Abstract
Unreasonable soil tillage measures have caused a sharp decline in the soil carbon (C) pool capacity of rice (Oryza sativa L.) paddy fields, have reduced soil fertility, and have threatened the safe production of rice. Based on long-term position–location experiments started in 2005, this paper systematically studied the effects of different soil tillage treatments (CT: no return of plowing straw to the field as control; CTS: return of plowing straw to the field; NTS: return of no-tillage straw to the field; RTS: return of rotary plowing straw to the field) on soil physical and chemical properties and soil organic carbon (SOC) accumulation characteristics in rice paddy fields, in order to clarify the impact of different long-term soil tillage measures on soil carbon cycle microecology in double-cropped rice paddy fields and provide a theoretical basis for soil SOC sequestration and the sustainable utilization of rice paddy fields in double-cropped rice paddy fields in southern China. The results were as follows: A total of 30.7–40.7% of the SOC stored in rice paddy fields was residue C derived from microorganisms, and 45.7–54.2% of SOC accumulation came from plant residue-derived C. Straw return treatments (CTS, RTS, and NTS) significantly increased soil lignin phenol content and promoted the accumulation of plant-derived SOC. Soil lignin phenol content in the RTS treatment was significantly higher than that in the CTS treatment (p < 0.05). Amino sugar content in rhizosphere soil was higher than that in non-rhizosphere soil. The measure of returning straw to the field increased amino sugar content in the rhizosphere and non-rhizosphere. C derived from plants was greater than that from microbial residues in double-cropped rice paddy fields in southern China. Hence, no-till/rotary tillage and straw return can improve the sequestration of soil SOC, which is of great significance for achieving “C neutrality” and alleviating the pressure on food security.
1. Introduction
Rice (Oryza sativa L.) is a major food crop in China. Double cropping, whereby early rice and late rice are grown successively in a single year, is important in rice production. Stabilizing and increasing the planting area and yield of double-cropped rice is of great strategic importance for ensuring national food security [1]. Globally, in the context of C neutrality goals and increasing food insecurity, research on soil C sequestration has rapidly developed in recent decades. Soil organic C (SOC) is a key indicator for evaluating soil fertility and an important indicator for measuring land degradation or restoration [2,3]. As more C is stored in the soil than in plants or the atmosphere, small changes in soil C sequestration ultimately affect global climate [4].
Global soil C reserves are approximately three times greater than atmospheric C reserves, and microorganisms affect the accumulation of soil C through in vitro modification (microbial extracellular enzymes reconstruct SOC molecules) and in vivo turnover (microbial residues re-synthesize C molecules) [5]. Soil microbial communities constitute the active pool of SOC, and their necrotic substances (microbial residue (MRC)) greatly contribute to the stable pool of SOC. MRC constitutes a persistent SOC pool [5,6], and determining its contribution to the SOC stock may provide a useful indicator.
In recent years, scientific research and technological breakthroughs have greatly improved our understanding of soil C sequestration [7,8]. However, gaps remain regarding methods for scientifically and effectively managing soil to improve C sequestration. Recent evidence has shown that SOC storage mainly originates from microorganisms and plants [9,10]. The classification of SOC into microbial and plant C can help us understand the pathways of C formation and the mechanisms of SOC persistence [5,11]. The energy of microorganisms is mainly supplied by plant C. The transformation of plant C into microbial C occurs according to the principle of microbial processing and assimilation [10]. Microbe-derived C is very close to and interacts with the soil mineral matrix, so unprocessed plant-derived C is more stable [10,12]. Microbial decomposition products, mainly necrotic substances and other by-products, contribute considerably to stabilizing SOC [13,14]. Therefore, the accumulation of microbe- and plant-derived C plays an important role in SOC pools and is affected by land use and related environmental conditions [9]. In recent years, SOC from plant and microbial sources has been comprehensively assessed by using lignin phenol and amino sugars [15].
Amino sugars are highly stable substances derived from microorganisms and are components of soil microbial cell walls. At present, four amino sugar monomers have been quantified in soil. Glucosamine is a fungal synthetic compound. It is the main component of deacylated chitin and the only component of chitin in fungi. Muramic acid is the only bacterial source and is a component of cell wall peptidoglycan and lipopolysaccharide in bacteria. Various inferences have been made regarding the origin of galactosamine, and it is generally believed to be a synthetic product of bacteria the source of which cannot be determined. Notably, its content is very low in soil, and it is rarely used in research on soil organic matter transformation [5,11].
Lignin phenols mainly originate from the side-chain oxidative degradation of lignin. Some studies have used gas chromatography to analyze the monocyclic phenolic compounds of CuO oxidation products to isolate lignin in the form of its basic structural units (vanillyl, syringyl, and cinnamyl) and their corresponding aldehyde groups, ketones, and acid substituents. These monocyclic phenolic compounds are often used as biochemical indicators of the origin and decomposition of lignin [16].
