The Effect of Ornamental Groundcover Habit and Irrigation Delivery on Dynamic Soil Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Preparation and Establishment
2.2. Irrigation
2.3. Plant Materials and Installation
2.4. Coverage Analysis
2.5. Weed Density and Biomass
2.6. Microbial Communities
2.7. Data Analysis
3. Results and Discussion
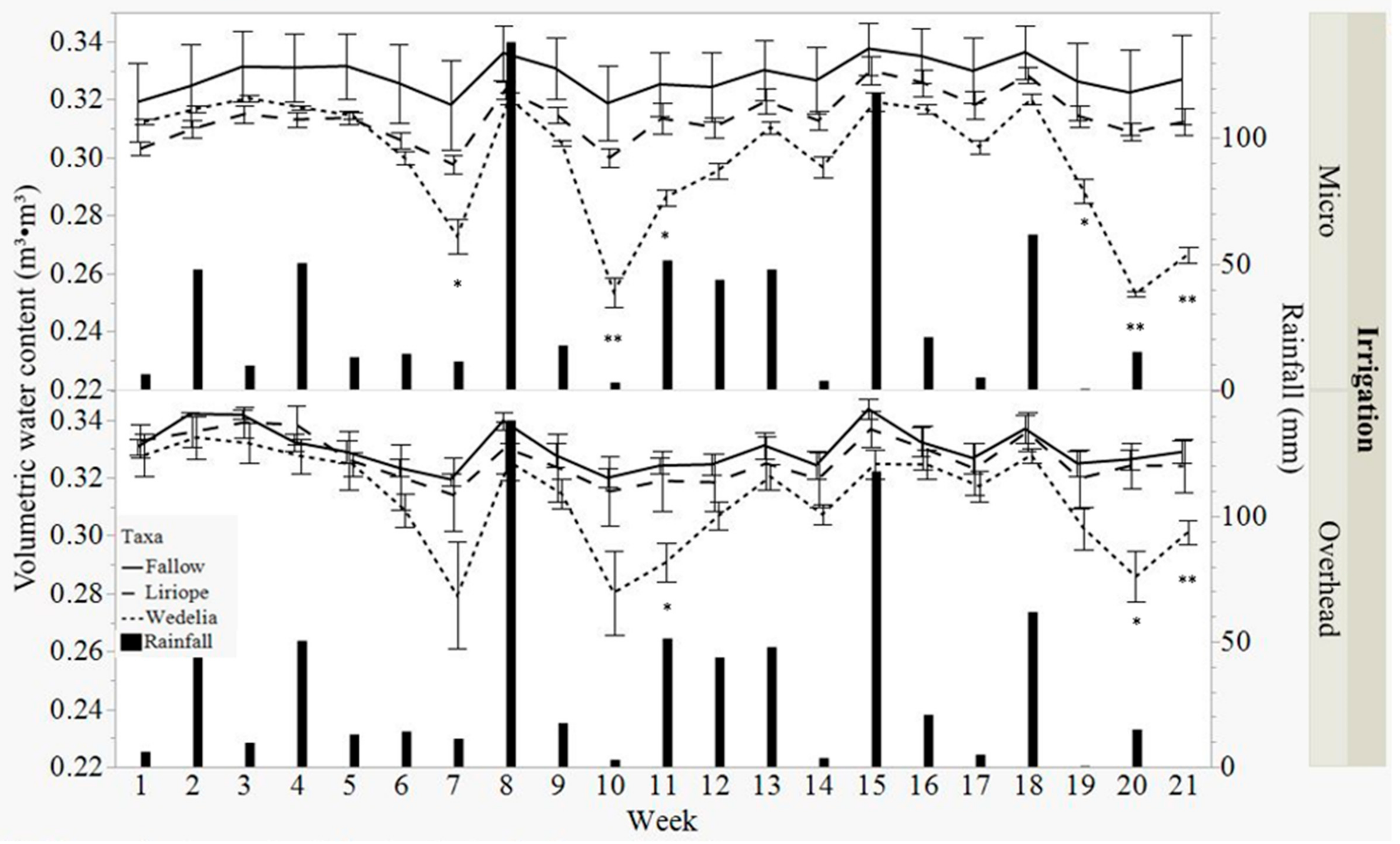
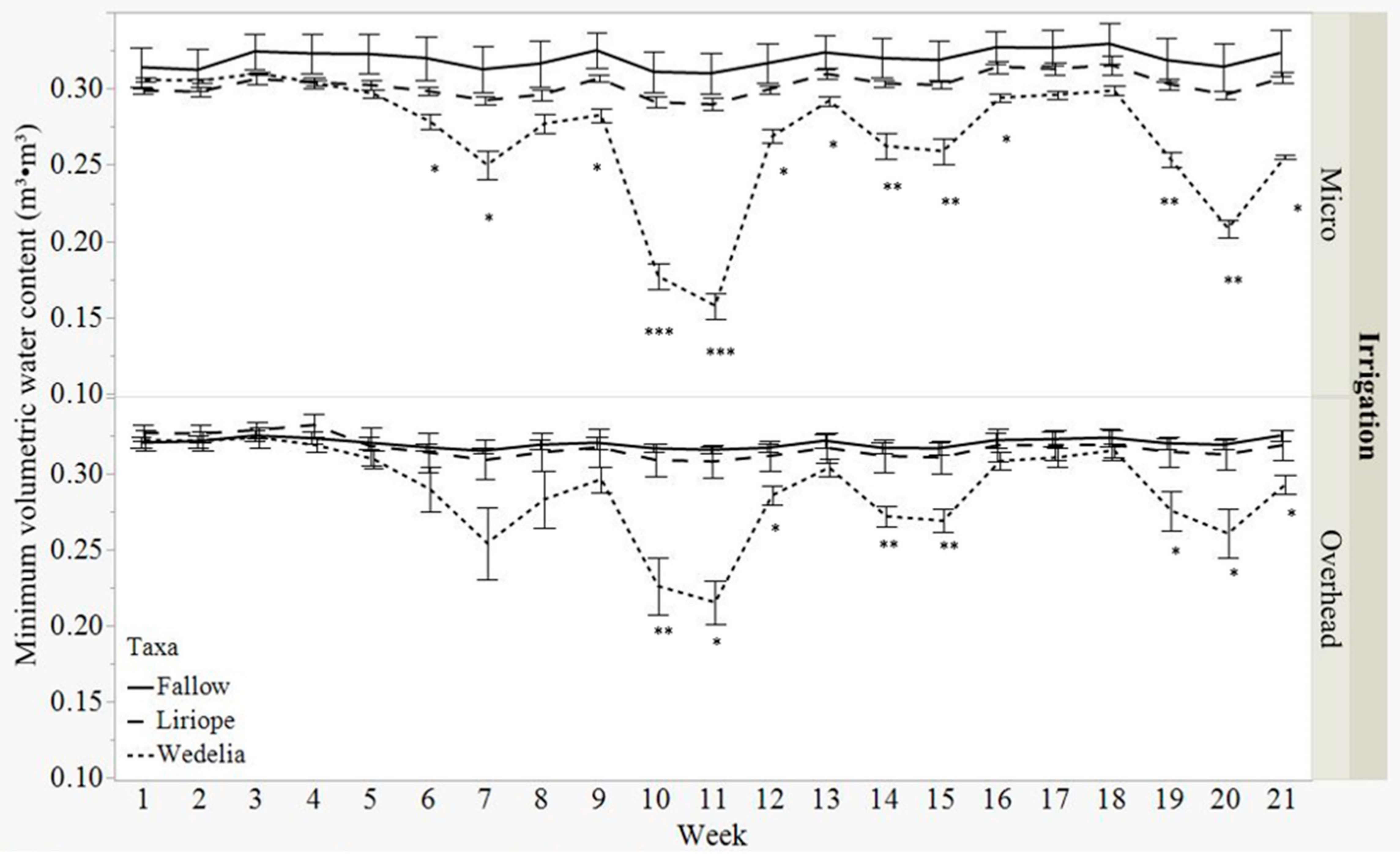
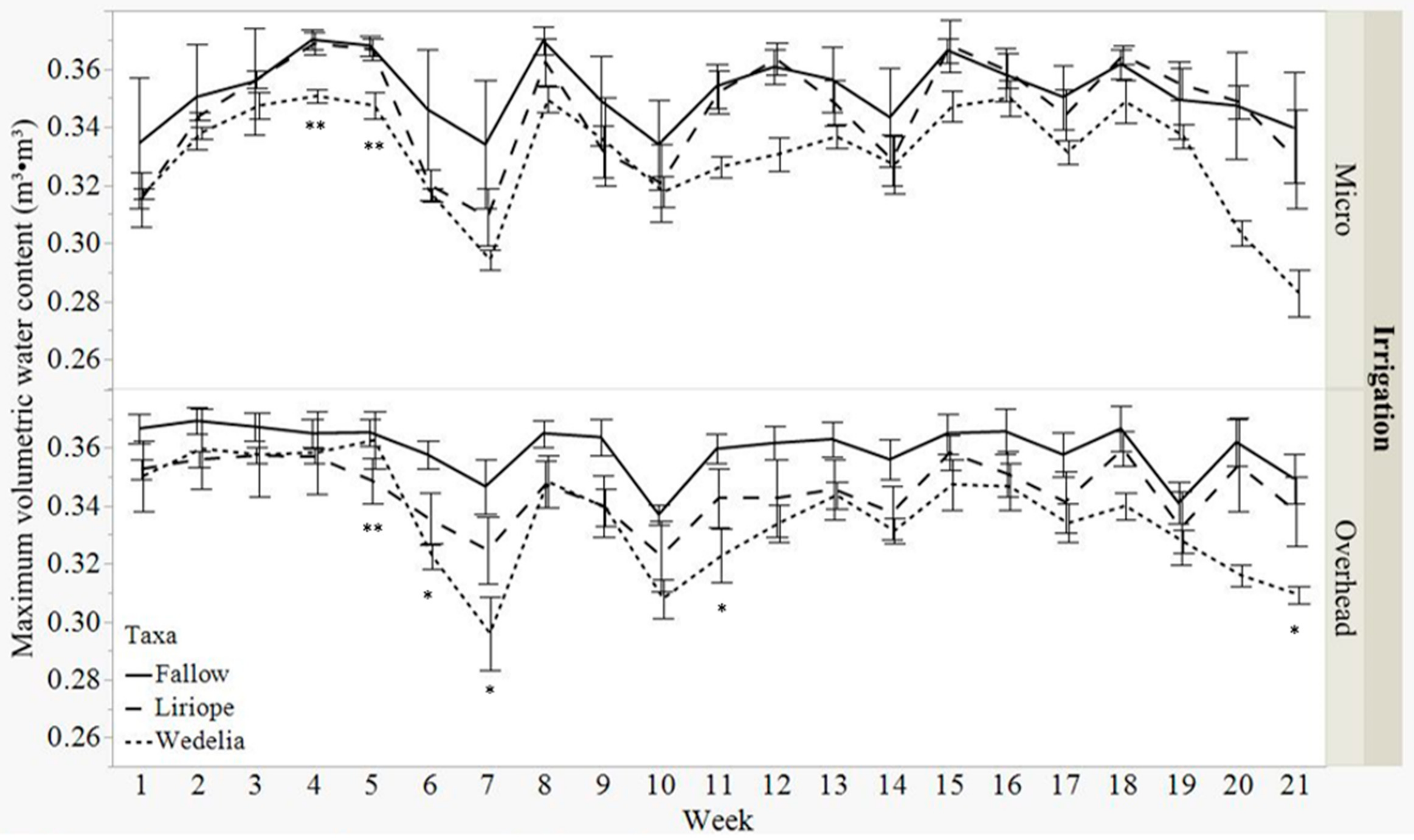
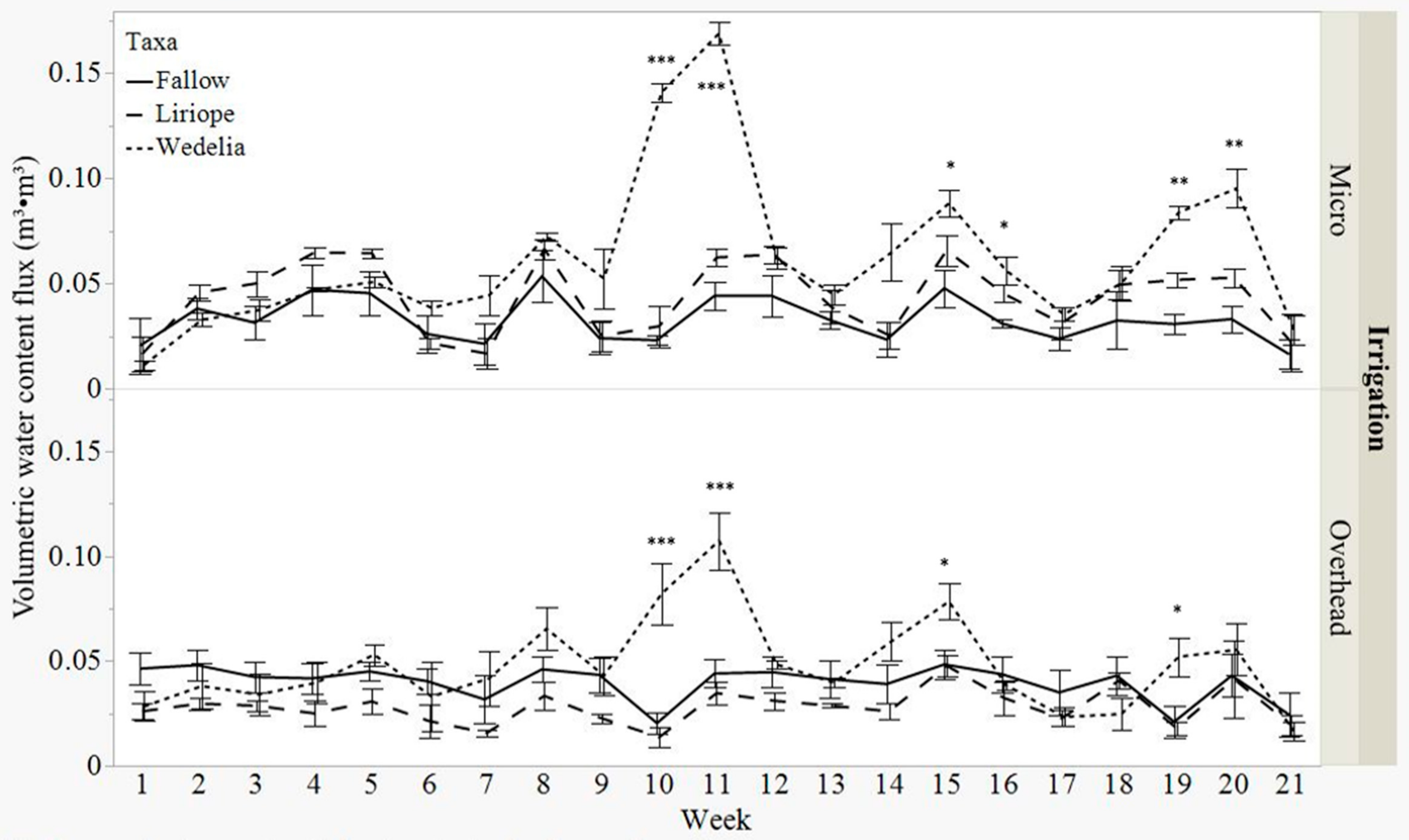
3.1. Volumetric Water Content
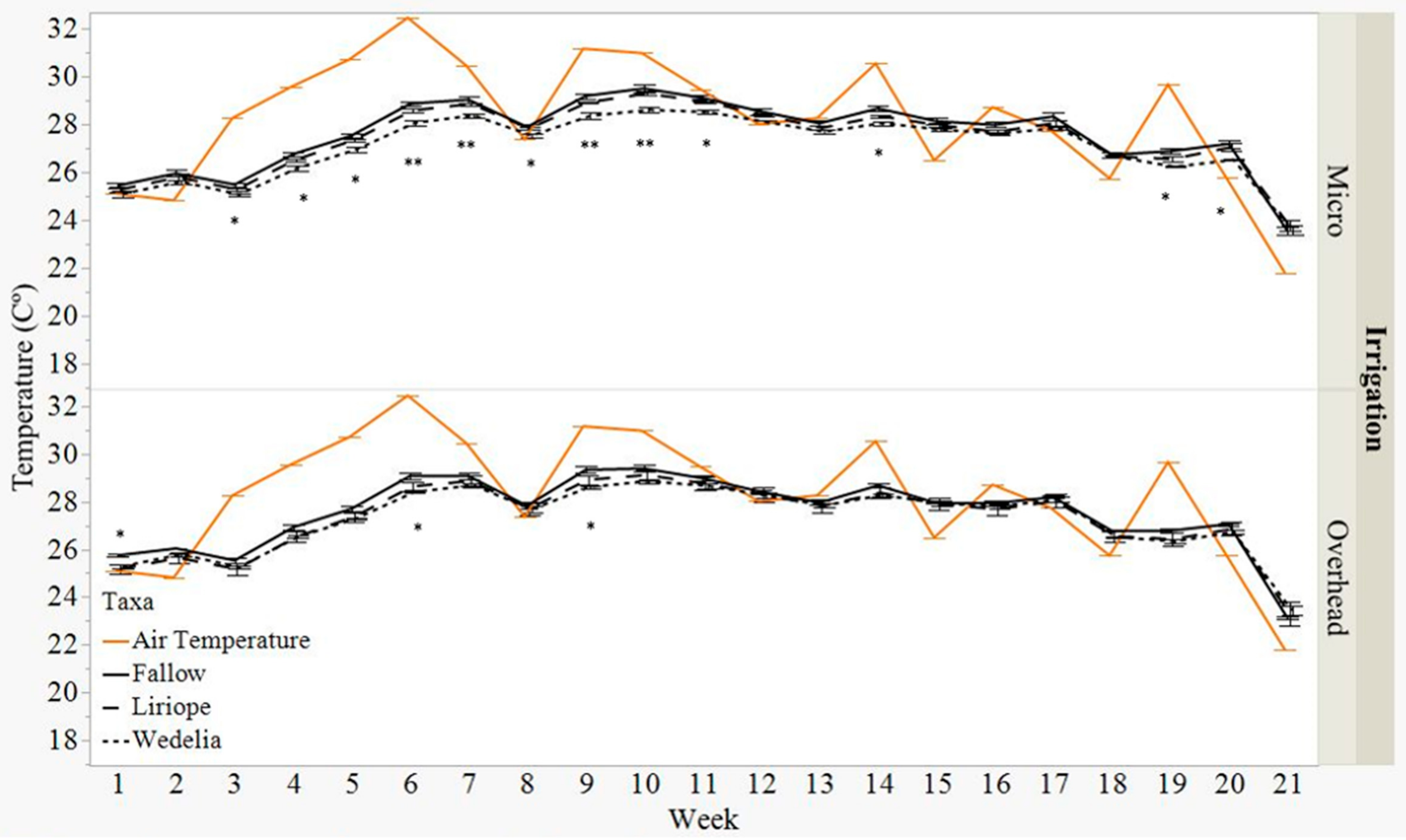
