Soil Organic Carbon Turnover Following Afforestation of a Savanna Revealed by Particle-Size Fractionation and Natural 13C Measurements in Ivory Coast
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site and Sampling Process
2.2. Particle-Size Fractionation
2.3. Organic Carbon and Stable Isotopes
2.4. Statistical Analysis
3. Results
3.1. SOM Content
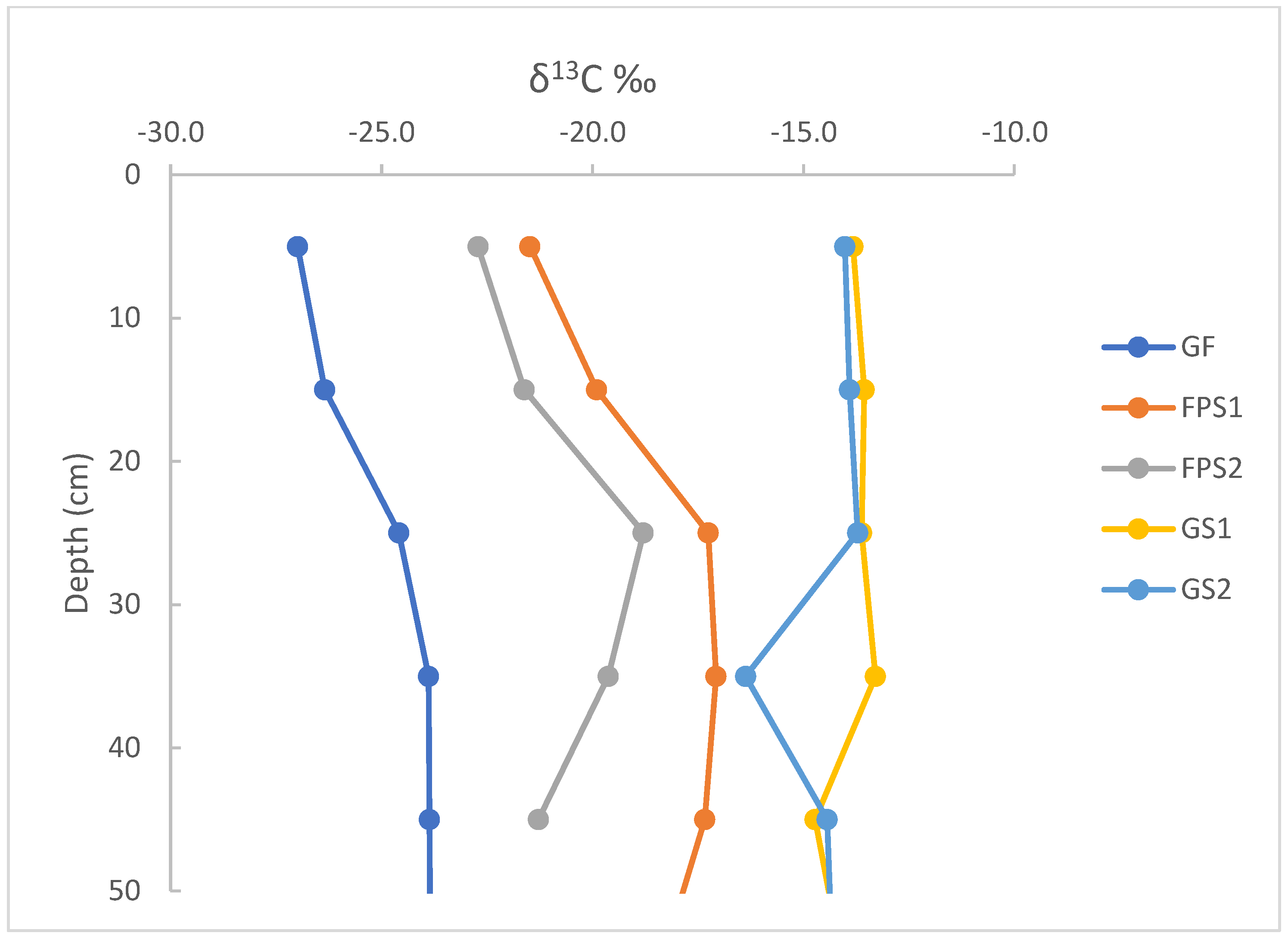
3.2. δ13 C Values of Litter and SOM
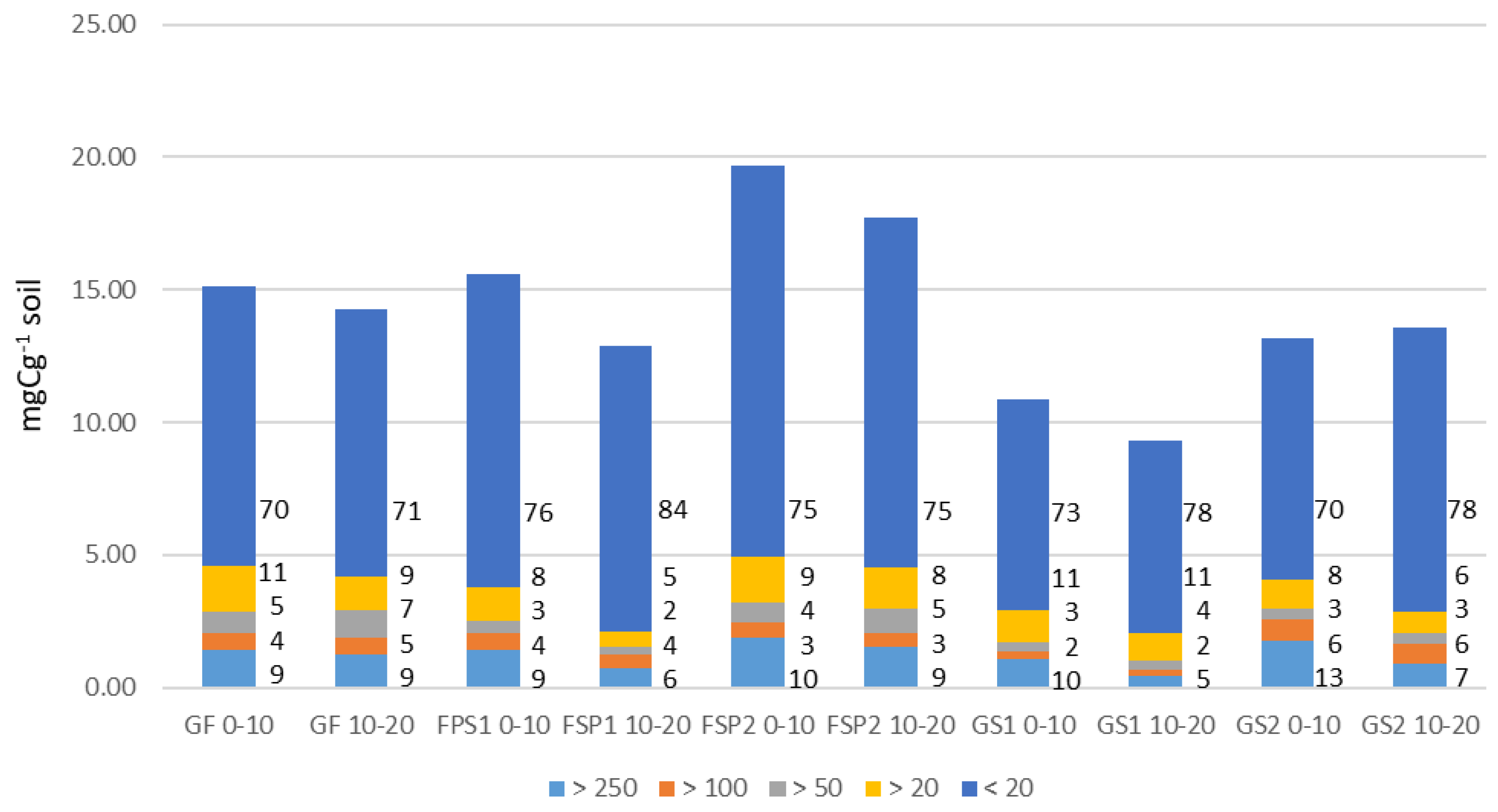
3.3. Distribution of Total C in SOM Fractions
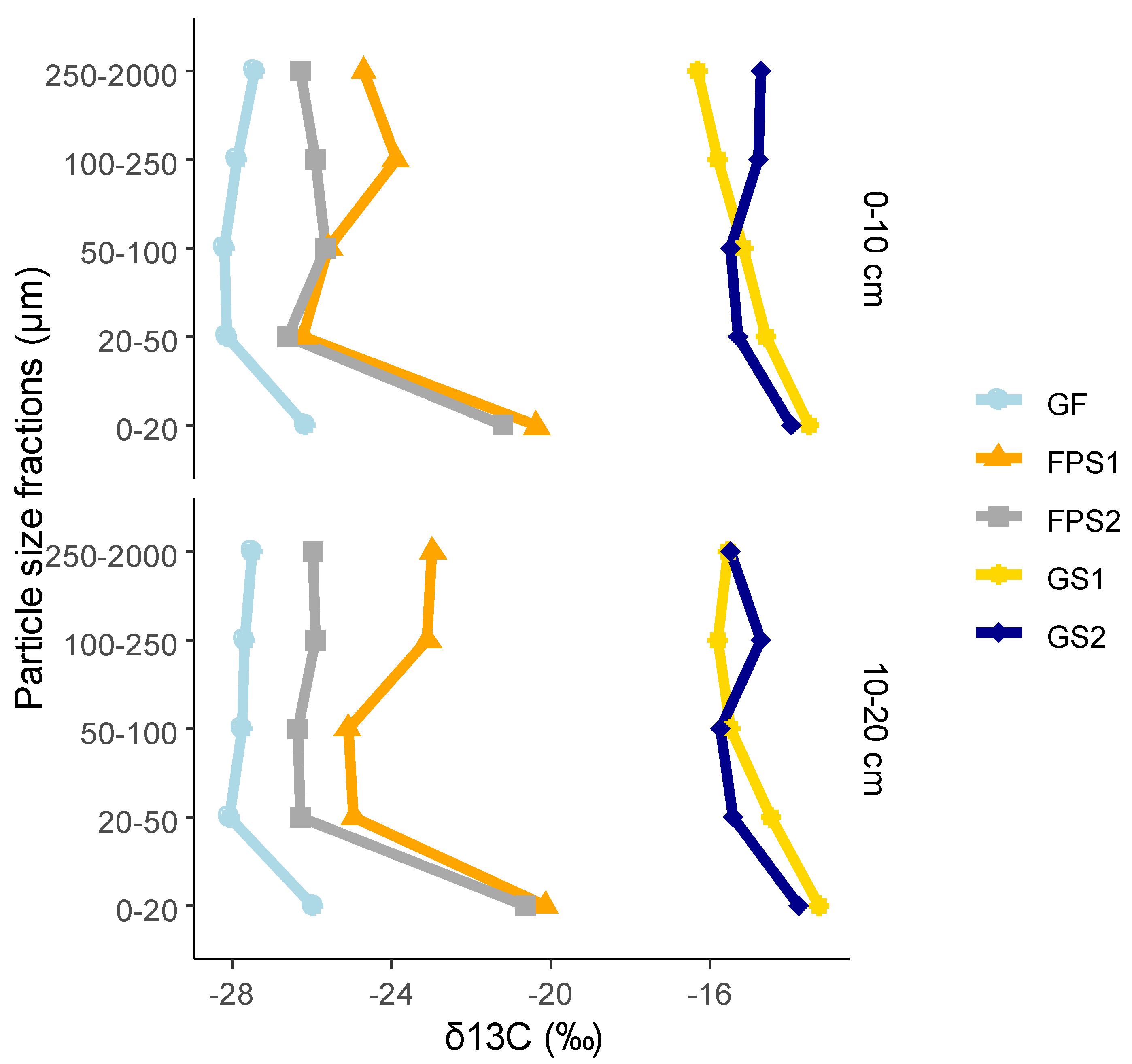
3.4. δ13C Values of Particle-Size Fractions
3.5. Percentages of C Derived from Savanna (C4) and Forest (C3) Material
4. Discussion
4.1. SOM Content
4.2. δ13C Values of Litter and SOM
4.3. Distribution of Total SOM in Particle-Size Fractions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The ecology of soil carbon: Pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Falloon, P.; Coleman, K.; Smith, J.; Piccolo, M.C.; Cerri, C.; Bernoux, M.; Jenkinson, D.; Ingram, J.; Szabo, J.; et al. Modeling soil carbon dynamics in tropical ecosystems. In Global Climate Change and Tropical Ecosystems; CRC Press: Boca Raton, FL, USA, 2019; pp. 341–364. [Google Scholar] [CrossRef]
- Greenland, D.J.; Wild, A.; Adams, D. Organic matter dynamics in soils of the tropics—From myth to complex reality. Myth. Sci. Soils Trop. 1992, 29, 17–33. [Google Scholar] [CrossRef]
- Santos, R.S.; Oliveira, F.C.; Ferreira, G.W.; Ferreira, M.A.; Araújo, E.F.; Silva, I.R. Carbon and nitrogen dynamics in soil organic matter fractions following eucalypt afforestation in southern Brazilian grasslands (Pampas). Agric. Ecosyst. Environ. 2020, 301, 106979. [Google Scholar] [CrossRef]
- Schimel, D.S. Terrestrial Ecosystems and the carbon cycle. Glob. Change Biol. 1995, 1, 77–91. [Google Scholar] [CrossRef]
- Trumbore, S.E. Comparison of carbon dynamics in tropical and temperate soils using radiocarbon measurements. Global Biogeochem. Cycles 1993, 7, 275–290. [Google Scholar] [CrossRef]
