Abstract
It has become clear that state-owned protected areas (PAs) are insufficient in preserving the world’s spatially heterogenous biodiversity. Private land conservation could contribute significantly to national conservation goals, without further burdening state resources. In South Africa, legislation has been introduced to incentivise private landowners to contribute to national biodiversity goals. In this study, we used camera trap arrays and hierarchical multi-species occupancy modelling to evaluate the impact of land-use on mammal (body mass >0.5 kg) diversity in the drylands of South Africa. Four hundred and fifty-one camera traps were deployed across a statutory PA, private PA and a neighbouring group of farmlands, covering ~2096 km2. Although trophic species richness were similar across all three land-uses, occurrence and detection probabilities of larger (>20 kg) species were low in the farmlands and highest in the private PA. In contrast, smaller species had higher occurrence probabilities in the farmlands, where large predators and megaherbivores have been extirpated. Differences in species-specific occurrence probabilities were primarily driven by land-use context, as opposed to fine-scale habitat attributes. These results highlight how a land-use matrix incorporating statutory PAs, private PAs and well-managed rangelands can benefit wildlife conservation, as long as these land-uses are included in carefully developed regional conservation planning.
1. Introduction
Habitat loss and fragmentation, largely due to expanding agriculture, are the leading drivers of terrestrial biodiversity loss [1,2]. Preserving large tracts of natural habitat is thus critical to achieving global conservation goals and this has largely been achieved through the establishment of state-run protected areas (PAs) [3]. Many statutory PAs are, however, situated in the least productive portions of the landscape, where anthropogenic activities are either unprofitable or non-viable [4,5]. This is particularly evident in Africa, with the consequence that the contemporary network of PAs fails to protect a representative sample of the continent’s ecosystems and biodiversity [6,7].
To bolster national conservation objectives, South Africa has introduced legislation that offers landowners commercial authority over wildlife on their property [8,9]. Although these laws typically prioritise economically viable species (e.g., gemsbok [Oryx gazella]), they encourage the husbandry of biodiversity on privately owned properties [10]. Consequently, there is increasing interest in conservation actions in the matrix of agricultural lands and private protected areas (hereafter called private PAs) surrounding existing statutory PAs [11,12]. Yet the preservation of wildlife is heavily dependent on the land-use and the perceived financial viability of each species [9,13]. Most larger species are heavily persecuted on commercial livestock farms, to reduce competition for grazing [14] and in retaliation for real or perceived livestock predation [12]. Abundant wildlife populations, particularly those of predators, are thus seldom tolerated on rangelands [15]. In contrast, private PAs, which seek to support profit-driven ventures (i.e., trophy hunting and/or eco-tourism]), often introduce charismatic extralimital species to inflate species richness and abundance [16,17,18,19]. Even private PAs established for reasons other than economic benefit may be ignorant of local ecosystem processes [20], unintentionally implementing practices that may degrade the ecosystem(s) they were established to preserve [21,22].
Recent evidence has shown, however, that when managed correctly, farmland used for low-intensity livestock grazing may encompass enough ecological variation to sustain diverse wildlife [23,24]. This is particularly notable in South Africa’s drylands, where commercial farmlands typically support sparse populations of cosmopolitan mammals [25,26], invertebrates [27] and birds [18,19]. Likewise, private PAs can effectively preserve biodiversity not subsumed by statutory PAs, as they often have the financial capabilities to manage larger, conflict-prone species [10,28,29].
Although a number of studies have examined the differences in farmland and statutory and private PAs in preserving biodiversity at a national/regional level in South Africa [8,30], relatively few have been conducted at the local scale (however, see [31,32]). A major challenge with such research is that it is seldom possible to control for environmental variation between land-uses. To address this limitation, here, we investigate dryland terrestrial mammal (>0.5 kg) communities across three land-uses (private and statutory PAs and a neighbouring cluster of commercial small-livestock farms), all of which are in proximity within South Africa’s Karoo region. We hypothesised that land-use would influence the target communities’ species richness, functional composition, and species-specific detection and occurrence. More specifically, we predicted that the overall mammal assemblage would be positively influenced by an increased level of protection. We expected that the richness of trophic guilds would be significantly modified in the farmland relative to both PAs, due to targeted removal of species from the carnivore and herbivore trophic guilds. We also anticipated that the reduced larger species occurrence outside of PAs would result in the increased occurrence of smaller indigenous mammals, whose abundance may be suppressed through complex top-down trophic cascades [33].
2. Materials and Methods
2.1. Land-Uses
Our study was carried out in the Karoo, a dryland region (characterised by low, unpredictable rainfall) found within the Western Cape Province of South Africa (GPS: 33°23′ S; 20°50′ E). The land-use includes three distinct types, namely commercial small-livestock farms (farmland), a statutory PA and a nearby private PA. The proximity of the three land-uses somewhat limits biogeographic variation (e.g., climate) and allows for a matched pseudo-experimental study design with land-use as the treatment. The farmland comprises 22 neighbouring small-livestock (predominantly sheep) farms in the Laingsburg Municipality District (Figure 1; [32]). Dorper and Merino are the primary sheep (Ovis aries) breeds used by the commercial livestock industry within the region, with a mean stocking rate of 144 breeding ewes/10 km2 [32]. Most farms are sub-divided into multiple ‘camps’ (characterised by low-level wire fencing) to allow for rotational grazing [34,35]. Some farmers have reintroduced fallow deer (Dama dama), an introduced European species, onto their properties, for trophy hunting. These farms cover approximately 800 km2 in the Nama-Karoo biome, which is characterised by perennial dwarf shrubs and grasses [36,37]. Meandering throughout this region are somewhat more productive drainage lines, with sturdier shrubs and small trees providing shade and forage for browsers [38].
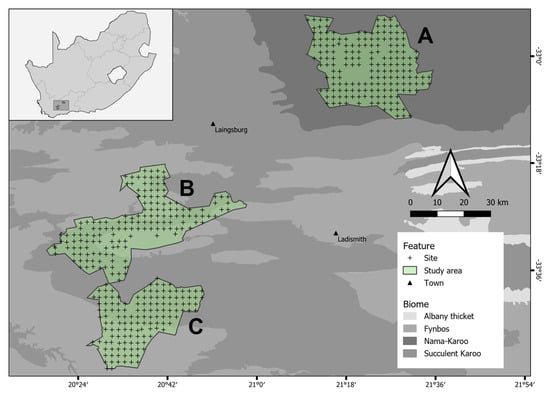
Figure 1.
The location of the three land-uses (farmland [A], statutory PA [B] and private PA [C]) within the Karoo and the major biomes in the region. Black and green polygons represent the cadastral boundaries of each land-use, and (+) shows the position of the camera traps (i.e., sites) within them. Camera traps were spaced approximately 2km apart. Areas within the farmland with no camera traps present indicate landowners unwilling to permit access or faulty camera traps, and gaps in the statutory and private PA represent inaccessible areas due to extreme terrain (i.e., cliffs) and faulty camera traps. Insert shows the three land-uses within South Africa.
Only 50 km south-west of the farmland is the Anysberg Nature Reserve. This large PA (±796.2 km2) is presently managed by Cape Nature, the provincial government entity responsible for conserving biodiversity in the Western Cape, South Africa (Figure 1; [39]). The PA contains elements of the Fynbos, Succulent Karoo and Albany thicket biomes [40]. Patterns of vegetation within the statutory PA are strongly influenced by rainfall, which varies predictably with the topography in the reserve [39]. Formally proclaimed as a statutory PA in 1990, the reserve is unique in that it is one of the few PAs which have not sought to reintroduce extirpated carnivores or megaherbivores (e.g., cheetah [Acinonyx jubatus] or African elephant [Loxodonta africana]). Instead, vegetation rehabilitation is a primary focus for the reserve, and as such, although reserve management has removed most internal fences, most of the boundary fences consist of a dilapidated 1.4 m tall jackal-proof fence [39].
Finally, Sanbona Wildlife Reserve is approximately 540 km2, with its northern border adjacent to the statutory PA (Figure 1). This private PA is the largest privately owned protected area in the region and includes the Fynbos and Succulent Karoo biomes [41]. Historically, the private PA comprised 19 privately owned small-livestock farms, with small areas set aside for crop production. However, as with the statutory PA, variable rainfall patterns and cumulative environmental damage reduced the profitability of agricultural ventures, and the land was purchased by the Mantis Collection in 2002 to establish a for-profit private PA. The non-consumptive tourism model employed by the Mantis Collection necessitated the reintroduction of large, charismatic carnivores (i.e., African lion [Panthera leo] and cheetah) and herbivores (i.e., African elephant, black wildebeest [Connochaetes gnou], plains zebra [Equus quagga], South African giraffe [Giraffa camelopardalis giraffa], white rhinoceros [Ceratotherium simum]), that had been previously extirpated from the region. To prevent these species from moving out of the reserve, the private PA maintains an electric 1–1.5 m game-proof fence [42]. Presently, the private PA is managed by the Caleo Foundation, a non-profit organisation which aims to promote conservation of vulnerable ecosystems ([41]).
2.2. Camera Trapping
To allow for direct comparisons between all three land-uses we followed the methods described by the authors of [32], who used camera trapping and a systematic sampling design (2 km2 grid), with a randomised starting point and orientation. This systematic sampling survey design ensures that key habitat features are sampled in proportion to their occurrence in the landscape [43,44,45] and prevents bias in detection as it targets no single species [46,47]. We therefore deployed Bushnell Trophy CAM HD (model #119437; Bushnell Outdoor Products, Overland Park, KS, USA) camera traps at 451 locations (Figure 1) within the farmland and statutory and private PAs (Table A1). We did not have sufficient camera traps to sample the all three land-uses concurrently; thus, cameras traps were sequentially deployed within each land-use (in two phases). Cameras were deployed between September 2012 and March 2013 in the farmland, between October 2013 and June 2014 in the statutory PA, and between August and November of 2015 in the private PA. To give the highest probability of obtaining photographs of a wide range of species, we placed camera traps within 100 m of the chosen centroid, choosing a micro placement which provided the least obstructed field of view and included signs of animal activity [48,49]. Camera traps were mounted on natural elements present in the landscape (i.e., small trees) in the statutory PA and farmland, and on metal poles approximately 30–50 cm above ground in the private PA. To minimise potential anthropogenic stimuli, we did not visit the camera traps until the end of each phase. All camera traps were programmed to take three pictures per trigger, with a 1 min delay between triggers.
