Thailand’s Urban Forestry Programs Are Assisted by Calculations of Their Ecological Properties and Economic Values
Abstract
1. Introduction
2. Materials and Methods
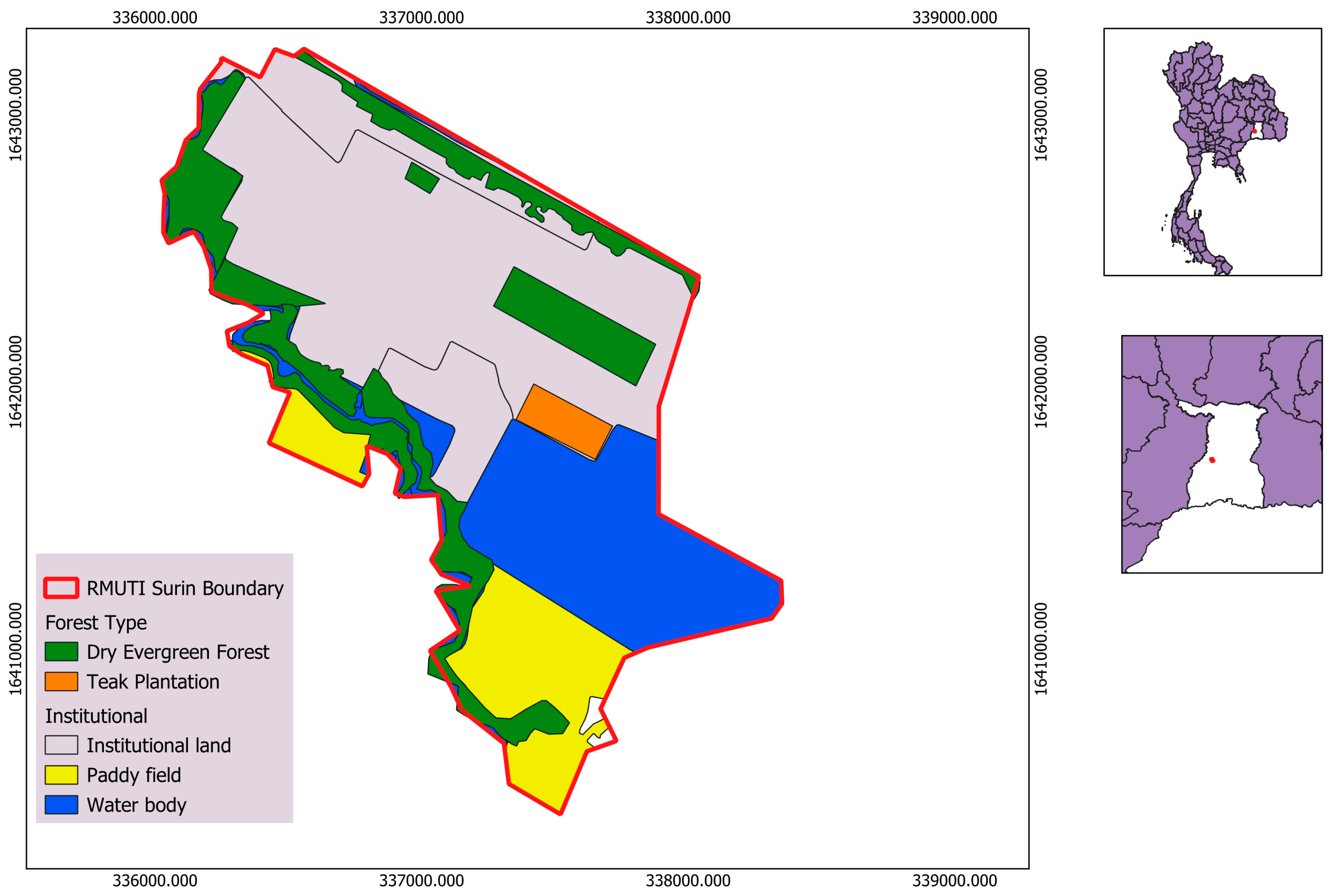
2.1. Research Area
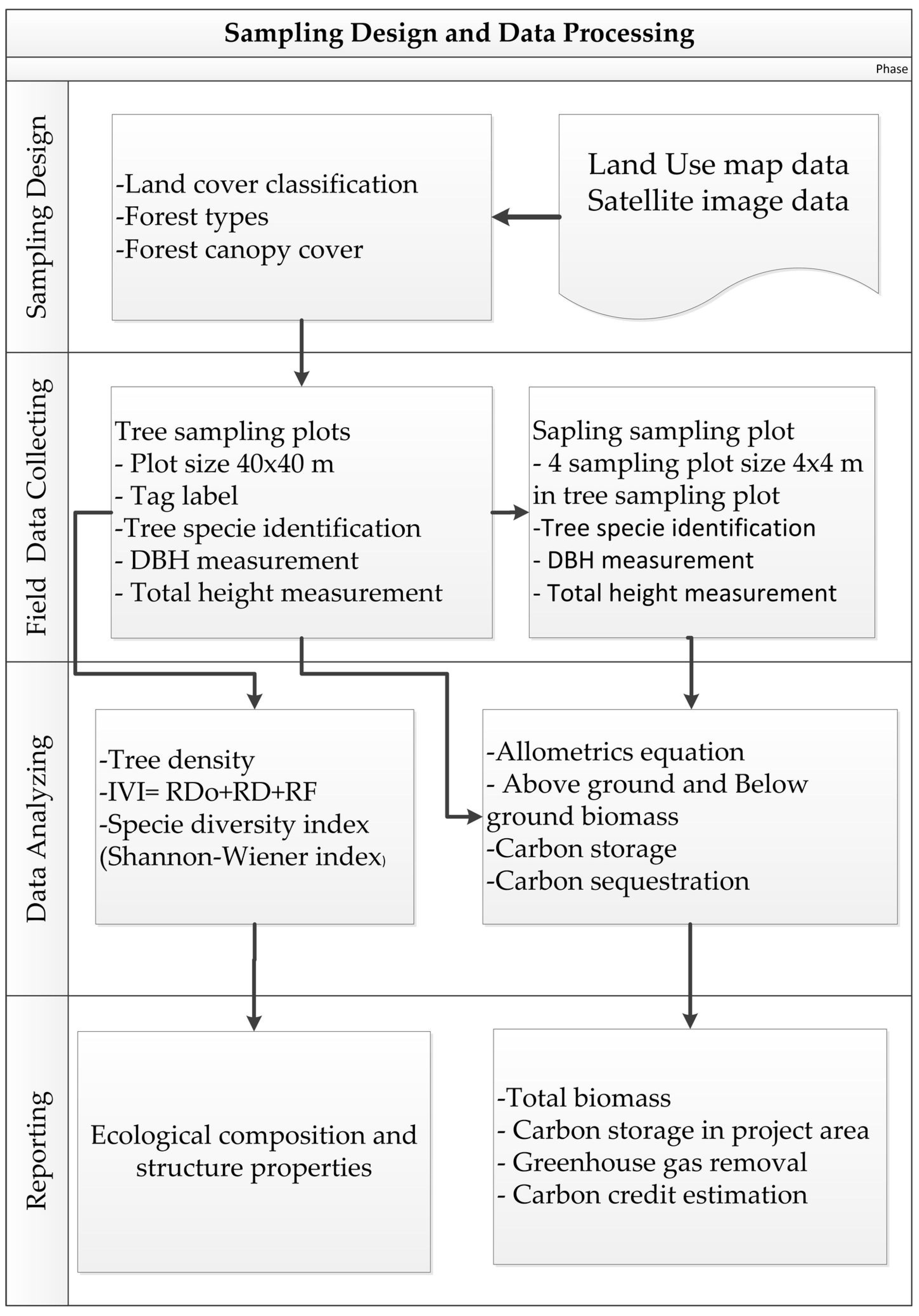
2.2. Data Collection and Analysis
2.2.1. Forest Boundary Delineation and Stratification
2.2.2. Plot Sampling Design and Layout
2.2.3. Plant Diversity Survey
2.2.4. Analysis of Biomass and Carbon Storage
2.3. Calculation of the Value from Carbon Credit Trading
3. Results and Discussion
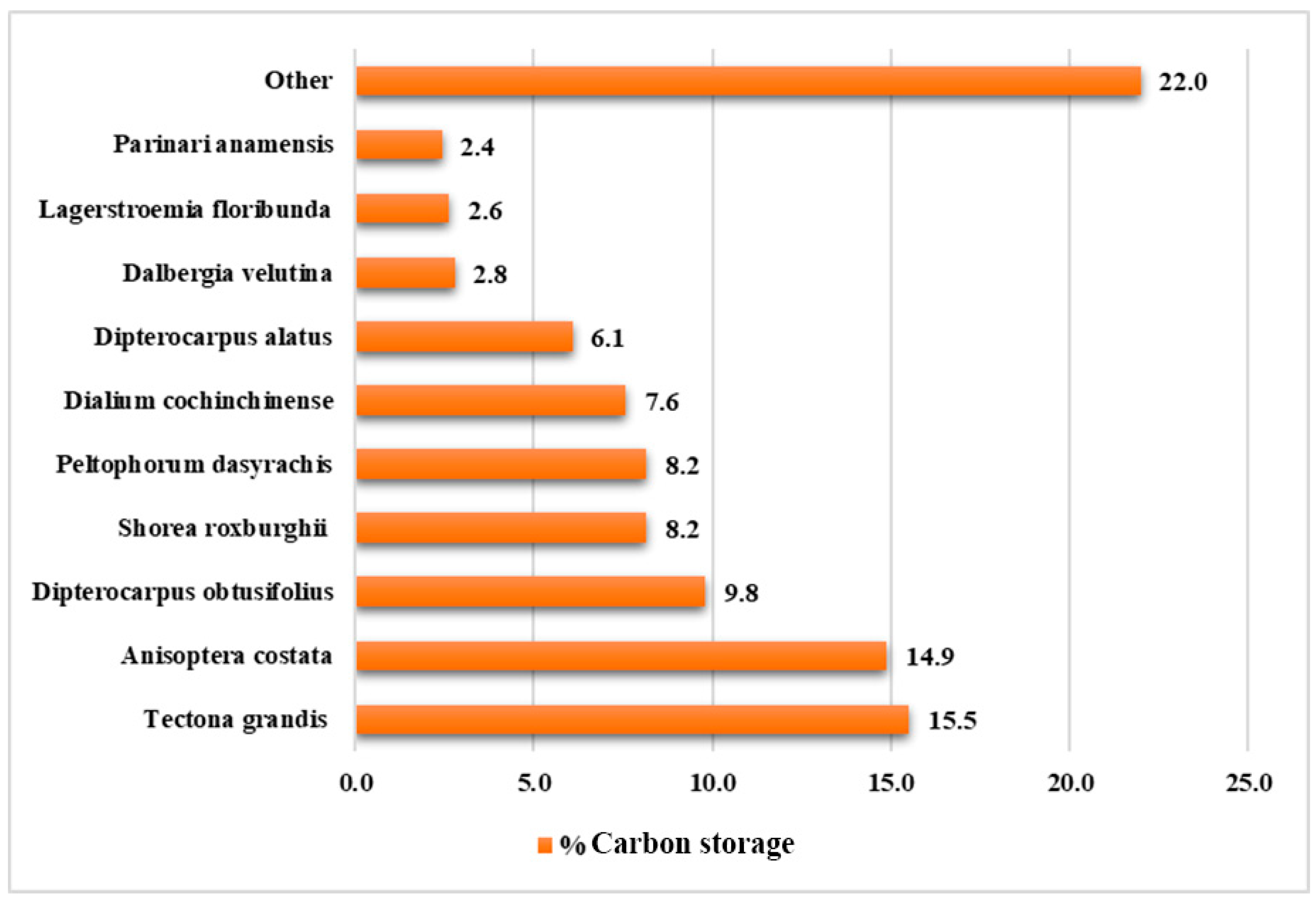
3.1. Plant Species Diversity
3.2. Biomass Quantity and Carbon Storage Studies
3.3. Calculation of the Value from Carbon Credit Trading
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Plant Species Diversity
| Family Name | Scientific Name | Number of Trees | Mean Density (Tree·ha−1) |
| Anacardiaceae | Buchanania lanzan Spreng | 8 | 0.128 |
| Gluta usitata (Wall.) Ding Hou. | 5 | 0.080 | |
| Lannea coromandelica (Houtt.) Merr. | 2 | 0.032 | |
| Mangifera caloneura Kurz | 4 | 0.064 | |
| Semecarpus albescens Kurz | 3 | 0.048 | |
| Annonaceae | Hubera cerasoides (Roxb.) Chaowasku | 1 | 0.016 |
| Melodorum fruticosum Lour | 132 | 2.112 | |
| Polyalthia viridis Craib | 1 | 0.016 | |
| Uvaria rufa Blume | 6 | 0.096 | |
| Xylopia vielana Pierre. | 4 | 0.064 | |
| Antalaceae | Scleropyrum pentandrum (Dennst.) Mabb. | 5 | 0.080 |
| Apocynaceae | Willughbeia edulis Roxb. | 45 | 0.720 |
| Wrightia arborea (Dennst.) Mabb | 12 | 0.192 | |
| Burseraceae | Canarium subulatum Guillaumin | 2 | 0.032 |
| Calophyllaceae | Mammea siamensis (T. Anderson) Kosterm | 4 | 0.064 |
| Cannabaceae | Trema orientalis (L.) Blume | 1 | 0.016 |
| Celastraceae | Celastrus paniculatus Willd | 1 | 0.016 |
| Chrysobalanaceae | Parinari anamensis Hance | 41 | 0.656 |
| Clusiaceae | Garcinia cowa Roxb. ex Choisy | 44 | 0.704 |
| Dilleniaceae | Dillenia ovata Wall. ex Hook. f.& Thomson | 1 | 0.016 |
| Dipterocarpaceae | Anisoptera costata Korth | 116 | 1.856 |
| Dipterocarpus alatus Roxb | 88 | 1.408 | |
| Dipterocarpus intricatus Dyer | 9 | 0.144 | |
