Abstract
This study evaluates the influence of land use and water stress on ecosystem resilience, using panel data for thirty-three European countries from 2007 to 2024, following the identification of a research gap in the literature on this topic. The dependent variable is the bioclimatic ecosystem resilience index (BER), and the explanatory variables are Agricultural Land Share (ALS), Forest Land Share (FLS), and the Level of Water Stress (WS). The estimated models are a fixed-effects panel regression with Driscoll-Kraay standard errors, robust to autocorrelation, heteroscedasticity, and spatial dependence, and a kernel-based regularized least squares model, which offers a new, nonlinear, heterogeneous, and sensitive to local contexts perspective on ecosystem resilience. The results indicate a significant positive effect of FLS on ecosystem resilience, ALS has a mixed influence, while WS has a negative impact. Robustness checks using cluster-robust standard errors and alternative model specifications confirmed the stability and direction of the estimated coefficients. The conclusions support the promotion of forest conservation policies, sustainable water resource management, and ecosystem-friendly agriculture practices as main directions for enhancing the capacity of ecosystems to respond to human and climate pressures.
1. Introduction
Biodiversity is essential to human well-being, a not diseased planet, security from natural disasters, food supply, and economic prosperity for human beings, as mentioned in the Convention on Biological Diversity [1]. Biodiversity refers not only to every living world’s species, plants, animals, microorganisms, insects, etc., but also genetic variability within populations, ecosystems, landscapes, or oceans [2]. Ecosystem services encompass the benefits that biodiversity provides to humans, such as healthy soils, clean water due to wetlands, clean air thanks to forests, crops pollinated with the help of insects, coastal areas protected from storms by mangrove forests, disease regulation, and more [3].
However, there is a significant number of studies that discuss the loss of biodiversity and the multiple barriers that hinder successfully stopping the biodiversity loss [4,5,6,7,8], among others. In 2022, around a quarter of the planet’s animal and plant species were threatened, emphasizing the essential need for actions to curb the loss of the planet’s biodiversity [1]. Swiss Re Institute assesses that natural ecosystems have decreased by 47% and emphasizes that the loss of biodiversity and ecosystem services will increasingly impact the world’s economies, from the availability of water and food to the global supply of products and services [9].
While the pressing need to protect natural habitats is agreed upon among researchers, the prioritization of regions where conservation actions are most needed has been a topic of ongoing debate in recent years. Some voices advocate for prioritizing the conservation of those vast areas that have been least affected by human impact to date [10,11], while others argue for prioritizing conservation measures in smaller regions that have already been heavily modified by human actions [12,13]. Additionally, other voices see the saving solution in combining both approaches, prioritizing efforts in both intact territories and those facing strong human pressures, based on habitats with high-value biodiversity [14].
Resilience refers to a system’s capability to adapt to any type of disruption and self-organize while undergoing transformation, remaining with its initial forms, identity, and feedback mechanism [15]. A resilient system tends to remain in its Safe Operating Space, despite change, and operationalizing it to ecosystems involves management of local stressors to mitigate the risk of climate change-induced shifts to states that offer fewer ecosystem services [16]. There is a strong link between the resilience of ecosystems and biodiversity. In the face of environmental crises caused by human actions and climate change, ecosystems are able to support biodiversity and ecosystem services through their resilience, and an ecosystem with high biodiversity is generally more resilient to environmental disturbances [17]. Some ecosystems have higher functional redundancy: if some species become extinct, others can keep the ecosystem working [18]. Among the mechanisms that support ecosystem resilience, biodiversity is a crucial one [19]. By conserving biodiversity, the genetic diversity of species is ensured, which ultimately secures the resilience of ecosystems [20]. The theme of ecosystem resilience has gained greater importance after 2022, when the Kunming–Montreal Global Biodiversity Framework took place, proposing a plan to transform the relationship between human societies and biodiversity, and one of the defined objectives was to restore the resilience, integrity, and connectivity of ecosystems by 2050 through a substantial increase in the area of natural ecosystems, or in other words, to minimize the impacts of climate change on biodiversity and build resilience [1].
To effectively influence global policies regarding biodiversity conservation, scientists are measuring the progress made towards achieving the goals set out in international conventions, using reliable indicators [8]. Species richness is a biodiversity measure often used in studies, while the measurement of biodiversity through the ability to maintain species diversity considering environmental disturbances, that is, with the help of ecosystem resilience, has been little exploited. First, indicators for measuring ecosystem resilience in the short term were developed, applicable to limited types of ecosystems, and later they were improved to more accurately measure the resilience of ecosystems [21], as is the case with the Bioclimatic Ecosystem Resilience Index (BER) that measures the resilience of ecosystems in terms of their ability to maintain species diversity in the face of ongoing climate changes [22].
Human actions significantly influence the planet’s biodiversity, and the causes of biodiversity loss attributed to human activities in order of their impact [23] are first, land use change, mainly for food production; second, the overexploitation of natural resources such as overfishing; and third, climate change and pollution contribute to the loss of biodiversity, followed by deforestation, as forests are rich in biodiversity and are being destroyed. Worryingly, ecosystems are under pressure from climate variation and human actions, having to endure the new conditions, as well as to adapt to them [24].
Forest land, grassland, waters, and construction land are the main types of land that affect changes in the ecosystem resilience indicator [25]. The relationship between land use and biodiversity is context dependent; specific land uses may sustain biodiversity [26], others may drive biodiversity loss and significant environmental change. This paper addresses the relationship between the ability to maintain species diversity, agricultural and forest land.
The importance of land use for the expansion of agriculture and food security, while conserving biodiversity of the habitat to the greatest extent possible, is mentioned in many studies [27,28,29], among others. There is an unresolved puzzle regarding the optimal strategy for protecting biodiversity while agricultural production expands. Some studies [30,31] argue that the ‘land sharing’ strategy, in which the intensity of agricultural processes used is low, requires larger areas, has a more complex landscape structure, and agricultural lands where biodiversity-friendly measures are applied are intermixed with patches for natural habitats, would be more suitable for biodiversity conservation in that area. Other studies strongly promote the benefits of the ‘land sparing’ strategy, in which agricultural processes are very intensive but in smaller areas, leaving space for undisturbed habitats, well separated from agricultural land [7,32]. Recent research findings emphasize the need to consider the specific context of the region in managing the balance between agricultural production and biodiversity conservation, as neither land-sharing nor land-sparing strategies can clearly claim to be better than the other [33].
As mentioned above, some of the human actions that have a significant impact on the conservation of the planet’s biodiversity are related to deforestation. In Europe, the forest land share remained nearly constant in the past 20 years, recording almost the same consumption and formation forest amounts [34]. But in 2024, worryingly, tropical forests decreased in area by 6.7 million hectares, an area nearly double that of 2023 and a record for the last few decades [35]. The cited report on the latest trends in global forest evolution points out that tropical forests are critical ecosystems for maintaining biodiversity, carbon storage, and water provision, and while the main cause of tropical forest loss after 2000 was always attributed to deforestation for permanent agricultural space, in 2024 the main cause of loss was represented by fires almost always caused by humans [35]. Thus, an essential perspective in understanding the resilience of ecosystems is provided by both the relationship between the resilience variable and the land converted for agricultural use, as well as its relationship with forest land share. Recently, Ref. [36] used a dynamic system model that incorporates land cover with vegetation, plant growth parameters, and the simulated interaction between species of insects, birds, and plants to study the impact of agricultural land conversion and deforestation on the resilience and stability of ecosystems. The results of the study indicate that the stability and resilience of an ecosystem significantly decrease when forest cover falls below 30%, while converting land to agricultural use can maintain ecosystem stability if good sustainable agricultural practices are followed, such as the existence of ecological buffer zones and limiting chemical use.
Water is essential for maintaining life on Earth, in agriculture and in all economic activities, and in fighting diseases and pandemics. Thus, in countries where it is under stress, the impact is significant, from limiting the availability of water for people’s personal needs to food insecurity [37]. The cited report discusses the importance of minimizing the pressure that human activities put on natural freshwater resources, pressure measured by the water stress indicator. It emphasizes that the current rate of progress towards achieving the Sustainable Development Goals’ (SDGs) six objectives needs to be doubled and, in some cases, increased by four times to meet the targets. At the same time, for the objective regarding the minimization of water stress, the report mentions that it is necessary to mobilize investments three times greater than those currently present in water-related infrastructure [37]. The water stress indicator estimates the pressure by all sectors on the country’s freshwater resources, representing the total freshwater withdrawn by agricultural, industrial, and municipal sectors, divided by total renewable freshwater resources, after considering environmental flow requirements necessary to sustain aquatic ecosystems [38].