Notably, SOC sequestration in agricultural soils is critical to mitigating climate warming, ensuring food security, and improving the sustainability of agricultural ecosystems [4,9]. However, due to land-use changes, intensive agricultural practices, and erosion, global farmland soils have lost approximately 40–133 Pg of C [17]. Despite the impacts of intensive human activities, paddy soils exhibit substantial SOC sequestration potential [18,19]. The SOC content in rice paddies is approximately 12–58% higher than that in dry land. Given the wide area covered by rice paddies worldwide (1.67 × 108 hm2), SOC sequestration in rice paddy ecosystems is very important the global climate as it improves soil C stabilization and stocks [20,21]. In China, rice paddies play an important role in agriculture. Owing to their high C density and C sequestration potential, rice paddy ecosystems are considered important potential sites for C sequestration, thus mitigating climate change. However, owing to long-term, unreasonable soil tillage treatments in the past, the soil C pool in large rice paddies has been markedly reduced. This has not only reduced soil fertility and threatened rice production but also increased greenhouse gas emissions and exacerbated global warming [22]. Therefore, there is an urgent need to study and promote scientific soil tillage treatments to improve the soil structure, improve soil C pools, increase soil fertility, and slow down global warming [23].
Soil tillage has an important impact on agricultural production. The mechanical power of tillage machinery is used to change the state of the gas, solid, and liquid phases of the soil, building a reasonable tillage layer. However, long-term plowing may damage the ecological environment. In the 1930s, following years of intensive farming practices, large-scale dust storms occurred in the United States, which eroded fertile surface soil and severely damaged the soil structure, greatly impacting agricultural production. Conservation tillage measures such as no-till and low-till treatments can reduce soil disturbance, increase surface cover, improve soil structure, increase soil nutrient content, increase soil C sequestration, and increase crop yield [24]. In recent years, the decline in cultivated land quality caused by unreasonable tillage treatments has attracted widespread attention [25]. Soil quality assessments are typically based on measurements of soil biological, chemical, and physical characteristics, which change in response to different management measures (soil tillage, crop rotation, and straw return). The depth and intensity of soil tillage affect crop yield by changing the chemical and physical properties of the soil, and plowing affects the compactness of topsoil to a certain extent due to mechanical force [26]. Studies have found that reducing tillage disturbances can increase soil stability and SOC content [27].
We hypothesize that (i) different soil tillage measures result in different total SOC, lignin phenol, and amino sugar contents and that (ii) different soil cultivation practices affect SOC sequestration in paddy soil. The main objectives of this study were to (1) analyze the total SOC, lignin phenol, and amino sugar contents in rice paddy soil under different tillage conditions and (2) evaluate SOC sequestration in paddy soils under different tillage management practices.
2. Materials and Methods
2.1. Study Site
The experiment was initiated in October 2005 and concluded in October 2023. The study site, soil type and texture, climate conditions, and cropping system of this experimental region were described in detail by Qi [28].
2.2. Experimental Design
The field trial plot covered an area of 66.7 m2. A random-block design was used, and four treatments were applied, with three replicates of each treatment. The treatments were as follows: (1) plowing, rotary tilling, and no straw return (control (CT)); (2) plowing, rotary tilling, and straw return (RTS); (3) no plowing, no tilling, and straw return (NTS); and (4) rotary tilling and straw return (CTS). Under CT, the field was first plowed once with a share plow and then tilled twice with a rotary tiller at a depth of approximately 15 cm, and all the straw was removed from the rice paddy. Under RTS, the field was first plowed with a share plow and then tilled twice with a rotary tiller at a depth of approximately 15 cm, and all straw was returned to the field. After the early-rice harvest, the straw was completely turned over and returned to the field, and after the late-rice harvest, the straw was covered and returned to the field before the seedlings of the early rice were planted in the following year. Under NTS, no soil preparation occurred, and no tillage was performed before the seedlings were planted. After the early rice was harvested, all the straw was returned to the field. Under CTS, the field was tilled with a rotary tiller four times at a depth of approximately 8 cm before the rice seedlings were planted, and all straw was returned to the field. All early-rice straw was returned to the field through rotary tillage, and late-rice straw was covered and returned to the field. Next year, early-rice straw was rotary-tilled into the field before the rice seedlings were planted. Each straw return treatment was approximately 12,500 kg·hm−2.
The variety and fertilizer were described in detail by Cheng [21].
2.3. Soil Sampling
During the maturity periods of early and late rice in 2023 (July and October), rhizosphere and non-rhizosphere soil samples were collected from each experimental plot. A sampling method was used to collect the rhizosphere samples. Five plants were selected from each treatment, the soil around the roots was shaken off, and the roots were taken back to the laboratory and washed with phosphate-buffered saline. A five-point sampling method was used to collect non-rhizosphere soil samples (sampled from the middle of the plants) from the 0–5 cm, 5–10 cm, and 10–20 cm soil layers. One part of the soil samples was used for SOC determination; the other part was placed was subject to refrigeration for the determination of soil microbial organic C accumulation markers. Additionally, six representative points were selected in each plot to measure the soil physical characteristics (permeability, moisture, and bulk density).