| Dipterocarpus obtusifolius Teijsm.ex Miq. | 46 | 0.736 | |
| Shorea roxburghii G.Don | 64 | 1.024 | |
| Ebenaceae | Diospyros castanea Fletcher | 20 | 0.320 |
| Diospyros ferrea (Willd.) Bakh. | 1 | 0.016 | |
| Diospyros filipendula Pierre ex Lecomte | 8 | 0.128 | |
| Diospyros oblonga Wall. ex G.Don. | 10 | 0.160 | |
| Diospyros rhodocalyx Kurz | 2 | 0.032 | |
| Elaeocarpaceae | Elaeocarpus sphaericus (Gaertn.) K.Schum. | 7 | 0.112 |
| Euphorbiaceae | Baccaurea ramiflora Lour. | 1 | 0.016 |
| Aporosa villosa (Wall. ex Lindl.) Bail | 5 | 0.080 | |
| Mallotus thorelii Gagnep. | 3 | 0.048 | |
| Suregada multiflorum (A.Juss.) Baill | 14 | 0.224 | |
| Fabaceae | Acacia auriculaeformis A. Cunn. ex Benth. | 25 | 0.400 |
| Acacia comosa Gangep. | 21 | 0.336 | |
| Albizia lebbeck Benth | 1 | 0.016 | |
| Albizia lebbeckoides (DC.) Benth. | 2 | 0.032 | |
| Butea superba Roxb. | 2 | 0.032 | |
| Dalbergia cochinchinensis Pierre | 18 | 0.288 | |
| Dalbergia nigrescens Kurz. | 12 | 0.192 | |
| Dalbergia velutina Benth. | 109 | 1.744 | |
| Dialium cochinchinense Pierre | 42 | 0.672 | |
| Leucaena leucocephala (Lam.) de Wit | 1 | 0.016 | |
| Peltophorum dasyrachis (Miq.) Kurz | 31 | 0.496 | |
| Pterocarpus macrocarpus Kurz | 4 | 0.064 | |
| Sindora siamensis Teijsm. ex Miq | 9 | 0.144 | |
| Xylia xylocarpa (Roxb.) Taub. | 12 | 0.192 | |
| Fagaceae | Lithocarpus sp. | 20 | 0.320 |
| Flacourtiaceae | Casearia grewiifolia Vent | 4 | 0.064 |
| Hypericaceae | Cratoxylum prunifolium Dyer | 11 | 0.176 |
| Irvingiaceae | Irvingia malayana Oliv. ex A. Benn. | 15 | 0.240 |
| Labiatae | Hymenopyramis parvifolia Moldenke | 6 | 0.096 |
| Vitex pinnata L. | 1 | 0.016 | |
| Lamiaceae | Tectona grandis Linn.f. | 194 | 3.104 |
| Lauraceae | Litsea glutinosa (Lour.) C.B.Robinson | 2 | 0.032 |
| Lecythidaceae | Barringtonia acutangula (L.) Gaertn. | 1 | 0.016 |
| Loganiaceae | Fagraea fragrans Roxb | 7 | 0.112 |
| Lythraceae | Lagerstroemia floribunda Jack | 25 | 0.400 |
| Lagerstroemia speciosa (L.) Pers | 1 | 0.016 | |
| Malvaceae | Bombax anceps Pierre | 2 | 0.032 |
| Melastomataceae | Memecylon edule Roxb. | 7 | 0.112 |
| Memecylon ovatum Smith | 11 | 0.176 | |