- Paustian, K.; Andrén, O.; Janzen, H.H.; Lal, R.; Smith, P.; Tian, G.; Tiessen, H.; Van Noordwijk, M.; Woomer, P.L. Agricultural Soils as a sink to mitigate CO2 emissions. Soil Use Manage. 1997, 13, 230–244. [Google Scholar] [CrossRef]
- Foley, J.A. Net primary productivity in the terrestrial biosphere. The application of a global model. J. Geophys. Res. 1994, 99, 20773–20783. [Google Scholar] [CrossRef]
- Dixon, R.K.; Winjum, J.K.; Andrasko, K.J.; Lee, J.J.; Schroeder, P.E. Integrated land-use systems assessment: Promising agroforest and alternative land-use practices to enhance carbon conservation and sequestration. Clim. Change 1994, 27, 71–92. [Google Scholar] [CrossRef]
- Cerri, C.; Feller, C.; Balesdent, J.; Victoria, R.; Plegnecassagne, A. Application du traçage isotopique naturel en 13C à l’étude de la dynamique de la matière organique dans les sols. C. R. Acad. Sci 1985, 300, 423–429. [Google Scholar]
- Fonkeng, E.E.; Chevallier, T.; Sauvadet, M.; Enock, S.; Rakotondrazafy, N.; Chapuis-Lardy, L.; Takoutsing, B.; Fritz, O.T.; Harmand, J.-M. Dynamics of soil organic carbon pools following conversion of savannah to cocoa agroforestry systems in the Centre region of Cameroon. Geoderma Reg. 2024, 36, e00758. [Google Scholar] [CrossRef]
- Sevink, J.; Obale-Ebanga, F.; Meijer, H.A.J. Land-use related organic matter dynamics in North Cameroon soils assessed by 13C analysis of soil organic matter fractions. Eur. J. Soil Sci. 2005, 56, 103–111. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Change Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Balesdent, J.; Besnard, E.; Arrouays, D.; Chenu, C. The dynamics of carbon in particle-size fractions of soil in a forest-cultivation sequence. Plant Soil 1998, 201, 49–57. [Google Scholar] [CrossRef]
- Six, J.; Guggenberger, G.; Paustian, K.; Haumaier, L.; Elliott, E.T.; Zech, W. Sources and composition of soil organic matter fractions between and within soil aggregates. Eur. J. Soil Sci. 2001, 52, 607–618. [Google Scholar] [CrossRef]