Soil penetration resistance was measured by using an SP1000 trocar penetration instrument [29]. The instrument can measure resistance at depths of 0–20 cm. The penetration resistance value (cm) was measured at intervals of possible penetration depths and was defined as the average value of each treatment.
The soil water content determination procedures were described in detail by Regalado [30].
2.4. Soil Laboratory Analysis
Soil Chemical Analysis
The methods for determining lignin phenol and amino sugar contents and impact on SOC sequestration sources were described in detail by Chen [14].
The content of lignin phenol was calculated as the sum of syringyl (S)-, cinnamyl (C)-, and vanillin (V)-type phenols. Two-thirds of the V phenol in the lignin structure is not oxidized and released by CuO, while the release efficiency of S phenol is 90%. The release efficiency of C phenol is assumed to be 100%. Plant-derived carbon in total organic carbon (P) is estimated with the following equation:
where C, S, and V represent the carbon contents (g·kg−1) in type C, S, and V phenols, respectively; 8% represents the lowest content of lignin in plant residues from major crops; and SOC represents total SOC content (g·kg−1).
2.5. Statistical Analysis
The results for each measurement item were expressed as the mean values and standard errors. Following standard procedures, we used one-way analysis of variance at a 5% probability level to analyze and compare the data for each treatment. All statistical analyses were carried out by using the SAS 9.3 software package. Differences in SOC-normalized biomarkers were tested by using Duncan’s test and one-way analysis of variance (p < 0.05). Levene’s test was used for uniformity of variance, and Shapiro’s test was used for the normal distribution of residuals. After natural logarithmic transformation of non-normally distributed data, the correlations between the biomarkers and environmental parameters were tested. The figures were drawn by using Origin 8.5.
3. Results
3.1. Effects on Soil Physical Properties
As shown in Table 1, compared with the CT treatment, the NTS, CTS, and RTS treatments increased soil water content in rice paddy soil and reduced soil resistance to infiltration. Soil anti-permeability decreased under each straw return treatment (CTS, RTS, and NTS) with an increase in soil volumetric water content. Soil permeability resistance under the CTS, RTS, and NTS treatments decreased by 6.34%, 32.09%, and 13.06%, respectively, in early rice and by 4.38%, 30.29%, and 12.77%, respectively, in late rice compared with that under CT treatment. The above results show that soil water content in rice paddy fields is closely related to straw return and soil tillage treatments.

Table 1.
Soil volumetric water content, permeability resistance, and bulk density in paddy fields under different tillage measures.
Different soil tillage treatments and straw return measures significant affected soil bulk density (Table 1). In contrast to the NTS treatment, the CT, CTS, and RTS treatments reduced soil bulk density. Soil bulk density was higher under the RTS treatment than under the CT and CTS treatments, but the difference was not significant (p > 0.05). Among the straw return treatments, soil bulk density under the NTS treatment was 25.45%, 28.97%, and 17.95% higher than that under the CT, CTS, and RTS treatments, respectively, in early rice and 23.21%, 27.78%, and 16.95% higher, respectively, in late rice (p < 0.05).
3.2. Effects on SOC
In the 0–20 cm soil layer, SOC content in rice paddies under each treatment showed a consistent decreasing trend with an increase in soil depth (Table 2). Compared with that under the CT treatment, SOC contents in early rice under the CTS, RTS, and NTS treatments increased in the 0–5 cm, 5–10 cm, and 10–20 cm soil layers. SOC contents in early rice at 0–5 cm under the CTS, NTS, and RTS treatments were 4.76%, 28.25%, and 6.67% higher than under the CT treatment. SOC contents in late rice at 0–5 cm under the CTS, NTS, and RTS treatments were 8.85%, 34.75%, and 13.77% higher than under the CT treatment. During the maturity period of early and late rice, SOC content under NTS was significantly higher than that under the other treatments in the 0–5 cm soil layer, whereas SOC content under CTS was significantly higher than that under NTS in the 5–10 cm and 10–20 cm soil layers.

Table 2.
Soil organic carbon content of paddy field under different soil tillage measures at maturity of rice (g·kg−1).
The impacts of different tillage treatments on total SOC in double-cropped rice paddies are shown in Figure 1. The outcomes indicate that SOC content in rhizosphere and non-rhizosphere soils under different tillage treatments ranged from 21.8 to 28.6 g·kg−1. Total rhizosphere SOC increased by 15.37%, 23.28%, and 19.68% under the CTS, RTS, and NTS treatments, respectively, compared with that under CT treatment. Further, non-rhizosphere SOC increased by 18.50%, 26.15%, and 22.78% under the CTS, RTS, and NTS treatments, respectively, compared with that under CT treatment. Under the same tillage conditions, total SOC contents in rhizosphere and non-rhizosphere soils were significantly lower under the CTS treatment than under the NTS treatment (p < 0.05). These results show that no-till treatments and straw return can increase total SOC content in soil to a certain extent.