| Meliaceae | Azadirachta indica A. Juss. | 5 | 0.080 |
| Moraceae | Streblus asper Lour | 206 | 3.296 |
| Myrtaceae | Eucalyptus globulus Labill. | 4 | 0.064 |
| Syzygium cinereum (Kurz) Chantar. & J. Parn. | 31 | 0.496 | |
| Tristaniopsis burmanica (Griff.) Peter G.Wilson & J.T.Waterh | 5 | 0.080 | |
| Ochnaceae | Gomphia serrata (Gaertn.) Kanis | 1 | 0.016 |
| Phyllanthaceae | Phyllanthus angkorensis Beille | 9 | 0.144 |
| Rhamnaceae | Ziziphus oenopolia (L.) Mill. | 2 | 0.032 |
| Zizyphus cambodiana Pierre | 1 | 0.016 | |
| Rhizophoraceae | Carallia brachiata (Lour.) Merr | 10 | 0.160 |
| Rubiaceae | Ixora ebarbata Craib | 6 | 0.096 |
| Mitragyna hirsuta Havil | 2 | 0.032 | |
| Oxyceros horridus Lour | 14 | 0.224 | |
| Salicaceae | Salix tetrasperma Roxb. | 1 | 0.016 |
| Sapindacea | Filicium decipiens (Wight & Arn.) Thwaites & Hook | 10 | 0.160 |
| Sladeniaceae | Sladenia celastrifolia Kurz | 4 | 0.064 |
| Theaceae | Schima wallichii (DC.) Korth. | 14 | 0.224 |
| Tiliaceae | Microcos tomentosa Smith | 141 | 2.256 |
| Total | 1778 | 28.448 |
Appendix B. The Quantitative Structure of Trees
| Scientific Name | RD | RDo | RF | IVI |
|---|---|---|---|---|
| Tectona grandis Linn.f. | 10.894 | 16.255 | 0.949 | 28.098 |
| Streblus asper Lour | 11.508 | 6.457 | 1.899 | 19.864 |
| Anisoptera costata Korth | 6.480 | 8.830 | 1.899 | 17.209 |
| Microcos tomentosa Smith | 7.877 | 5.671 | 2.848 | 16.396 |
| Melodorum fruticosum Lour | 7.374 | 3.620 | 1.899 | 12.893 |
| Shorea roxburghii G.Don | 3.575 | 6.125 | 2.215 | 11.915 |
| Dalbergia velutina Benth. | 6.089 | 3.469 | 2.215 | 11.774 |
| Dipterocarpus alatus Roxb | 4.916 | 5.665 | 0.949 | 11.531 |
| Dipterocarpus obtusifolius Teijsm.ex Miq. | 2.570 | 5.846 | 1.899 | 10.314 |
| Dialium cochinchinense Pierre | 2.346 | 4.593 | 1.582 | 8.522 |
| Peltophorum dasyrachis (Miq.) Kurz | 1.732 | 3.832 | 1.899 | 7.462 |
| Parinari anamensis Hance | 2.291 | 2.387 | 2.215 | 6.893 |
| Lagerstroemia floribunda Jack | 1.397 | 1.970 | 2.215 | 5.581 |
| Syzygium cinereum (Kurz) Chantar. & J. Parn. | 1.732 | 1.453 | 2.215 | 5.400 |
| Garcinia cowa Roxb. ex Choisy | 2.458 | 1.568 | 1.266 | 5.292 |