The effects of increased water stress have been addressed in the literature. Among others, Ref. [39] demonstrated through experiments in controlled environments that the effects of rising temperatures under conditions of water stress on several grass species caused between 95% and 100% failure in the transition of seeds from the germination stage to the survival stage, thus highlighting the adverse effects of water stress conditions for ecosystems in the increasingly arid areas of the planet.
Under the conditions of resilience and sensitivity of natural ecosystems in 2018, the most vulnerable areas on Earth in the future will be found in the upper part of the Northern Hemisphere [40]. Since the Northern Hemisphere includes the vast majority of European countries, the results of the aforementioned study justify the choice of the study area in our work, meaning the European countries that will increasingly be exposed to climate vulnerability in the future. Additionally, Europe is an intensively used continent, having the largest proportion of land used for production systems in agriculture and other sectors, for infrastructure, and for settlements, compared to the other continents of the globe [41].
All these aspects justify the choice of water stress variable, forest land share, and agricultural land share as determinants of the resilience of the ecosystems studied in our paper.
The scientific puzzle that this study aimed to solve is to what extent and which different types of land use and water resource pressures strengthen the resilience of ecosystems and which weaken it, in the European continent with diverse policy and environmental backgrounds. We apply panel data and Kernel-Based Regularized Least Squares (KRLS) models, attempting to identify the main drivers of ecosystem stability. Then we suggest actionable policy recommendations for sustainable land and water management based on the evidence. We chose to analyze this set of European countries due to the diversity in land use practices, the different degrees of water stress, and the existence of comparable environmental EU policies, which allowed a coherent transnational analysis.
We formulated the following three research hypotheses:
H1:
Forest land share has a positive effect on ecosystem resilience.
H2:
Water stress has a negative influence on ecosystem resilience.
H3:
Agricultural land share has a positive impact on ecosystem resilience.
Under the dome of the Kunming–Montreal Global Biodiversity Framework, with its ambitious plan to transform the relationship between human actions and biodiversity, it is crucial to deeply analyze the relationships between biodiversity loss drivers. Existing studies have exposed relevant measurement indicators, methods, and results at the regional and country level regarding ecosystem resilience and its determinants, but there are few studies that explore the relationship between ecosystem resilience, land use, and water stress. Most existing studies adopt linear models, ignoring the complexity of the driving factors’ influence, especially in the context of diverse policy and environmental backgrounds in the European continent. Such simplified analysis may lead to policy misjudgments and leave a gap in the literature that our study fills. Based on the above research perspectives, this study brings some elements of novelty: (1) a new perspective on ecosystem resilience, nonlinear, heterogeneous, and sensitive to local contexts, argued with the KRLS model; (2) a deeper understanding of the mixed role played by agricultural land expansion on ecological resilience, clarifying that it can strengthen resilience when land expansion for agriculture is performed with the application of land-friendly practices or, conversely, it can erode resilience; and (3) an in-depth panel data analysis of anthropogenic drivers of ecosystem resilience in Europe.
The paper is organized as follows. Section 2 contains the methodological part, the panel econometric models, and Kernel-Based Regularized Least Squares. Section 3 presents the empirical results, input data description, followed by the fixed-effects panel techniques and the nonlinear KRLS analysis. Section 4 and Section 5 conclude with the main findings, discussion, policy implications, and connections to international biodiversity and sustainability frameworks.
2. Materials and Methods
This study applies panel data models to study the impact of ALS, FLS, and WS on bioclimatic ecosystem resilience for 33 European countries during 2007–2024. The empirical approach includes a fixed-effect model with Driscoll-Kraay (DC) standard errors [42] to correct heteroskedasticity and autocorrelation in the panel data. The cluster-robust standard errors (CRSE) [43] model was used as a robustness check to verify the consistency of the results. The methodological flowchart is presented in Figure 1.
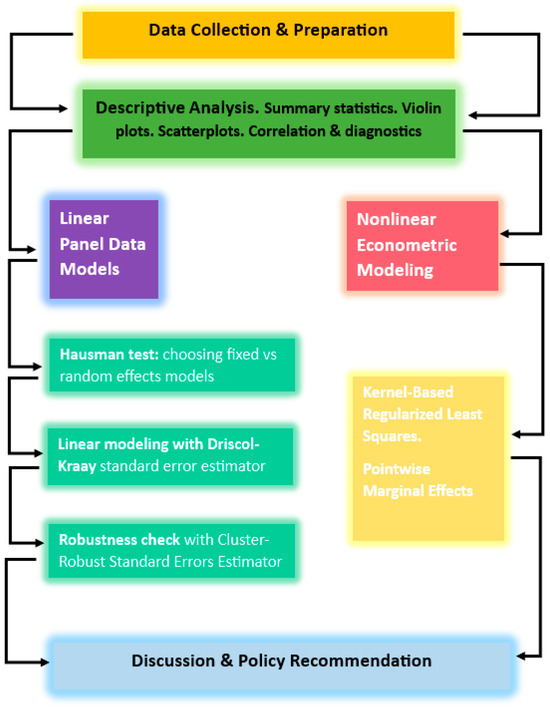
Figure 1.
Methodological flowchart.
2.1. Fixed and Random-Effects Models
Panel data models account for unobserved heterogeneity by reflecting time-invariant characteristics across units. They combine cross-sectional and time variation [44], increasing the accuracy of the estimations. The general linear panel model can be expressed as follows:
In Equation (1), is the dependent variable for cross-section i at time t, represents the cross-section specific effects which are time invariant, is the vector of explanatory variables, is the transposed vector of coefficients, and is the error term.
The random effects model can be formulated as follows:
In Equation (2), is the individual random component. In contrast with the fixed-effects model (1), the random-effects model assumes that is not correlated with the vector of explanatory variables [44]. To choose between the fixed and random-effects models, we apply the Hausman test [45]. The null hypothesis (NH) of the Hausman test is:
NH: The random-effects model is preferred, i.e., .
Versus the following alternative hypothesis:
AH: The fixed-effects model is preferred, i.e., .
If the p-value of the Hausman test is less than 5%, then the NH is rejected, pointing to the choice of the fixed-effects model. If the p-values exceed 5%, then the NH is not rejected, indicating that the random-effects model is more consistent.
The Hausman test statistic is a -type statistic:
In Equation (3), and are the estimated vectors of coefficients for the random-effects and fixed-effects models, respectively.
2.2. The Driscoll–Kraay Estimator
The Driscoll–Kraay (DC) estimator provides robust standard errors for panel models, dealing with heteroskedasticity, autocorrelation, and cross-sectional dependence in datasets for which the number of periods T is relatively small and the number of cross-sections N is large.
We start from the well-known formulation of the panel regression model in matrix form:
is the vector of observations for the dependent variable. K is the number of explanatory variables. is the matrix of explanatory variables, demeaned if using fixed effects.
is the vector of regression coefficients, is the error term. The coefficients are estimated using Ordinary Least Squares (OLS):
The robust standard errors are expressed by the variance–covariance matrix of [42]:
where is the autocovariance matrix at lag s, k(s, L) is a kernel function, L is the maximum lag, is the residual at time t. The sum in Equation (6) comprises both serial correlation and cross-sectional dependence. The DC estimator uses a variant of the Newey–West standard errors [46] aggregated at the cross-section level. This makes it suitable for generalized panel models.
In this study, all estimations were performed in EViews 12 and Stata 17.0 [47].
2.3. Cluster-Robust Standard Errors Estimator
The theoretical framework for the CRSE estimator builds upon the results of [48], which were later developed by [43]. Sometimes panel data have error correlation within clusters (cross-sections, here countries observed over time). The assumption of independently and identically distributed errors is violated when observations in the same cluster have unobserved characteristics. Therefore, we apply the CRSE estimator, robust to the intra-cluster correlation.
In the model framework (3), we assume that the data are grouped into G clusters (for example, countries). The CRSE estimator of the variance–covariance matrix of has the following formula:
In Equation (7), is the regressor matrix for cluster g, are the residuals for cluster g, where g = 1, …, G is the cluster index. It is assumed that the clusters are independent, but they can have their own structure and variance. The CRSE estimator is consistent when the number of clusters . G should exceed 30. If G is too small, then small-sample corrections should be applied [49].