2.3. Single-Season Multi-Region Community Occurrence Model
We adopted the hierarchical multi-region community occurrence model described by Tenan et al. (2016), and expanded upon by Oberosler et al. (2020), to assess how different land-uses (r = 3, i.e., farmland, statutory PA and private PA) influenced community- and species-level attributes [50,51]. This model accounts for both imperfect detection and regional heterogeneity in occurrence [52]. We defined each occasion (hereafter denoted by k) as a pooled 6-day (144 h) period. For each target species i the observed data consisted of a site by occasion matrix (‘detection history’ [53]), whereby at each site j, for each occasion k, a species was either recorded as detected (‘1′) or not detected (‘0′). Species detected in each land-use were assigned to one of four guilds g: carnivore, herbivore, insectivore and omnivore [51]. In this manner, g was known for all detected species, but unknown for unobserved species (with nr being the total number of observed species in each land-use). We defined species-, site- and land-use-specific occurrence as the binary variable zijr, whereby zijr =1 if species i occurs at site j in land-use r (and zero otherwise [52]). zijr is assumed to be the outcome of a Bernoulli random variable, such that:
where ψijr is the probability of occurrence of species i at site j in land-use r, and wir is a binary variable indicating whether species i is present (wir = 1) or absent (wir = 0) in land-use r. We estimated the number of species in the three communities (Nr) that were unobserved through the method of ‘data augmentation’ [54,55,56]. We augmented the detection data such that the total number of possible species in any land-0use was = 80, with the proportion of undetected species (i.e., –nr) estimated to exist in Nr being Ωr. wir is therefore governed by the hyper-parameter Ωr:
This is specified as:
where λgr is the expected variation in guild- and land-use-specific species richness, and is assumed to be the outcome of a Poisson process [50,51]. We adopted a guild indicator variable that allows for the estimation of guild membership for each undetected species:
with πr being the sum of πgr (the derived probability that species i from land-use r belongs to guild g), which is defined as:
Due to imperfect detection [53], zij is not known for unobserved species. We thus specified a detection model for the observed data (yijr) as:
where Kjr is the total number of occasions k at site j in region r, and pijr is the detection probability of species i at site j in land-use r, conditional on its presence (i.e., zijr = 1 [57]). Both the occurrence (Ψ) and detection (p) probabilities for species i were modelled as a function of environmental covariates [51]. We expected ψijr to vary based on three environmental covariates, namely distance from drainage lines, vegetation productivity and terrain ruggedness. We firstly included the linear distance (m) from each site to the nearest drainage line (DIST). Drainage lines were obtained from the World Wildlife Fund (WWF) 3-arc-second HydroSHEDS, which calculates flow accumulation based on high-resolution SRTM data. Flow accumulation values >80 were considered in this study [58]. We also included both the Modified Secondary Soil-Adjusted Vegetation Index (MSAVI2) and Terrain Ruggedness Index (TRI) as proxies for vegetation productivity and habitat complexity, respectively [59]. For this study, MSAVI2 was sampled every 16 days at 250 m spatial resolution and averaged within a 1 km radius of all camera sites. We derived TRI from 30 m raster elevation data from the Shuttle Radar Topography Mission (SRTM) [60]. The TRI at each site was calculated as the average mean difference in elevation (m) between the central pixel and its eight neighbours from within a 500 m buffer of each site [61]. All covariates were scaled to have a mean of 0 and a variance of 1. The occurrence model for species i at site j in region r (on the logit scale) is thus specified as:
We assumed that pijr varied based on vegetation structure, whereby denser foliage limited the sensitivity of the camera traps. We thus modelled pijr (on the logit scale) as:
Finally, the occurrence and detection models are connected through an additional hierarchal component, in which species- and land-use-specific parameters (e.g., β0ir) are random effects that are derived from community-level distribution. For example:
where μβ0r is the mean occurrence probability of land-use r’s community, and σβ0r is the associated standard deviation amongst all land-use r’s species. Finally, as species’ abundance may impact detection probabilities, we included a correlation structure ρr between β0ir and α0ir [57].
All modelling was carried out in a Bayesian framework using JAGS [62], executed through ‘R v.4.2.1′ [63], using the package ‘jagsUI’ [64]. For most parameters, we used uninformative priors of normal distributions [0,0.01] for the means, and uniform distributions over the interval of [0,10] for the standard deviations. Posterior distributions were obtained using 3 chains of 100,000 iterations, after first discarding a burn-in sample of 10,000 iterations, with a thinning rate of 10. Model convergence was assessed through a combination of Geweke statistics (Z; where −1.96 < Z < 1.96 indicates adequate convergence within single chains [65]), Ȓ statistics (where Ȓ < 1.1 indicates convergence across all chains [66]) and visual examination of the chains through trace plots.
3. Results
The final dataset resulted in a total of 8261 pooled independent detections of indigenous mammal species (>0.5 kg), with 43 species from 19 families being recorded across all three land-uses (Table A1). Six of the species are categorised by the International Union for Conservation of Nature (IUCN) as being ‘globally threatened’ (vulnerable or endangered). Of these, four were exclusively found in the private PA (as expected due to reintroductions; African elephant, African lion, cheetah and South African giraffe), whereas leopard (Panthera pardus) and Cape mountain zebra (Equus zebra) were detected in both the statutory and private PA but not farmland. The only extralimital species recorded (black wildebeest, South African giraffe and white rhinoceros [Ceratotherium simum]) were unique to the private PA. Fallow deer were only detected in the farmland.
One species known to occur throughout the region (based on discussions with landowners and official species lists) was not detected, namely the riverine rabbit (Bunolagus monticularis). At the land-use level, we failed to detect three additional species in the private PA (African buffalo [Syncerus caffer], striped polecat [Ictonyx striatus] and vervet monkey [Chlorocebus pygerythrus]), one in the statutory PA (yellow mongoose [Cynictis penicillata]) and one in farmland (water mongoose [Atilax paludinosus]). Additionally, although red hartebeest were detected in the farmland at two camera sites, software failure meant that the date and time of these detections were not recorded, and these data were subsequently removed from further analysis.
Estimates of species richness differed substantially between the three land-uses (Figure 2), with a mean species richness of 27.92 (95% BCI = [27.01, 31.23]) in the farmland, 31.10 in the statutory PA (95% BCI = [30.05, 35.49]) and 38.88 in the private PA (95% BCI = [36,46]). Although species richness within each of the four guilds was similar between the three land-uses (Figure 3), carnivore and herbivore species richness were highest in the private PA, omnivore species richness was lowest in the private PA and insectivore lowest in the statutory PA.
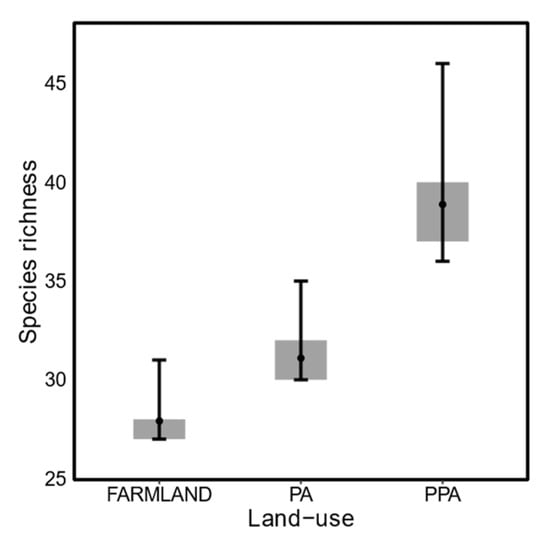
Figure 2.
Estimated mammalian (>0.5 kg) species richness in the small-livestock farmland (Farmland), protected area (PA), and private protected area (PPA) (black dots: mean, grey boxes: 50% BCI, and black bars: 95% BCI).
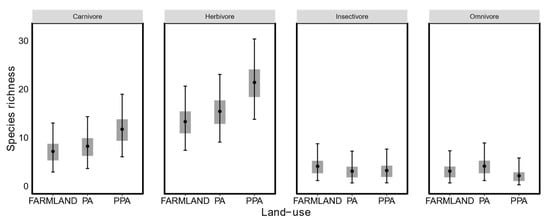
Figure 3.
Estimated mammalian (>0.5 kg) species richness for each functional guild in the small-livestock farmland (Farmland), protected area (PA), and private protected area (PPA) (black dots: mean, grey boxes: 50% BCI, and black bars: 95% BCI).
After excluding species not common across paired land-uses, species-specific mean occurrence (Ψ) was similar between all three land-uses and most similar between the statutory and private PA (adjusted r2 = 0.40, F [1,19] = 1.49, p < 0.00), and least similar between the farmland and both the farmland and private PA (Figure 4). The highest overall probability of use across all three land-uses was obtained for chacma baboon (Papio ursinus), whereas the lowest was for striped polecat. Large herbivores, such as greater kudu (Tragelaphus strepsiceros), gemsbok (Oryx gazella) and Cape eland (Taurotragus oryx), were more likely to occur within the private PA as opposed to either the statutory PA or farmland (Figure 4). Although more large carnivores (such a brown hyena [Parahyaena brunnea]) were more widespread throughout the private PA, leopards had higher rates of occurrence within the statutory PA. Smaller, generalist species (e.g., steenbok [Raphicerus campestris]) tended to occupy a greater proportion of farmland relative to both protected areas (Figure 4). Aardvark (Orycteropus afer) and African wildcat (Felis silvestris) had similar occurrence patterns in the private PA and farmland, whereas black-backed jackal (Canis mesomelas) had almost equitable occurrence probabilities between the statutory PA and farmland. As with occurrence, the detection probability of all species across all three land-uses was low, with over 80% of species having mean detection probabilities of less than 0.3 per sampling occasion (Figure 4). Species-specific mean detection was remarkably similar across all three land-uses, although it differed the most between the statutory and private PA (Figure 4). The highest overall detection probabilities across all land-uses were obtained for Chacma baboon, whereas the lowest were for bushpig (Potamochoerus larvatus).
