Abstract
The wetlands of Sardinia (Italy) supply food and shelter for many waterbird species that migrate along the central–eastern Mediterranean bird flyway. Despite many different policies and laws (the Birds and Habitats Directives, the European Water Framework Directive, and the Ramsar Convention), the Sardinian wetlands are seriously threatened by human activities and climate change, which in turn menace the associated avifauna. In this study, we (a) inventoried (four sampling dates) the avian metacommunity of the largest coastal wetlands in Sardinia during the crucial period of the year for the avifauna (August–September), (b) explored the spatiotemporal dynamics in bird species assemblage, and (c) used results to refine planning for bird habitat management and bird diversity conservation. We recorded 60 bird species, of which 54 were migratory and 21 belonged to Annex I of the Birds Directive. During August–September, (a) α, β, and γ avian diversity showed no significant temporal trends, (b) the contributions of space (wetlands) and time (dates of sampling) in determining the presence/absence of the waterbird species were comparable, (c) wetlands formed three statistically significant clusters with regard to the species richness, (d) a significant increase in the number of the species belonging to the “mixed” migration guild, and “divers from the surface” foraging guild, occurred, (e) there was a statistically significant chronological succession of the occurrence of waterbird species, (f) twenty-five species made use of the Sardinian wetlands all summer long, while ten further species were present in three sampling dates out of four, (g) the spatial distributions of the waterbird species in the Sardinian wetlands were significantly different between the sampling dates, (h) the Little Egret, the Grey Heron, and the Greater Flamingo were primarily responsible for the observed difference in the spatial distributions of species between the sampling dates, (i) Is Brebeis, Pilo, and S. Giovanni were the wetlands that changed their species composition the most during the studied period, (j) twenty-two waterbird species resulted at high priority for conservation, and thirteen species at medium priority. Based on these results, we have proposed new strategies for the conservation of the waterbird species of the Sardinian wetlands during the post-breeding migration period.
1. Introduction
Sardinia is the second largest island in the Mediterranean, with an area of 24,094 km2, and is part of the European Mediterranean biogeographical region [1]. Its wetlands are located along the Sardinia–Corsica corridor of the central–eastern Mediterranean bird flyway [2]; accordingly, they act as a natural bridge between Europe and Africa for numerous bird species confronted with travelling across the Mediterranean sea. In addition, they support resident waterbirds that do not migrate [3].
Sardinia is also home to 1.6 million people, and hosts around 3 million tourists annually [4]. The recent anthropization process has led to severe threats to the Sardinian wetlands. During 1990–2012, the surroundings of the largest wetlands in Sardinia saw an increase in artificial areas and a decrease in both agricultural and natural areas [5]. Although the Natura 2000 policies preserved these wetlands from shrinkage during 1990–2012 [5], their surroundings experienced intense processes of degradation and artificialization [6]. Several anthropogenic threats (recreational and tourism activities, water salinization, pollutants from urban, agricultural, and aquaculture systems, and excessive water depth due to artificial water regulation for fishing and harvesting aquatic resources [7]) are now endangering many waterbird species in these wetlands; this is particularly true in summer when tourism pressure is most elevated, corresponding with the crucial span of time for waterbirds [8]. It has been demonstrated that the avian diversity in these wetlands is destined to: (a) decrease due to the most likely increase in the most important drivers of avian diversity (water salinity, water discharges, and tourism pressure); (b) halve in a worst possible scenario where all these drivers degenerate simultaneously [9]. However, it has been also evidenced that the structure of the avian metacommunity (i.e., the set of interacting communities that are linked by the dispersal of multiple, potentially interacting species) of the Sardinian wetlands would allow for pro-active conservation measures to counteract the effects of current and future threats [8]. In fact, in summer, this avian metacommunity significantly nested, and is considerably dependent on wetland attributes [8]. In a nested metacommunity, the species present on a few wetlands can be found on those wetlands with higher levels of species diversity, which assigns high priority to the conservation of those wetlands with a larger number of species [10,11]. The dependence on wetland attributes implies that environmental control for species diversity is predominant, which implies that appropriate interventions on these wetlands will have considerable beneficial effects on the avifauna [12]. In fact, mitigation and compensation measures like restrictions on tourism activities, water desalination, prevention of future saltwater intrusions, and the prohibition of water discharges could remarkably increase the level of avian diversity present [8].
In this study, we explored the space-time dynamics in bird species assembly in the coastal wetlands of Sardinia during the summer period, proposing to add new information for the conservation of the waterbirds and migration routes along the Mediterranean bird flyway.
2. Materials and Methods
2.1. Study Area and Bird Surveys
The study area encompassed the natural coastal wetlands larger than 10 hectares in Sardinia (Figure 1; Table S1). Tartanelle and Tortoli apart, all wetlands belonged to the Natura 2000 network, i.e., the largest network of protected areas worldwide, which requires management plans and conservation measures as the basis for the preservation of species and habitats [13].