The dependence equation of our model is as follows:
i is the cross-section index, i = 1, …, 33 (countries), t is the time index, t = 1, …, 18 years.
is the total error which can be decomposed as [45]:
is the unit-specific error (fixed or random), and is the idiosyncratic error term, varying across time and countries. The total error variance is the sum of the variances of the two components from (9):
reflects the influence of latent factors that are stable in time (e.g., stable environmental laws, geographic characteristics, institutional aspects).
reflects annual effects (climate shocks, droughts, etc.)
The intra-class coefficient ρ is as follows:
It can be interpreted as the proportion of total variance due to differences between countries.
From now on, we work with the logarithmic transformation of the variables to reduce heteroskedasticity, to stabilize the variance, and to interpret the estimated coefficients as elasticities [50].
2.4. Kernel-Based Regularized Least Squares (KRLS)
The panel regression models mentioned above assume linearity and additivity properties. In an ecological context, the effects of land use and WS on ecosystem resilience may be nonlinear and heterogeneous. To deal with these limitations, we applied the KRLS model introduced by [51]. The KRLS specification is as follows:
where , is a vector of predictors (ALS, FLS, WS), and is the error term:
f(.) is an unknown function, i = 1, …, 33, t = 1, …, 18. KRLS uses kernel methods to approximate f(.) and applies a regularization penalty to avoid overfitting. Instead of assuming that f(.) is linear, KRLS represents it in a reproducing kernel Hilbert space (RKHS). The kernel is defined as:
where is the squared Euclidean distance between two observations, is the bandwidth parameter controlling smoothness. The estimator takes the following form:
with the coefficients , K is the kernel matrix with entries , is the regularization parameter to prevent overfitting, and y is the vector of outcomes.
We also estimate pointwise marginal effects (PMEs).
The KRLS algorithm is implemented using the KRLS R package with default Gaussian kernels, in the RStudio 2023.12 environment. PME distributions are also computed.
The PME predictor at observation (i, t) is as follows:
measures the marginal contribution of a given variable at a certain point in the dataset. It quantifies the heterogeneity.
PMEs are averaged across all observations:
In the empirical part, we presented PMEs as boxplots and density plots.
The combination of panel econometric models and KRLS suits our research objectives. Heteroskedasticity, autocorrelation, and cross-sectional dependence are corrected by DK and CRSE regressions, which account for unobserved heterogeneity across countries. At the same time, KRSL reflects nonlinear, heterogeneous effects for countries. This dual approach leads to robust and context-sensitive results on ecosystem resilience.
3. Results
3.1. Input Data
The dataset includes yearly observations for 33 European countries from the period 2007–2024 extracted from several databases, as indicated in Table 1. Bioclimatic Ecosystem Resilience Index (BER) is reported by Commonwealth Scientific and Industrial Research Organisation (CSIRO) [52] and measures the resilience of ecosystems in terms of their ability to maintain species diversity in the face of ongoing climate changes, based on the ecological similarity of species composition between two pairs of locations, calculated with the help of several environmental variables such as minimum, maximum, monthly temperatures and water deficit, soil pH and clay proportion, annual precipitation, actual and potential evaporation, amphibians, birds, mammals, vascular plants, and other species occurrence, land use maps with impacts on local retention of species, greenhouse-gas concentration [22]. Agricultural Land Share (ALS) and Forest Land Share (FLS) are disseminated annually by the Food and Agriculture Organization of the United Nations (FAO) [53] as part of land use statistics, the human use of the land area for economic activities, and different purposes. The water stress indicator is made available to the public by FAO along with other Sustainable Development Goals indicators [54] and estimates the pressure by all sectors on the country’s freshwater resources, representing the total freshwater withdrawn by agricultural, industrial, and municipal sectors, divided by total renewable freshwater resources, after considering environmental flow requirements necessary to sustain aquatic ecosystems [38]. The time of collection for the datasets employed in our study is the 2024 releases, accessed in July 2025.

Table 1.
Variables and their sources.
Table 2 contains the descriptive statistics of the logarithmic values of the variables. This transformation allows the comparison of the variables with different units of measurement and reduces skewness [50]. Therefore, the econometric analysis is facilitated. As variables in Table 2 are log-transformed, the reported values are unitless and therefore not directly comparable to the original measurement units shown in Table 1. BER, ALS, and FLS have moderate standard deviations, and WS is the most volatile. All variables are negatively skewed. FLS and ALS have the lowest skewness, thus a longer left tail and few countries with very low land share. FLS and ALS have the largest kurtosis, corresponding to leptokurtic distributions with heavy tails and outliers. BER distribution is closer to the normal one, while WS has relatively low skewness and kurtosis. Therefore, the use of robust estimation methods is justified.

Table 2.
Descriptive statistics of log-transformed variables.
The choropleth map in Figure 2 illustrates regional differences in ecosystem resilience among the 33 analyzed countries. Northern European countries (Finland, Sweden, Norway, and Iceland) record the highest average BER, while Central and Eastern Europe show lower resilience values. The classification of the countries has been performed by grouping the BER mean values in four groups delimited by the three quartiles. Table A1 in Appendix A complements the map by offering the explicit comparison. Group 1 includes countries with the lowest resilience (≤14.54), such as Poland, Hungary, and Romania, while Group 4 includes those with the highest resilience (>38.75), such as Slovenia, Switzerland, and the Nordic countries. Our findings suggest that Europe is characterized by a strong North–South and West–East divide in ecosystem resilience.
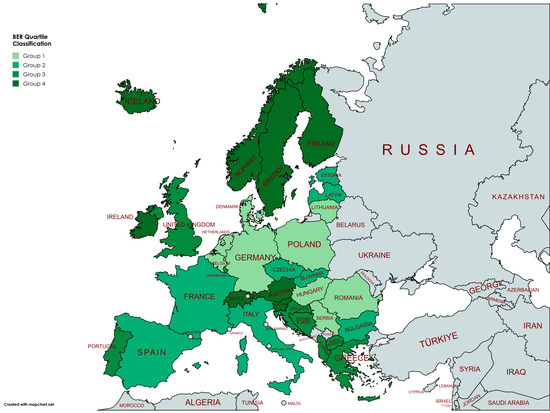
Figure 2.
Bioclimatic Ecosystem Resilience (BER) in 33 European countries, average for 2007–2024. Group 1, the lowest resilience. Group 4, the highest resilience.
Figure 3 displays the distributions of original variables as violin plots with embedded boxplots. BER distribution is right-skewed, and most countries are clustered at lower to mid resilience values. The BER long tail extends toward high resilience. ALS distribution has limited variability, concentrated in the upper range. The few observations at the lower end indicate countries with relatively low ALS. FLS distribution is multimodal, reflecting variation. The FLS’s long left tail reflects the countries with very low forest share. WS distribution is right-skewed.
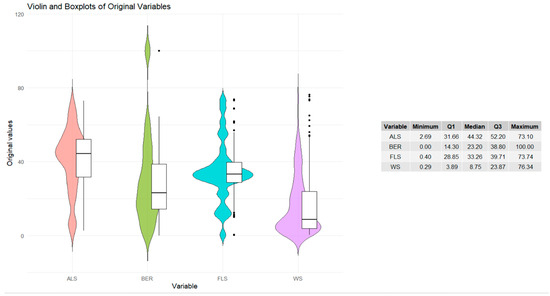
Figure 3.
Violin plots of original variables.
Figure 4 presents scatterplots with fitted regression lines of BER against ALS, FLS, and WS. The shaded confidence bands represent 95% confidence intervals for the regression estimates; they reflect the uncertainty in patterns.
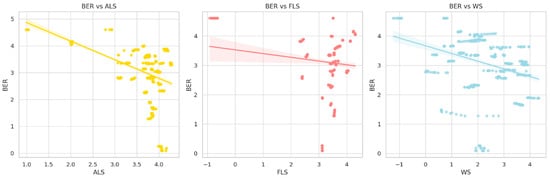
Figure 4.
Scatterplots of BER against ALS, FLS, and WS.
The results suggest that land use in agriculture and forests, as well as water stress, are essential factors to consider when designing strategies targeting ecosystem resilience improvements and biodiversity conservation.