| Willughbeia edulis Roxb. | 2.514 | 1.328 | 1.266 | 5.108 |
| Dalbergia cochinchinensis Pierre | 1.006 | 0.838 | 2.215 | 4.059 |
| Lithocarpus sp. | 1.117 | 1.069 | 1.582 | 3.769 |
| Diospyros castanea Fletcher | 1.117 | 0.499 | 1.899 | 3.515 |
| Acacia auriculaeformis A. Cunn. ex Benth. | 1.397 | 1.377 | 0.633 | 3.407 |
| Dipterocarpus intricatus Dyer | 0.503 | 1.000 | 1.899 | 3.401 |
| Irvingia malayana Oliv. ex A. Benn. | 0.838 | 0.497 | 1.899 | 3.234 |
| Wrightia arborea (Dennst.) Mabb | 0.670 | 0.636 | 1.899 | 3.205 |
| Diospyros oblonga Wall. ex G.Don. | 0.559 | 0.330 | 2.215 | 3.103 |
| Sindora siamensis Teijsm. ex Miq | 0.503 | 0.581 | 1.899 | 2.982 |
| Dalbergia nigrescens Kurz. | 0.670 | 0.727 | 1.582 | 2.980 |
| Acacia comosa Gangep. | 1.173 | 0.827 | 0.949 | 2.950 |
| Carallia brachiata (Lour.) Merr | 0.559 | 0.610 | 1.582 | 2.751 |
| Cratoxylum prunifolium Dyer | 0.615 | 0.457 | 1.582 | 2.654 |
| Suregada multiflorum (A.Juss.) Baill | 0.782 | 0.588 | 1.266 | 2.636 |
| Buchanania lanzan Spreng | 0.447 | 0.495 | 1.582 | 2.524 |
| Oxyceros horridus Lour | 0.782 | 0.351 | 1.266 | 2.399 |
| Xylia xylocarpa (Roxb.) Taub. | 0.670 | 0.453 | 1.266 | 2.389 |
| Gluta usitata (Wall.) Ding Hou. | 0.279 | 0.670 | 1.266 | 2.216 |
| Elaeocarpus sphaericus (Gaertn.) K.Schum. | 0.391 | 0.504 | 1.266 | 2.161 |
| Schima wallichii (DC.) Korth. | 0.782 | 0.410 | 0.949 | 2.142 |
| Filicium decipiens (Wight & Arn.)Thwaites & Hook | 0.559 | 0.444 | 0.949 | 1.952 |
| Memecylon ovatum Smith | 0.615 | 0.371 | 0.949 | 1.935 |
| Diospyros filipendula Pierre ex Lecomte | 0.447 | 0.205 | 1.266 | 1.917 |
| Ixora ebarbata Craib | 0.335 | 0.242 | 1.266 | 1.843 |
| Fagraea fragrans Roxb | 0.391 | 0.499 | 0.949 | 1.839 |
| Memecylon edule Roxb. | 0.391 | 0.165 | 1.266 | 1.822 |
| Mangifera caloneura Kurz | 0.223 | 0.563 | 0.949 | 1.736 |
| Xylopia vielana Pierre. | 0.223 | 0.205 | 1.266 | 1.694 |
| Aporosa villosa (Wall. ex Lindl.) Bail | 0.279 | 0.135 | 1.266 | 1.680 |
| Calophyllum saigonense. Pierre. | 0.223 | 0.143 | 1.266 | 1.632 |
| Phyllanthus angkorensis Beille | 0.503 | 0.360 | 0.633 | 1.496 |
| Pterocarpus macrocarpus Kurz | 0.223 | 0.321 | 0.949 | 1.494 |