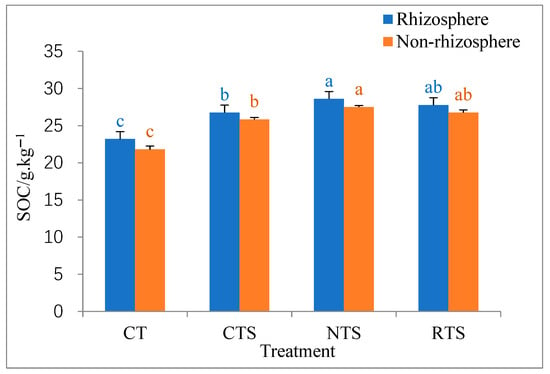
Figure 1.
Effects of different tillage treatments on total SOC contents in rhizosphere and non-rhizosphere soils. Note: Different lowercase letters in the same color of data in the figure indicate that the difference between treatments reaches the significant level of 5%. The same applies below. These results were obtained after the late-rice harvest in 2023.
3.3. Effects on Lignin Phenol and Amino Sugar Contents
Under different tillage treatments, the proportion of lignin phenol in rhizosphere and non-rhizosphere SOC ranged from 27.9 to 46.4 mg·g−1 SOC. In rhizosphere and non-rhizosphere soils, lignin phenol content was significantly higher under the CTS, RTS, and NTS treatments than under the CT treatment (Figure 2), indicating that returning all straw to the field can significantly increase lignin phenol content in soil and promote the accumulation of plant-derived SOC. However, soil lignin phenol content was significantly higher under RTS than under CTS, indicating that traditional plowing was not conducive to the accumulation of plant-derived SOC.
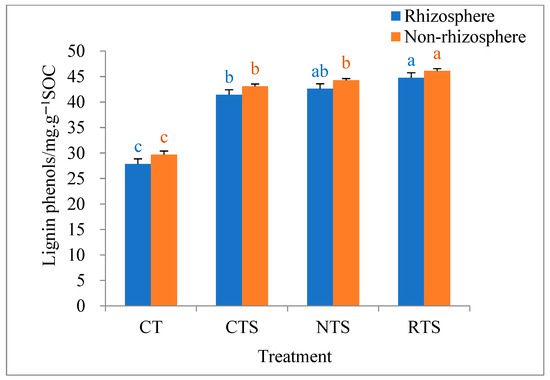
Figure 2.
Effects of different tillage treatments on lignin phenols in rhizosphere and non-rhizosphere soils. Note: Different lowercase letters in the same color of data in the figure indicate that the difference between treatments reaches a the significant level of 5%.
The influences of different tillage treatments on soil microbial amino sugar content in double-cropped rice paddies are shown in Figure 3. The results show that amino sugar contents in the non-rhizosphere and rhizosphere soils ranged from 41.5 to 57.4 mg·g−1 SOC under different tillage treatments. In rhizosphere and non-rhizosphere soils, amino sugar content was significantly higher under the CTS, RTS, and NTS treatments than under the CT treatment. In rhizosphere soil, amino sugar content increased by 14.2%, 19.0%, and 17.9% under the CTS, RTS, and NTS treatments, respectively, compared with that under the CT treatment. In non-rhizosphere soil, amino sugar content increased by 17.0%, 20.0%, and 20.1% under the CTS, RTS, and NTS treatments, respectively, compared with that under the CT treatment. Overall, straw return increased amino sugar content, and amino sugar content in rhizosphere soil was higher than that in non-rhizosphere soil.
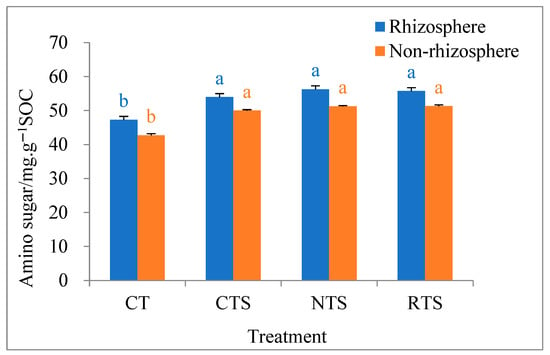
Figure 3.
Effects of different tillage treatments on amino sugar content in rhizosphere and non-rhizosphere soils. Note: Different lowercase letters in the same color of data in the figure indicate that the difference between treatments reaches a the significant level of 5%.
The impacts of different tillage treatments on soil amino sugar fungal markers in double-cropped rice paddies are shown in Figure 4. The results show that the amino sugar fungal marker content in rhizosphere and non-rhizosphere soils ranged from 23.1 to 32.0 mg·kg−1 under different tillage treatments, and the amino sugar fungal markers were more abundant in rhizosphere soil than in non-rhizosphere soil. In rhizosphere soil, amino sugar fungal marker content increased by 14.01%, 21.33%, and 17.55% under the CTS, RTS, and NTS treatments, respectively, compared with that under the CT treatment. In non-rhizosphere soil, soil amino sugar fungal marker content increased by 21.24%, 31.64%, and 27.31% under CTS, RTS, and NTS, respectively, compared with that under CT. Among the straw return treatments, amino sugar fungal marker contents in rhizosphere and non-rhizosphere soils were significantly lower under CTS than under NTS (p < 0.05). In summary, no-till or rotary tillage treatments combined with straw return can increase the content of soil amino sugar fungal markers to a certain extent.
