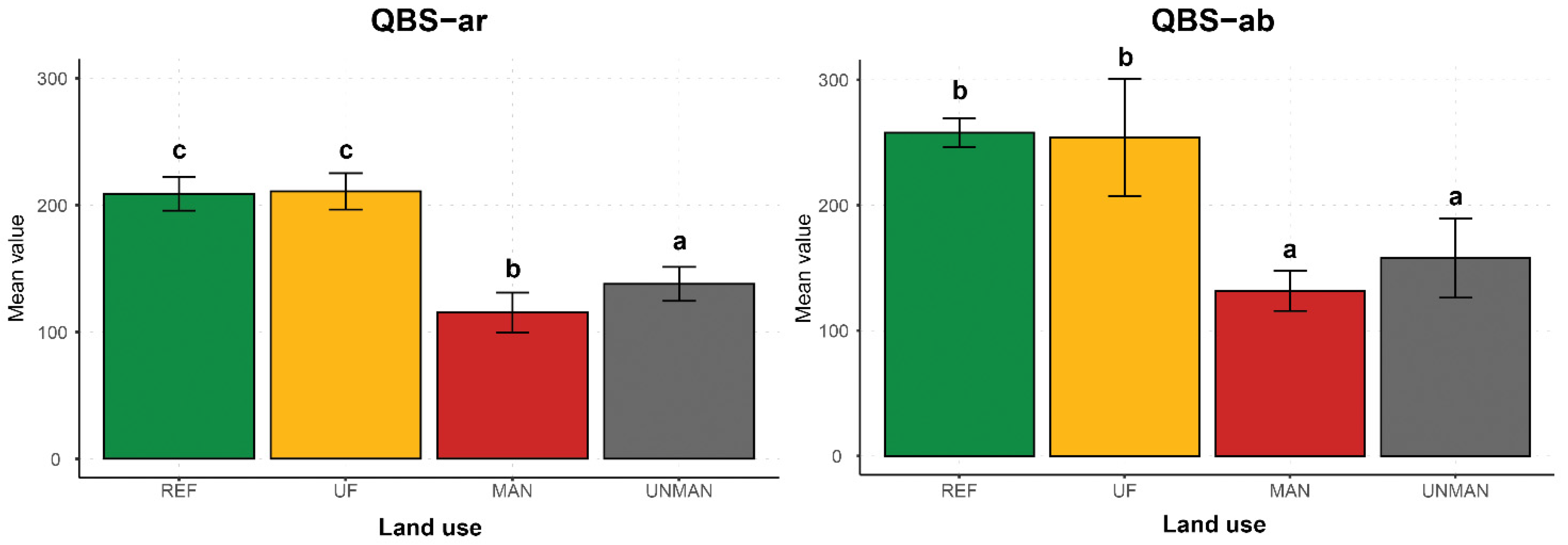
| QBS-ar | 137.75 ± 4.21 a | 115.25 ± 4.92 b | 210.75 ± 4.50 c | 208.75 ± 4.15 c | ||
| QBS-ab | 157.92 ± 9.95 a | 131.715 ± 5.03 a | 254.01 ± 14.68 b | 257.82 ± 3.64 b |
| Final Model | Estimate | SE | t-Value | p-Value | R2 |
|---|---|---|---|---|---|
| H0 (richness) | 0.87 | ||||
| Compaction | −2.649 | 0.663 | −3.993 | 0.001 | |
| CEC | 1.674 | 0.663 | 2.523 | 0.03 | |
| H1 (expShannon) | 0.66 | ||||
| EVI | 1.038 | 0.198 | 5.254 | 0.0001 | |
| H2 (1/Simpson) | 0.49 | ||||
| EVI | 0.548 | 0.151 | 3.630 | <0.01 | |
| Density | 0.57 | ||||
| Compaction | −4499.000 | 1804.000 | 2.494 | 0.03 | |
| CEC | 4967.000 | 2266.000 | 2.192 | 0.05 | |
| QBS-ar | 0.82 | ||||
| Compaction | −0.171 | 0.034 | −4.962 | <0.001 | |
| pH | −0.121 | 0.036 | −3.335 | <0.01 | |
| QBS-ab | 0.88 | ||||
| Compaction | −0.020 | 0.008 | −2.442 | 0.03 | |
| CEC | 0.021 | 0.007 | 3.107 | <0.01 | |
| Soil temperature | −0.027 | 0.008 | −3.383 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardini, P.; Fattorini, S.; Audisio, P.; Sabatelli, S. Influence of Different Land-Use Types on Soil Arthropod Communities in an Urban Area: A Case Study from Rome (Italy). Land 2025, 14, 714. https://doi.org/10.3390/land14040714
Gardini P, Fattorini S, Audisio P, Sabatelli S. Influence of Different Land-Use Types on Soil Arthropod Communities in an Urban Area: A Case Study from Rome (Italy). Land. 2025; 14(4):714. https://doi.org/10.3390/land14040714
Chicago/Turabian StyleGardini, Pietro, Simone Fattorini, Paolo Audisio, and Simone Sabatelli. 2025. "Influence of Different Land-Use Types on Soil Arthropod Communities in an Urban Area: A Case Study from Rome (Italy)" Land 14, no. 4: 714. https://doi.org/10.3390/land14040714
APA StyleGardini, P., Fattorini, S., Audisio, P., & Sabatelli, S. (2025). Influence of Different Land-Use Types on Soil Arthropod Communities in an Urban Area: A Case Study from Rome (Italy). Land, 14(4), 714. https://doi.org/10.3390/land14040714