- Balesdent, J.; Mariotti, A.; Guillet, B. Natural 13C abundance as a tracer for soil organic matter dynamics studies. Soil Biol. Biochem. 1987, 19, 25–30. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Six, J.; Kaiser, M.; Benbi, D.; Chenu, C.; Cotrufo, M.F.; Derrien, D.; Gioacchini, P.; Grand, S.; et al. Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils—A comprehensive method comparison. Soil Biol. Biochem. 2018, 125, 10–26. [Google Scholar] [CrossRef]
- Epron, D.; Marsden, C.; Thongo M’Bou, A.; Saint-André, L.; d’Annunzio, R.; Nouvellon, Y. Soil carbon dynamics following afforestation of a tropical savannah with Eucalyptus in Congo. Plant Soil 2009, 323, 309–322. [Google Scholar] [CrossRef]
- Balesdent, J.; Wagner, G.H.; Mariotti, A. Soil organic matter turnover in long-term field experiments as revealed by carbon-13 natural abundance. Soil Sci. Soc. Am. J. 1988, 52, 118–124. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Change Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Fujisaki, K.; Perrin, A.-S.; Desjardins, T.; Bernoux, M.; Balbino, L.C.; Brossard, M. From forest to cropland and pasture systems: A critical review of soil organic carbon stocks changes in Amazonia. Glob. Change Biol. 2015, 21, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Sandoval López, D.M.; Arturi, M.F.; Goya, J.F.; Pérez, C.A.; Frangi, J.L. Eucalyptus grandis plantations: Effects of management on soil carbon, nutrient contents and yields. J. For. Res. 2020, 31, 601–611. [Google Scholar] [CrossRef]
- Martin, A.; Mariotti, A.; Balesdent, J.; Lavelle, P.; Vuattoux, R. Estimate of organic matter turnover rate in a savanna soil by 13C natural measurements. Soil Biol. Biochem. 1990, 22, 517–523. [Google Scholar] [CrossRef]
- Menaut, J.-C.; César, J. Structure and primary productivity of Lamto savannas, Ivory Coast. Ecology 1979, 60, 1197–1210. [Google Scholar] [CrossRef]
- Diawara, A.; Yoroba, F.; Kouadio, K.Y.; Kouassi, K.B.; Assamoi, E.M.; Diedhiou, A.P.; Assamoi, P. Climate Variability in the Sudano-Guinean Transition Area and Its Impact on Vegetation: The Case of the Lamto Region in Côte D’Ivoire. Adv. Meteorol. 2014, 2014, 831414. [Google Scholar] [CrossRef][Green Version]