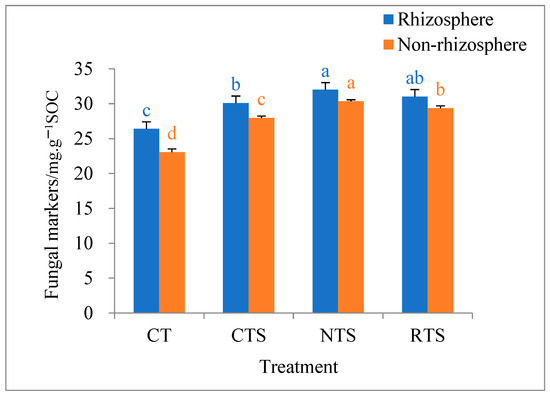
Figure 4.
Effects of different tillage treatments on content of fungal markers in rhizosphere and non-rhizosphere soils. Note: Different lowercase letters in the same color of data in the figure indicate that the difference between treatments reaches a the significant level of 5%.
The effects of different tillage treatments on soil amino sugar bacterial markers in double-cropped rice paddies are shown in Figure 5. The results show that amino sugar bacterial marker content in rhizosphere and non-rhizosphere soils under different tillage treatments ranged from 2.2 to 2.7 mg·kg−1, and amino sugar bacterial marker content was lower in rhizosphere soil than in non-rhizosphere soil. In rhizosphere soil, amino sugar bacterial marker content increased by 16.38%, 22.10%, and 19.63% under the CTS, RTS, and NTS treatments, respectively, compared with that under the CT treatment. In non-rhizosphere soil, amino sugar bacterial marker content increased by 17.37%, 20.99%, and 20.85% under the CTS, RTS, and NTS treatments, respectively, compared with that under the CT treatment. When the straw was returned to the field, amino sugar bacterial marker contents in non-rhizosphere and rhizosphere soils were significantly lower under the CTS treatment than under the NTS treatment (P < 0.05). These results show that no-till methods combined with straw return can increase the content of soil amino sugar bacterial markers to a certain extent.
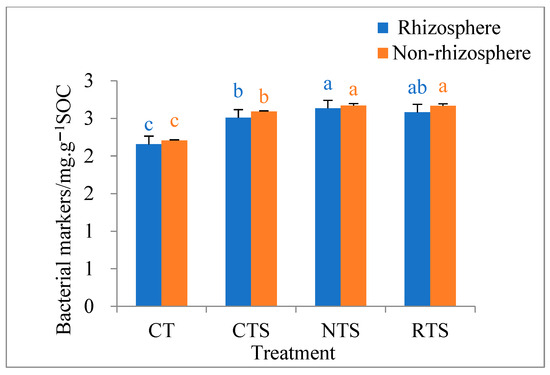
Figure 5.
Effects of different tillage treatments on contents of bacterial markers in rhizosphere and non-rhizosphere soils. Note: Different lowercase letters in the same color of data in the figure indicate that the difference between treatments reaches a the significant level of 5%.
As shown in Figure 6, examining the ratio of fungal to bacterial markers revealed that the ratio in rhizosphere soil was significantly higher than that in non-rhizosphere soil (p < 0.05). However, there was no significant difference in the ratio in rhizosphere soil among different tillage treatments, and the ratio of fungal to bacterial markers in non-rhizosphere soil was significantly higher under NTS than under CT.
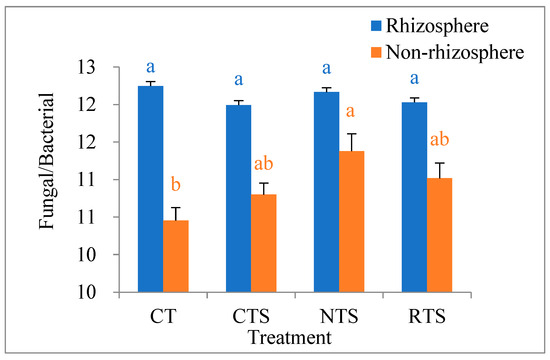
Figure 6.
Effects of different tillage treatments on ratio of fungal and bacterial markers between rhizosphere and non-rhizosphere. Note: Different lowercase letters in the same color of data in the figure indicate that the difference between treatments reaches a the significant level of 5%.
3.4. Impact on SOC Sequestration Sources in Soil
To study the sources of SOC sequestration in the soil of double-cropped rice paddies under different tillage treatments, we used the biomarkers of lignin phenol and amino sugars in rhizosphere and non-rhizosphere soils to calculate the amount of C derived from either plant or microbial residues (Figure 7). We found that 45.7–54.2% of SOC accumulation originated from plant sources, while 30.7–40.7% of the SOC stored in rice paddies was from microbial sources. In double-cropped rice paddies, the amount of C derived from plants was greater than that derived from microorganisms. In rhizosphere soil, the amount of C derived from plant residues was 12.22%, 11.64%, 8.26%, and 11.69% higher under the CT, CTS, RTS, and NTS treatments, respectively, than that derived from microorganisms. In non-rhizosphere soil, the C content derived from plant residues was 17.99%, 15.20%, 10.59%, and 15.80% higher under the CT, CTS, RTS, and NTS treatments, respectively, than that derived from microorganisms.