3.2. Soil Temperature
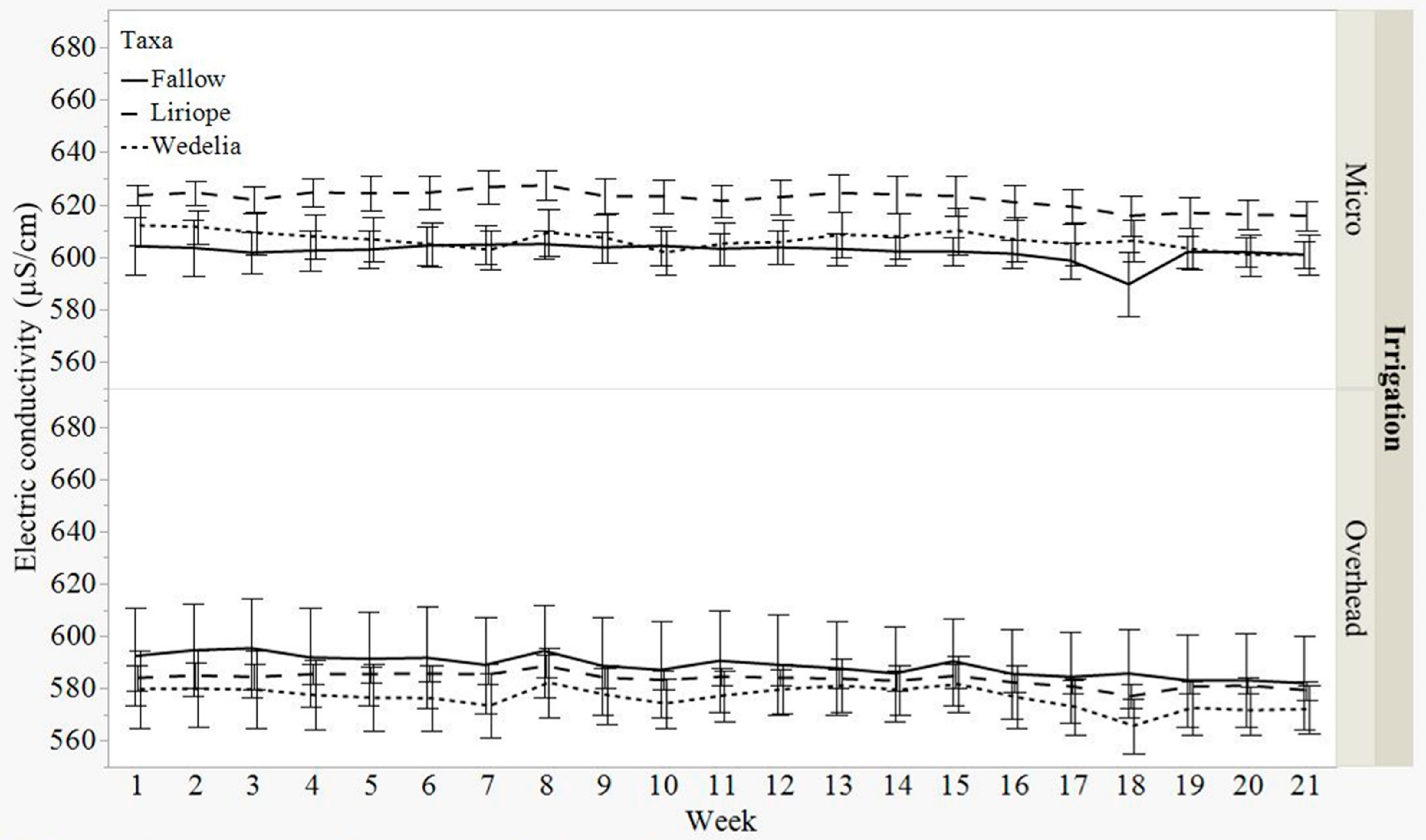
3.3. Electric Conductivity
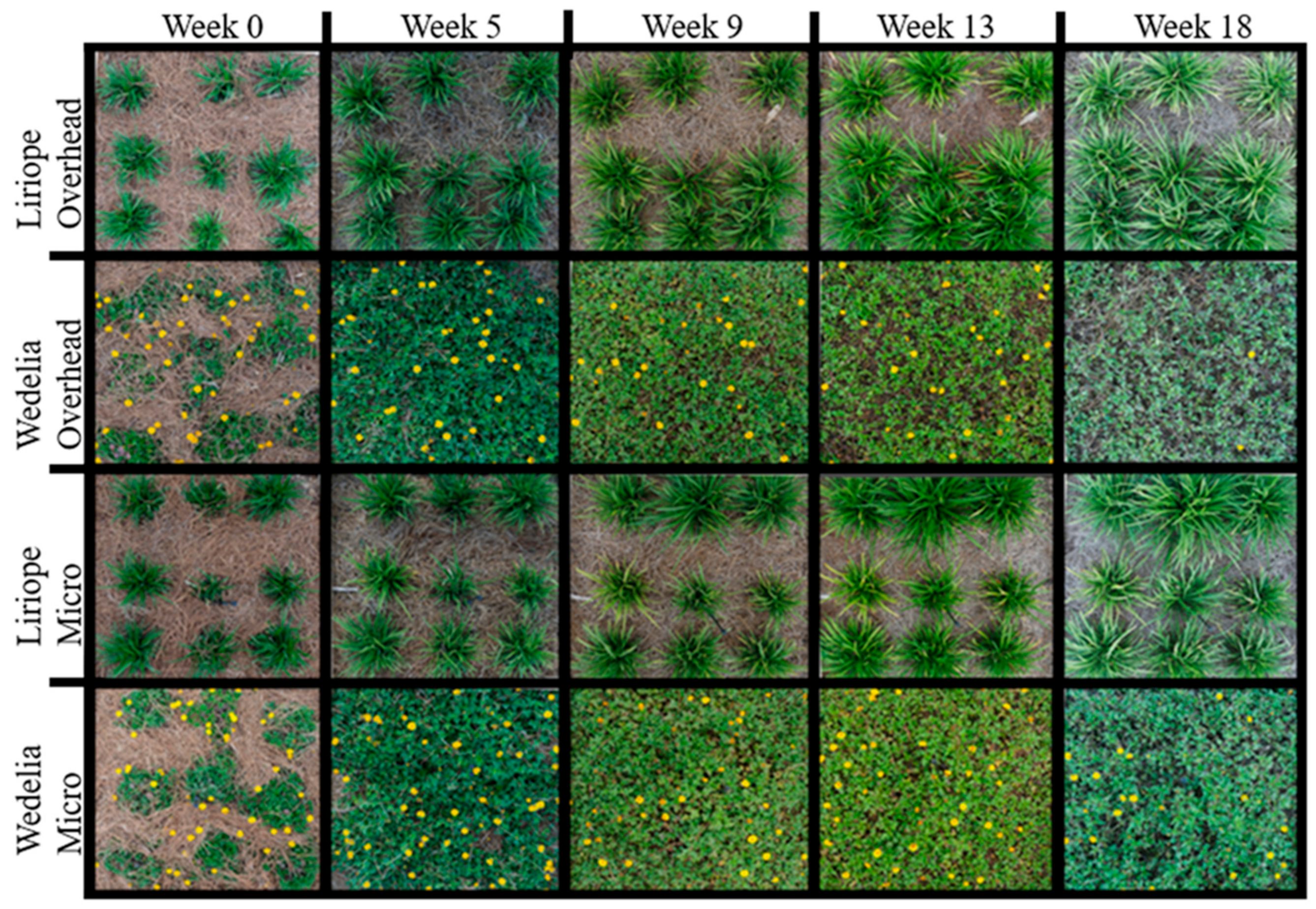
3.4. Vegetative Coverage
3.5. Weed Biomass and Density
3.6. Microbial Communities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saksa, K.A.; Ilvento, T.W.; Barton, S.S. Interpretation of Sustainable Landscapes Affects Student Perception of Sustainable Landscape Features. Horttechnology 2012, 22, 402–409. [Google Scholar] [CrossRef]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Staats, D.; Klett, J.E. Water Conservation