- César, J.; Menaut, J.C. Le peuplement végétal de la savane de Lamto. Analyse d’un écosystème tropical humide: La savane de Lamto. Bull. Liaison Cherch. Lamto 1974, 2, 1–161. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Italy, 2014; No. 106. [Google Scholar]
- Devineau, J.L.; Lecordier, C.; Vuattoux, R. Evolution de la diversité spécifique du peuplement ligneux dans une succession préforestière de colonisation d’une savane protégée des feux (Lamto, Côte d’Ivoire). Candollea 1984, 39, 103–134. [Google Scholar]
- Elliott, E.T.; Cambardella, C.A. Physical separation of soil organic matter. Agric. Ecosyst. Environ. 1991, 34, 407–419. [Google Scholar] [CrossRef]
- Anderson, D.W.; Saggar, S.; Bettany, R.; Stewart, J.W.B. Particle-size fractions and their use in studies of soil organic matter. 1. The nature and distribution of forms of carbon, nitrogen, and sulfur. Soil Sci. Soc. Am. J. 1981, 45, 767–772. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Evol. Syst. 1987, 18, 293–320. Available online: https://www.jstor.org/stable/2097134 (accessed on 24 February 2025). [CrossRef]
- Feller, C.; Beare, M.H. Physical control of soil organic matter dynamics in the tropics. Geoderma 1997, 79, 69–116. [Google Scholar] [CrossRef]
- Koné, A.W.; Kassi, S.P.A.Y.; Bernard, Y.; Koffi, B.Y.; Masse, D.; Maïga, A.A.; Tondoh, J.E.; Kisaka, O.M.; Touré, G.-P.T. Chromolaena odorata (L.) K&R (Asteraceae) invasion effects on soil microbial biomass and activities in a forest-savanna mosaic. Catena 2021, 207, 105619. [Google Scholar] [CrossRef]
- Silue, B.K.; Koné, A.W.; Masse, D.; Moulin-Esmard, P.; Kotaix, A.J.A.; Chapuis-Lardy, L. Contrasted effects of shade tree legumes on soil organic carbon stock and carbon balance in 20-year cacao agroforestry, Ivory Coast. Geoderma Reg. 2024, 37, e00807. [Google Scholar] [CrossRef]
- Otieno, D.O.; K’Otuto, G.O.; Jakli, B.; Schröttle, P.; Maina, J.N.; Jung, E.; Onyango, J.C. Spatial heterogeneity in ecosystem structure and productivity in a moist Kenyan savanna. Plant Ecol. 2011, 212, 769–783. [Google Scholar] [CrossRef]