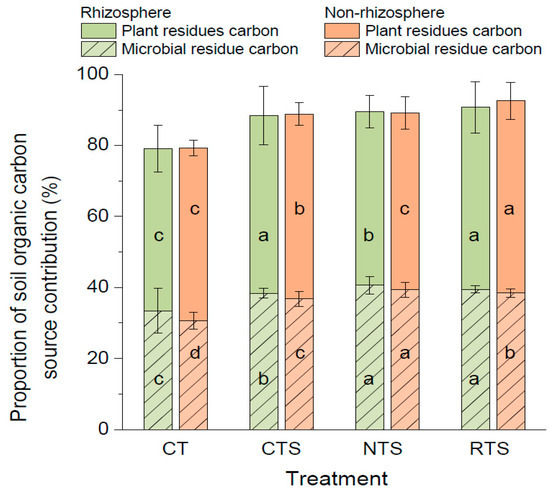
Figure 7.
Effects of different tillage measures on content of amino sugar biomarkers in rhizosphere and non-rhizosphere soils. Note: Different lowercase letters in the same color of data in the figure indicate that the difference between treatments reaches a the significant level of 5%.
4. Discussion
The term “rhizosphere” was coined by Hiltner in 1904 to describe the part of the soil in which microorganisms participate in processes that are influenced by the roots. Rhizosphere function is crucial for crop growth and development and soil health and quality. As the roots secrete compounds, the biomass and activity of microorganisms improve [31]. As rhizosphere plant–microbial interactions are of great importance to C sequestration and nutrient cycling in agricultural ecosystems [32], it is crucial to study the rhizosphere microenvironment.
Irrespective of the tillage treatments adopted, the organic C content in the rhizosphere soil of rice paddies exceeded than that in non-rhizosphere soil. In the absence of tillage, rice roots are prevented from growing deeper into the soil, leading to more root C being obtained from the surface layer. In addition, the anoxic environment in rice caused by flooding restricts microbial activities, reduces the production of oxidase involved in the decomposition of plant residues, and limits other related processes in which O2 replaces electron acceptors [33]. Therefore, organic matter remains in rice paddies for a long time. Overall, the differences in SOC under different tillage practices may be attributed to corresponding differences in the non-rhizosphere and rhizosphere microecology.
Carbon sequestration is one of the key soil functions, and the increase in its SOC is an important indicator of ecosystem restoration, because it can serve as a substrate of soil microbial communities to support other ecosystem service functions. SOC sequestration in agricultural soils is crucial to mitigating climate warming, food security, and the sustainability of agricultural ecosystems. Reducing soil tillage will increase soil stability and SOC content, and on the premise of reducing soil disturbance, SOM content, microporosity, and soil infiltration rate can be effectively improved. SOC content is strongly affected by tillage practices and straw return to the field. Reasonable soil tillage treatments can improve the soil structure of rice fields, significantly increase soil carbon pool levels, improve soil fertility, and slow down global warming.
The main characteristic of SOC in rice paddies is that the proportion of C derived from plants is high, whereas the proportion of C derived from microorganisms is low. Compared with the results of previous studies, in this study, 30.7–40.7% of the SOC in rice fields came from microbial residues, which is lower than that usually found in forest soil (33–62%) [14], fertilized dry land (36–86%) [34], and alpine grassland (34–53%). In rice paddies, microbial anabolic metabolism due to oxygen restriction is weak, which reduces the composition of microbial residues while promoting the retention and accumulation of plant residues. As a result, rice paddies can fix SOC more effectively, primarily because of the accumulation characteristics of plant-derived C.
The different sources of SOC in paddy soils are crucial for its stability. Numerous studies have confirmed that microbe-derived C might be steadied and more long-lasting than plant-derived C owing to it is greater tendency to be physically protected in the organic mineral mix [10,13]. We found that the accumulated SOC in rice paddies is the result of rich, activated C from plants. For plant-derived C, a decrease in the C/V and S/V ratios of lignin phenol signifies an increase in the microbial change phase [16]. Therefore, a lower (Ad/Al)S ratio and a higher C/V ratio represent a lower degree of oxidative decomposition, indicating that plant-derived C decomposes less than microbe-derived C in paddy soil [35]. For microbe-derived C, bacterial biomarkers represented by muramic acid decompose more easily after cell death than fungal biomarkers. Therefore, the ratio of fungal glucosamine to bacterial muramic acid in rice paddy soil is low, indicating that microbe-derived C is less stable in rice paddy fields. Accordingly, the accumulation of SOC in rice paddies may be unsteady and vulnerable to further disturbances.