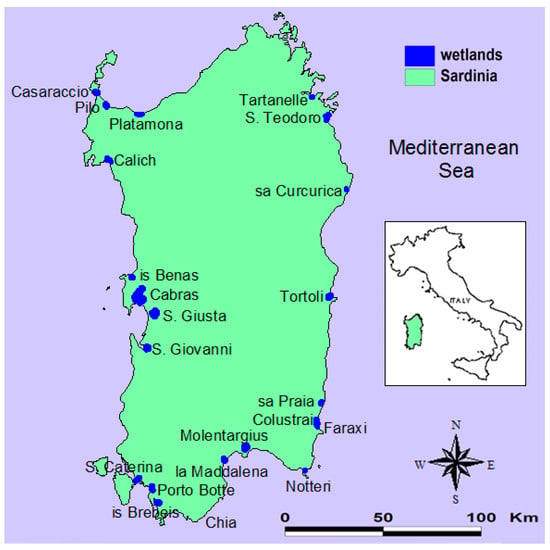
Figure 1.
The 22 coastal wetlands (total surface area: 5545 hectares) under study in Sardinia (Italy).
For each wetland, during August–September 2016, we performed four sampling sessions of avian diversity at intervals of 10–15 days between sessions. The sampling sessions corresponded to the first (A1) and second (A2) half of August and the first (S1) and second (S2) half of September. We employed sampling points with a 200 m minimum distance to minimize spatial autocorrelation [14]. As a result, for each sampling session, we used 144 sampling points of avian diversity (Table S2), where we employed the point count sampling method [15], i.e., all bird sightings were documented through 15-min point counts with a 100 m radius at each survey site.
We assigned two functional traits to each bird species recorded during field surveys: foraging (divers from flight, divers from the surface, intermediate waders, large waders, small waders, surface feeders) and migratory (Mediterranean, mixed, sedentary, trans-Saharan) guilds [16]. We also assigned the SPEC category to each bird species [17]: SPEC 1 = species of global conservation concern (i.e., critically endangered, endangered, vulnerable, or near threatened at global level); SPEC 2 = species whose global population is concentrated in Europe and which is classified as regionally extinct, critically endangered, endangered, vulnerable, or near threatened at European level, or as declining, depleted, or rare in Europe; SPEC 3 = species whose global population is not concentrated in Europe, but which is classified as regionally extinct, critically endangered, endangered, vulnerable, or near threatened at European level or as declining, depleted, or rare in Europe; non-SPEC = species whose European population status is currently considered to be secure.
Our study required neither ethical approval nor permission for fieldwork.
2.2. Analytical Framework
2.2.1. Metacommunity
For each sampling date, we computed α diversity (mean number of bird species per wetland), β diversity (ratio between regional and local species diversity [18]; Appendix A), γ diversity (total number of bird species in the wetlands under study), and matrix fill (percent of 1s in the presence–absence matrix). We used the Mann–Kendall trend test [19,20] to detect significant increasing or decreasing trends of these metrics during August–September. This test can be used to find trends with few as four samples, under the null hypothesis H0 that there is no monotonic trend in the series. This test returns S (negative for a decreasing trend, zero for no trend, and positive for an increasing trend), whose p-value was calculated through the Z statistic from the cumulative normal distribution. p-values < 0.05 were considered statistically significant.
For each sampling date, we built the species-by-site matrix (Tables S3–S6). We then conducted the test of homogeneity of multivariate dispersion (PERMDISP; ref. [21]) to examine the distinct contributions of space (twenty-two wetlands) and time (four dates of sampling) in determining the distribution of species in the metacommunity during August–September. The null hypothesis H0 was that the contributions of space and time were not significantly different, while the alternate hypothesis H1 was that one factor overwhelmed the other. In essence, PERMDISP tests for equal dispersion in two or more groups (dates of sampling) of multivariate data (species-by-site matrices). This involved calculating the distance of each wetland to its group centroid with regard to species presences/absences (within–group distance; i.e., contribution of wetlands to species distributions in the metacommunity), and then testing whether those distances differed significantly among the groups (between–group distance; i.e., contribution of dates of sampling to species distributions in the metacommunity). We used the Bray–Curtis distance, and statistical significance was assessed based on permutation tests with 9999 runs [21].
2.2.2. Communities
We built the matrix of species richness for the twenty-two wetlands in the four sampling dates (Table S7). We used hierarchical cluster analysis with the unweighted pair-group method with arithmetic mean (UPGMA; at each step, the nearest two clusters are joined based on the average distance between all members in the two groups; ref. [22]) and Euclidean distance to group wetlands with regard to the species richness during August–September. We used the bootstrap technique, i.e., we computed the percentage of bootstrap replicates where each node was still supported after 9999 runs, and retained node splits with support > 50% after bootstraps.
2.2.3. Guilds
We built the two matrices of the number of waterbird species belonging to the (a) six foraging guilds and (b) four migratory guilds in the four sampling dates (A1, A2, S1, S2). We then used the Mann–Kendall trend test to discover, if any, significantly increasing or decreasing trends in the amount of bird species belonging to these guilds.
2.2.4. Species
We created the matrix of presences–absences of the waterbird species in the Sardinian wetlands in the four dates of sampling (Table S8). We then used seriation analysis [23] to arrange bird species into a temporal sequence. The seriation routine operated a reordering of rows (species) such that the presences were concentrated along the diagonal. A perfect seriation score was C = 1, while less perfect seriations generate lower C. We used constrained optimization where only taxa (rows) were free to move, while dates of sampling (columns) were fixed. We then used Monte Carlo simulations to generate and arrange 103 random matrices with the same number of occurrences within each taxon, and compared them to the original matrix to check if it had higher C than random ones. We used the Z statistic to calculate the p-value associated to C from the cumulative normal distribution of the randomized C; p-values < 0.05 were considered statistically significant. Based on these results, we grouped species into different clusters corresponding to different temporal uses of the Sardinian wetlands during August–September.