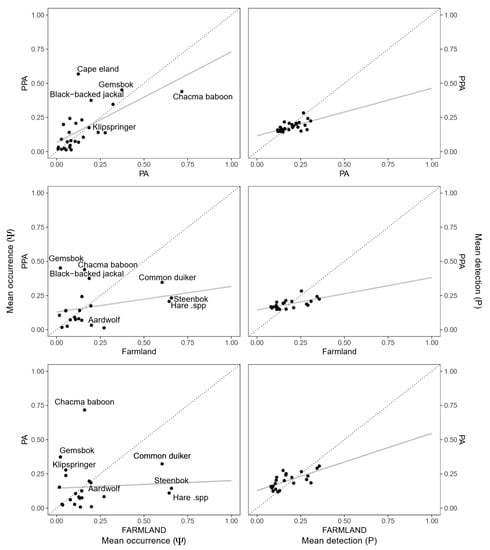
Figure 4.
Species-specific mean occurrence (left) and detection (right) probabilities of mammal species between the small-livestock farmland (Farmland), protected area (PA), and private protected area (PPA). Species not found in any of the land-uses were excluded. Solid grey lines illustrate the regression line for each relationship, and the dotted black line the 1–1 line. Species with notably higher occurrence (>0.25) on the Y or X axis are labelled.
None of the modelled covariates had a significant negative effect (i.e., 95% BCIs included zero; Table 1) on either community-level habitat use or detection in the farmland. However, vegetation productivity (MSAVI2) had a significant effect on community-level detection in the statutory (negative) and private PA (positive). Interestingly, only eight species-specific detection probabilities on farmland and the statutory PA were affected by vegetation productivity (Figure A3). Both vegetation productivity (positive) and terrain ruggedness (TRI, negative) exerted a significant influence over community-level habitat use in the statutory and private PA. Distance to drainage line (m) had a significant negative impact in the private PA only. Most species-specific occurrence probabilities were not significantly impacted by distance to drainage or vegetation productivity, but >50% of species were negatively associated with increasing terrain ruggedness (Figure A6).

Table 1.
Mean and associated 95% Bayesian credible intervals (BCIs) of community-level hyper-parameters hypothesised to influence the probability of use and detection of mammal species (>0.5 kg) across three land-uses (farmland, PA and PPA). Bold denotes covariates with significant effects. Parameters include distance to drainage line (m), vegetation productivity (MSAVI2) and terrain ruggedness (TRI) on the occurrence probability, and MSAVI2 on the detection probability.
4. Discussion
Identifying the potential contribution private land can make to national conservation objectives is imperative, given the limited resources available to biodiversity preservation [67]. Our study revealed minimal, yet crucial, differences in a mammal community between commercial farmland and both state-owned and privately owned PAs in the Karoo region of South Africa—highlighting the important role private landowners play in bolstering South Africa’s statutory PA network.
In line with our predictions, the private PA had higher species richness than both the statutory PA and farmland (with the latter having the lowest), a dissimilarity likely driven by the high number of unique species present in the private PA (n = 7). As mammalian diversity in African PAs is positively related to visitor attendance (and thus increased profits [68]), the private PA in our study is incentivised to augment its wildlife populations. The private PA has, since its inception, bolstered the abundance of select large charismatic species through introductions (e.g., African elephant) [69]. In contrast, the statutory PA has only introduced one species (Cape mountain zebra), and the gemsbok population was culled in 2013 to limit the impacts of over-grazing on the unique local flora [39]. In addition to active removal, the statutory PA’s boundary fence is porous in many parts, allowing species to move into the surrounding farmlands, whereas the private PA’s fence restricts the movement of larger species (e.g., African lion) [69]. The high costs of installing and maintaining adequate perimeter fencing (e.g., ±$25,972.23 per annum to maintain 100 km of game fencing [70]) and anti-poaching operations effectively precludes most PAs from supporting such species [11].
Despite concerns that reintroductions of larger species would have detrimental impacts on smaller mammal richness [17], we found that the composition of trophic guilds did not differ significantly between the land-uses. The similarity between the three land-uses also suggests that decades of sedentary pastoralism in the Karoo [71] and the subsequent protection in statutory or private PAs have had minimal impact upon the region’s medium-sized mammal community (Figure 3). Although not significant, carnivore and herbivore species richness was higher in both PAs than in farmland. This likely reflects the long history of reintroductions in PAs and targeted removal by commercial farmers of large species [31,72,73]. Yet, the farmland in our study did not differ in overall species richness from that of the statutory PA, a result congruent with other studies on extensive livestock farms in Africa; [32,42,74,75,76]). This result is not exclusive to Africa; low-intensity farming in West Bengal [77] and Brazil [78] has been shown to be similarly compatible with local wildlife. Many smaller, cosmopolitan species present in the farmland in our study are ecologically elastic, allowing them to adjust to persistent persecution.
Although species richness was similar across all three land-uses, species-specific occurrence probabilities varied substantially. Unsurprisingly, the greatest differences were observed between protected areas and farmland, with minimal differences between the statutory and private PA (Figure 4). Most observed dissimilarities were driven primarily by increased occurrence of large herbivore species in the private PA relative to both the statutory PA and farmland. Furthermore, the occurrence of most species >20 kg, many of which are herbivores in direct competition with domestic livestock for sparse resources [25], was artificially suppressed in the farmland through hunting. Interestingly, smaller herbivores, such as common duiker and hare spp. (Lepus saxatilis and Lepus capensis), had significantly higher occurrence probabilities in the farmland than in either PA (Figure 4). Owen-Smith et al. (2017) suggested that the declining densities of small ungulates in PAs is because of their confinement within increasingly homogenous habitats, depriving them of their need for fine-scale patchy habitats [79]. Well-managed small-livestock farming maintains this heterogenous habitat over large spatial scales, and is compatible with the many adaptable, gregarious antelope species [80,81,82]. It is also possible that the abundance of small herbivores in the Karoo’s PAs, both private and statutory, is suppressed through ecological top-down effects (i.e., predation) by a full suite of large- and medium-sized carnivores that is incomplete in farmlands due to the absence of large carnivores [83]. Costs associated with predator avoidance reduce the foraging efficiency of herbivores, diminishing their overall fitness [84,85]. For many small herbivores, open habitat, such as that present on commercial rangelands, can drastically improve visibility, thus lowering predation risk [33,86]. In addition, in rangelands, the abundance of mostly unguarded, easily catchable sheep stocked just under carrying capacity (as in our system) may act as a buffer for small wild ungulates against predation, by forming an important part of the mesopredators’ diet without outcompeting small wild ungulates for resources [87].
The impact of abiotic environmental factors (i.e., distance to drainage lines, vegetation productivity and terrain ruggedness) was similar across all three land-uses, albeit with varying degrees of importance. None had a significant impact on the community-level probability of occurrence for target mammal species in the commercial small-livestock farmlands. Most of the species present throughout the farmland are adaptable, generalist mammals able to persist in landscapes with substantial anthropogenic impacts. In the Karoo, the prevalence of artificial water sources (typically one per camp [88]) has likely reduced wildlife’s reliance on riparian zones and associated natural water sources. The overall lacklustre response to vegetation productivity is, however, not unprecedented in the Karoo—there is prior evidence of its inconsequential effect in the farmland [32]. It is possible that high stocking rates of livestock and very low rainfall have greatly reduced the productivity of drainage lines, making the distinction between riverine vegetation and the surrounding plant communities less apparent [38]. Finally, it may be that anthropogenic pressures not measured in this study (e.g., hunting) are far more important in determining species’ occurrence within the landscape.
In the both PAs, community-level probability of occurrence was significantly influenced both by vegetation productivity and terrain ruggedness. Terrain ruggedness, in particular, was important for 40% (n = 13) of all detected species in the statutory PA and 38% (n = 14) in the private PA, with only klipspringer and Smith’s red rock rabbit having a positive association with more rugged terrain (Figure A6). Globally, species richness, diversity and distribution all vary predictably for vertebrates along elevation gradients [89]. Lower elevations are typically less rugged, have richer soils and increased water availability, and are associated with higher primary productivity [90], all of which are important resources in determining species occurrence [91]. The private PA annual census data has historically been used to map several large antelope and carnivore landscape preferences, whereby species favoured lower-lying areas [69]. Distance to drainage lines was only a significant driver of community-level probability of occurrence in the private PA. This might be the result of the influence of introduced extralimital species, which are not drylands specialists, on the community’s response in the private PA. Despite being in close proximity, observed differences between land-uses were almost certainly confounded by land-use-specific differences not accounted for in this study. For example, the farmland was located within the Nama-Karoo biome, whereas both the statutory and private PA consisted largely of succulent Karoo flora. Similar to anthropogenic impacts, small-scale habitat use may be impacted by the change in vegetation structure associated with these biomes.
5. Conclusions
Through single-season multi-region community occurrence analyses, we have shown that the mosaic of land-uses present in South Africa’s Karoo region supports a diverse community of mammals, thus playing a crucial role in the preservation of dryland wildlife. With the current challenges of a growing human population and its associated need for increased resources [92,93], there is little opportunity to expand the network of statutory PAs, and those already established are coming under increasing pressure for access to their natural resources [3,6], despite clear evidence of their global importance in preserving mammals [94,95]. Many existing statutory PAs are also too small or poorly connected to provide resilient protection for all mammal species [96]. When managed correctly, multi-tenure landscapes increase landscape connectivity between state-owned PAs, providing corridors for wildlife and preserving a wider array of environments and the whole suite of mammals present within them [29,97,98,99]. They also support various economic models and human-centric activities. Encouragingly, the farmland in this study still maintains considerable ecosystem functionality, while allowing for food production and local employment, and, although reduced, its mammal assemblage is somewhat comparable to both PAs. In contrast, the newly established private PA has the economic capacity to preserve larger species that many statutory PAs lack.