| Azadirachta indica A. Juss. | 0.279 | 0.242 | 0.949 | 1.471 |
| Uvaria rufa Blume | 0.335 | 0.152 | 0.949 | 1.436 |
| Bombax anceps Pierre | 0.112 | 0.283 | 0.949 | 1.344 |
| Sladenia celastrifolia Kurz | 0.223 | 0.139 | 0.949 | 1.312 |
| Mammea siamensis (T. Anderson) Kosterm | 0.223 | 0.111 | 0.949 | 1.284 |
| Semecarpus albescens Kurz | 0.168 | 0.154 | 0.949 | 1.271 |
| Hymenopyramis parvifolia Moldenke | 0.335 | 0.299 | 0.633 | 1.267 |
| Casearia grewiifolia Vent | 0.223 | 0.093 | 0.949 | 1.266 |
| Mitragyna hirsuta Havil | 0.112 | 0.148 | 0.949 | 1.209 |
| Mallotus thorelii Gagnep. | 0.168 | 0.091 | 0.949 | 1.208 |
| Tristaniopsis burmanica (Griff.) Peter G.Wilson & J.T.Waterh | 0.279 | 0.239 | 0.633 | 1.151 |
| Dillenia ovata Wall. ex Hook. f.& Thomson | 0.112 | 0.078 | 0.949 | 1.139 |
| Diospyros rhodocalyx Kurz | 0.112 | 0.074 | 0.949 | 1.136 |
| Eucalyptus globulus Labill. | 0.223 | 0.237 | 0.633 | 1.094 |
| Scleropyrum pentandrum (Dennst.) Mabb. | 0.279 | 0.157 | 0.633 | 1.069 |
| Albizia lebbeck Benth | 0.056 | 0.354 | 0.633 | 1.042 |
| Albizia lebbeckoides (DC.) Benth. | 0.112 | 0.244 | 0.633 | 0.988 |
| Canarium subulatum Guillaumin | 0.112 | 0.153 | 0.633 | 0.897 |
| Ardisia helferiana Kurz. | 0.168 | 0.092 | 0.633 | 0.893 |
| Cansjera rheedii J.F. Gmelin | 0.168 | 0.083 | 0.633 | 0.884 |
| Lannea coromandelica (Houtt.) Merr. | 0.112 | 0.129 | 0.633 | 0.873 |
| Litsea glutinosa (Lour.) C.B.Robinson | 0.112 | 0.122 | 0.633 | 0.867 |
| Ziziphus oenopolia (L.) Mill. | 0.112 | 0.059 | 0.633 | 0.804 |
| Butea superba Roxb. | 0.112 | 0.049 | 0.633 | 0.794 |
| Salix tetrasperma Roxb. | 0.056 | 0.083 | 0.633 | 0.772 |
| Polyalthia viridis Craib | 0.056 | 0.074 | 0.633 | 0.763 |
| Trema orientalis (L.) Blume | 0.056 | 0.061 | 0.633 | 0.749 |
| Leucaena leucocephala (Lam.) de Wit | 0.056 | 0.054 | 0.633 | 0.743 |
| Vitex pinnata L. | 0.056 | 0.052 | 0.633 | 0.741 |
| Celastrus paniculatus Willd | 0.056 | 0.047 | 0.633 | 0.735 |
| Zizyphus cambodiana Pierre | 0.056 | 0.040 | 0.633 | 0.729 |
| Barringtonia acutangula (L.) Gaertn. | 0.056 | 0.037 | 0.633 | 0.725 |