We used one-way PERMANOVA (permutational multivariate analysis of variance; ref. [24]) to test whether the distributions of the waterbird species in the Sardinian wetlands (species-by-site matrices; Tables S3–S6) were significantly different between the sampling dates. PERMANOVA is a non-parametric test of significant difference between two or more groups (the four sampling dates), based on any distance measure, under the null hypothesis that the centroids and dispersion of the groups are equivalent for all groups. A rejection of H0 means that either the centroids and/or the spread of the species around the centroids is significantly different between the groups. PERMANOVA calculated an F value whose significance was computed by permutation of group membership with 9999 replicates. We used the Bray–Curtis distance, and the option “repeated measures” as samples (i.e., wetlands) were the same in all the sampling dates.
We then used SIMPER (Similarity Percentage; ref. [25]) analysis with Bray–Curtis distance for assessing which taxa and wetlands were primarily responsible for the observed difference in the spatial distributions of species between the sampling dates. We sought to determine (a) which species showed high/low site (wetland) fidelity and (b) which wetlands had similar/dissimilar species composition during August–September. The SIMPER procedure attempts to disentangle the contribution (average dissimilarity; AD) of individual variables (species and wetlands in our study) from the dissimilarity between groups (sampling dates). The percent contribution (C%) was simply the normalization of AD to 100. This allowed us to identify species and wetlands that were the major contributors to differences between the four sampling dates.
Finally, we built the decision space for species prioritization in the form of a biplot for cross-tabulation of (a) species persistence (X-axis) and (b) site infidelity (Y-axis) during August–September. For each species, the former index was simply calculated as the percentage of dates of sampling where the species was present in the Sardinian wetlands. Because we employed four dates of sampling, this index could be equal to 25% (i.e., the species was present just once out of four dates of sampling), 50% (2 out of 4), 75% (3 out of 4), and 100% (4 out of 4). The site infidelity index was simply the result of SIMPER analysis. High priority was then assigned to species with elevated persistence and low site infidelity (elevated site fidelity), i.e., the waterbird species that depended unremittingly on the very same wetlands during summer, being thus at higher risk of impacts by the human alteration of wetlands and ongoing climate change. Medium priority was assigned to species with both elevated persistence and site infidelity (low site fidelity). These species depended unremittingly on the Sardinian wetlands during summer, but were able to frequently change their spatial distribution, thus showing that they can more easily colonize new wetlands in case of negative impacts by human alterations and climate change upon the wetlands where they dwell. Following the same logic, low/no priorities were assigned to the waterbird species with low persistence during August–September, and low/elevated infidelity, respectively.
3. Results
3.1. Metacommunity
We recorded 60 bird species, of which 54 were migratory (Table S9), 21 belonged to Annex I of the Birds Directive, 3 belonged to the SPEC category 1, 11 to the SPEC category 2, and 38 to the SPEC category 3 (Table S10).
The metacommunity metrics showed no significant trends (p-values > 0.05) between the sampling dates (Table 1), thus they should be regarded as constant during August–September.

Table 1.
Results of the Mann–Kendall trend test applied to the metacommunity metrics.
The results of the PERMDISP test showed that the contributions of space (twenty-two wetlands) and time (four dates of sampling) in determining the distribution of the waterbird species in the metacommunity during August–September were not significantly different (i.e., mean sum of squares between the groups approximately equal to mean sum of squares within the groups; p-value > 0.05; Table 2).

Table 2.
Results of the test for multivariate dispersion (PERMDISP). Because groups corresponded to the four dates of sampling, the term “between groups” should be regarded as the variability in species composition (species-by-site matrix) between dates of sampling, and “within groups” as the variability in species composition within dates of sampling.
3.2. Communities
The highest species richness (22) was recorded at the Pilo wetland in the second half of August, and at S. Giovanni in the second half of September (Table S7).
UPGMA individuated three significant (i.e., >50% support after 9999 bootstraps) clusters with regard to the species richness during August–September (Figure 2).
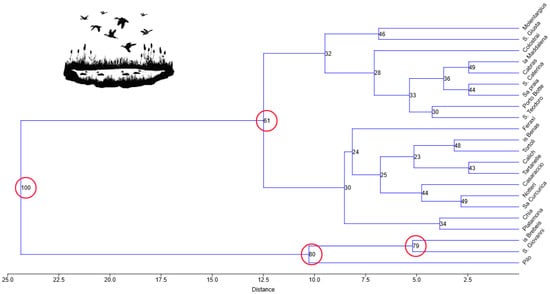
Figure 2.
Results of the hierarchical cluster analysis with unweighted pair-group average (UPGMA) method and Euclidean distance. Numbers at node splits indicate the percentage of replicates where each node is still supported after 9999 bootstrap replicates. Red circles shows node splits with support > 50% after bootstraps.