- Riou, G. Les sols de la savane de Lamto. In Analyse d’un Ecosystème Tropical Humide: La Savane de Lamto (Côte d’lvoire); Bulletin Liaison Chercheurs Lamto; Publications Laboratoire Zoologie ENS: Paris, France, 1974; Volume 1, pp. 3–38. [Google Scholar]
- Gillon, M.; Barbecot, F.; Gibert, E.C.; Plain, C.; Corcho-Alvarado, J.-A.; Massault, M. Controls on 13C and 14C variability in soil CO2. Geoderma 2012, 189–190, 431–441. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.; Lei, Y.; Yang, F.; Zhang, D.; Zhang, K.; Zhang, Q.; Cheng, X. Agricultural land use change impacts soil CO2 emission and its 13C-isotopic signature in central China. Soil Till. Res. 2018, 177, 105–112. [Google Scholar] [CrossRef]
- Kang, X.; Liu, T.; Wang, G.; Hao, L.; Duan, L.; Tong, X.; Singh, V.P.; Wu, R. Control of hydrologic conditions and vegetation composition on carbon dynamics characterized by 13C enrichment in a dune-meadow cascade ecosystem in a semi-arid region. Catena 2023, 231, 107286. [Google Scholar] [CrossRef]
- Diels, J.; Vanlauwe, B.; Sanginga, N.; Coolen, E.; Merckx, E.R. Temporal variations in plant d13C values and implications for using the 13C technique in long-term soil organic matter studies. Soil Biol. Biochem. 2001, 33, 1245–1251. [Google Scholar] [CrossRef]
- Balesdent, J.; Basile-Doelsch, I.; Chadoeuf, J.; Cornu, S.; Derrien, D.; Fekiacova, Z.; Hatté, C. Atmosphere–soil carbon transfer as a function of soil depth. Nature 2018, 559, 599–602. [Google Scholar] [CrossRef]
- Brüggemann, N.; Gessler, A.; Kayler, Z.; Keel, S.G.; Badeck, F.; Barthel, M.; Boeckx, P.; Buchmann, N.; Brugnoli, E.; Esperschütz, J.; et al. Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: A review. Biogeosciences 2011, 8, 3457–3489. [Google Scholar] [CrossRef]
- Paul, A.; Balesdent, J.; Hatté, C. 13C-14C relations reveal that soil 13C-depth gradient is linked to historical changes in vegetation 13C. Plant Soil 2020, 447, 305–317. [Google Scholar] [CrossRef]