An SOC-increasing strategy aimed at quantifying the specific contributions of plant and microbial C was proposed [7]. Microbe-derived C is firmer but saturated when covered by mineral combinations (mineral-related organic matter), whereas plant-derived C (particulate organic matter) is less protected in soil and can accumulate infinitely. Approximately 98% of C losses occur in subtropical and warmer tropical climates, with plant-derived C accounting for 90% of the total C losses. Therefore, it is necessary to protect the loss of SOC in rice paddies, especially the C from plant sources.
The amino sugar content in rice paddy soil is relatively stable, but the lignin phenol content changes considerably. Thus, the part of microbe-derived C in the formation of organic C is more reactionary, because it relies less on environmental components and agricultural management than on plant residues [16]. Different soil environmental conditions can directly affect the ratio of plant-derived C to organic C, and their effect is relatively stronger in paddy soils. In summary, dividing microbial residue C into bacterial and fungal C is more conducive to explaining the different microbial trail of SOC formation in rice paddies [34].
5. Conclusions
Compared with the CT treatment, the NTS, CTS, and RTS treatments increased soil water content and reduced soil permeability in rice paddies. Straw return and no-till measures increased total SOC content, and SOC content in non-rhizosphere and rhizosphere soils under different treatments ranged from 21.8 to 28.6 mg·kg−1. We determined that 30.7–40.7% of the SOC stored in rice paddies was derived from microorganisms, while 45.7–54.2% of SOC was derived from plant residues. Returning straw to the field significantly increased lignin phenol content in soil and promoted the accumulation of plant-derived SOC. Soil lignin phenol content under the RTS treatment was significantly higher than that under the CTS treatment, indicating that traditional plowing is not favorable to the accumulation of plant-derived SOC. Amino sugar content in rhizosphere soil was higher than that in non-rhizosphere soil, and returning straw to the field increased amino sugar content. The results of this study clarify the response of plant and microbial C sources to different tillage treatments. Hence, no-till/rotary tillage and straw return can improve the sequestration of soil SOC, which is of great significance for achieving “C neutrality” and alleviating the pressure on food security.
Author Contributions
H.T. set up the experiment, and C.L. conducted the experiment, L.L., H.L. and J.L. analyzed of the data, and L.W. and S.P. provided the material for this experiment. K.C. wrote this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Fund for the Director of Hunan Soil and Fertilizer Research Institute (2020tfs201). Agricultural Science and Technology Innovation Fund Project of Hunan Province (2023CX103). National Key Research and Development Project of China (2023YFD2301403).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
This study was thanks to the scholars Fu Chen and Hailin Zhang for their contribution to this field experiment.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Tang, H.M.; Xiao, X.P.; Li, C.; Tang, W.G.; Cheng, K.K.; Pan, X.C.; Wang, K.; Li, W.Y. Effects of different soil tillage systems on soil carbon management index under double-cropping rice paddy field in southern China. Agron. J. 2019, 111, 440–447. [Google Scholar] [CrossRef]
- Ghosh, B.N.; Meena, V.S.; Singh, R.J.; Alam, N.M.; Patra, S.; Bhattacharyya, R.; Sharma, N.K.; Dadhwal, K.S.; Mishra, P.K. Effects of fertilization on soil aggregation, carbon distribution and carbon management index of maize-wheat rotation in the north-western Indian Himalayas. Ecol. Indic. 2019, 105, 415–424. [Google Scholar] [CrossRef]
- Lei, K.J.; Dai, W.X.; Wang, J.; Li, Z.W.; Cheng, Y.; Jiang, Y.J.; Yin, W.Q.; Wang, X.Z.; Song, X.D.; Tang, Q. Biochar and Straw Amendments over a Decade Divergently Alter Soil Organic Carbon Accumulation Pathways. Agronomy 2024, 14, 2176. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jackson, R.D.; DeLucia, E.H.; Tiedje, J.M.; Liang, C. The soil microbial carbon pump: From conceptual insights to empirical assessments. Glob. Chang. Biol. 2020, 26, 6032–6039. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef]
- Joergensen, R.G. Amino sugars as specific indices for fungal and bacterial residues in soil. Biol. Fert. Soils 2018, 54, 559–568. [Google Scholar] [CrossRef]