The first group (support = 80%; wetlands Pilo, S. Giovanni, Is Brebeis) was characterized by higher levels of species richness in nearly all the sampling dates (Table S7). Within this first cluster, the sub-group formed by the wetlands Is Brebeis and S. Giovanni (support = 79%) had high species richness in all the sampling dates, while Pilo scored a lower level of species richness in the first half of August. The second group (19 wetlands) was qualified by lower levels of species richness in almost all the sampling dates (Table S7). Two sub-groups emerged (support = 61%), of which one (ten wetlands) was characterized by the lowest levels of species richness, in particular Notteri and Sa Curcurica; Table S7), and the other (nine wetlands) scored average levels in nearly all the sampling dates, with the exception of the Molentargius wetland, which hosted 17 species in the second half of September (Table S7).
3.3. Guilds
We found a significant increase in the number of species belonging to the “mixed” (p = 0.042) migration guild, and “divers from the surface” (p = 0.042) foraging guild during August–September (Figure 3; Table S11). The other foraging and migration guilds showed no significant temporal trends (Table S11).
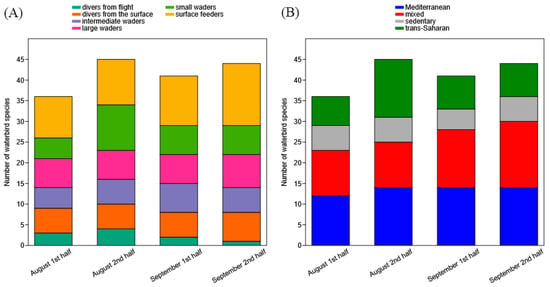
Figure 3.
For each sampling date (X-axis), the number of waterbird species belonging to the foraging (A) and migration (B) guilds are shown (Y-axis).
3.4. Species
We found a statistically significant (C = 0.96; Z = −2.28; p = 0.02) temporal sequence of the occurrence of waterbird species in the Sardinian wetlands during August–September (Figure 4). Two species (Squacco Heron and Little Tern; group 1) were only early dwellers (A1), while four species (group 12) were only late dwellers (S2). Twenty-five species utilized the Sardinian wetlands all summer long (group 4), and ten species (groups 3, 5, and 8) were present in three sampling dates out of four.
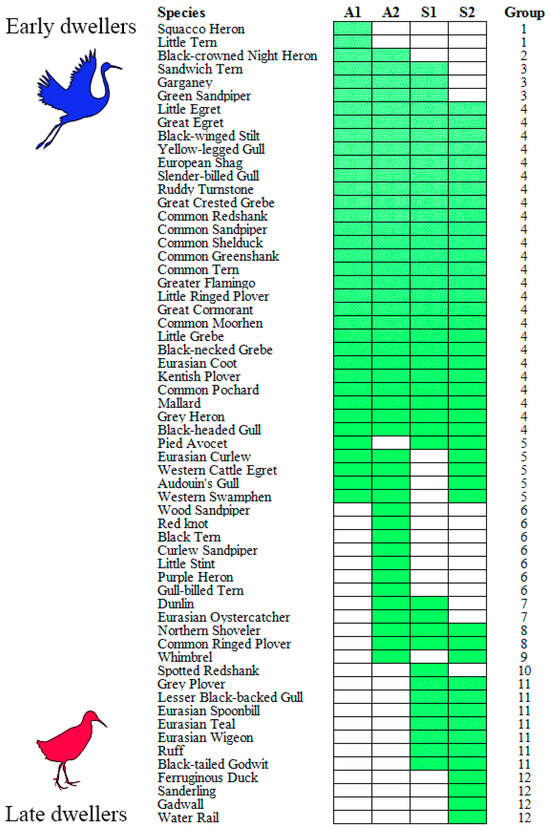
Figure 4.
Results of the seriation analysis. Waterbird species are ordered downward from early dwellers (group 1) to late dwellers (group 12). A1 and A2: first and second half of August; S1 and S2: first and second half of September. Green squares indicate species presences, white ones stand for species absences.
The distributions of the waterbird species (Tables S3–S6) in the Sardinian wetlands were significantly different between the four sampling dates (p < 0.01; Table 3).

Table 3.
Results of the one-way PERMANOVA analysis with repeated measures.
Between A1 and A2 (Table S12), the species that most changed their spatial distribution were the Grey Heron (AD = 4.18; C% = 6.08%) and the Little Egret (AD = 4.00; C% = 5.83%), while 12 species occupied the same wetlands (AD = 0; C% = 0%). Between A2 and S1 (Table S13), the Grey Heron (AD = 4.38; C% = 6.30%) and the Little Egret (AD = 3.96; C% = 5.70%) were again the species with highest site infidelity; in contrast, six species kept their spatial distribution constant (AD = 0; C% = 0%). Between S1 and S2 (Table S14), the Little Egret (AD = 3.89; C% = 5.97%) and the Great Egret (AD = 3.62; C% = 5.55%) were the species with highest site infidelity, while 10 species did not change their spatial distribution (AD = 0; C% = 0%). Overall, the Little Egret (AD = 4.09, C% = 6.06%), the Grey Heron (AD = 3.92, C% = 5.80%), and the Greater Flamingo (AD = 3.58, C% = 5.29%) were the bird species with highest site infidelity during August–September (Figure 5). In contrast, the Water Rail (AD = 0.09, C% = 0.13%), the Red Knot (AD = 0.78, C% = 0.11%), and the Curlew Sandpiper (AD = 0.78, C% = 0.11%) were the bird species with highest site fidelity (Figure 5).