| Hubera cerasoides (Roxb.) Chaowasku | 0.056 | 0.035 | 0.633 | 0.724 |
| Lagerstroemia speciosa (L.) Pers | 0.056 | 0.025 | 0.633 | 0.714 |
| Diospyros ferrea (Willd.) Bakh. | 0.056 | 0.024 | 0.633 | 0.713 |
| Baccaurea ramiflora Lour. | 0.056 | 0.023 | 0.633 | 0.712 |
| Gomphia serrata (Gaertn.) Kanis | 0.056 | 0.020 | 0.633 | 0.709 |
References
- Li, J.; Sun, Z.; Zhou, J.; Sow, Y.; Cui, X.; Chen, H.; Shen, Q. The Impact of the Digital Economy on Carbon Emissions from Cultivated Land Use. Land 2023, 12, 665. [Google Scholar] [CrossRef]
- Prudil, J.; Pospíšilová, L.; Dryšlová, T.; Barančíková, G.; Smutný, V.; Sedlák, L.; Ryant, P.; Hlavinka, P.; Trnka, M.; Halas, J.; et al. Assessment of carbon sequestration as affected by different management practices using the RothC model. Plant Soil Environ. 2023, 69, 532–544. [Google Scholar] [CrossRef]
- Dagiliūtė, R.; Kazanavičiūtė, V. Impact of Land-Use Changes on Climate Change Mitigation Goals: The Case of Lithuania. Land 2024, 13, 131. [Google Scholar] [CrossRef]
- Zhang, R.X.; Qu, Z.Y.; Yang, W.; Wang, L.P.; Zhang, D.L.; Liu, L.; Li, J.J.; Zhang, Z.M. Biochar addition enhances annual carbon stocks and ecosystem carbon sink intensity in saline soils of the Hetao Irrigation District, Inner Mongolia. Plant Soil Environ. 2024, 70, 263–275. [Google Scholar] [CrossRef]
- Gabric, A.J. The Climate Change Crisis: A Review of Its Causes and Possible Responses. Atmosphere 2023, 14, 1081. [Google Scholar] [CrossRef]
- Uttaruk, Y.; Khoa, P.V.; Laosuwan, T. A guideline for greenhouse gas emission reduction and carbon sequestration in forest sector based on Thailand Voluntary Emission Reduction Programme. Sains Malays. 2024, 53, 477–486. [Google Scholar] [CrossRef]
- Tokeeree, Y.; Jarat, C.; Novacek, D.; Saraphi, N. Carbon storage in biomass of perennial plants at ban sangtawan community forest, Surin province. PSRU J. Sci. Technol. 2020, 5, 23–36. [Google Scholar]
- Citizenthaipbs. Carbon Neutrality. Available online: https://thecitizen.plus/node/86607 (accessed on 2 May 2024).
- IPST. Photosynthesis. Available online: https://www.ipst.ac.th/learning/21712/20220310-photosynthesis.html (accessed on 5 May 2024).