Potential and Quality of Non-turf Groundcovers versus Kentucky Bluegrass under Increasing Levels of Drought Stress. J. Environ. Hortic. 1995, 13, 181–185. [Google Scholar] [CrossRef]
- Carey, R.O.; Hochmuth, G.J.; Martinez, C.J.; Boyer, T.; Nair, V.D.; Dukes, M.; Toor, G.S.; Shober, A.; Cisar, J.L.; Trenholm, L.E.; et al. A Review of Turfgrass Fertilizer Management Practices: Implications for Urban Water Quality. Horttechnology 2012, 22, 280–291. [Google Scholar] [CrossRef]
- Campbell, J.; Rihn, A.; Khachatryan, H. Factors Influencing Home Lawn Fertilizer Choice in the United States. Horttechnology 2020, 30, 296–305. [Google Scholar] [CrossRef]
- Milesi, C.; Running, S.W.; Elvidge, C.D.; Dietz, J.B.; Tuttle, B.T.; Nemani, R.R. Mapping and Modeling the Biogeochemical Cycling of Turf Grasses in the United States. Environ. Manag. 2005, 36, 426–438. [Google Scholar] [CrossRef]
- Larson, K.L.; Brumand, J. Paradoxes in landscape management and water conservation: Examining neighborhood norms and institutional forces. Cities Environ. 2014, 7, 6. Available online: https://digitalcom-mons.lmu.edu/cate/vol7/iss1/6 (accessed on 2 October 2022).