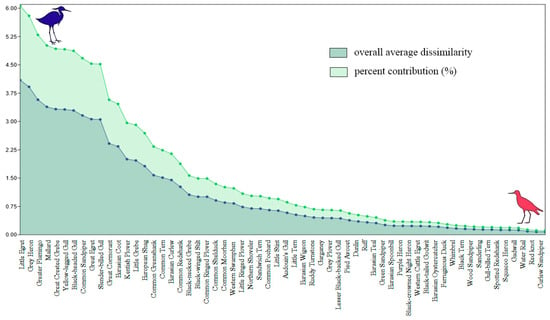
Figure 5.
Results of the application of SIMPER analysis to the waterbird species. Species are arranged from left to right in order of their change in spatial distribution (overall average dissimilarity; AD) during August–September. The percent contribution is the normalization of AD to 100.
Is Brebeis (AD = 9.53; C% = 11.73%), Pilo (AD = 9.29; C% = 11.43%), and S. Giovanni (AD = 8.33; C% = 10.25%) were the wetlands that changed their species composition the most during August-September (Figure 6); in contrast, Notteri (AD = 1.16; C% = 1.43%), Casaraccio (AD = 0.87; C% = 1.07%), and Sa Curcurica (AD = 0.08; C% = 0.09%) changed their species composition the least (Figure 6).
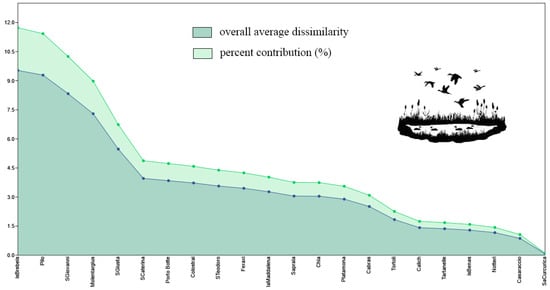
Figure 6.
Results of the application of SIMPER analysis to the wetlands. Wetlands are arranged from left to right in order of their change in species composition (overall average dissimilarity; AD) during August–September. The percent contribution is the normalization of AD to 100.
The biplot between the species persistence and site infidelity (Figure 7) individuated 22 waterbird species at high priority for conservation (dark red square), 13 species at medium priority (light red square), 25 species at low priority (light green square), and no species at no priority (dark green square).
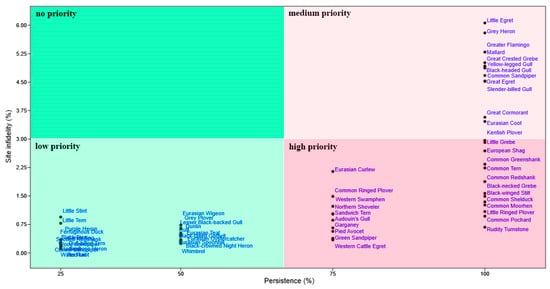
Figure 7.
Prioritization for conservation of the waterbirds species recorded in the Sardinian wetlands. The biplot shows the composite indices of persistence (%) on the X-axis and site infidelity (%) on the Y-axis. Refer to the main text for their meaning.
4. Discussion
The alarming consequences of human and climate threats on wetlands, and the associated avifauna, have been subject of many scientific studies [26,27,28,29]. Threats range from tourist and recreational activities, water discharges, and water diversion for agricultural activities up to saltwater intrusions caused by climate-induced sea level rise, and freshwater shortage owing to warmer and dryer summer periods [30,31,32,33].
Waterbirds, especially migratory ones, are largely influenced by habitat changes caused by anthropogenic land conversions and human disturbance in wetlands, which affect their migrating breeding and foraging [34,35,36]. The Sardinian wetlands are no exception. In fact, around 90% of these wetlands, especially along the coast, have been destroyed, or heavily modified, during the last century to eradicate malaria and for agricultural and industrial uses [37]. More recently, tourism development has become another major threatening factor [6].
The conservation of the avifauna in the remnant wetlands of Sardinia requires rigorous knowledge of the use that waterbirds make of them, in particular during the critical summer period. In this study, we found that during August–September: (a) α, β, and γ avian diversity presented no significant temporal trends, (b) the contributions of space and time in determining the presence/absence of the waterbird species were comparable, (c) wetlands formed three statistically significant clusters regarding the species richness, (d) there was a significant increase in the number of the species belonging to the “mixed” migration guild and “divers from the surface” foraging guild, (e) a statistically significant temporal sequence of the presence/absence of waterbird species occurred, (f) twenty-five species utilized the Sardinian wetlands all summer long, and ten further species were present in three sampling dates out of four, (g) the spatial distributions of the waterbird species were significantly different between the sampling dates, (h) the Little Egret, the Grey Heron, and the Greater Flamingo were primarily responsible for such observed difference, (i) the wetlands Is Brebeis, Pilo, and S. Giovanni changed their species composition the most, and (j) twenty-two waterbird species were deemed at high priority for conservation and thirteen species at medium priority.