- Dortzbach, D.; Pereira, M.G.; Blainski, E.; González, A.P. Estoque de C e abundância natural de 13C em razão da conversão de areas de floresta e pastagem em bioma Mata Atlântica. R. Bras. Ci. Solo 2015, 39, 1643–1660. [Google Scholar] [CrossRef][Green Version]
- Andriollo, D.D.; Redin, C.G.; José Miguel Reichert, J.M.; Silva, L.S. Soil carbon isotope ratios in forest-grassland toposequences to identify vegetation changes in southern Brazilian grasslands. Catena 2017, 159, 126–135. [Google Scholar] [CrossRef]
- Xu, T.; Chen, X.; Hou, Y.; Zhu, B. Changes in microbial biomass, community composition and diversity, and functioning with soil depth in two alpine ecosystems on the Tibetan plateau. Plant Soil 2021, 459, 137–153. [Google Scholar] [CrossRef]
- Wang, C.; Dippold, M.A.; Blagodatskaya, E.; Dorodnikov, M. Oxygen matters: Short- and medium-term effects of aeration on hydrolytic enzymes in a paddy soil. Geoderma 2022, 407, 115548. [Google Scholar] [CrossRef]
- Mbé, B.; Monga, O.; Pot, V.; Otten, W.; Hecht, F.; Raynaud, X.; Nunan, N.; Chenu, C.; Baveye, P.C.; Garnier, P. Scenario modelling of carbon mineralization in 3D soil architecture at the microscale: Toward an accessibility coefficient of organic matter for bacteria. Eur. J. Soil Sci. 2021, 73, e13144. [Google Scholar] [CrossRef]
- Nuñez, J.; Moinet, G.Y.K.; Graham, S.L.; Turnbull, M.H.; Grelet, G.-A.; Whitehead, D. Addition of sorptive mineral phases to soils decreases short-term organic matter decomposition by reducing microbial access to substrates. Eur. J. Soil Sci. 2021, 73, e13176. [Google Scholar] [CrossRef]
- Desjardins, T.; Barros, E.; Sarrazin, M.; Girardin, C.; Mariotti, A. Effects of forests conversion to pasture soil carbon content and dynamics in Brazilian Amazonia. Agric. Ecosyst. Environ. 2004, 103, 365–373. [Google Scholar] [CrossRef]
- Koutika, L.S.; Choné, T.; Andreux, F.; Cerri, C.C. Carbon decomposition of the topsoils and soil fractions under forest and pasture in the western Brazilian Amazon basin, Rondônia. Biol. Fertil. Soils 2000, 30, 284–287. [Google Scholar] [CrossRef]