- Jin, Y.; Liu, C.; Qian, S.S.; Luo, Y.; Zhou, R.; Tang, J.; Bao, W. Large-scale patterns of understory biomass and its allocation across China’s forests. Sci. Total Environ. 2022, 804, 150169. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Chen, X.; Tian, X.; Wang, X.; Luo, G. Biomass allocation patterns across China’s terrestrial biomes. PLoS ONE 2014, 9, e93566. [Google Scholar] [CrossRef]
- Temwut, S.; Chaitieng, T. Biomass and carbon stock of trees in the plant genetic conservation and returning of wild animals project in kalasin university (namon area). J. Res. Unit Sci. Technol. Environ. Learn. 2024, 12, 150–159. [Google Scholar]
- Timilsina, N.; Staudhammer, L.C.; Escobedo, J.; Escobedo, F.J.; Lawrence, A. Tree biomass, wood waste yield, and carbon storage changes in an urban forest. Landsc. Urban Plan. 2014, 127, 18–27. [Google Scholar] [CrossRef]
- Thongmee, T.; Som-ard, J.; Jitsukka, W. Evaluation of above-ground carbon sequestration of forest in mahasarakham university using remote sensing data. J. Sci. Technol. Mahasarakham Univ. 2019, 38, 586–597. [Google Scholar]
- Uttaruk, Y.; Laosuwan, T. Development of prototype project for carbon storage and greenhouse gas emission reduction from Thailand’s agricultural sector. Sains Malays. 2019, 48, 2083–2092. [Google Scholar] [CrossRef]
- Laosuwan, T.; Uttaruk, Y.; Sangpradid, S.; Butthep, C.; Leammanee, S. The Carbon Sequestration Potential of Silky Oak (Grevillea robusta A.Cunn. ex R.Br.), a High-Value Economic Wood in Thailand. Forests 2023, 14, 1824. [Google Scholar] [CrossRef]
- Carboncredits. The Ultimate Guide to Understanding Carbon Credits. 2020. Available online: https://carboncredits.com/the-ultimate-guide-to-understanding-carbon-credits/ (accessed on 11 January 2024).
- Laosuwan, T.; Uttaruk, Y.; Rotjanakusol, T. Atmospheric Environment Monitoring in Thailand via Satellite Remote Sensing: A Case Study of Carbon Dioxide. Pol. J. Environ. Stud. 2023, 32, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- TGO. The Concept of the Carbon Offsetting. Available online: https://thaicarbonlabel.tgo.or.th/index.php?lang=EN&mod=WTI5dVkyVndkRjl2Wm1aelpYUjBhVzVu (accessed on 20 February 2024).
- TGO. Carbon Credit Mechanism. Available online: https://www.thailand-energy-academy.org/assets/upload/coursedocument/file/01%20Carbon%20Credit%20Mechanism.pdf (accessed on 21 February 2024).
- TGO. Thailand Greenhouse Gas Management Organization (Public Organization). Available online: https://www.tgo.or.th/2023/index.php/th/ (accessed on 22 February 2024).
- TGO. What Is T-VER? Available online: https://ghgreduction.tgo.or.th/en/what-is-t-ver/what-is-t-ver.html (accessed on 22 February 2024).
- Adegun, O.B. When green is grievous: Downsides of human-nature interactions in informal urban settlements. J. Urban. 2018, 11, 329–346. [Google Scholar] [CrossRef]
- FAO. Urban Forests: Aglobal Perspective. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/c27b6b7f-52fc-4706-a485-c53df42dd6d2/content (accessed on 25 February 2024).
- Chishaleshale, M.; Shackleton, C.M.; Gambiza, J.; Gumbo, D. The prevalence of planning and management frameworks for trees and green spaces in urban areas of South Africa. Urban For. Urban Green. 2015, 14, 817–825. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, Z.; She, X.; Gao, J. Quantifying the Potential Contribution of Urban Forest to PM2.5 Removal in the City of Shanghai, China. Atmosphere 2021, 12, 1171. [Google Scholar] [CrossRef]
- Fujimoto, M.; Puangchit, L.; Sugawara, F.; Sripraram, D.; Jiamjeerakul, W.; Kato, H. Carbon Sequestration Estimation of Urban Trees in Parks and Streets of Bangkok Metropolitan, Thailand. Thai J. For. 2016, 35, 30–41. [Google Scholar]
- Singkran, N. Carbon sink capacity of public parks and carbon sequestration efficiency improvements in a dense urban landscape. Environ. Monit. Assess. 2022, 194, 750. [Google Scholar] [CrossRef]
- TGO. Thailand Voluntary Emission Reduction Program Reference Manual: Forestry and Agriculture Sector. Available online: http://conference.tgo.or.th/download/TGO_InfoCenter/Section9/Book/TVER_ForestryArgiculture.pdf (accessed on 22 February 2024).
- TGO. T-VER-S-TOOL-01-01. Available online: https://tver.tgo.or.th/database/public/tools/1?lang=en (accessed on 28 February 2024).
- Whittaker, R.H. Communities and Ecosystems; Macmillan, Collier Macmillan Ltd.: London, UK, 1970. [Google Scholar]
- Shannon, C.E.; Weaver, W.W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, E.C. Ecological Diversity; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Tsutsumi, T.; Yoda, K.; Sahunalu, P.; Dhanmanonda, P.; Prachaiyo, B. Forest: Felling, Burning and Regeneration. In Shifting Cultivation. An Experiment at Nam Phrom, Thailand and Its Implications for Upland Farming in the Monsoon Tropics; Kyuma, K., Pairintra, C., Eds.; Kyoto University, Faculty of Agriculture: Tokyo, Japan, 1983. [Google Scholar]
- Viriyabuncha, C.; Peawsa-ad, K. Assessment of the Potentiality of Re-Afforestation Activities in Climate Change Mitigation, Annual Report April, 2001–March, 2002; Forest Research Office, Royal Forest Department: Bangkok, Thailand, 2002. [Google Scholar]
- Visaratana, T.; Chernkhuntod, C. Floristic composition and aboveground trees biomass in dry evergreen forest. In Proceedings of the Forestry and Climate Change: Forests and Climate Change, Bangkok, Thailand, 16–17 August 2004. [Google Scholar]
- SCB. Economic Intelligence Center: Key Challenges in Thailand’s Carbon Credit Market. Available online: https://www.scbeic.com/en/detail/file/product/8952/gl13e47v9s/SCB-EIC_Future-Perspective_Carbon-credit_EN_20230420.pdf (accessed on 8 March 2024).