We can see from Figure 4 a strong negative association between ALS and BER. It means that the more land is taken up by agriculture, the more biodiversity and natural habitat are negatively impacted [55,56,57]. This reflects the potential negative impact of agricultural expansion on biodiversity and natural habitat when analyzed in isolation from other influencing factors.
A negative bivariate association can be noticed between FLS and BER in Figure 4. This relationship could be misleading since other drivers, such as ALS and WS, are not involved. Once these indicators are included in the econometric model, FLS has a significant positive effect on BER. This is in line with FAO [58], which asserts that forests contribute more to ecological stability than secondary regrowth or monoculture. Resilience is determined not only by FLS but also by structural complexity, species diversity, and connectivity [18]. Haddad et al. [59] suggest that critical thresholds of habitat loss can determine whether changes in forest cover have marginal or significant effects on resilience.
The negative relation between BER and WS noticed in Figure 4 suggests that ecosystems with water scarcity show limited resilience. This idea is supported by [60], which shows that ecosystems with higher WS have a limited capacity for transpiration and photosynthesis.
Repeated droughts in water-scarce environments amplify ecological stress; therefore, their capacity to resist disturbances is limited [61]. In semi-arid and water-scarce areas, ecosystems have lower drought resilience and face difficulties in regaining their operability after the dry periods [62].
First, we tested for serial correlation using the Wooldridge test [63] and for multicollinearity using the variance inflation factor (VIF). The Wooldridge test has the NH: there is no first-order autocorrelation, versus the AH: there is first-order autocorrelation. The results indicate an F-statistic of 27.67, with a p-value = 0.000, less than 5%. It leads to the rejection of the NH; therefore, first-order autocorrelation is present.
The VIF values in Table 3 are below 0.5, indicating no multicollinearity in the model. The mean VIF of 1.32 suggests that the predictors are not highly correlated.

Table 3.
Variance inflation factor.
Figure 5 displays the correlation matrix. BER shows a moderate negative correlation with ALS and with WS. Its correlation with FLS is very weak. ALS is moderately positively correlated with WS, implying that areas with greater agricultural land use also tend to face higher WS. ALS is very weakly negatively correlated with FLS. FLS has a weak positive correlation with WS.
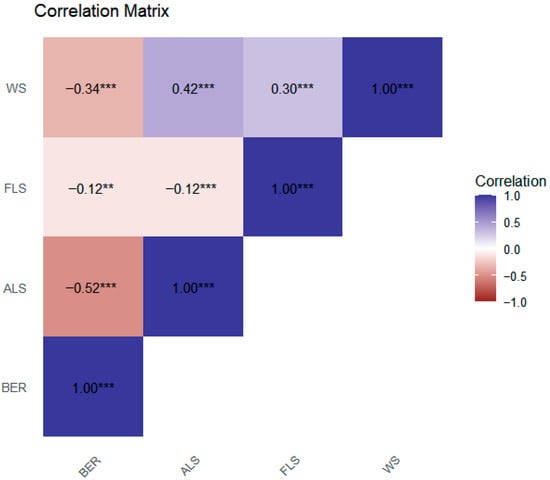
Figure 5.
Correlation matrix. Asterisks denote significance levels: ** p < 0.05, *** p < 0.01.
The above findings justify the use of robust standard errors in panel regressions to correct for heteroskedasticity.
3.2. Fixed Effects Estimators
To choose between the fixed- and random-effects models, we conduct the Hausman test. It yielded a chi-square statistic of 16.10 with 3 degrees of freedom and a p-value of 0.0011, less than 5%. Hence, we reject the NH that the random-effects model is consistent, choosing the fixed-effects model (FEM).
Pesaran’s test of cross-sectional dependence (CD) [64] is applied to verify if the residuals of FEM are correlated. The test yielded a statistic of 14.103, with the p-value < 0.0001. It follows that the NH of CD is rejected. Therefore, common shocks of spillovers can affect the countries in the panel. Thus, we can apply the DK standard errors model in Table 4.

Table 4.
Fixed effects Driscoll–Kraay regression.
One can see from Table 4 that FLS exhibits a strong positive effect on BER, confirming hypothesis H1, that forest coverage enhances resilience.
As seen in Table 4, WS has a significant negative effect on BER, supporting H2. It means that higher levels of WS reduce the adaptive capacity of ecosystems.
As seen in Table 4, ALS is marginally significant at the 10% level, being positively associated with BER, confirming H3.
The overall model is statistically significant (F(3, 17) = 34.61, p < 0.0001), with a within R-squared of 0.2012, showing that about 20% of the within-country variance is explained by the model.
The robustness check is performed by means of the CRSE estimator to account for potential intra-cluster correlation in the error term. In our context, the clusters are countries observed for several years. The assumption of independent and identically distributed errors is relaxed in the CRSE model. Observations in the same cluster can be correlated. In environmental panel data, intra-cluster dependence can be created by unobserved shocks or policy interventions. The CRSE estimator provides more conservative results compared to the DK model. In Table 5, the sign of the coefficients remains consistent with the DK estimation, even if their significance levels decline. This decline in significance level suggests that some part of the explanatory power of the baseline estimates may be driven by within-country persistence. In Europe, ecological resilience is determined by land use practices, long-term policies, and water management practices. The fact that none of the explanatory variables are statistically significant at the 5% level reflects the increased uncertainty in coefficient estimates when accounting for within-cluster correlation. The within R-squared remains unchanged at 0.2012.

Table 5.
Fixed effects with cluster-robust standard errors.
Additional parameters are reported together with the results from Table 5. =0.9846 is the standard deviation of the unobserved individual effects (country-specific heterogeneity). = 0.0211 is the standard deviation of the idiosyncratic error term. ρ = 0.9995 suggests that most of the variance in the error term is due to unobserved heterogeneity rather than random noise. Since most variation is between countries and not over time, it justifies the use of a fixed-effects model. The high ρ value points to unobservable country-level factors that influence ecosystem resilience.
In the European context, latent influences may include implementation of biodiversity targets and water management protocols [65], agroecological regimes of legacy crop diversity and soil conservation [66], and long-term water resource stress trajectories caused by climatic and hydrological conditions [67].
3.3. KRLS Results
To complement the fixed effects panel regression, we estimate a non-parametric KRLS model. This method relaxes linearity assumptions and reflects potential nonlinearities and heterogeneity in the relation between BER and its determinants.
Figure 6 represents the density of PMEs for each predictor. The dashed line indicates the average marginal effect. ALS distribution is bimodal, with peaks around −1 and 1. This reflects strong heterogeneity. Negative PMEs suggest that ALS diminishes ecosystem stability, while positive PMEs indicate that ALS contributes to ecosystem stability. It means that H3 is partially supported.
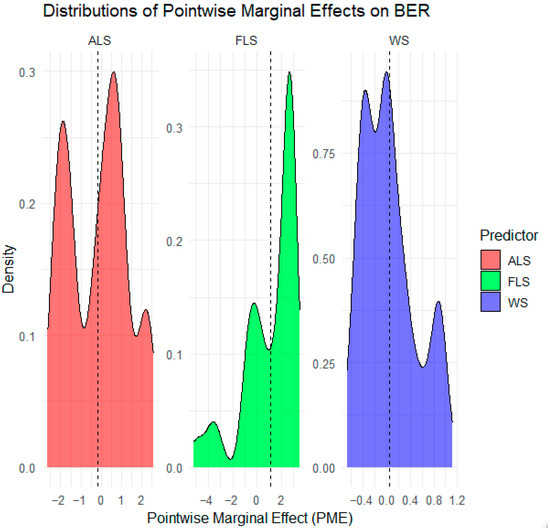
Figure 6.
Distributions of pointwise marginal effects (PMEs) of ALS, FLS, and WS on BER.
FLS distribution is positively skewed; most PME values are above 0 and concentrated near 2. The density bulk shows that FLS increases resilience, supporting H1.
WS distribution is centered around negative values. This indicates that WS has an adverse effect on resilience, confirming H2.
Figure 7 uses box-and-whisker plots to summarize the distribution of PMEs for each predictor. Each box represents the interquartile range (IOR), while the bottom and top edges correspond to the first quartile (Q1) and the third quartile (Q3). The line inside the box represents the median PME. The whiskers extend to the lower and upper adjacent values.