- Solomon, D.; Fritzsche, F.; Lehmann, J.; Tekalign, M.; Zech, W. Soil organic matter dynamics in the subhumid agroecosystems of the Ethiopian highlands: Evidence from natural C-13 abundance and particle-size fractionation. Soil Sci. Soc. Am. J. 2002, 66, 969–978. [Google Scholar] [CrossRef]
- Amelung, W.; Zech, W.; Zhang, X.; Follet, R.F.; Tiessen, H.; Knox, E.; Flach, K.W. Carbon, nitrogen, and sulfur pools in particle-size fractions as influenced by climate. Soil Sci. Soc. Am. J. 1998, 62, 172–181. [Google Scholar] [CrossRef]
- Solomon, D.; Lehmann, J.; Zech, W. Land use effects on soil organic matter properties of chromic Luvisols in the semiarid tropics: Carbon, nitrogen, lignin and carbohydrates. Agric. Ecosyst. Environ. 2000, 78, 203–213. [Google Scholar] [CrossRef]
- Desjardins, T.; Folgarait, P.J.; Pando-Bahuon, A.; Girardin, C.; Lavelle, P. Soil organic matter dynamics along a rice chronosequence in north-eastern Argentina: Evidence from natural 13C abundance and particle size fractionation. Soil Biol. Biochem. 2006, 38, 2753–2761. [Google Scholar] [CrossRef]
- Christensen, B.T. Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur. J. Soil Sci. 2001, 52, 345–353. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Marschner, B.; Brodowski, S.; Dreves, A.; Gleixner, G.; Gude, A.; Pieter, M.; Grootes, P.M.; Hamer, U.; Heim, A.; Jandl, G.; et al. How relevant is recalcitrance for the stabilization of organic matter in soils? J. Plant Nutr. Soil Sci. 2008, 171, 91–110. [Google Scholar] [CrossRef]
| Depth | GS1 | GS2 | FPS1 | FPS2 | GF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | C mg·g−1 | C/N | C mg·g−1 | C/N | C mg·g−1 | C/N | C mg·g−1 | C/N | C mg·g−1 | C/N |
| 0–10 | 12.0 ± 1.9 d | 18.6 ± 0.4 a | 16.6 ± 1.3 c | 18.6 ± 0.6 a | 15.6 ± 1.6 c | 14.2 ± 0.5 b | 22.1 ± 1.8 a | 14.1 ± 0.2 b | 18.0 ± 2.4 b | 11.3 ± 0.2 c |
| 10–20 | 10.2 ± 0.8 d | 18.3 ± 0.4 a | 15.8 ± 1.8 b | 18.0 ± 0.4 a | 13.0 ± 1.4 c | 14.6 ± 0.4 b | 19.0 ± 1.6 a | 14.3 ± 0.4 b | 13.0 ± 1.3 c | 11.1 ± 0.2 c |
| 20–30 | 8.2 | 16.9 | 15.8 | 16.0 | 10.5 | 15.4 | 15.5 | 13.9 | 11.5 | 11.5 |