The Karoo is experiencing substantial shifts in land-use, some of which will have a positive influence on biodiversity (e.g., private PAs [100]), but some of which may have negative impacts (e.g., hydraulic fracturing [71]). Thus, encouraging a land-use matrix incorporating multiple land-uses can benefit wildlife conservation, as long as these land-uses are included in carefully developed regional conservation planning.
Author Contributions
Conceptualisation, Z.W., M.D. and M.J.O.; methodology, Z.W. and G.D.; formal analysis, Z.W.; investigation, Z.W.; resources, M.J.O.; data curation, Z.W.; writing—original draft preparation, Z.W.; writing—review and editing, M.D., G.D. and M.J.O.; funding acquisition, M.J.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by WWF Nedbank Green Trust, grant number GT 2251 and Koos and Rona Rupert Opvoedkundige Trust (via the Endangered Wildlife Trust [EWT]).
Data Availability Statement
The data used in this study can be obtained upon request from the first author.
Acknowledgments
We thank the Karoo farmers, CapeNature and Sanbona Wildlife reserve (L. Eichenberger) for their participation and allowing us to access their properties. We are especially grateful to B. Conradie and C. Bragg for introducing us to the Karoo community. We also wish to thank M. Pretorius for her assistance in early analyses. Finally, we wish to thank the two anonymous reviewers for their valuable comments on the original manuscript. This article is based on original research conducted as part of the PhD thesis of the lead author, Z.W. (2021), available at: http://hdl.handle.net/20.500.12143/8608 (accessed on 23 December 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Summary of the camera trap surveys conducted on the farmland, protected area (PA) and private protected area (PPA) in the Karoo, South Africa. Area is the total extent (km2) of each land-use. Camera trap failure indicates the proportion of camera traps that failed, due to software malfunction or physical damage, in each survey. Effort is the total number of days (24 h) camera traps were active in each land-use. The total independent detections is the sum of independent photographs (≥30 min) of all target species per survey, and the overall trapping rate is the number of independent detections divided by the total number of trap nights.
Table A1.
Summary of the camera trap surveys conducted on the farmland, protected area (PA) and private protected area (PPA) in the Karoo, South Africa. Area is the total extent (km2) of each land-use. Camera trap failure indicates the proportion of camera traps that failed, due to software malfunction or physical damage, in each survey. Effort is the total number of days (24 h) camera traps were active in each land-use. The total independent detections is the sum of independent photographs (≥30 min) of all target species per survey, and the overall trapping rate is the number of independent detections divided by the total number of trap nights.
| Land-se | Area (km2) | Sampling Period | Total No. Sites | Effort | Total Independent Detections | Overall Trapping Rate | Total Detected Species | Mean No. Species per Site |
|---|---|---|---|---|---|---|---|---|
| Farmland | 754 | September–March 2012/2013 | 156 | 10,842 | 3480 | 0.27 | 27 1 | 10.8 |
| PA | 802 | October–June 2013/2014 | 176 | 9538 | 2966 | 0.28 | 30 | 10.8 |
| PPA | 540 | August–November 2015 | 119 | 5951 | 2484 | 0.38 | 36 | 11.4 |
| Total | 2096 | 451 | 15,489 | 8930 |
This should be 26, but the presented number includes detections of red hartebeest (Alcelaphus buselaphus caama) that had no associated date or time stamp due to software failure, and so were not included in multi-species occupancy analysis.

Table A2.
General results of the camera trapping surveys conducted on the farmland, presented per family and species. Shaded in light grey, the species/families that are absent or were not detected. Shaded in blue, the species/families that are true (i.e., known) absences. Total independent detections indicate the sum of independent detections, and the naïve occupancy is the proportion of camera trap sites at which the species was detected. The detection frequency (or relative abundance index [RAI]) is the camera trapping rate.
Table A2.
General results of the camera trapping surveys conducted on the farmland, presented per family and species. Shaded in light grey, the species/families that are absent or were not detected. Shaded in blue, the species/families that are true (i.e., known) absences. Total independent detections indicate the sum of independent detections, and the naïve occupancy is the proportion of camera trap sites at which the species was detected. The detection frequency (or relative abundance index [RAI]) is the camera trapping rate.
| Family Common Name Species | Total Independent Detections | Total Camera Trap Sites | Naïve Occupancy | Detection Frequency |
|---|---|---|---|---|
| Bovidae | ||||
| Black Wildebeest Connochaetes gnou | 0 | 0 | 0.00 | 0.00 |
| Blesbok Damaliscus pygargus | 27 | 10 | 0.06 | 0.25 |
| Cape eland Taurotragus oryx | 0 | 0 | 0.00 | 0.00 |
| Cape grysbok Raphicerus melanotis | 0 | 0 | 0.00 | 0.00 |
| Common duiker Sylvicapra grimmia | 957 | 107 | 0.69 | 8.83 |
| Gemsbok Oryx gazella | 20 | 3 | 0.02 | 0.18 |
| Greater kudu Tragelaphus strepsiceros | 124 | 27 | 0.17 | 1.24 |
| Grey rhebuck Pelea capreolus | 69 | 20 | 0.13 | 0.64 |
| Klipspringer Oreotragus oreotragus | 50 | 14 | 0.09 | 0.46 |
| Red hartebeest Alcelaphus buselaphus caama | 2 | 1 | * | * |
| Springbok Antidorcas marsupialis | 86 | 14 | 0.09 | 0.79 |
| Steenbok Raphicerus campestris | 700 | 116 | 0.74 | 6.46 |
| Canidae | ||||
| Bat-eared fox Otocyon megalotis | 218 | 41 | 0.26 | 2.01 |
| Black-backed jackal Canis mesomelas | 107 | 34 | 0.22 | 0.99 |
| Cape fox Vulpes chama | 16 | 11 | 0.07 | 0.15 |
| Cercopithecidae | ||||
| Chacma baboon Papio ursinus | 95 | 36 | 0.23 | 0.88 |
| Vervet monkey Chlorocebus pygerythrus | 8 | 6 | 0.04 | 0.07 |
| Cervidae | ||||
| Fallow deer Dama dama | 18 | 4 | 0.03 | 0.16 |
| Elephantidae | 0.00 | |||
| African elephant Loxodonta africana | 0 | 0 | 0.00 | 0.00 |
| Equidae | ||||
| Cape mountain zebra Equus zebra zebra | 0 | 0 | 0.00 | 0.00 |
| Plains zebra Equus quagga | 0 | 0 | 0.00 | 0.00 |
| Felidae | ||||
| African Lion Panthera leo | 0 | 0 | 0.00 | 0.00 |
| African wildcat Felis silvestris | 10 | 10 | 0.06 | 0.09 |
| Caracal Caracal caracal | 23 | 19 | 0.12 | 0.21 |
| Cheetah Acinonyx jubatus | 0 | 0 | 0.00 | 0.00 |
| Leopard Panthera pardus | 0 | 0 | 0.00 | 0.00 |
| Giraffidae | ||||
| South African giraffe Giraffa camelopardalis giraffa | 0 | 0 | 0.00 | 0.00 |
| Herpestidae | ||||
| Cape grey mongoose Herpestes pulverulentus | 37 | 27 | 0.17 | 0.34 |
| Meerkat Suricata suricatta | 3 | 3 | 0.02 | 0.02 |
| Water mongoose Atilax paludinosus | 0 | 0 | 0.00 | 0.00 |
| Yellow mongoose Cynictis penicillata | 16 | 12 | 0.08 | 0.15 |
| Hyaenidae | ||||
| Aardwolf Proteles cristata | 100 | 49 | 0.31 | 0.92 |
| Brown hyena Parahyaena brunnea | 0 | 0 | 0.00 | 0.00 |
| Hystricidae | ||||
| Porcupine Hystrix africaeaustralis | 65 | 36 | 0.23 | 0.60 |
| Leporiade | ||||
| Hare spp. Lepus saxatilis and Lepus capensis | 556 | 114 | 0.73 | 5.12 |
| Smith’s red rock rabbit Pronolagus rupestris | 30 | 2 | 0.01 | 0.28 |
| Mustelidae | ||||
| Honey badger Mellivora capensis | 0 | 0 | 0.00 | 0.00 |
| Striped polecat Ictonyx striatus | 36 | 25 | 0.16 | 0.33 |
| Orycteropodidae | ||||
| Aardvark Orycteropus afer | 43 | 24 | 0.15 | 0.40 |
| Procaviidae | ||||
| Rock hyrax Procavia capensis | 14 | 5 | 0.03 | 0.13 |
| Rhinocerotidae | ||||
| White rhinoceros Ceratotherium simum | 0 | 0 | 0.00 | 0.00 |
| Suidae | ||||
| Bushpig Potamochoerus larvatus | 0 | 0 | 0.00 | 0.00 |
| Viverridae | ||||
| Small spotted genet Genetta genetta | 42 | 24 | 0.15 | 0.39 |

Table A3.
General results of the camera trapping surveys conducted on the statutory protected area, presented per family and species. Shaded in light grey, the species/families that are absent or were not detected. Shaded in blue, the species/families that are true (i.e., known) absences. Total independent detections indicate the sum of independent detections, and the naïve occupancy is the proportion of camera trap sites at which the species was detected. The detection frequency (or relative abundance index [RAI]) is the camera trapping rate.
Table A3.