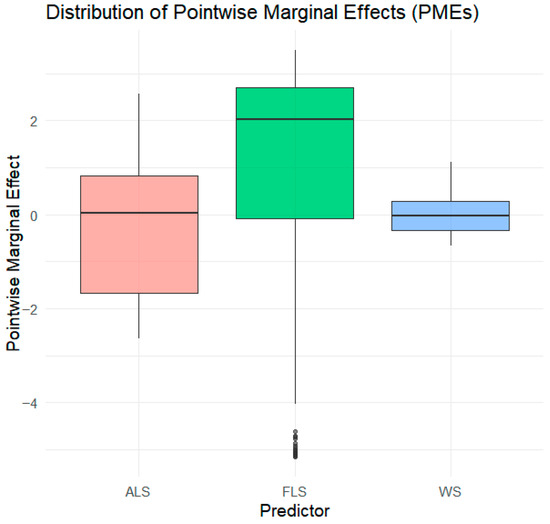
Figure 7.
Pointwise marginal effects (PMEs) on BER using box-and-whisker plots.
The dots under FLS represent outliers, indicating heterogeneity. FLS has a positive effect on BER, with its median and IOR being above 0. This supports hypothesis H1. WS has a negative effect on resilience. Both the median and the WS distribution are concentrated below zero, with little variation. This indicates that higher levels of WS reduce BER, in line with hypothesis H2.
ALS has mixed effects. The box spans both negative and positive values, reflecting variation; the ALS median is close to zero. This suggests that ALS can either support or undermine BER depending on the context. Therefore, hypothesis H3 is only partially confirmed.
4. Discussion
Starting from the reported results, the key research findings on the determinants of ecosystem resilience in Europe are discussed as follows:
- (1)
- BER distribution concentrated in the upper part, as shown in Figure 3, highlights that many European countries have high ecosystem resilience scores. This result is in line with the Ecosystem Vitality index scores reported for 180 countries in the world [68]. It is notable that out of the top 20 positions in the 2024 Ecosystem Vitality ranking, countries in Europe occupy 18 of them, with Luxembourg, Germany, Poland, Slovakia, and the Czech Republic earning the top five positions. Serbia, Bosnia and Herzegovina, and Montenegro record the lowest ecosystem vitality scores among the countries of Europe, ranking 55th, 76th, and 85th, while there are no European countries in the lower half of the ranking. The Ecosystem Vitality indicator [69] measures how well countries are protecting, preserving, and enhancing ecosystems and the services they provide.
- (2)
- The results in Table 4 and Figure 6 and Figure 7 confirm hypothesis H1, that forest land share has a positive effect on ecosystem resilience. In recent studies, the critical role of forest structural diversity in ecosystem resilience is emphasized. Ref. [70] shows that stand structural diversity, measured by the Gini coefficient of tree heights, contributes to balanced carbon storage and resilience. This balance can be optimized by means of silvicultural thinning strategies. Ref. [71] proves that in the context of even-aged Norway spruce stands, structural diversity maximizes stand density, volume yield, and growth. Therefore, it has stabilizing ecosystem effects. Protected area expansion and forest management policies can improve forest conservation and recovery [72,73]. However, the perspective of [74] is cautionary. Even if forests contribute to climate change mitigation, their resilience is affected by fires, pests, etc.
- (3)
- Hypothesis H2 is confirmed: water stress negatively influences ecosystem resilience, as justified by Table 4, and Figure 6 and Figure 7. Water scarcity can reduce vegetation productivity by restricting transpiration and photosynthetic activity [60]. This way, biodiversity and habitat quality are reduced. Ref. [75] found that in the Yellow River Basin in China, ecosystem resilience when there are droughts varies in space. Forests resist better but recover more slowly compared to grasslands. The same study found that precipitation, temperature, and plant biodiversity are the main drivers of resilience. Ref. [76] recently assessed the intensity of climate stress, analyzing relevant environmental data from 1996 to 2024, and identified among the key stress factors an increase in water deficits (by +100 mm/year), as well as an increase in maximum temperatures (by +1.50C) during the analyzed period.
- (4)
- Hypothesis H3, agricultural land share has a positive impact on ecosystem resilience, is partially supported. Ecological multifunctionality is increased through sustainable management of grassland and cropland [77]. Agricultural resilience is not determined by what happens on a single farm or field, but on multiple scales [78]. A higher ecological and economic resilience is obtained by producers who set specific goals for a stronger environmental performance [79]. Regenerative or intensive farming profiles tend to be less economically resilient compared to adaptive or sustainable models [80]. There is also evidence against hypothesis H3. Scherzinger et al. [77] prove that intensive land use reduces ecological multifunctionalities, in contrast to sustainable regimes. Jian et al. [81] notice a conflict between agricultural output and supporting ecosystem services under different scenarios on China’s Loess Plateau. They conclude that even “sustainable” land expansion can undermine resilience.
- (5)
- The main result of the KRLS model is that the effect of ALS on BER is nonlinear and heterogeneous. The PME distributions in Figure 5 and Figure 6 show a dual pattern: in some contexts, ALS exerts a negative effect on BER, reducing ecosystem stability, while in other contexts it can exert a positive effect on BER, supporting resilience. This mixed role of ALS nuances the results from the fixed-effects model, which only indicated a marginally positive average effect. The KRLS analysis deepens the understanding of why the literature often reports conflicting findings on agriculture–resilience linkages. This duality addresses our third hypothesis (H3), showing that it is only partially confirmed. The BER is appropriate for cross-country comparison, but it conceals local variations in ecological resilience. This limitation is relevant to the KRLS model, which captures heterogeneous relationships. We included both a choropleth map (Figure 2) and a quartile-based classification table (Table A1) to provide a clearer spatial dimension to the econometric analysis. Furthermore, this nonlinear evidence allows us to return explicitly to the research question posed in the Introduction: To what extent do land use and water stress determine ecosystem resilience in European countries? The KRLS findings show that while forest land consistently strengthens resilience (H1 supported) and water stress consistently undermines it (H2 supported), agricultural land represents a contingent driver whose impact depends on the underlying management regime. This insight is central to our study’s contribution, as it emphasizes that policies aimed at expanding agricultural areas must be conditional on adopting ecosystem-friendly practices (e.g., agroecology, buffer zones, and soil conservation).
- (6)
- The results of our study highlight that ecosystem resilience and biodiversity are strongly connected. In our study, with the help of BER, we quantify resilience as the ability of ecosystems to preserve species diversity under climate change. If biodiversity is lost, ecosystems become less resilient and are likely to collapse when faced with WS or changes in land use [57].
- (7)
- We can formulate some policy directions based on our findings. Reforestation programs and forest management policies can improve ecosystems’ resilience. Water management policies should consider boosting investments in protecting water-related ecosystems to strengthen the resilience of ecosystems and thus their biodiversity. Agricultural policies should support agroecological practices, and farmers should implement biodiversity-based strategies to be more supportive of nature.
The scatterplot in Figure 4 indicates a negative association between ALS and BER, while the DK fixed-effects estimates in Table 4 indicate a marginally significant positive coefficient. This apparent contradiction arises because the bivariate plot in Figure 4 captures only the unconditional correlation between the two variables. The econometric model controls for other determinants, FLS and WS, and unobserved country-specific effects. The partial effect of ALS is thereby isolated. From an econometric viewpoint, this can be seen as a suppression effect or the resolution of omitted variable bias [63]. The positive correlation between ALS and WS, combined with their negative correlation with BER (see Figure 5), can be a plausible explanation for the sign-change ALS-BER relation when moving from Figure 4 to Table 4. This sign-change is consistent with an omitted-variable effect.
Our study provides evidence that the resilience of ecosystems is influenced by land use for agriculture and forestry, but it has remained outside its scope to analyze the influence of green spaces within urban environments on ecosystem resilience due to the limitations of the available data for the countries and the period analyzed. Urban green spaces, like parks and green corridors, improve air and water quality and support biodiversity [82,83]. From the resilience point of view, urban green spaces have the role of a micro-refuge for species. They regulate ecosystems in densely populated areas [84].