- Nowak, D.J.; Hoehn, R.E., III; Crane, D.E.; Stevens, J.C.; Walton, J.T. Assessing Urban Forest Effects and Values, Washington, D.C.‘s’ Urban Forest; Resour. Bull. NRS-1; Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2006. [Google Scholar]
- USDA. Urban Forests and Climate Change. Available online: https://www.fs.usda.gov/ccrc/topics/urban-forests (accessed on 1 June 2024).
- Locosselli, G.M.; Camargo, E.P.; Moreira, T.C.L.; Todesco, E.; Andrade, M.F.; André, C.D.S.; André, P.A.; Singer, M.J.; Ferreira, L.S.; Saldiva, P.H.N.; et al. The role of air pollution and climate on the growth of urban trees. Sci. Total Environ. 2019, 666, 652–661. [Google Scholar] [CrossRef]
| Plant Part | Biomass Amount (kg) | Carbon Storage Amount (kg) | % | ||
|---|---|---|---|---|---|
| Average/ha | Per Forest Area | Average/ha | Per Forest Area | ||
| Ws | 88,706.81 | 454,178.88 | 41,692.19 | 213,464.00 | 59.44 |
| Wb | 27,761.69 | 142,139.84 | 13,048.00 | 66,805.76 | 18.60 |
| Wl | 4687.69 | 24,000.96 | 2203.19 | 11,280.32 | 3.14 |
| Wr | 28,069.25 | 143,714.56 | 13,192.56 | 67,545.92 | 18.81 |
| Total | 149,225.44 | 764,034.24 | 70,135.94 | 359,096.03 | 100 |
| Plant Parts | Biomass Amount (kg) | Carbon Storage Amount (kg) | % | ||
|---|---|---|---|---|---|
| Average/ha | Per Forest Area | Average/ha | Per Forest Area | ||
| Ws | 141,397.00 | 7,895,608.48 | 66,456.56 | 3,710,935.99 | 63.76 |
| Wb | 45,025.50 | 2,514,223.92 | 21,162.00 | 1,181,685.24 | 20.30 |
| Wl | 3581.69 | 200,001.43 | 1683.38 | 94,000.67 | 1.62 |
| Wr | 31,767.25 | 1,773,883.24 | 14,930.63 | 833,725.13 | 14.32 |
| Total | 221,771.44 | 12,383,717.07 | 104,232.56 | 5,820,347.03 | 100 |
| Plant Parts | Biomass Amount (kg) | Carbon Storage Amount (kg) | % | ||
|---|---|---|---|---|---|
| Average/ha | Per Forest Area | Average/ha | Per Forest Area | ||
| Ws | 222.56 | 1139.52 | 104.63 | 535.68 | 33.75 |
| Wb | 290.38 | 186.72 | 136.50 | 698.88 | 44.03 |
| Wl | 37.25 | 190.72 | 17.50 | 89.6 | 5.65 |
| Wr | 109.31 | 559.68 | 51.38 | 263.04 | 16.57 |
| Total | 659.50 | 2076.64 | 310.00 | 1587.20 | 100 |
| Plant Parts | Biomass Amount (kg) | Carbon Storage Amount (kg) | % | ||
|---|---|---|---|---|---|
| Average/ha | Per Forest Area | Average/ha | Per Forest Area | ||
| Ws | 251.88 | 14,064.70 | 118.38 | 6610.41 | 42.39 |
| Wb | 173.19 | 9670.79 | 81.38 | 4545.27 | 29.15 |
| Wl | 42.88 | 2394.14 | 20.13 | 1125.25 | 7.21 |
| Wr | 126.25 | 7049.80 | 59.38 | 3313.41 | 21.25 |
| Total | 594.19 | 33,179.43 | 279.25 | 15,594.34 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uttaruk, Y.; Laosuwan, T.; Sangpradid, S.; Butthep, C.; Rotjanakusol, T.; Sittiwong, W.; Nilrit, S. Thailand’s Urban Forestry Programs Are Assisted by Calculations of Their Ecological Properties and Economic Values. Land 2024, 13, 1440. https://doi.org/10.3390/land13091440
Uttaruk Y, Laosuwan T, Sangpradid S, Butthep C, Rotjanakusol T, Sittiwong W, Nilrit S. Thailand’s Urban Forestry Programs Are Assisted by Calculations of Their Ecological Properties and Economic Values. Land. 2024; 13(9):1440. https://doi.org/10.3390/land13091440
Chicago/Turabian StyleUttaruk, Yannawut, Teerawong Laosuwan, Satith Sangpradid, Chetphong Butthep, Tanutdech Rotjanakusol, Wuttichai Sittiwong, and Sutthicha Nilrit. 2024. "Thailand’s Urban Forestry Programs Are Assisted by Calculations of Their Ecological Properties and Economic Values" Land 13, no. 9: 1440. https://doi.org/10.3390/land13091440
APA StyleUttaruk, Y., Laosuwan, T., Sangpradid, S., Butthep, C., Rotjanakusol, T., Sittiwong, W., & Nilrit, S. (2024). Thailand’s Urban Forestry Programs Are Assisted by Calculations of Their Ecological Properties and Economic Values. Land, 13(9), 1440. https://doi.org/10.3390/land13091440