General results of the camera trapping surveys conducted on the statutory protected area, presented per family and species. Shaded in light grey, the species/families that are absent or were not detected. Shaded in blue, the species/families that are true (i.e., known) absences. Total independent detections indicate the sum of independent detections, and the naïve occupancy is the proportion of camera trap sites at which the species was detected. The detection frequency (or relative abundance index [RAI]) is the camera trapping rate.
| Family Common Name Species | Total Independent Detections | Total Camera Trap Sites | Naïve Occupancy | Detection Frequency |
|---|---|---|---|---|
| Bovidae | ||||
| Black Wildebeest Connochaetes gnou | 0 | 0 | 0.00 | 0.00 |
| Blesbok Damaliscus pygargus | 0 | 0 | 0.00 | 0.00 |
| Cape eland Taurotragus oryx | 64 | 25 | 0.14 | 0.67 |
| Cape grysbok Raphicerus melanotis | 0 | 0 | 0.00 | 0.00 |
| Common duiker Sylvicapra grimmia | 320 | 63 | 0.36 | 3.36 |
| Gemsbok Oryx gazella | 441 | 68 | 0.39 | 4.62 |
| Greater kudu Tragelaphus strepsiceros | 80 | 18 | 0.10 | 0.84 |
| Grey rhebuck Pelea capreolus | 73 | 19 | 0.11 | 0.77 |
| Klipspringer Oreotragus oreotragus | 193 | 49 | 0.28 | 2.02 |
| Red hartebeest Alcelaphus buselaphus caama | 115 | 19 | 0.11 | 1.21 |
| Springbok Antidorcas marsupialis | 91 | 22 | 0.13 | 0.95 |
| Steenbok Raphicerus campestris | 103 | 37 | 0.21 | 1.08 |
| Canidae | ||||
| Bat-eared fox Otocyon megalotis | 7 | 1 | 0.01 | 0.07 |
| Black-backed jackal Canis mesomelas | 237 | 51 | 0.29 | 2.48 |
| Cape fox Vulpes chama | 0 | 0 | 0.00 | 0.00 |
| Cercopithecidae | ||||
| Chacma baboon Papio ursinus | 536 | 112 | 0.64 | 5.62 |
| Vervet monkey Chlorocebus pygerythrus | 16 | 5 | 0.03 | 0.17 |
| Cervidae | ||||
| Fallow deer Dama dama | 0 | 0 | 0.00 | 0.00 |
| Elephantidae | ||||
| African elephant Loxodonta africana | 0 | 0 | 0.00 | 0.00 |
| Equidae | ||||
| Cape mountain zebra Equus zebra zebra | 20 | 10 | 0.06 | 0.21 |
| Plains zebra Equus quagga | 0 | 0 | 0.00 | 0.00 |
| Felidae | ||||
| African Lion Panthera leo | 0 | 0 | 0.00 | 0.00 |
| African wildcat Felis silvestris | 97 | 44 | 0.25 | 1.02 |
| Caracal Caracal caracal | 9 | 5 | 0.03 | 0.09 |
| Cheetah Acinonyx jubatus | 0 | 0 | 0.00 | 0.00 |
| Leopard Panthera pardus | 23 | 15 | 0.09 | 0.24 |
| Giraffidae | ||||
| South African giraffe Giraffa camelopardalis giraffa | 0 | 0 | 0.00 | 0.00 |
| Herpestidae | ||||
| Cape grey mongoose Herpestes pulverulentus | 49 | 21 | 0.12 | 0.51 |
| Meerkat Suricata suricatta | 0 | 0 | 0.00 | 0.00 |
| Water mongoose Atilax paludinosus | 1 | 1 | 0.01 | 0.01 |
| Yellow mongoose Cynictis penicillata | 0 | 0 | 0.00 | 0.00 |
| Hyaenidae | ||||
| Aardwolf Proteles cristata | 23 | 16 | 0.09 | 0.24 |
| Brown hyena Parahyaena brunnea | 15 | 9 | 0.05 | 0.16 |
| Hystricidae | ||||
| Porcupine Hystrix africaeaustralis | 112 | 36 | 0.20 | 1.17 |
| Leporidae | ||||
| Hare spp. Lepus saxatilis and Lepus capensis | 137 | 25 | 0.14 | 1.44 |
| Smith’s red rock rabbit Pronolagus rupestris | 99 | 28 | 0.16 | 1.04 |
| Mustelidae | ||||
| Honey badger Mellivora capensis | 17 | 9 | 0.05 | 0.18 |
| Striped polecat Ictonyx striatus | 1 | 1 | 0.01 | 0.01 |
| Orycteropodidae | ||||
| Aardvark Orycteropus afer | 28 | 23 | 0.13 | 0.29 |
| Procaviidae | ||||
| Rock hyrax Procavia capensis | 37 | 4 | 0.02 | 0.39 |
| Rhinocerotidae | ||||
| White rhinoceros Ceratotherium simum | 0 | 0 | 0.00 | 0.00 |
| Suidae | ||||
| Bushpig Potamochoerus larvatus | 4 | 4 | 0.02 | 0.04 |
| Viverridae | ||||
| Small spotted genet Genetta genetta | 18 | 13 | 0.07 | 0.19 |

Table A4.
General results of the camera trapping surveys conducted on the private protected area, presented per family and species. Shaded in light grey, the species/families that are absent or were not detected. Shaded in blue, the species/families that are true (i.e., known) absences. Total independent detections indicate the sum of independent detections, and the naïve occupancy is the proportion of camera trap sites at which the species was detected. The detection frequency (or relative abundance index [RAI]) is the camera trapping rate.
Table A4.
General results of the camera trapping surveys conducted on the private protected area, presented per family and species. Shaded in light grey, the species/families that are absent or were not detected. Shaded in blue, the species/families that are true (i.e., known) absences. Total independent detections indicate the sum of independent detections, and the naïve occupancy is the proportion of camera trap sites at which the species was detected. The detection frequency (or relative abundance index [RAI]) is the camera trapping rate.
| Family Common Name Species | Total Independent Detections | Total Camera Trap Sites | Naïve Occupancy | Detection Frequency |
|---|---|---|---|---|
| Bovidae | ||||
| Black Wildebeest Connochaetes gnou | 24 | 4 | 0.03 | 0.51 |
| Blesbok Damaliscus pygargus | 0 | 0 | 0.00 | 0.00 |
| Cape eland Taurotragus oryx | 460 | 69 | 0.58 | 7.10 |
| Cape grysbok Raphicerus melanotis | 2 | 1 | 0.01 | 0.03 |
| Common duiker Sylvicapra grimmia | 159 | 44 | 0.37 | 2.45 |
| Gemsbok Oryx gazella | 231 | 55 | 0.46 | 3.56 |
| Greater kudu Tragelaphus strepsiceros | 121 | 30 | 0.25 | 1.87 |
| Grey rhebuck Pelea capreolus | 33 | 9 | 0.08 | 0.51 |
| Klipspringer Oreotragus oreotragus | 81 | 19 | 0.16 | 1.25 |
| Red hartebeest Alcelaphus buselaphus caama | 25 | 6 | 0.05 | 0.39 |
| Springbok Antidorcas marsupialis | 68 | 11 | 0.09 | 1.05 |
| Steenbok Raphicerus campestris | 89 | 31 | 0.26 | 1.37 |
| Canidae | ||||
| Bat-eared fox Otocyon megalotis | 7 | 4 | 0.03 | 0.11 |
| Black-backed jackal Canis mesomelas | 134 | 51 | 0.43 | 2.07 |
| Cape fox Vulpes chama | 0 | 0 | 0.00 | 0.00 |
| Cercopithecidae | ||||
| Chacma baboon Papio ursinus | 228 | 54 | 1.92 | 3.52 |
| Vervet monkey Chlorocebus pygerythrus | 0 | 0 | 0.00 | 0.00 |
| Cervidae | ||||
| Fallow deer Dama dama | 0 | 0 | 0.00 | 0.00 |
| Elephantidae | ||||
| African elephant Loxodonta africana | 24 | 6 | 0.05 | 0.37 |
| Equidae | ||||
| Cape mountain zebra Equus zebra zebra | 1 | 1 | 0.01 | 0.02 |
| Plains zebra Equus quagga | 165 | 32 | 0.27 | 2.55 |
| Felidae | ||||
| African Lion Panthera leo | 42 | 10 | 0.08 | 0.65 |
| African wildcat Felis silvestris | 32 | 20 | 0.17 | 0.49 |
| Family Common name Species | Total independent detections | Total camera traps | Naïve occupancy | Detection frequency (RAI) |
| Caracal Caracal caracal | 21 | 12 | 0.10 | 0.32 |
| Cheetah Acinonyx jubatus | 10 | 5 | 0.04 | 0.15 |
| Leopard Panthera pardus | 6 | 4 | 0.03 | 0.09 |
| Giraffidae | ||||
| South African giraffe Giraffa camelopardalis giraffa | 42 | 12 | 0.10 | 0.65 |
| Herpestidae | ||||
| Cape grey mongoose Herpestes pulverulentus | 14 | 9 | 0.08 | 0.22 |
| Meerkat Suricata suricatta | 0 | 0 | 0.00 | 0.00 |
| Water mongoose Atilax paludinosus | 2 | 2 | 0.02 | 0.03 |
| Yellow mongoose Cynictis penicillata | 3 | 3 | 0.03 | 0.05 |
| Hyaenidae | ||||
| Aardwolf Proteles cristata | 2 | 1 | 0.01 | 0.03 |
| Brown hyena Parahyaena brunnea | 49 | 27 | 0.23 | 0.76 |
| Hystricidae | ||||
| Porcupine Hystrix africaeaustralis | 55 | 24 | 0.20 | 0.85 |
| Leporidae | ||||
| Hare spp. Lepus saxatilis and Lepus capensis | 244 | 28 | 0.24 | 3.77 |
| Smith’s red rock rabbit Pronolagus rupestris | 41 | 14 | 0.12 | 0.63 |
| Mustelidae | ||||
| Honey badger Mellivora capensis | 3 | 3 | 0.03 | 0.05 |
| Striped polecat Ictonyx striatus | 0 | 0 | 0.00 | 0.00 |
| Orycteropodidae | ||||
| Aardvark Orycteropus afer | 19 | 11 | 0.09 | 0.29 |
| Procaviidae | ||||
| Rock hyrax Procavia capensis | 5 | 2 | 0.02 | 0.08 |
| Rhinocerotidae | ||||
| White rhinoceros Ceratotherium simum | 12 | 4 | 0.03 | 0.19 |
| Suidae | ||||
| Bushpig Potamochoerus larvatus | 0 | 0 | 0.00 | 0.00 |
| Viverridae | ||||
| Small spotted genet Genetta genetta | 30 | 18 | 0.15 | 0.46 |
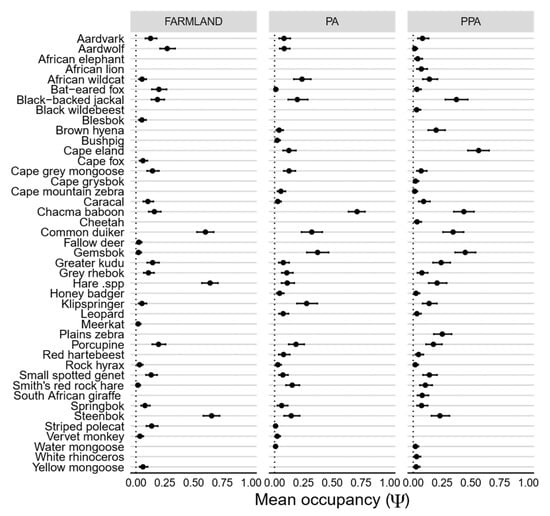
Figure A1.