5. Conclusions
This study approached biodiversity conservation and ecosystem resilience for thirty-three European countries. The empirical analysis proved that forest ecosystems sustain biodiversity and act as buffers against climate and environmental stressors, while scarcity of freshwater resources weakens ecosystems’ resilience, and agricultural expansion can either support or undermine ecosystem resilience, depending on the practices adopted. Although Europe as a continent is not classified as a region of high-water scarcity, some countries or regions within it (particularly Southern and Eastern European countries such as Spain, Italy, Greece, and parts of Hungary and Romania) face alarming levels of water stress, particularly during dry seasons or in areas that rely heavily on agricultural irrigation and rising temperatures linked to climate change.
One of the confirmed policy directions based on the results of the work is that of biodiversity actions for resilient and sustainable agriculture. From an applied perspective, we propose a policy to create biodiversity areas on at least 7% of grassland and arable land, while respecting the condition of forming connected semi-natural habitats, thus extending at the European continent scale the successful results of the pilot project [85] carried out between 2020 and 2023 in one of the European countries emphasized in our study as having the strongest ecosystem resilience, Austria. Regarding the direction of protecting, restoring, and enlarging Europe’s forests, a direction confirmed by our study’s results as being beneficial for strengthening ecosystem resilience, we propose the following policies: (1) the extension across all European countries of the policy proven to be effective in Sweden [86], namely detecting illegal tree cutting using information gathered from satellite imagery, as well as the policy already used in Portugal [86] of employing artificial intelligence to provide in real-time forest inventories and health status of trees; (2) a policy recognizing and rewarding individuals, forest owners, companies, and associations involved to participate in the planting of billions of additional trees over the next decade. Ultimately, the results of our paper argue for the necessity of sustainable water resource management to facilitate the improvement of ecosystem resilience. In this regard, we propose intensifying investment programs in water-saving technologies across Europe, as well as enhancing policies for the collection and treatment of urban wastewater, so that other European countries can follow the good practices implemented in Austria, Germany, the Netherlands, or Sweden, countries that have already achieved a 100% proportion of urban wastewater that meets sustainability requirements [87].
The implications of our findings, starting from the hypotheses tested in this paper, are significant. They show that land and water factors are the main determinants of ecological stability. The KRLS model proved that ecosystem resilience could be understood as a nonlinear and heterogeneous process, sensitive to local contexts. This means the necessity of designing flexible policies that balance agricultural expansion, water use, and forest and biodiversity conservation.
On a global scale, these conclusions support the objectives of the Kunming–Montreal Global Biodiversity Framework and the Sustainable Development Goals, centered on having nature on a recovery path, for the benefit of Earth and people. The results of our study can serve the European institutions involved in the design of ecosystem resilience policies, as well as relevant stakeholders beyond the European framework.
Author Contributions
Conceptualization, M.B. and I.G.; methodology, I.G.; software, I.G.; validation, M.B. and I.G.; formal analysis, M.B. and I.G.; investigation, M.B. and I.G.; resources, M.B. and I.G.; data curation, M.B. and I.G.; writing—original draft preparation, M.B. and I.G.; writing—review and editing, M.B. and I.G.; visualization, M.B. and I.G.; supervision, M.B. and I.G.; project administration, I.G.; funding acquisition, I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Mean Bioclimatic Ecosystem Resilience index (BER) in 33 European countries (2007–2024) and quartile classification.
Table A1.
Mean Bioclimatic Ecosystem Resilience index (BER) in 33 European countries (2007–2024) and quartile classification.
| Country | BER Mean (2007–2024) | Quartile Group |
|---|---|---|
| Denmark | 0.00 | Group 1 (lowest) |
| Hungary | 1.19 | Group 1 (lowest) |
| Lithuania | 4.01 | Group 1 (lowest) |
| Poland | 5.36 | Group 1 (lowest) |
| Belgium | 6.66 | Group 1 (lowest) |
| Serbia | 9.62 | Group 1 (lowest) |
| Romania | 10.20 | Group 1 (lowest) |
| The Netherlands | 13.27 | Group 1 (lowest) |
| Germany | 14.54 | Group 1 (lowest) |
| Latvia | 16.17 | Group 2 |
| Italy | 16.48 | Group 2 |
| Estonia | 16.58 | Group 2 |
| Czech Republic | 17.16 | Group 2 |
| Spain | 20.66 | Group 2 |
| France | 21.37 | Group 2 |
| Bulgaria | 22.80 | Group 2 |
| Slovakia | 23.19 | Group 2 |
| United Kingdom | 27.94 | Group 3 |
| Greece | 28.35 | Group 3 |
| Albania | 28.46 | Group 3 |
| Croatia | 28.84 | Group 3 |
| Luxembourg | 30.52 | Group 3 |
| Portugal | 35.62 | Group 3 |
| North Macedonia | 37.46 | Group 3 |
| Bosnia and Herzegovina | 38.75 | Group 3 |
| Slovenia | 45.71 | Group 4 (highest) |
| Ireland | 47.08 | Group 4 (highest) |
| Austria | 47.26 | Group 4 (highest) |
| Switzerland | 54.04 | Group 4 (highest) |
| Finland | 57.85 | Group 4 (highest) |
| Sweden | 63.16 | Group 4 (highest) |
| Iceland | 100.00 | Group 4 (highest) |
| Norway | 100.00 | Group 4 (highest) |
References
- Convention on Biological Diversity. Kunming-Montreal Global Biodiversity Framework. Fifteenth Meeting of the Conference of the Parties (COP 15). 2022. Available online: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-04-en.pdf (accessed on 2 August 2025).
- National Research Council US. Committee on Noneconomic and Economic Value of Biodiversity. Perspectives on Biodiversity: Valuing Its Role in an Everchanging World; National Academies Press (US): Washington, DC, USA, 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK224405/ (accessed on 10 August 2025).
- Mace, G.; Norris, K.; Fitter, A. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef]
- Giam, X. Global Biodiversity Loss from Tropical Deforestation. Proc. Natl. Acad. Sci. USA 2017, 114, 5775–5777. [Google Scholar] [CrossRef]
- Murray, D.L.; Peers, M.J.L.; Majchrzak, Y.N.; Wehtje, M.; Ferreira, C.; Pickles, R.S.A.; Row, J.R.; Thornton, H.D. Continental divide: Predicting climate-mediated fragmentation and biodiversity loss in the boreal forest. PLoS ONE 2017, 12, e0176706. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yiew, T.; Habibullah, M.S.; Chen, J.; Mat Kamal, S.N.; Saud, N.A. Research trends in biodiversity loss: A bibliometric analysis. Environ. Sci. Pollut. Res. 2023, 30, 2754–2770. [Google Scholar] [CrossRef] [PubMed]
- Bateman, I.; Balmford, A. Current conservation policies risk accelerating biodiversity loss. Nature 2023, 618, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, E.; Collen, B.; Barausse, A.; Blanchard, J.L.; Costelloe, B.T.; Sullivan, K.M.E.; Underwood, F.M.; Burn, R.W.; Fritz, Z.; Jones, L.P.G.; et al. Making robust policy decisions using global biodiversity indicators. PLoS ONE 2012, 7, e41128. [Google Scholar] [CrossRef]
- Swiss Re Institute. Biodiversity and Ecosystems Services Index: Measuring the Value of Nature. 2025. Available online: https://www.swissre.com/institute/research/topics-and-risk-dialogues/climate-and-natural-catastrophe-risk/expertise-publication-biodiversity-and-ecosystems-services.html#/countries/fr (accessed on 1 August 2025).