Entailments for the Preservation of the Avian Species
Unlike the Sicilian wetlands [38,39,40], the second half of September did not exhibit a decrease in the biodiversity metrics in our study, which would have suggested a decline in the support to the waterbird species by the Sardinian wetlands. Thus, it was not possible to propose a reduction in the time period when conservation measures (restrictions on tourist and recreational activities in the close surroundings of the wetlands, water desalination, prevention of saltwater intrusions, prohibition of water discharges from the surrounding urban and farm areas; refs. [5,6,7,8]) should be obligatory during summer.
Three wetlands (Pilo, S. Giovanni, Is Brebeis) were characterized by higher levels of species richness in nearly all the sampling dates. These three wetlands cover the whole latitudinal range of Sardinia from north (Pilo) to south (Is Brebeis) passing through the central belt (S. Giovanni) (Figure 1). In addition, these wetlands changed their species composition the most during August-September, thus showing that they can support, in terms of habitat and food, a large variety of waterbird species in different time spans. These three wetlands and, in general, the western coast of Sardinia hosted most of the waterbird species in summer, and thus should be given the highest priority for interventions. This result provides conservation managers a solution to assign priority to a limited number of wetlands, therefore enhancing the feasibility and effectiveness of conservation strategies.
Because the contributions of space and time in determining the presence/absence of the waterbird species were equally important, it follows that the conservation measures for the avifauna should be not only be wetland-dependent, but also time-dependent during the summer period. The significant increase (from A1 to S2) in the number of the species belonging to the “divers from the surface” foraging guild and the “mixed” migration guild has consequences for species conservation. The divers from the surface are favored by higher water levels [7]; on the contrary, such levels highly disadvantage small and intermediate waders that choose shallow water to forage [7]. However, the mean water level in the Sardinian wetlands is >100 cm in seven wetlands (Calich, Casaraccio, Feraxi, S. Giovanni, Sa praia, and Tortoli) and >50 cm in six wetlands (Cabras, la Maddalena, Porto Botte, S. Caterina, S. Giusta) for human activities (fishing and aquaculture; Table S1) all summer long [8]. Our results indicate that artificial water regulation would be highly desirable in these wetlands to produce water-level fluctuations that can benefit small and intermediate waders by lowering water levels in August, as well as divers from the surface by raising water levels in September when they significantly increase in number. In addition, in the Sardinian wetlands, water salinity severely impacts the waterbird species belonging to the “mixed” migration guild [7]. Drinking highly saline water induces dehydration, reduces the waterproofing of feathers in these species, and increases the energy costs of thermoregulation [41]. Because mixed migrants significantly grow in number from A1 to S2, conservation strategies based on water desalination and prevention of saltwater intrusions become more necessary as summer progresses (particularly in September) in the twelve Sardinian wetlands where saltwater intrusions are permanent due to artificial connections to the sea (Table S1). Another cost-effective solution could be the cultivation of plant species for phytoremediation activities. Plants adapted to local climate conditions, with high biomass and elevated phytoextraction of salt from wetland water, are perfect candidates for this use [42].
We individuated twenty-two waterbird species with both elevated persistence and elevated site fidelity, i.e., species that depended unremittingly on the very same wetlands during summer. This group of species comprised seven waterbird species (Audouin’s Gull, Black-winged Stilt, Common Tern, European Shag, Pied Avocet, Sandwich Tern, Western Swamphen) that belong to Annex I of the Birds Directive (i.e., bird species which are protected due to being in danger of extinction, vulnerable to specific changes in their habitats, or considered rare due to small populations or restricted local distribution), and three species (Audouin’s Gull, Common Pochard, Eurasian Curlew) belonging to the SPEC category 1. These species, besides their elevated conservation interest, showed to be: (a) dependent on the Sardinian wetlands all summer long and (b) less able than other more generalist species to colonize new wetlands in case of negative impacts by human alterations and climate change upon the wetlands where they dwell. These species should be thus considered at highest priority of conservation in the Sardinian wetlands. In addition, their populations trends should be used as proactive indicators of the ecological status of these wetlands because these species are highly sensitive to wetland traits [8].
5. Conclusions
Our study stressed sharp spatiotemporal patterns in bird species assemblages in the coastal wetlands of Sardinia during the summer period. Results indicate that ad hoc conservation strategies (restrictions on tourism activities, water desalination, prevention of future saltwater intrusions, and the prohibition of water discharges) are necessary to support the avian diversity of these wetlands in summer, because all the actors involved (wetlands, waterbird species, time spans) tightly interact to produce complex dynamics that are both wetland- and species-dependent, as well as time-dependent.
We employed an in-depth statistical framework to untangle such complexity, and used results to refine the conservation strategies of these waterbird species. Because most wetlands examined in this study belong to the Natura 2000 network, these strategies should be included in their management plans. In addition, our study suggests that updating avian diversity data of the Sardinian wetlands on a regular basis is worthy of being recommended to the local administrations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/land13122193/s1, Table S1: Description of the wetlands under study in Sardinia (Italy); Table S2: List of the 144 sampling points used in the Sardinian wetlands; Table S3: Species-by-site matrix in the first half of August (A1); Table S4: Species-by-site matrix in the second half of August (A2); Table S5: Species-by-site matrix in the first half of September (S1); Table S6: Species-by-site matrix in the second half of September (S2); Table S7: Matrix of species richness (number of avian species) recorded in the coastal wetlands of Sardinia during the four sampling sessions; Table S8: Matrix of presences–absences of waterbird species recorded in the Sardinian wetlands during the four dates of sampling; Table S9: Functional (foraging, migration) traits of the bird species recorded in the coastal wetlands of Sardinia; Table S10: List of the avian species recorded in the coastal wetlands of Sardinia; Table S11: Results of the Mann–Kendall trend test applied to the migratory and foraging guilds in the four sampling dates; Table S12: Results of SIMPER analysis between the two sampling dates A1 and A2; Table S13: Results of SIMPER analysis between the two sampling dates A2 and S1; Table S14: Results of SIMPER analysis between the two sampling dates S1 and S2.