Caterpillar plots showing posterior means of occurrence (Ψ) probabilities per species, as estimated under a hierarchical multi-species occupancy model, in farmland, PA and PPA. Error bars represent 95% Bayesian credible intervals.
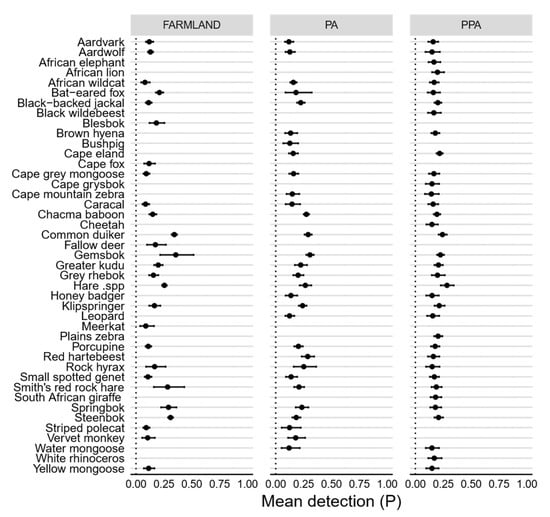
Figure A2.
Caterpillar plots showing posterior means of detection (P) probabilities per species, as estimated under a hierarchical multi-species occupancy model, in farmland, PA and PPA. Error bars represent 95% Bayesian credible intervals.
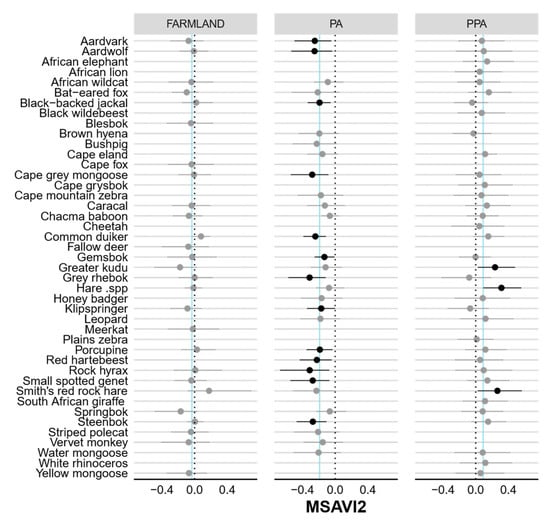
Figure A3.
Caterpillar plots showing the standardised beta coefficients and 95% Bayesian credible intervals for the influence of Modified Soil-Adjusted Vegetation Index (MSAVI2) on the detection probability (P) of each species in the farmland, PA and PPA. Confidence intervals in bold do not overlap 0 (dashed line), indicating significant predictors of occupancy. Vertical blue lines indicate community means.
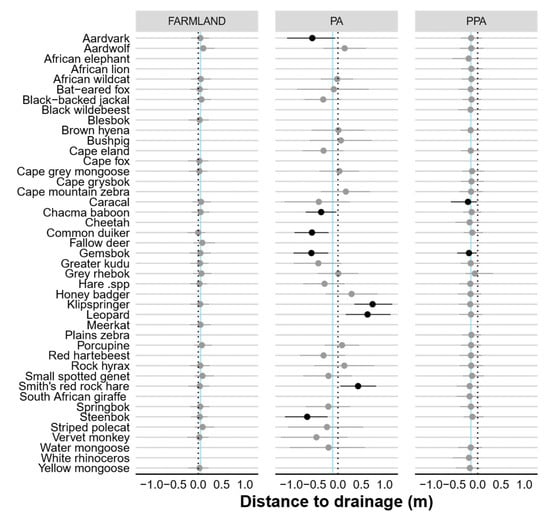
Figure A4.
Caterpillar plots showing the standardised beta coefficients and 95% Bayesian credible intervals for the influence of distance to drainage (m) on the occurrence probability (Ψ) of each species in the farmland, PA and PPA. Confidence intervals in bold do not overlap 0 (dashed line), indicating significant predictors of detection. Vertical blue lines indicate community means.
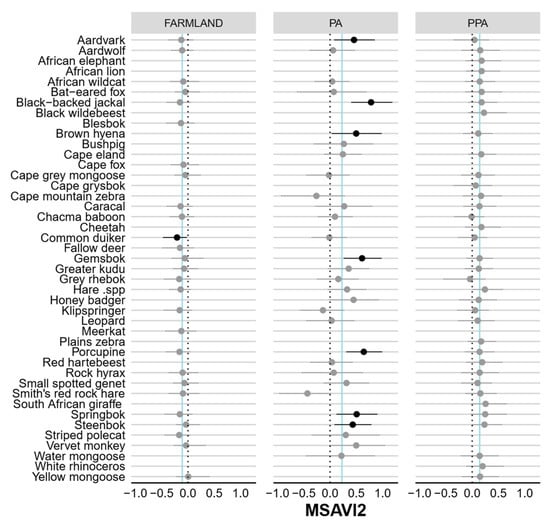
Figure A5.
Caterpillar plots showing the standardised beta coefficients and 95% Bayesian credible intervals for the influence of Modified Soil-Adjusted Vegetation Index (MSAVI2) on the occurrence probability (Ψ) of each species in the farmland, PA and PPA. Confidence intervals in bold do not overlap 0 (dashed line), indicating significant predictors of detection. Vertical blue lines indicate community means.
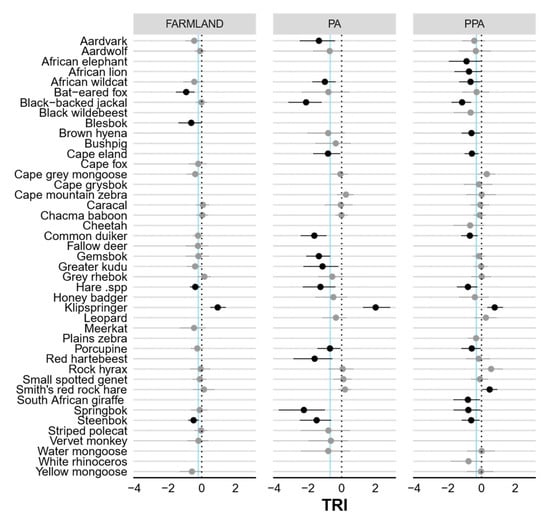
Figure A6.
Caterpillar plots showing the standardised beta coefficients and 95% Bayesian credible intervals for the influence of terrain ruggedness (TRI) on the occurrence probability (Ψ) of each species in the farmland, PA and PPA. Confidence intervals in bold do not overlap 0 (dashed line), indicating significant predictors of detection. Vertical blue lines indicate community means.