- Watson, J.E.M.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, C.; Thompson, I.; Ray, J.C.; Murray, K.; Salazar, A.; et al. The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef]
- Di Marco, M.; Ferrier, S.; Harwood, T.D.; Hoskins, A.J.; Watson, J.E.M. Wilderness areas halve the extinction risk of terrestrial biodiversity. Nature 2019, 573, 582–585. [Google Scholar] [CrossRef]
- Veach, V.; Moilanen, A.; Di Minin, E. Threats from urban expansion, agricultural transformation and forest loss on global conservation priority areas. PLoS ONE 2017, 12, e0188397. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef]
- Mokany, K.; Ferrier, S.; Harwood, T.D.; Ware, C.; Di Marco, M.; Grantham, H.S.; Venter, O.; Hoskins, A.J.; Watson, J.E. Reconciling global priorities for conserving biodiversity habitat. Proc. Natl. Acad. Sci. USA 2020, 117, 9906–9911. [Google Scholar] [CrossRef]
- Walker, B.H.; Holling, C.S.; Carpenter, S.R.; Kinzig, A.P. Resilience, adaptability and transformability in social—Ecological systems. Ecol. Soc. 2004, 9, 5. [Google Scholar] [CrossRef]
- Sterk, M.; van de Leemput, I.; Peeters, E. How to conceptualize and operationalize resilience in socio-ecological systems? Curr. Opin. Env. Sust. 2017, 28, 108–113. [Google Scholar] [CrossRef]
- Cantarello, E.; Newton, A.C.; Martin, P.A.; Evans, P.M.; Gosal, A.; Lucash, M.S. Quantifying resilience of multiple ecosystem services and biodiversity in a temperate forest landscape. Ecol. Evol. 2017, 7, 9661–9675. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.; Mackey, B.; McNulty, S.; Mosseler, A. Forest Resilience, Biodiversity, and Climate Change: A Synthesis of the Biodiversity/Resilience/Stability Relationship in Forest Ecosystems. Secretariat of the Convention on Biological Diversity. 2009. Available online: https://www.cbd.int/doc/publications/cbd-ts-43-en.pdf (accessed on 16 September 2025).
- Oliver, T.; Heard, M.; Isaac, N.; Roy, D.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, D. The role of biodiversity in ecosystem resilience. IOP Conf. Series. Earth Environ. Sci. 2022, 1072, 012012. [Google Scholar] [CrossRef]
- Bryant, T.; Waring, K.; Sánchez, M.A.; Bradford, J.A. Framework for Quantifying Resilience to Forest Disturbance. Front. Forests Glob. Change 2019, 2, 56. [Google Scholar] [CrossRef]
- Ferrier, S.; Harwood, T.D.; Ware, C.; Hoskins, A.J. A globally applicable indicator of the capacity of terrestrial ecosystems to retain biological diversity under climate change: The bioclimatic ecosystem resilience index. Ecol. Ind. 2020, 117, 106554. [Google Scholar] [CrossRef]
- RS The Royal Society. How Do Humans Affect Biodiversity? 2025. Available online: https://royalsociety.org/news-resources/projects/biodiversity/human-impact-on-biodiversity/ (accessed on 1 August 2025).
- Harwood, T. Landscape Connectedness Under Climate Change in Oxfordshire Implications of the Bioclimatic Ecosystem Resilience Index, Oxford: Environmental Change Institute, University of Oxford. 2024. Available online: https://naturerecovery.ox.ac.uk/wp-content/uploads/2024/08/Landscape-Connectedness-Under-Climate-Change-in-Oxfordshire.pdf (accessed on 17 September 2025).
- Liao, L.; Ma, E.; Long, H.; Peng, X. Land use transition and its ecosystem resilience response in China during 1990–2020. Land 2023, 12, 141. [Google Scholar] [CrossRef]
- Haines-Young, R. Land use and biodiversity relationships. Land Use Policy 2009, 26, S178–S186. [Google Scholar] [CrossRef]
- Ma, S.; Wang, L.J.; Jiang, J.; Zhao, Y.G. Direct and indirect effects of agricultural expansion and landscape fragmentation processes on natural habitats. Agric. Ecossyst. Env. 2023, 353, 108555. [Google Scholar] [CrossRef]
- Lanz, B.; Dietz, S.; Swanson, T. The Expansion of Modern Agriculture and Global Biodiversity Decline: An Integrated Assessment. Ecol. Econ. Env. 2018, 144, 260–277. [Google Scholar] [CrossRef]
- Gonthier, D.J.; Ennis, K.K.; Farinas, S.; Hsieh, H.Y.; Iverson, A.L.; Batáry, P.; Rudolphi, J.; Tscharntke, T.; Cardinale, B.J.; Perfecto, I. Biodiversity conservation in agriculture requires a multi-scale approach. Proc. Biol. Sci. 2014, 281, 20141358. [Google Scholar] [CrossRef]
- Perfecto, I.; Vandermeer, J. The agroecological matrix as alternative to the land-sparing/agriculture intensification model. Proc. Natl. Acad. Sci. USA 2010, 107, 5786–5791. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C. Reframing the land-sparing/land-sharing de bate for biodiversity conservation. Ann. N. Y. Acad. Sci. 2015, 1355, 52–76. [Google Scholar] [CrossRef] [PubMed]
- Loconto, A.; Desquilbet, M.; Moreau, T.; Couvet, D.; Dorin, B. The land sparing—Land sharing controversy: Tracing the politics of knowledge. Land Use Policy 2020, 96, 103610. [Google Scholar] [CrossRef]
- Augustiny, E.; Frehner, A.; Green, A.; Mathys, A.; Rosa, F.; Pfister, S.; Muller, A. Empirical evidence supports neither land sparing nor land sharing as the main strategy to manage agriculture–biodiversity tradeoffs. PNAS Nexus 2025, 4, pgaf251. [Google Scholar] [CrossRef]
- Ivits, E.; Orlitova, E.; Milego, R.; Maucha, G.; Kosztra, B.; Mancosu, E.; Fons, J.; Gregor, M.; Löhnertz, M.; Hazeu, G. Twenty Years of Land Accounts in Europe. Land 2024, 13, 1350. [Google Scholar] [CrossRef]
- WRI World Resources Institute’s Global Forest Watch Platform. Fires Drove Record-Breaking Tropical Forest Loss in 2024. Data Created and Updated by Peter Potapov, Svetlana Turubanova and Sasha Tyukavina—University of Maryland’s GLAD Lab. 2025. Available online: https://gfr.wri.org/latest-analysis-deforestation-trends?utm_campaign=tcl2024&utm_medium=bitly&utm_source=GFWHomepage (accessed on 3 August 2025).
- Peng, Z.; Duan, Z.; Wang, J. Balancing agricultural productivity and ecosystem resilience: A multi-species interaction and simulation framework. E3S Web Conf. 2025, 630, 02020. [Google Scholar] [CrossRef]
- FAO and UN Water Progress on Level of Water Stress. Global Status and Acceleration Needs for SDG Indicator 6.4.2; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Incorporating Environmental Flows into “Water Stress” Indicator 6.4.2—Guidelines for a Minimum Standard Method for Global Reporting; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; 32p. [Google Scholar]
- Lewandrowski, W.; Stevens, J.C.; Webber, B.L.; Dalziell, E.L.; Trudgen, M.S.; Bateman, A.M.; Erickson, T.E. Global change impacts on arid zone ecosystems: Seedling establishment processes are threatened by temperature and water stress. Ecol. Evol. 2021, 11, 8071–8084. [Google Scholar] [CrossRef]
- Li, D.; Wu, S.; Liu, L.; Zhang, Y.; Li, S. Vulnerability of the global terrestrial ecosystems to climate change. Global Change Biol. 2018, 24, 4095–4106. [Google Scholar] [CrossRef] [PubMed]
- EEA European Environment Agency. Land Use. 2020. Available online: https://www.eea.europa.eu/themes/landuse/intro (accessed on 2 August 2025).
- Driscoll, J.C.; Kraay, A.C. Consistent covariance matrix estimation with spatially dependent panel data. Rev. Econ. Stat. 1998, 80, 549–560. [Google Scholar] [CrossRef]
- Cameron, A.C.; Miller, D.L. A practitioner’s guide to cluster-robust inference. J. Human Res. 2015, 50, 317–372. [Google Scholar] [CrossRef]
- Baltagi, B.H. Econometric Analysis of Panel Data, 4th ed.; John Wiley & Sons Ltd.: Chichester, UK, 2008. [Google Scholar]
- Hausman, J.A. Specification tests in econometrics. Econometrica 1978, 46, 1251–1271. [Google Scholar] [CrossRef]
- Newey, W.K.; West, K.D. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica 1987, 55, 703–708. [Google Scholar] [CrossRef]
- Hoechle, D. Robust standard errors for panel regressions with cross-sectional dependence. Stata J. 2007, 7, 281–312. [Google Scholar] [CrossRef]
- Liang, K.Y.; Zeger, S.L. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Angrist, J.D.; Pischke, J.-S. Mostly Harmless Econometrics: An Empiricist’s Companion, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2009. [Google Scholar]
- Lütkepohl, H.; Xu, F. The role of the log transformation in forecasting economic variables. Empir. Econ. 2012, 42, 619–638. [Google Scholar] [CrossRef]
- Hainmueller, J.; Hazlett, C. Kernel regularized least squares: Reducing misspecification bias with a flexible and interpretable machine learning approach. Political Anal. 2014, 22, 143–148. [Google Scholar] [CrossRef]
- CSIRO Commonwealth Scientific and Industrial Research Organisation. BERI v2: Bioclimatic Ecosystem Resilience Index: 30s Global Time Series. 2024. Available online: https://data.csiro.au/collection/csiro:54238 (accessed on 20 July 2025).