- Steinberg, T. Lawn and Landscape in World Context, 1945–2000. OAH Mag. Hist. 2005, 19, 62–69. [Google Scholar] [CrossRef]
- Yue, C.; Cui, M.; Watkins, E.; Patton, A. Investigating Factors Influencing Consumer Adoption of Low-input Turfgrasses. Hortscience 2021, 56, 1213–1220. [Google Scholar] [CrossRef]
- Burayu, W.; Umeda, K. Versatile Native Grasses and a Turf-Alternative Groundcover for the Arid Southwest United States. J. Environ. Hortic. 2021, 39, 160–167. [Google Scholar] [CrossRef]
- Garber, M.; Bondari, K. Landscape Maintenance Firms: II. Pest Management Practices. J. Environ. Hortic. 1996, 14, 58–61. [Google Scholar] [CrossRef]
- Roka, F.M.; Rouse, R.E.; Miavitz-Brown, E. Economic considerations from using perennial peanut in urban landscapes. Proc. Fla. State Hortic. Soc. 2003, 116, 339–341. [Google Scholar]
- Masierowska, M.; Stawiarz, E.; Rozwałka, R. Perennial ground cover plants as floral resources for urban pollinators: A case of Geranium species. Urban For. Urban Green. 2018, 32, 185–194. [Google Scholar] [CrossRef]
- Marble, S.C.; Koeser, A.K.; Hasing, G. A Review of Weed Control Practices in Landscape Planting Beds: Part II—Chemical Weed Control Methods. Hortscience 2015, 50, 857–862. [Google Scholar] [CrossRef]
- Marble, S.C.; Pickens, J.M. Tolerance of Three Deep South Non-turf Ornamental Groundcovers to Applications of Postemergence Herbicides1. J. Environ. Hortic. 2020, 38, 91–100. [Google Scholar] [CrossRef]
- Marble, S.C.; Koeser, A.K.; Hasing, G.; McClean, D.; Chandler, A. Efficacy and Estimated Annual Cost of Common Weed Control Methods in Landscape Planting Beds. Horttechnology 2017, 27, 199–211. [Google Scholar] [CrossRef]
- Doxon, L.E. Landscape Sustainability: Environmental, Human, and Financial Factors. Horttechnology 1996, 6, 362–365. [Google Scholar] [CrossRef]
- Arnold, C.L., Jr.; Gibbons, C.J. Impervious Surface Coverage: The Emergence of a Key Environmental Indicator. J. Am. Plan. Assoc. 1996, 62, 243–258. [Google Scholar] [CrossRef]
- Shuster, W.D.; Bonta, J.; Thurston, H.; Warnemuende, E.; Smith, D.R. Impacts of impervious surface on watershed hydrology: A review. Urban Water J. 2005, 2, 263–275. [Google Scholar] [CrossRef]
- Brabec, E.; Schulte, S.; Richards, P.L. Impervious Surfaces and Water Quality: A Review of Current Literature and Its Implications for Watershed Planning. J. Plan. Lit. 2002, 16, 499–514. [Google Scholar] [CrossRef]
- Li, J.; Rao, M. Sprinkler water distributions as affected by winter wheat canopy. Irrig. Sci. 2000, 20, 29–35. [Google Scholar] [CrossRef]
- Kaspar, T.; Singer, J. The use of cover crops to manage soil. In Soil Management: Building a Stable Base for Agriculture; Hatfield, J.L., Sauer, T.J., Eds.; American Society of Agronomy and Soil Science Society of America: 5585 Guilford Road, Madison, WI, USA, 2011. [Google Scholar] [CrossRef]
- Rosario-Lebron, A.; Leslie, A.W.; Yurchak, V.L.; Chen, G.; Hooks, C.R. Can winter cover crop termination practices impact weed suppression, soil moisture, and yield in no-till soybean [Glycine max (L.) Merr.]? Crop Prot. 2019, 116, 132–141. [Google Scholar] [CrossRef]
- Strock, J.S.; Porter, P.M.; Russelle, M.P. Cover Cropping to Reduce Nitrate Loss through Subsurface Drainage in the Northern U.S. Corn Belt. J. Environ. Qual. 2004, 33, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, S.R.; Delaune, R.D. Soil Oxidation-Reduction in Wetlands and Its Impact on Plant Functioning. Biology 2012, 1, 196–221. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Brandsaeter, L.O.; Calegari, A.; Neto, F.S. Cover crops and weed management. In Non-Chemical Weed Management: Principles, Concepts and Technology; United States Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2007. [Google Scholar]
- Merriam, J.L.; Keller, J. Farm Irrigation System Evaluation: A Guide for Management; Utah State University: Logan, UT, USA, 1978. [Google Scholar]
- CardiNa, J.; Sparrow, D.H.; McCoy, E.L. Spatial Relationships Between Seedbank and Seedling Populations of Common Lambsquarters (Chenopodium album) and Annual Grasses. Weed Sci. 1996, 44, 298–308. [Google Scholar] [CrossRef]
- Quideau, S.A.; McIntosh, A.C.; Norris, C.E.; Lloret, E.; Swallow, M.J.; Hannam, K. Extraction and Analysis of Microbial Phospholipid Fatty Acids in Soils. J. Vis. Exp. 2016, 114, e54360. [Google Scholar] [CrossRef]
- Merwin, I.A.; Stiles, W.C.; Van Es, H.M. Orchard Groundcover Management Impacts on Soil Physical Properties. J. Am. Soc. Hortic. Sci. 1994, 119, 216–222. [Google Scholar] [CrossRef]
- Kahimba, F.C.; Ranjan, R.S.; Froese, J.; Entz, M.; Nason, R. Cover Crop Effects on Infiltration, Soil Temperature, and Soil Moisture Distribution in the Canadian Prairies. Appl. Eng. Agric. 2008, 24, 321–333. [Google Scholar] [CrossRef]