References
- O’Bryan, C.J.; Garnett, S.T.; Fa, J.E.; Leiper, I.; Rehbein, J.A.; Fernández-Llamazares, Á.; Jackson, M.V.; Jonas, H.D.; Brondizio, E.S.; Burgess, N.D.; et al. The Importance of Indigenous Peoples’ Lands for the Conservation of Terrestrial Mammals. Conserv. Biol. 2021, 35, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Zungu, M.M.; Maseko, M.S.T.; Kalle, R.; Ramesh, T.; Downs, C.T. Factors Affecting the Occupancy of Forest Mammals in an Urban-Forest Mosaic in EThekwini Municipality, Durban, South Africa. Urban For. Urban Green. 2020, 48, 126562. [Google Scholar] [CrossRef]
- Hoffmann, S.; Beierkuhnlein, C. Climate Change Exposure and Vulnerability of the Global Protected Area Estate from an International Perspective. Divers. Distrib. 2020, 26, 1496–1509. [Google Scholar] [CrossRef]
- Jenkins, C.N.; Joppa, L. Expansion of the Global Terrestrial Protected Area System. Biol. Conserv. 2009, 142, 2166–2174. [Google Scholar] [CrossRef]
- Venter, O.; Magrach, A.; Outram, N.; Klein, C.J.; Possingham, H.P.; Di Marco, M.; Watson, J.E.M. Bias in Protected-Area Location and Its Effects on Long-Term Aspirations of Biodiversity Conventions. Conserv. Biol. 2018, 32, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hoveka, L.N.; van der Bank, M.; Davies, T.J. Evaluating the Performance of a Protected Area Network in South Africa and Its Implications for Megadiverse Countries. Biol. Conserv. 2020, 248, 108577. [Google Scholar] [CrossRef]
- Sarkar, S.; Pressey, R.L.; Faith, D.P.; Margules, C.R.; Fuller, T.; Stoms, D.M.; Moffett, A.; Wilson, K.A.; Williams, K.J.; Williams, P.H.; et al. Biodiversity Conservation Planning Tools: Present Status and Challenges for the Future. Annu. Rev. Environ. Resour. 2006, 31, 123–159. [Google Scholar] [CrossRef]
- Clements, H.S.; Kerley, G.I.H.; Cumming, G.S.; De Vos, A.; Cook, C.N. Privately Protected Areas Provide Key Opportunities for the Regional Persistence of Large- and Medium-Sized Mammals. J. Appl. Ecol. 2019, 56, 537–546. [Google Scholar] [CrossRef]
- Lindsey, P.A.; Romañach, S.S.; Davies-Mostert, H.T. The Importance of Conservancies for Enhancing the Value of Game Ranch Land for Large Mammal Conservation in Southern Africa. J. Zool. 2009, 277, 99–105. [Google Scholar] [CrossRef]
- Taylor, W.A.; Child, M.F.; Lindsey, P.A.; Nicholson, S.K.; Relton, C.; Davies-Mostert, H.T. South Africa’s Private Wildlife Ranches Protect Globally Significant Populations of Wild Ungulates. Biodivers. Conserv. 2021, 30, 4111–4135. [Google Scholar] [CrossRef]
- Spierenburg, M.; Brooks, S. Private Game Farming and Its Social Consequences in Post-Apartheid South Africa: Contestations over Wildlife, Property and Agrarian Futures. J. Contemp. African Stud. 2014, 32, 151–172. [Google Scholar] [CrossRef]
- Faure, J.P.B.; Swanepoel, L.H.; Cilliers, D.; Venter, J.A.; Hill, R.A. Estimates of Carnivore Densities in a Human-Dominated Agricultural Matrix in South Africa. Oryx 2021, 56, 774–781. [Google Scholar] [CrossRef]
- Taylor, W.A.; Lindsey, P.A.; Nicholson, S.K.; Relton, C.; Davies-Mostert, H.T. Jobs, Game Meat and Profits: The Benefits of Wildlife Ranching on Marginal Lands in South Africa. Biol. Conserv. 2020, 245, 108561. [Google Scholar] [CrossRef]
- Gordon, I.J.; Hester, A.J.; Festa-Bianchet, M. REVIEW: The Management of Wild Large Herbivores to Meet Economic, Conservation and Environmental Objectives. J. Appl. Ecol. 2004, 41, 1021–1031. [Google Scholar] [CrossRef]
- Boronyak, L.; Jacobs, B.; Wallach, A.; McManus, J.; Stone, S.; Stevenson, S.; Smuts, B.; Zaranek, H. Pathways towards Coexistence with Large Carnivores in Production Systems. Agric. Hum. Values 2022, 39, 47–64. [Google Scholar] [CrossRef]
- Carter, E.; Adams, W.M.; Hutton, J. Private Protected Areas: Management Regimes, Tenure Arrangements and Protected Area Categorization in East Africa. Oryx 2008, 42, 177–186. [Google Scholar] [CrossRef]
- Cousins, J.A.; Sadler, J.P.; Evans, J. Exploring the Role of Private Wildlife Ranching as a Conservation Tool in South Africa: Stakeholder Perspectives. Ecol. Soc. 2008, 13, 43. [Google Scholar] [CrossRef]
- Maciejewski, K.; Kerley, G.I.H.H. Understanding Tourists’ Preference for Mammal Species in Private Protected Areas: Is There a Case for Extralimital Species for Ecotourism? PLoS ONE 2014, 9, e88192. [Google Scholar] [CrossRef]
- Palfrey, R.; Oldekop, J.; Holmes, G. Conservation and Social Outcomes of Private Protected Areas. Conserv. Biol. 2021, 35, 1098–1110. [Google Scholar] [CrossRef]
- Barany, M.E.; Hammett, A.L.; Shillington, L.J.; Murphy, B.R. The Role of Private Wildlife Reserves in Nicaragua’s Emerging Ecotourism Industry. J. Sustain. Tour. 2001, 9, 95–110. [Google Scholar] [CrossRef]
- de Santo, E.M. From Paper Parks to Private Conservation: The Role of NGOs in Adapting Marine Protected Area Strategies to Climate Change. J. Int. Wildl. Law Policy 2012, 15, 25–40. [Google Scholar] [CrossRef]
- Gooden, J.; ‘t Sas-Rolfes, M. A Review of Critical Perspectives on Private Land Conservation in Academic Literature. Ambio 2020, 49, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, D.; Puigdefabregas, J.; White, R.; Lal, R.; Winslow, M.; Ziedler, J.; Prince, S.; Archer, E.; King, C.; Shaprio, B.; et al. Dryland Systems. In Ecosystems and Human Well-Being: Current State and Trends; Hassan, R., Scholes, R., Neville, A., Eds.; Island Press: Washington, DC, USA, 2005; pp. 623–662. [Google Scholar]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Kiffner, C.; Wenner, C.; LaViolet, A.; Yeh, K.; Kioko, J. From Savannah to Farmland: Effects of Land-Use on Mammal Communities in the Tarangire-Manyara Ecosystem, Tanzania. Afr. J. Ecol. 2015, 53, 156–166. [Google Scholar] [CrossRef]
- Woodgate, Z.; Distiller, G.; O’Riain, J. Variation in Mammal Species Richness and Relative Abundance in the Karoo. Afr. J. Range Forage Sci. 2018, 35, 325–334. [Google Scholar] [CrossRef]
- Fabricius, C.; Burger, M.; Hockey, P.A.R. Comparing Biodiversity between Protected Areas and Adjacent Rangeland in Xeric Succulent Thicket, South Africa: Arthropods and Reptiles. J. Appl. Ecol. 2003, 40, 392–403. [Google Scholar] [CrossRef]
- Greve, M.; Chown, S.L.; van Rensburg, B.J.; Dallimer, M.; Gaston, K.J. The Ecological Effectiveness of Protected Areas: A Case Study for South African Birds. Anim. Conserv. 2011, 14, 295–305. [Google Scholar] [CrossRef]
- Shumba, T.; De Vos, A.; Biggs, R.; Esler, K.J.; Ament, J.M.; Clements, H.S. Effectiveness of Private Land Conservation Areas in Maintaining Natural Land Cover and Biodiversity Intactness. Glob. Ecol. Conserv. 2020, 22, e00935. [Google Scholar] [CrossRef]
- Ament, J.M.; Cumming, G.S. Scale Dependency in Effectiveness, Isolation, and Social-Ecological Spillover of Protected Areas. Conserv. Biol. 2016, 30, 846–855. [Google Scholar] [CrossRef]
- Curveira-Santos, G.; Sutherland, C.; Santos-Reis, M.; Swanepoel, L.H. Responses of Carnivore Assemblages to Decentralized Conservation Approaches in a South African Landscape. J. Appl. Ecol. 2021, 58, 92–103. [Google Scholar] [CrossRef]
- Drouilly, M.; Clark, A.; O’Riain, M.J. Multi-Species Occupancy Modelling of Mammal and Ground Bird Communities in Rangeland in the Karoo: A Case for Dryland Systems Globally. Biol. Conserv. 2018, 224, 16–25. [Google Scholar] [CrossRef]
- Wells, H.B.M.; Kimuyu, D.M.; Odadi, W.O.; Dougill, A.J.; Stringer, L.C.; Young, T.P. Wild and Domestic Savanna Herbivores Increase Smaller Vertebrate Diversity, but Less than Additively. J. Appl. Ecol. 2021, 58, 953–963. [Google Scholar] [CrossRef]
- Dean, W.R.J.; Milton, S.L. The Karoo: Ecological Patterns and Processes; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Milton, S.J. Studies of Herbivory and Vegetation Change in Karoo Shrublands. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1993; p. 146. [Google Scholar]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland. In Strelitzia 19; South African National Biodiversity Institute: Pretoria, South Africa, 2006; pp. 1–30. ISBN 978-1-919976-21-1. [Google Scholar]
- Timm Hoffman, M.; Skowno, A.; Bell, W.; Mashele, S. Long-Term Changes in Land Use, Land Cover and Vegetation in the Karoo Drylands of South Africa: Implications for Degradation Monitoring. Afr. J. Range Forage Sci. 2018, 35, 209–221. [Google Scholar] [CrossRef]
- Allsopp, N.; Gaika, L.; Knight, R.; Monakisi, C.; Hoffman, M.T. The Impact of Heavy Grazing on an Ephemeral River System in the Succulent Karoo, South Africa. J. Arid Environ. 2007, 71, 82–96. [Google Scholar] [CrossRef]
- Brand, M.; Schutte-Vlok, A.; Huisamen, J. Anysberg Nature Reserve and World Heritage Site. Protected Area Management Plan 2018–2028. Cape Nature: Cape Town, South Africa, 2018; 170, Unpublished Internal Report. [Google Scholar]