- FAO Food and Agriculture Organization of the United Nations. Data. Land Use. 2025. Available online: https://www.fao.org/faostat/en/#data/RL (accessed on 1 July 2025).
- FAO Food and Agriculture Organization of the United Nations. Data. SDG Indicators. 2025. Available online: https://www.fao.org/faostat/en/#data/SDGB (accessed on 1 July 2025).
- Tscharnkte, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; et al. (Eds.) Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Zenodo: Geneva, Switzerland, 2019. [Google Scholar] [CrossRef]
- FAO Food and Agriculture Organization of the United Nations. Global Forest Resources Assessment 2020: Main Report; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Townsend, J.R.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Ciais, P.; Wigneron, J.P.; Gentine, P.; Feldman, A.F.; Makowski, D.; Viovy, N.; Kemanian, A.R.; Goll, D.S.; Stoy, P.C.; et al. Global critical soil moisture thresholds of plant water stress. Nat. Commun. 2024, 15, 4826. [Google Scholar] [CrossRef] [PubMed]
- Moss, W.E.; Crausbay, S.D.; Rangwala, I.; Wason, J.W.; Trauernicht, C.; Stevens-Rumann, C.S.; Sala, A.; Rottler, C.M.; Pederson, G.T.; Miller, B.W. Drought as an emergent driver of ecological transformation in the twenty-first century. BioScience 2024, 74, 524–538. [Google Scholar] [CrossRef]
- Fatecha, B.V.; Martínez-Vilalta, J.; Mencuccini, M.; Poyatos, R. Multi-biome assessment of tree water use resilience to drought. Agric. For. Meteorol. 2025, 372, 110666. [Google Scholar] [CrossRef]
- Wooldridge, J.M. Econometric Analysis of Cross Section and Panel Data, 2nd ed.; MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Pesaran, M.H. General Diagnostic Tests for Cross Section Dependence in Panels; Cambridge Working Papers in Economics No. 0435; University of Cambridge: Cambridge, UK, 2004. [Google Scholar]
- Osuma, G.; Yusuf, N. Towards an optimal renewable energy mix for the European Union: Enhancing energy security and sustainability. J. Knowl. Econ. 2025. [Google Scholar] [CrossRef]
- Machefer, M.; Zampieri, M.; van der Velde, M.; Dentener, F.; Claverie, M.; d’Andrimont, R. Earth observation based multi-scale analysis of crop diversity in the European Union: First insights for agro-environmental policies. arXiv 2024. [Google Scholar] [CrossRef]
- EEA European Environment Agency. Europe’s State of Water 2024: The Need for Improved Water Resilience; EEA Report 07/2024; European Environment Agency: Copenhagen, Denmark, 2024. [CrossRef]
- EPI. Environmental Performance Index. Available online: https://epi.yale.edu/downloads/2024-epi-report-20250106.pdf (accessed on 6 August 2025).
- Block, S.; Emerson, J.W.; Esty, D.C.; de Sherbinin, A.; Wendling, Z.A.; Kurczynski, K.; Lin, F.; Xu, C.; Wu, N.; Harwood, J.; et al. Environmental Performance Index; Yale Center for Environmental Law & Policy: New Haven, CT, USA, 2024. [Google Scholar]
- Pretzsch, H.; Hilmers, T. Structural diversity and carbon stock of forest stands: Tradeoff as modified by silvicultural thinning. Eur. J. Forest Res. 2024, 144, 775–796. [Google Scholar] [CrossRef]
- Pretzsch, H.; Hilmers, T.; del Río, M. The effect of structural diversity on the self-thinning line, yield level, and density-growth relationship in even-aged stands of Norway spruce. Forest. Ecol. Manag. 2024, 556, 121736. [Google Scholar] [CrossRef]
- Georgescu, I.; Kinnunen, J.; Nica, I. Assessing Forest Conservation for Finland: An ARDL-Based Evaluation. Sustainability 2024, 16, 612. [Google Scholar] [CrossRef]
- Georgescu, I.; Nica, I. Evaluating the Determinants of Deforestation in Romania: Empirical Evidence from an Autoregressive Distributed Lag Model and the Bayer–Hanck Cointegration Approach. Sustainability 2024, 16, 5297. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Trugman, A.T.; Badgley, G.; Anderson, C.M.; Bartuska, A.M.; Ciais, P.; Cullenward, D.; Field, C.B.; Freeman, J.; Goetz, S.J.; et al. Climate-driven risks to the climate mitigation potential of forests. Science 2020, 368, eaaz7005. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, S.; Singh, V.P.; Huang, Q.; Zhang, H.; Leng, G.; Gao, L.; Li, P.; Guo, W.; Peng, J. Terrestrial ecosystem resilience to drought stress and driving mechanisms thereof in the Yellow River Basin, China. J. Hydrol. 2025, 649, 132480. [Google Scholar] [CrossRef]
- Santibáñez, P.; Zamora, R.; Franchi, J.; Montaner-Fernández, D.; Santibáñez, F. Bioclimatic stress index: A tool to evaluate climate change impact on Mediterranean arid ecosystems. J. Arid Environ. 2025, 229, 105376. [Google Scholar] [CrossRef]
- Scherzinger, F.; Schädler, M.; Reitz, T.; Yin, R.; Auge, H.; Merbach, I.; Roscher, C.; Harpole, W.S.; Blagodatskaya, E.; Siebert, J.; et al. Sustainable land management enhances ecological and economic multifunctionality under ambient and future climate. Nat. Comm. 2024, 15, 4930. [Google Scholar] [CrossRef]
- Arndt, M.; Helming, K. Agricultural diversification across spatial levels—A contribution to resilience and sustainability? Agric. Ecosyst. Environ. 2025, 385, 109547. [Google Scholar] [CrossRef]
- Yletyinen, J.; Kuhmonen, I.; Stahlmann-Brown, P. Resilient and sustainable natural resource production: How are farmers and foresters coping? Ecol. Soc. 2024, 29, 6. [Google Scholar] [CrossRef]
- Volkov, A.; Morkūnas, M.; Žičkienė, A.; Rudienė, E. Will European agriculture be resilient? Assessment of the share and economic resilience levels of the future European farmer profiles: Evidence from Lithuania. Agric. Food Econ. 2025, 13, 16. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, H.; Zhang, J.; Dong, B. Trade-offs between agricultural production and ecosystem services under different land management scenarios in the Loess Plateau of China. Sci. Rep. 2025, 15, 21385. [Google Scholar] [CrossRef]
- Kabisch, N.; Qureshi, S.; Haase, D. Human–environment interactions in urban green spaces—A systematic review of contemporary issues and prospects for future research. Environ. Impact Assess. Rev. 2015, 50, 25–34. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Barton, D.N. Classifying and valuing ecosystem services for urban planning. Ecol. Econ. 2013, 86, 235–245. [Google Scholar] [CrossRef]
- Elmqvist, T.; Setälä, H.; Handel, S.N.; van der Ploeg, S.; Aronson, J.; Blignaut, J.N.; Gómez-Baggethun, E.; Nowak, D.J.; Kronenberg, J.; de Groot, R. Benefits of restoring ecosystem services in urban areas. Curr. Opin. Environ. Sustain. 2015, 14, 101–108. [Google Scholar] [CrossRef]
- EU Cap Network. Biodiversity Actions at Scale—Inspiring Examples from Member States. Output of TG on Enhancing Biodiversity on Farmland for Improved Resilience 2025. Available online: https://eu-cap-network.ec.europa.eu/publications/biodiversity-actions-scale-inspiring-examples-member-states_en#section--resources (accessed on 22 September 2025).
- European Commission. New EU Forest Strategy for 2030. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52021DC0572 (accessed on 22 September 2025).
- WISE Freshwater Information System for Europe. Country Profiles on Urban Waste Water Treatment 2020. Available online: https://water.europa.eu/freshwater/countries/uwwt (accessed on 22 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).