Author Contributions
Conceptualization, A.F., C.C. and M.G.; methodology, A.F.; software, A.F.; validation, C.C. and M.G.; formal analysis, A.F.; investigation, A.F.; resources, C.C.; data curation, M.G.; writing—original draft preparation, A.F.; writing—review and editing, A.F., C.C. and M.G.; visualization, A.F.; supervision, C.C. and M.G.; project administration, C.C. and M.G.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
Field surveys and geoprocessing were supported by the MAVA Foundation (“Mediterranean Mosaics II”, project MAVA, contract 15038 Mediterranean basin C2/2015) for the period 2016–2019 and analyses by LIPU-UK in 2024.
Data Availability Statement
Data are available in the Supplementary Materials and also from the first author on request.
Acknowledgments
We thank for the help with field surveys: Sergio Nissardi, Carla Zucca, Fabio Cherchi, Davide De Rosa, Ilaria Fozzi, Walter Piras, Jessica Atzori, and Lara Bassu. All individuals included in this section have consented to the acknowledgment. We acknowledge the helpful comments and suggestions provided by the Editor and four anonymous reviewers.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Beta diversity was computed as [18]
where T is the total number of species occurrences, ei is the number of wetlands containing the waterbird species i, and αk is the number of species at wetland k.
References
- European Commission. The Natura 2000 Biogeographical Regions. 2016. Available online: http://ec.europa.eu/environment/nature/natura2000/biogeog_regions/ (accessed on 29 August 2024).
- ISPRA. Introduction to the Italian Bird Migration Atlas; ISPRA Editore: Rome, Italy, 2008. [Google Scholar]
- Berthold, P. Bird Migration: A General Survey; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Fois, M.; Fenu, G.; Bacchetta, G. Estimating land market values from real estate offers: A replicable method in support of biodiversity conservation strategies. Ambio 2019, 48, 312–323. [Google Scholar] [CrossRef]
- Ferrarini, A.; Gustin, M.; Celada, C. Twenty-three years of land-use changes induced considerable threats to the main wetlands of Sardinia and Sicily (Italy) along the Mediterranean bird flyways. Diversity 2021, 13, 240. [Google Scholar] [CrossRef]
- Ferrarini, A.; Celada, C.; Gustin, M. Preserving the Mediterranean bird flyways: Assessment and prioritization of 38 main wetlands under human and climate threats in Sardinia and Sicily (Italy). Sci. Total Environ. 2020, 751, 141556. [Google Scholar] [CrossRef] [PubMed]
- Salafsky, N.; Margoluis, R. Threat reduction assessment: A practical and cost-effective approach to evaluating conservation and development projects. Conserv. Biol. 1999, 13, 830–841. [Google Scholar] [CrossRef]
- Ferrarini, A.; Gustin, M.; Celada, C. Wetland attributes significantly affect patterns of bird species distribution in the Sardinian wetlands (Italy): An uncertain future for waterbird conservation. J. Appl. Ecol. 2023, 60, 650–660. [Google Scholar] [CrossRef]
- Ferrarini, A.; Gustin, M.; Celada, C. Simulation modeling unveils the unalike effects of alternative strategies for waterbird conservation in the coastal wetlands of Sardinia (Italy). Biology 2023, 12, 1440. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.D.; Atmar, W. Nested subsets and the structure of insular mammalian faunas and archipelagos. Biol. J. Linn. Soc. Lond. 1986, 28, 65–82. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P.; Melian, C.J.; Olesen, J.M. The nested assembly of plant–animal mutualistic networks. Proc. Natl. Acad. Sci. USA 2003, 100, 9383–9387. [Google Scholar] [CrossRef]
- Legendre, P.; Galzin, R.; Harmelin-Vivien, M.L. Relating behavior to habitat: Solutions to the fourth-corner problem. Ecology 1997, 78, 547–562. [Google Scholar] [CrossRef]
- Evans, D. Building the European Union’s Natura 2000 network. Nat. Conserv. 2012, 1, 11–26. [Google Scholar] [CrossRef]
- Griffith, D.A. Spatial Autocorrelation: A Primer; Association of American Geographers: Washington, DC, USA, 1987. [Google Scholar]
- Hutto, R.L.; Pletschet, S.M.; Hendricks, P.A. fixed-radius point count method for nonbreeding and breeding season use. Auk 1986, 103, 593–602. [Google Scholar] [CrossRef]
- Cramp, S.E.; Simmons, K.E.L.; Brooks, D.J.; Perrins, C.M. Handbook of the Birds of Europe, the Middle East and North Africa: The Birds of the Western Palearctic (Vol. 1–9); 1977–1994; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Burfield, I.J.; Rutherford, C.A.; Fernando, E.; Grice, H.; Piggott, A.; Martin, R.W.; Balman, M.; Evans, M.I.; Staneva, A. Birds in Europe 4: The fourth assessment of Species of European Conservation Concern. Bird Conserv. Int. 2023, 33, e66. [Google Scholar] [CrossRef]