- Yunusa, I.A.M.; Zolfaghar, S.; Zeppel, M.J.B.; Li, Z.; Palmer, A.R.; Eamus, D. Fine Root Biomass and Its Relationship to Evapotranspiration in Woody and Grassy Vegetation Covers for Ecological Restoration of Waste Storage and Mining Landscapes. Ecosystems 2012, 15, 113–127. [Google Scholar] [CrossRef]
- Prasad, R. A linear root water uptake model. J. Hydrol. 1988, 99, 297–306. [Google Scholar] [CrossRef]
- Gerrits, A.M.J.; Pfister, L.; Savenije, H.H.G. Spatial and temporal variability of canopy and forest floor interception in a beech forest. Hydrol. Process. 2010, 24, 3011–3025. [Google Scholar] [CrossRef]
- Holmes, R. Taylor’s Master Guide to Gardening; Houghton Mifflin Harcourt: Boston, MA, USA, 2001. [Google Scholar]
- Rowe, D.B.; Kolp, M.R.; Greer, S.E.; Getter, K.L. Comparison of irrigation efficiency and plant health of overhead, drip, and sub-irrigation for extensive green roofs. Ecol. Eng. 2014, 64, 306–313. [Google Scholar] [CrossRef]
- Folorunso, O.; Rolston, D.E.; Prichard, T.; Louie, D.T. Cover crops lower soil surface strength, may improve soil permeability. Calif. Agric. 1992, 46, 26–27. [Google Scholar] [CrossRef]
- Krohn, N.; Ferree, D. Effects of Low-growing Perennial Ornamental Groundcovers on the Growth and Fruiting of ‘Seyval blanc’ Grapevines. Hortscience 2005, 40, 561–568. [Google Scholar] [CrossRef]
- Chalise, K.S.; Singh, S.; Wegner, B.R.; Kumar, S.; Pérez-Gutiérrez, J.D.; Osborne, S.L.; Nleya, T.; Guzman, J.; Rohila, J.S. Cover Crops and Returning Residue Impact on Soil Organic Carbon, Bulk Density, Penetration Resistance, Water Retention, Infiltration, and Soybean Yield. Agron. J. 2019, 111, 99–108. [Google Scholar] [CrossRef]
- Irmak, S.; Odhiambo, L.O.; Kranz, W.L.; Eisenhauer, D.E. Irrigation Efficiency and Uniformity, and Crop Water Use Efficiency. The Board of Regents of the University of Nebraska: Lincoln, NE, USA, 2011. Available online: https://digitalcommons.unl.edu/bio-sysengfacpub/451 (accessed on 25 October 2022).
- Horton, R.E. An Approach Toward a Physical Interpretation of Infiltration-Capacity. Soil Sci. Soc. Am. J. 1941, 5, 399–417. [Google Scholar] [CrossRef]
- Turner, D.P.; Sumner, M.E. The influence of initial soil moisture content on field measured infiltration rates. Water SA 1978, 4, 18–24. [Google Scholar]
- Michelsen-Correa, S.; Scull, P. The impact of reforestation on soil temperature. Middl. States Geograp. 2005, 38, 39–44. [Google Scholar]
- Van Huyssteen, L.; Van Zyl, J.; Koen, A. The Effect of Cover Crop Management on Soil Conditions and Weed 6ontrol in a Colombar Vineyard in Oudtshoorn. S. Afr. J. Enol. Vitic. 2017, 5, 7–17. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, D.; Zhang, H.; Li, G.; Jin, Y.; Li, Q. Effects of vegetation height and density on soil temperature variations. Chin. Sci. Bull. 2013, 58, 907–912. [Google Scholar] [CrossRef]
- Bavougian, C.M.; Read, P.E. Mulch and groundcover effects on soil temperature and moisture, surface reflectance, grapevine water potential, and vineyard weed management. PeerJ 2018, 6, e5082. [Google Scholar] [CrossRef] [PubMed]
- Calkins, J.B.; Swanson, B.T. Comparison of Conventional and Alternative Nursery Field Management Systems: Soil Physical Properties. J. Environ. Hortic. 1998, 16, 90–97. [Google Scholar] [CrossRef]
- Wu, J.-H.; Tang, C.-S.; Shi, B.; Gao, L.; Jiang, H.-T.; Daniels, J.L. Effect of Ground Covers on Soil Temperature in Urban and Rural Areas. Environ. Eng. Geosci. 2014, 20, 225–237. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Liu, J.; Wang, J.; Zhang, Z.; Xiao, N. Changes of Soil Water and Heat Transport and Yield of Tomato (Solanum lycopersicum) in Greenhouses with Micro-Sprinkler Irrigation under Plastic Film. Agronomy 2022, 12, 664. [Google Scholar] [CrossRef]
- Yang, X.; Reynolds, W.; Drury, C.; Reeb, M. Cover crop effects on soil temperature in a clay loam soil in southwestern Ontario. Can. J. Soil Sci. 2021, 101, 761–770. [Google Scholar] [CrossRef]
- Verma, J.K.; Sharma, A.; Paramanick, K.K. To evaluate the values of electrical conductivity and growth parameters of apple saplings in nursery fields. Int. J. Appl. Sci. Eng. Res. 2015, 4, 321–332. [Google Scholar]
- Grisso, R.D.; Alley, M.M.; Holshouser, D.L.; Thomason, W.E. Precision Farming Tools. Soil Electrical Conductivity; Virginia Tech Cooperative Extension: Blacksburg, VA, USA, 2005; pp. 442–508. [Google Scholar]
- Li, Y.; Li, J.; Gao, L.; Tian, Y. Irrigation has more influence than fertilization on leaching water quality and the potential environmental risk in excessively fertilized vegetable soils. PLoS ONE 2018, 13, e0204570. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Corwin, D.; Lesch, S. Apparent soil electrical conductivity measurements in agriculture. Comput. Electron. Agric. 2005, 46, 11–43. [Google Scholar] [CrossRef]