- Liu, X.; Ma, T.; Zhang, H.G.; Yan, K.; Zhou, S.W. The accumulation of fungal not bacterial residue carbon is management-dependent under conventional and organic practices in apple-orchard soil. Geoderma 2024, 448, 116968. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, P. The Microbial Eficiency-Matrix S tabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter. Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Chen, X.B.; Xia, Y.H.; Rui, Y.; Ning, Z.; Hu, Y.J.; Tang, H.M.; He, H.B.; Li, H.X.; Kuzyakov, Y.; Ge, T.D.; et al. Microbial carbon use efficiency, biomass turnover, and necromass accumulation in paddy soil depending on fertilization. Agric. Ecosyst. Environ. 2020, 292, 106816. [Google Scholar] [CrossRef]
- Crow, S.E.; Lajtha, K.; Filley, T.R.; Swanston, C.W.; Bowden, R.D.; Caldwell, B.A. Sources of plant-derived carbon and stability of organic matter in soil: Implications for global change. Glob. Chang. Biol. 2009, 15, 2003–2019. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.C.; Luo, J.F.; Lindsey, S.; Zhou, F.; Xie, H.T.; Li, Y.; Zhu, P.; Wang, L.C.; Shi, Y.L.; et al. Differential accumulation of microbial necromass and plant lignin in synthetic versus organic fertilizer-amended soil. Soil Biol. Biochem. 2020, 149, 107967. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- Pan, G.; Li, L.; Wu, L.; Zhang, X. Storage and sequestration potential of topsoil organic carbon in China’s paddy soils. Glob. Chang. Biol. 2004, 10, 79–92. [Google Scholar] [CrossRef]
- Tian, K.; Zhao, Y.; Xu, X.; Hai, N.; Huang, B.; Deng, W. Effects of long-term fertilization and residue management on soil organic carbon changes in paddy soils of China: A meta-analysis. Agric. Ecosyst. Environ. 2015, 204, 40–50. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ge, T.D.; Zhu, Z.K.; Liu, S.L.; Luo, Y.; Li, Y.; Wang, P.; Gavrichkova, O.; Xu, X.L.; Wang, J.K.; et al. Carbon input and allocation by rice into paddy soils: A review. Soil Biol. Biochem. 2019, 133, 97–107. [Google Scholar] [CrossRef]
- Cheng, K.K.; Tang, H.M.; Li, C.; Tang, W.G.; Xiao, X.P.; Yi, Z.Y. Impact of long-term tillage management on utilization of microbial carbon sources in rhizosphere and non-rhizosphere soils under a double-cropping rice paddy field. Environ. Sci. Pollut. Res. 2022, 29, 15205–15214. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.Y.; Cheng, C.; Wang, J.J.; Luo, K.Z.; Zeng, Y.G.; Yang, X.X. Net global warming potential, greenhouse gas intensity and carbon footprint as affected by different tillage systems from Chinese double-cropping paddy fields. Soil Till. Res. 2021, 209, 104947. [Google Scholar] [CrossRef]
- Wang, X.; Jing, Z.H.; He, C.; Liu, Q.Y.; Qi, J.Y.; Zhao, X.; Xiao, X.P.; Zhang, H.L. Temporal variation of SOC storage and crop yield and its relationship-A fourteen year field trial about tillage practices in a double paddy cropping system, China. Sci. Total Environ. 2021, 759, 143494. [Google Scholar] [CrossRef]
- Choudhary, S.; Gajanand, M.C.; Choudhary, M.S. Conservation Agriculture and its Impact on Physical, Chemical and Biological Properties of Soil: A Review. IJBS 2021, 8, 113–122. [Google Scholar] [CrossRef]
- Crittenden, S.J.; Poot, N.; Heinen, M. Soil physical quality in contrasting tillage systems in organic and conventional tillage. Soil Till. Res. 2015, 154, 136–144. [Google Scholar] [CrossRef]
- Govaerts, B.; Mezzalama, M.; Sayre, K.D.; Crossa, J.; Nicol, J.; Deckers, J. Long-term consequences of tillage, residue management, and crop rotation on maize/wheat root rot and nematode populations in subtropical highlands. Appl. Soil Ecol. 2006, 32, 305–315. [Google Scholar] [CrossRef]
- Van Groenigen, K.J.; Bloem, J.; Bååth, E.; Boeckx, P.; Rousk, J.; Bodé, S.; Forristal, D.; Jones, M.B. Abundance, production and stabilization of microbial biomass under conventional and reduced tillage. Soil Biol. Biochem. 2010, 42, 48–55. [Google Scholar] [CrossRef]
- Qi, J.Y.; Wang, X.; Zhao, X.; Pu, C.; Kan, Z.R.; Li, C.; Liu, P.; Xiao, X.P.; Rattan, L.; Zhang, H.L. Temporal variability of soil organic C in paddies during 13-year conservation tillage. Land Degrad. Dev. 2019, 30, 1840–1850. [Google Scholar] [CrossRef]
- Klute, A. Physical and mineralogical methods. Am. Soc. Agron. Soil Sci. Soc. Am. J. 1986, 2, 463–478. [Google Scholar]
- Regalado, C.M.; Carpena, R.M.; Socorro, A.R.; Moreno, J.M.H. Time domain reflectometry models as a tool to understand the dielectric response of volcanic soils. Geoderma 2003, 117, 313–330. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Chen, X. Economic potential of biomass supply from crop residues in China. Appl. Energy 2016, 166, 141–149. [Google Scholar] [CrossRef]
- Ye, G.; Lin, Y.; Kuzyakov, Y.; Liu, D.; Ding, W. Manure over crop residues increases soil organic matter but decreases microbial necromass relative contribution in upland Ultisols: Results of a 27-year field experiment. Soil Biol. Biochem. 2019, 134, 15–24. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Kögel, I. Estimation and decomposition pattern of the lignin component in forest humus layers. Soil Biol. Biochem. 1986, 18, 589–594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).