- Routledge, R.D. On Whittaker’s components of diversity. Ecology 1977, 38, 1120–1127. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods, 4th ed.; Charles Griffin: London, UK, 1975. [Google Scholar]
- Mann, H.B. Non-parametric tests against trend. Econometrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Michener, C.D.; Sokal, R.R. A quantitative approach to a problem of classification. Evolution 1957, 11, 490–499. [Google Scholar] [CrossRef]
- Sokal, R.R.; Sneath, P.H.A. Principles of Numerical Taxonomy; W. H. Freeman: San Francisco, CA, USA, 1963. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Yang, R.; Li, D.; Qiu, Y.; Lu, K.; Cao, X.; Chen, Q. Spatiotemporal Dynamics of Constructed Wetland Landscape Patterns during Rapid Urbanization in Chengdu, China. Land 2024, 13, 806. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, S.; Tian, G.; Dong, N.; Lei, Y. Coupling Biodiversity and Human Pressures to Indicate Conservation Priorities for Threatened Waterfowl Species: A Case in the Henan Yellow River Wetland National Nature Reserve. Land 2023, 12, 1250. [Google Scholar] [CrossRef]
- Hammana, C.; Pereña-Ortiz, J.F.; Meddad-Hamza, A.; Hamel, T.; Salvo-Tierra, Á.E. The Wetlands of Northeastern Algeria (Guelma and Souk Ahras): Stakes for the Conservation of Regional Biodiversity. Land 2024, 13, 210. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Y.; Zhang, Y.; Lu, X.; Cao, J. Comparative Assessment of the Spatiotemporal Dynamics and Driving Forces of Natural and Constructed Wetlands in Arid and Semiarid Areas of Northern China. Land 2023, 12, 1980. [Google Scholar] [CrossRef]
- Giovacchini, P.; Battisti, C.; Marsili, L. Evaluating the Effectiveness of a Conservation Project on Two Threatened Birds: Applying Expert-Based Threat Analysis and Threat Reduction Assessment in a Mediterranean Wetland. Diversity 2022, 14, 94. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, L.; Xia, S.; Zhou, J. Impact of Urbanisation Intensity on Bird Diversity in River Wetlands around Chaohu Lake, China. Animals 2022, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.M.; Adam, P. Climate Change and Intertidal Wetlands. Biology 2013, 2, 445–480. [Google Scholar] [CrossRef]
- Battisti, C.; Perchinelli, M.; Vanadia, S.; Giovacchini, P.; Marsili, L. Monitoring Effectiveness of an Operational Project on Two Threatened Landbirds: Applying a Before–After Threat Analysis and Threat Reduction Assessment. Land 2023, 12, 464. [Google Scholar] [CrossRef]
- Blake-Bradshaw, A.G.; Lancaster, J.D.; O’Connell, J.R.; Matthews, J.W.; Eichholz, M.W.; Hagy, H.M. Suitability of Wetlands for Migrating and Breeding Waterbirds in Illinois. Wetlands 2020, 40, 1993–2010. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.Y.; Li, Y.F.; Dong, B.; Qiu, C.Q.; Yang, J.L.; Zong, Y.; Chen, H.; Zhao, Y.Q.; Zhang, Y.A. Study on habitat suitability and environmental variable thresholds of rare waterbirds. Sci. Total Environ. 2021, 785, 147316. [Google Scholar] [CrossRef]
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef]
- Lai, S.; Leone, F.; Zoppi, C. Land cover changes and environmental protection: A study based on transition matrices concerning Sardinia (Italy). Land Use Policy 2017, 67, 126–150. [Google Scholar] [CrossRef]
- Ferrarini, A.; Celada, C.; Gustin, M. Anthropogenic Pressure and Climate Change Could Severely Hamper the Avian Metacommunity of the Sicilian Wetlands. Diversity 2022, 14, 696. [Google Scholar] [CrossRef]
- Ferrarini, A.; Celada, C.; Gustin, M. Spatiotemporal Dynamics in Bird Species Assembly in the Coastal Wetlands of Sicily (Italy): A Multilevel Analytical Approach to Promote More Satisfactory Conservation Planning. Land 2024, 13, 1333. [Google Scholar] [CrossRef]
- Ferrarini, A.; Celada, C.; Gustin, M. Waterbird Species are Highly Sensitive to Wetland Traits: Simulation-Based Conservation Strategies for the Birds of the Sicilian Wetlands (Italy). Biology 2024, 13, 242. [Google Scholar] [CrossRef]
- Rubega, M.A.; Robinson, J.A. Water salinization and shorebirds: Emerging issues. Intern. Wader Stud. 1997, 9, 45–54. [Google Scholar]
- Dezvareh, G.A.; Nabavi, E.; Shamskilani, M.; Darban, A.K. Water salinity reduction using the phytoremediation method by three plant species and analyzing their behavior. Water Air Soil Pollut. 2023, 234, 90. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).