- Ni, J.-J.; Cheng, Y.-F.; Bordoloi, S.; Bora, H.; Wang, Q.-H.; Ng, C.-W.; Garg, A. Investigating plant root effects on soil electrical conductivity: An integrated field monitoring and statistical modelling approach. Earth Surf. Process. Landforms 2018, 44, 825–839. [Google Scholar] [CrossRef]
- Nesom, G.L. Overview of Liriope and Ophiopogon (Ruscaceae) naturalized and commonly cultivated in the USA. Phytoneuron 2010, 56, 1–31. [Google Scholar]
- Si, C.-C.; Dai, Z.-C.; Lin, Y.; Qi, S.-S.; Huang, P.; Miao, S.-L.; Du, D.-L. Local adaptation and phenotypic plasticity both occurred in Wedelia trilobata invasion across a tropical island. Biol. Invasions 2014, 16, 2323–2337. [Google Scholar] [CrossRef]
- Clement, C.; DeFrank, J. The Use of Ground Covers during the Establishment of Heart-of-Palm Plantations in Hawaii. Hortscience 1998, 33, 814–815. [Google Scholar] [CrossRef]
- Haramoto, E.R.; Brainard, D.C. Strip Tillage and Oat Cover Crops Increase Soil Moisture and Influence N Mineralization Patterns in Cabbage. Hortscience 2012, 47, 1596–1602. [Google Scholar] [CrossRef]
- Workayehu, T.; Mazengia, W.; Hidoto, L. Growth habit, plant density and weed control on weed and root yield of sweet potato (Ipomoea batatas L.) Areka, Southern Ethiopia. J. Hortic. For. 2011, 3, 251–258. [Google Scholar]
- Yeganehpoor, F.; Salmasi, S.Z.; Abedi, G.; Samadiyan, F.; Beyginiya, V. Effects of cover crops and weed management on corn yield. J. Saudi Soc. Agric. Sci. 2015, 14, 178–181. [Google Scholar] [CrossRef]
- Pons, T.L. Induction of Dark Dormancy in Seeds: Its Importance for the Seed Bank in the Soil. Funct. Ecol. 1991, 5, 669. [Google Scholar] [CrossRef]
- Wesson, G.; Wareing, P.F. The Induction of Light Sensitivity in Weed Seeds by Burial. J. Exp. Bot. 1969, 20, 414–425. [Google Scholar] [CrossRef]
- Foo, C.; Harrington, K.; MacKay, M. Weed suppression by twelve ornamental ground cover species. N. Z. Plant Prot. 2011, 64, 149–154. [Google Scholar] [CrossRef]
- Xi, N.; Wu, Y.; Weiner, J.; Zhang, D.-Y. Does weed suppression by high crop density depend on crop spatial pattern and soil water availability? Basic Appl. Ecol. 2022, 61, 20–29. [Google Scholar] [CrossRef]
- Stewart, C.; Marble, S.; Pearson, B.J.; Wilson, P. Impact of Container Nursery Production Practices on Weed Growth and Herbicide Performance. Hortscience 2017, 52, 1593–1600. [Google Scholar] [CrossRef]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant–microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, E.; Benizri, E.; Guckert, A. Impact of artificial root exudates on the bacterial community structure in bulk soil and maize rhizosphere. Soil Biol. Biochem. 2003, 35, 1183–1192. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Buyer, J.S.; Roberts, D.P.; Russek-Cohen, E. Soil and plant effects on microbial community structure. Can. J. Microbiol. 2002, 48, 955–964. [Google Scholar] [CrossRef]
- Cregger, M.A.; Schadt, C.W.; McDowell, N.G.; Pockman, W.T.; Classen, A. Response of the Soil Microbial Community to Changes in Precipitation in a Semiarid Ecosystem. Appl. Environ. Microbiol. 2012, 78, 8587–8594. [Google Scholar] [CrossRef]
- Ettema, C.H. Spatial soil ecology. Trends Ecol. Evol. 2002, 17, 177–183. [Google Scholar] [CrossRef]
- Packer, A.; Clay, K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 2000, 404, 278–281. [Google Scholar] [CrossRef]
| Weeks after Planting | 0 WAP | 5 WAP | 9 WAP | 13 WAP | 18 WAP |
|---|---|---|---|---|---|
| Irrigation | % Plant Cover | % Plant Cover | % Plant Cover | % Plant Cover | % Plant Cover |
| Micro | 32.5 a i | 42.0 a | 57.5 a | 68.4 a | 67.3 a |
| Overhead | 31.2 a | 43.2 a | 56.2 a | 63.0 a | 60.4 a |
| p-valueirr | 0.7593 ii | 0.7694 | 0.9021 | 0.3809 | 0.0860 |
| Taxa | |||||
| Liriope | 26.0 b | 37.3 b | 40.1 b | 58.5 b | 65.5 b |
| Wedelia | 37.8 a | 47.9 a | 73.6 a | 72.9 a | 62.1 a |
| p-valuetaxa | 0.0001 | 0.0002 | <0.0001 | 0.0058 | 0.4319 |
| Interaction | |||||
| Wedelia-Micro | 40.6 a | 48.8 a | 75.5 a | 78.4 a | 67.4 a |
| Wedelia-Overhead | 35.0 a | 47.0 ab | 71.1 a | 67.3 ab | 67.1 a |
| Liriope-Overhead | 27.5 b | 39.3 bc | 40.7 b | 58.6 b | 63.9 a |
| Liriope-Micro | 24.5 b | 35.2 c | 39.6 b | 58.3 b | 56.9 a |
| p-valueirrxplot | 0.0211 | 0.1366 | 0.1096 | 0.1620 | 0.3525 |
| Irrigation | Plot | 2 WAP i | 4 WAP | 7 WAP | 10 WAP | 13 WAP | 17 WAP | 19 WAP |
|---|---|---|---|---|---|---|---|---|
| no./m2 | no./m2 | no./m2 | no./m2 | no./m2 | no./m2 | no./m2 | ||
| Overhead | Fallow | 12.5 ab ii | 25.5 a | 28.6 a | 23.8 a | 23.8 a | 15.8 a | 21.0 a |