| 30–40 | 7.2 | 15.7 | 14.1 | 17.9 | 8.3 | 14.8 | 13.3 | 12.4 | 8.7 | 10.8 |
| 40–50 | 7.1 | 15.3 | 9.6 | 14.3 | 6.3 | 11.9 | 10.9 | 11.6 | 6.6 | 10.0 |
| Particle Size (μm) | GS1 | GS2 | FPS1 | FPS2 | GF |
|---|---|---|---|---|---|
| 250–2000 | 63.9 | 53.7 | 41.1 | 37.2 | 5.8 |
| 100–250 | 8.9 | 13.0 | 16.1 | 13.9 | 42.4 |
| 50–100 | 3.4 | 8.5 | 8.1 | 9.8 | 12.5 |
| 20–50 | 4.4 | 5.8 | 8.3 | 8.7 | 12.0 |
| 0–20 | 19.5 | 19.0 | 26.6 | 30.4 | 27.4 |
| Particle Size Fractions | FPS1 | FSP2 | ||
|---|---|---|---|---|
| (μm) | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm |
| X (C4) % | X (C4) % | X (C4) % | X (C4) % | |
| 250–2000 | 20.1 | 37.8 | 9.7 | 12.7 |
| 100–250 | 31.6 | 37.0 | 15.6 | 14.0 |
| 50–100 | 20.2 | 21.8 | 19.3 | 11.9 |
| 20–50 | 14.9 | 23.8 | 11.6 | 13.7 |
| 0–20 | 46.6 | 46.8 | 40.1 | 42.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desjardins, T.; Henry Des Tureaux, T.; Mandeng-Yogo, M.; Cetin, F. Soil Organic Carbon Turnover Following Afforestation of a Savanna Revealed by Particle-Size Fractionation and Natural 13C Measurements in Ivory Coast. Land 2025, 14, 535. https://doi.org/10.3390/land14030535
Desjardins T, Henry Des Tureaux T, Mandeng-Yogo M, Cetin F. Soil Organic Carbon Turnover Following Afforestation of a Savanna Revealed by Particle-Size Fractionation and Natural 13C Measurements in Ivory Coast. Land. 2025; 14(3):535. https://doi.org/10.3390/land14030535
Chicago/Turabian StyleDesjardins, Thierry, Thierry Henry Des Tureaux, Magloire Mandeng-Yogo, and Fethiye Cetin. 2025. "Soil Organic Carbon Turnover Following Afforestation of a Savanna Revealed by Particle-Size Fractionation and Natural 13C Measurements in Ivory Coast" Land 14, no. 3: 535. https://doi.org/10.3390/land14030535
APA StyleDesjardins, T., Henry Des Tureaux, T., Mandeng-Yogo, M., & Cetin, F. (2025). Soil Organic Carbon Turnover Following Afforestation of a Savanna Revealed by Particle-Size Fractionation and Natural 13C Measurements in Ivory Coast. Land, 14(3), 535. https://doi.org/10.3390/land14030535