| Liriope | 5.3 ab | 11.5 a | 11.5 a | 9.8 a | 11.3 a | 9.0 a | 6.1 a | |
| Wedelia | 4.6 b | 7.0 a | 7.5 a | 6.0 a | 7.5 a | 3.0 a | 3.3 a | |
| Micro | Fallow | 2.5 a | 2.1 a | 5.6 a | 2.8 a | 5.1 a | 3.3 a | 6.0 a |
| Liriope | 5.8 ab | 11.8 a | 9.8 a | 7.3 a | 7.3 a | 4.0 a | 2.6 a | |
| Wedelia | 5.3 ab | 8.3 a | 2.6 a | 5.5 a | 5.5 a | 3.1 a | 3.6 a | |
| Irrigationp-value | 0.1106 iii | 0.2510 | 0.1516 | 0.1300 | 0.0680 | 0.0600 | 0.2856 | |
| Plotp-value | 0.4491 iv | 0.7125 | 0.3487 | 0.4850 | 0.3263 | 0.2160 | 0.2839 | |
| Irrxplotp-value | 0.0317 v | 0.1986 | 0.3838 | 0.2162 | 0.2475 | 0.2293 | 0.5121 | |
| g/m2 | g/m2 | g/m2 | g/m2 | g/m2 | g/m2 | g/m2 | ||
| Overhead | Fallow | 10.5 a | 2.5 a | 5.5 a | 3.7 a | 10.9 a | 8.1 a | 3.2 a |
| Liriope | 8.1 a | 1.3 a | 2.5 a | 4.0 a | 7.1 ab | 4.0 a | 1.1 a | |
| Wedelia | 7.5 a | 1.0 a | 1.9 a | 1.9 a | 4.3 b | 0.7 a | 0.8 a | |
| Micro | Fallow | 8.2 a | 0.4 a | 1.5 a | 0.7 a | 4.2 b | 5.5 a | 1.5 a |
| Liriope | 8.0 a | 0.6 a | 2.3 a | 2.6 a | 5.5 ab | 3.9 a | 0.4 a | |
| Wedelia | 7.5 a | 0.3 a | 2.1 a | 1.4 a | 4.2 b | 1.0 a | 0.8 a | |
| Irrigationp-value | 0.3036 | 0.0222 | 0.2020 | 0.0445 | 0.0139 | 0.6862 | 0.2808 | |
| Plotp-value | 0.1156 | 0.3900 | 0.4678 | 0.2259 | 0.0523 | 0.0748 | 0.1201 | |
| Irrxplotp-value | 0.3308 | 0.3723 | 0.1928 | 0.4080 | 0.0415 | 0.8125 | 0.6365 |
| Week 6 | Actinomycetes | Arbuscular Mycorrhizae | Eukaryotes | Fungi | Gram (+) Bacteria | GM (−) Bacteria | Protozoa | |
|---|---|---|---|---|---|---|---|---|
| Irrigation | Plot | nmol/g | nmol/g | nmol/g | nmol/g | nmol/g | nmol/g | nmol/g |
| Micro | Fallow | 2.95 a i | 6.63 a | 42.58 a | 57.94 a | 37.07 a | 12.93 a | 1.62 a |
| Liriope | 4.23 a | 14.36 a | 57.45 a | 96.03 a | 53.07 a | 22.90 a | 2.84 a | |
| Wedelia | 3.22 a | 9.93 a | 70.32 a | 199.16 a | 64.80 a | 28.00 a | 0.89 a | |
| Overhead | Fallow | 3.68 a | 7.23 a | 50.72 a | 82.88 a | 41.71 a | 18.66 a | 2.11 a |
| Liriope | 1.40 a | 9.14 a | 63.05 a | 73.04 a | 34.58 a | 14.75 a | 0.31 a | |
| Wedelia | 3.87 a | 12.63 a | 63.24 a | 114.81 a | 62.78 a | 29.43 a | 0.65 a | |
| Irrigationp-value | 0.7005 | 0.9343 | 0.8818 | 0.3455 | 0.6377 | 0.9537 | 0.2683 | |
| Plotp-value | 0.8854 | 0.5356 | 0.5359 | 0.0568 | 0.1951 | 0.1824 | 0.3969 | |
| Irrxplotp-value | 0.4236 | 0.6083 | 0.9024 | 0.3138 | 0.6826 | 0.5940 | 0.1857 | |
| Week 20 | Actinomycetes | Arbuscular Mycorrhizae | Eukaryotes | Fungi | Gram (+) bacteria | GM (−) bacteria | Protozoa | |
| Irrigation | Plot | nmol/g | nmol/g | nmol/g | nmol/g | nmol/g | nmol/g | nmol/g |
| Micro | Fallow | 3.23 a | 9.91 a | 19.79 a | 38.32 a | 33.22 a | 13.18 a | 2.30 a |
| Liriope | 2.75 a | 43.89 a | 40.07 a | 172.81 a | 44.28 a | 29.78 a | 2.98 a | |
| Wedelia | 2.95 a | 23.69 a | 19.49 a | 70.00 a | 34.99 a | 28.59 a | 2.91 a | |
| Overhead | Fallow | 2.25 a | 7.75 a | 23.89 a | 5.21 a | 36.93 a | 17.05 a | 8.88 × 10−16 a |
| Liriope | 4.83 a | 35.74 a | 77.56 a | 108.33 a | 57.50 a | 34.49 a | 2.94 a | |
| Wedelia | 7.50 a | 58.42 a | 71.48 a | 179.46 a | 83.01 a | 73.81 a | 6.53 a | |
| Irrigationp-value | 0.2679 ii | 0.6031 | 0.2887 | 0.6816 | 0.2786 | 0.2004 | 0.8763 | |
| Actinomycetes | Arbuscular Mycorrhizae | Eukaryotes | Fungi | Gram (+) bacteria | GM (−) bacteria | Protozoa | ||
| Plotp-value | 0.4785 iv | 0.1899 | 0.5686 | 0.2849 | 0.5949 | 0.1255 | 0.5705 | |
| Irrxplotp-value | 0.4067 v | 0.4827 | 0.7793 | 0.3804 | 0.6195 | 0.3757 | 0.6714 | |
| Datep-value | 0.5012 | 0.0170 | 0.3271 | 0.9987 | 0.9512 | 0.116 | 0.2766 | |
| Datexirrp value | 0.2554 | 0.5949 | 0.3695 | 0.4029 | 0.9512 | 0.2155 | 0.6718 | |
| Datexplotp-value | 0.6580 | 0.2774 | 0.8112 | 0.3860 | 0.8839 | 0.4277 | 0.4008 | |
| Datexirrxplotp-value | 0.3731 | 0.6012 | 0.7202 | 0.2051 | 0.6371 | 0.4286 | 0.5914 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKeown, T.M.; Fields, J.S.; Abdi, D.E. The Effect of Ornamental Groundcover Habit and Irrigation Delivery on Dynamic Soil Conditions. Land 2023, 12, 1119. https://doi.org/10.3390/land12061119
McKeown TM, Fields JS, Abdi DE. The Effect of Ornamental Groundcover Habit and Irrigation Delivery on Dynamic Soil Conditions. Land. 2023; 12(6):1119. https://doi.org/10.3390/land12061119
Chicago/Turabian StyleMcKeown, Thomas M., Jeb S. Fields, and Damon E. Abdi. 2023. "The Effect of Ornamental Groundcover Habit and Irrigation Delivery on Dynamic Soil Conditions" Land 12, no. 6: 1119. https://doi.org/10.3390/land12061119
APA StyleMcKeown, T. M., Fields, J. S., & Abdi, D. E. (2023). The Effect of Ornamental Groundcover Habit and Irrigation Delivery on Dynamic Soil Conditions. Land, 12(6), 1119. https://doi.org/10.3390/land12061119