- Cowling, R.M.; Pressey, R.L.; Lombard, A.T.; Desmet, P.G.; Ellis, A.G. From Representation to Persistence: Requirements for a Sustainable System of Conservation Areas in the Species-Rich Mediterranean-Climate Desert of Southern Africa. Divers. Distrib. 1999, 5, 51–71. [Google Scholar] [CrossRef]
- Hoffman, M.T.; Madden, C.F.; Erasmus, K.; Saayman, N.; Botha, J.C. The Impact of Indigenous Ungulate Herbivory over Five Years (2004–2008) on the Vegetation of the Little Karoo, South Africa. Afr. J. Range Forage Sci. 2009, 26, 169–179. [Google Scholar] [CrossRef]
- Mann, G.K.H.; Lagesse, J.V.; O’Riain, M.J.; Parker, D.M. Beefing Up Species Richness? The Effect of Land-Use on Mammal Diversity in an Arid Biodiversity Hotspot. Afr. J. Wildl. Res. 2015, 45, 321–331. [Google Scholar] [CrossRef]
- Cusack, J.J.; Dickman, A.J.; Rowcliffe, J.M.; Carbone, C.; Macdonald, D.W.; Coulson, T. Random versus Game Trail-Based Camera Trap Placement Strategy for Monitoring Terrestrial Mammal Communities. PLoS ONE 2015, 10, e0126379. [Google Scholar] [CrossRef]
- Rovero, F.; Ahumada, J. The Tropical Ecology, Assessment and Monitoring (TEAM) Network: An Early Warning System for Tropical Rain Forests. Sci. Total Environ. 2017, 574, 914–923. [Google Scholar] [CrossRef]
- Rich, L.N.; Miller, D.A.W.; Muñoz, D.J.; Robinson, H.S.; McNutt, J.W.; Kelly, M.J. Sampling Design and Analytical Advances Allow for Simultaneous Density Estimation of Seven Sympatric Carnivore Species from Camera Trap Data. Biol. Conserv. 2019, 233, 12–20. [Google Scholar] [CrossRef]
- Hofmeester, T.R.; Cromsigt, J.P.G.M.; Odden, J.; Andrén, H.; Kindberg, J.; Linnell, J.D.C. Framing Pictures: A Conceptual Framework to Identify and Correct for Biases in Detection Probability of Camera Traps Enabling Multi-Species Comparison. Ecol. Evol. 2019, 9, 2320–2336. [Google Scholar] [CrossRef] [PubMed]
- Larrucea, E.S.; Brussard, P.F.; Jaeger, M.M.; Barrett, H.R. Cameras, Coyotes, and the Assumption of Equal Detectability. J. Wildl. Manag. 2007, 71, 1682–1689. [Google Scholar] [CrossRef]
- Meek, P.D.; Ballard, G.; Claridge, A.; Kays, R.; Moseby, K.; O’Brien, T.; O’Connell, A.; Sanderson, J.; Swann, D.E.; Tobler, M.; et al. Recommended Guiding Principles for Reporting on Camera Trapping Research. Biodivers. Conserv. 2014, 23, 2321–2343. [Google Scholar] [CrossRef]
- O’Brien, T.G. Wildlife Picture Index and Biodiversity Monitoring: Issues and Future Directions. Anim. Conserv. 2010, 13, 350–352. [Google Scholar] [CrossRef]
- Tenan, S.; Brambilla, M.; Pedrini, P.; Sutherland, C. Quantifying Spatial Variation in the Size and Structure of Ecologically Stratified Communities. Methods Ecol. Evol. 2017, 8, 976–984. [Google Scholar] [CrossRef]
- Oberosler, V.; Tenan, S.; Zipkin, E.F.; Rovero, F. Poor Management in Protected Areas Is Associated with Lowered Tropical Mammal Diversity. Anim. Conserv. 2020, 23, 171–181. [Google Scholar] [CrossRef]
- Sutherland, C.; Brambilla, M.; Pedrini, P.; Tenan, S. A Multiregion Community Model for Inference about Geographic Variation in Species Richness. Methods Ecol. Evol. 2016, 7, 783–791. [Google Scholar] [CrossRef]
- Mackenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Andrew, J.; Langtimm, C.A. Estimating Site Occupancy Rates When Detection Probabilities Are Less Than One. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- Dorazio, R.M.; Royle, J.A.; Söderström, B.; Glimskär, A. Estimating Species Richness and Accumulation by Modeling Species Occurrence and Detectability. Ecology 2006, 87, 842–854. [Google Scholar] [CrossRef]
- Kéry, M.; Royle, J.A. Hierarchical Bayes Estimation of Species Richness and Occupancy in Spatially Replicated Surveys. J. Appl. Ecol. 2008, 45, 589–598. [Google Scholar] [CrossRef]
- Tingley, M.W.; Nadeau, C.P.; Sandor, M.E. Multi-Species Occupancy Models as Robust Estimators of Community Richness. Methods Ecol. Evol. 2020, 11, 633–642. [Google Scholar] [CrossRef]
- Dorazio, R.M.; Royle, J.A. Estimating Size and Composition of Biological Communities by Modeling the Occurrence of Species. J. Am. Stat. Assoc. 2005, 100, 389–398. [Google Scholar] [CrossRef]
- Lehner, B.; Verdin, K.; Jarvis, A. New Global Hydrography Derived From Spaceborne Elevation Data. Eos Trans. Am. Geophys. Union 2008, 89, 93. [Google Scholar] [CrossRef]
- Petracca, L.S.; Funston, P.J.; Henschel, P.; Cohen, J.B.; Maclennan, S.; Frair, J.L. Modeling Community Occupancy from Line Transect Data: A Case Study with Large Mammals in Post-War Angola. Anim. Conserv. 2020, 23, 420–433. [Google Scholar] [CrossRef]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L.; et al. The Shuttle Radar Topography Mission. Rev. Geophys. 2007, 45, RG2004. [Google Scholar] [CrossRef]
- Sappington, J.M.; Longshore, K.M.; Thompson, D.B. Quantifying Landscape Ruggedness for Animal Habitat Analysis: A Case Study Using Bighorn Sheep in the Mojave Desert. J. Wildl. Manag. 2007, 71, 1419–1426. [Google Scholar] [CrossRef]
- Plummer, M. JAGS: A Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling JAGS: Just Another Gibbs Sampler. In Proceedings of the 3rd International Workshop on Distributed Statistical Computing, Vienna, Austria, 20–22 March 2003; pp. 1–10. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2022. Available online: https://www.bibsonomy.org/bibtex/7469ffee3b07f9167cf47e7555041ee7 (accessed on 28 February 2023).
- Kellner, K. JagsUI: A Wrapper around Rjags to Streamline JAGS Analyses. R Package Version 2015, 1. [Google Scholar]
- Geweke, J. Comment: Inference and Prediction in the Presence of Uncertainty and Determinism. Stat. Sci. 1992, 7, 94–101. [Google Scholar] [CrossRef]
- Gelman, A.; Hwang, J.; Vehtari, A. Understanding Predictive Information Criteria for Bayesian Models. Stat. Comput. 2014, 24, 997–1016. [Google Scholar] [CrossRef]
- Davies-Mostert, H.T. Overcoming Barriers to Understanding the Biodiversity Contribution of Private Ranchlands. Anim. Conserv. 2014, 17, 399–400. [Google Scholar] [CrossRef]
- Arbieu, U.; Grünewald, C.; Martín-López, B.; Schleuning, M.; Böhning-Gaese, K. Large Mammal Diversity Matters for Wildlife Tourism in Southern African Protected Areas: Insights for Management. Ecosyst. Serv. 2018, 31, 481–490. [Google Scholar] [CrossRef]
- Lynch, K.; Vorster, L.; Vorster, P. Sanbona Wildlife Reserve: Environmental Management Plan. Unpublished Internal Report. 2015; 27, Unpublished. [Google Scholar]
- Lindsey, P.A.; Masterson, C.L.; Beck, A.L.; Romañach, S. Ecological, Social and Financial Issues Related to Fencing as a Conservation Tool in Africa. In Fencing for Conservation; Somers, M.J., Hayward, M., Eds.; Springer: New York, NY, USA, 2012; pp. 215–234. ISBN 9781461409021. [Google Scholar]
- Milton, S.J.; Dean, W.R.J. Anthropogenic Impacts and Implications for Ecological Restoration in the Karoo, South Africa. Anthropocene 2021, 36, 100307. [Google Scholar] [CrossRef]
- Ramesh, T.; Kalle, R.; Rosenlund, H.; Downs, C.T. Native Habitat and Protected Area Size Matters: Preserving Mammalian Assemblages in the Maputaland Conservation Unit of South Africa. For. Ecol. Manag. 2016, 360, 20–29. [Google Scholar] [CrossRef]
- Van der Weyde, L.K.; Tobler, M.W.; Gielen, M.C.; Cozzi, G.; Weise, F.J.; Adams, T.; Bauer, D.; Bennitt, E.; Bowles, M.; Brassine, A.; et al. Collaboration for Conservation: Assessing Countrywide Carnivore Occupancy Dynamics from Sparse Data. Divers. Distrib. 2022, 28, 917–929. [Google Scholar] [CrossRef]
- Tyrrell, P.; Toit, J.T.; Macdonald, D.W. Conservation beyond Protected Areas: Using Vertebrate Species Ranges and Biodiversity Importance Scores to Inform Policy for an East African Country in Transition. Conserv. Sci. Pract. 2020, 2, e136. [Google Scholar] [CrossRef]
- Verschueren, S.; Briers-Louw, W.D.; Monterroso, P.; Marker, L. Local-Scale Variation in Land Use Practice Supports a Diverse Carnivore Guild on Namibian Multiple-Use Rangeland. Rangel. Ecol. Manag. 2021, 79, 64–76. [Google Scholar] [CrossRef]
- Harris, N.C.; Mills, K.L.; Harissou, Y.; Hema, E.M.; Gnoumou, I.T.; VanZoeren, J.; Abdel-Nasser, Y.I.; Doamba, B. First Camera Survey in Burkina Faso and Niger Reveals Human Pressures on Mammal Communities within the Largest Protected Area Complex in West Africa. Conserv. Lett. 2019, 12, e12667. [Google Scholar] [CrossRef]
- Thapa, A.; Pradhan, P.K.; Joshi, B.D.; Mukherjee, T.; Thakur, M.; Chandra, K.; Sharma, L.K. Non-Protected Areas Demanding Equitable Conservation Strategies as of Protected Areas in the Central Himalayan Region. PLoS ONE 2021, 16, e0255082. [Google Scholar] [CrossRef]
- Bellón, B.; Henry, D.; Renaud, P.C.; Roque, F.d.O.; Santos, C.C.; Melo, I.; Arvor, D.; de Vos, A. Landscape Drivers of Mammal Habitat Use and Richness in a Protected Area and Its Surrounding Agricultural Lands. Agric. Ecosyst. Environ. 2022, 334, 107989. [Google Scholar] [CrossRef]
- Owen-Smith, N.; Cromsigt, J.P.G.M.; Le Roux, E. Smaller Ungulates Are First to Incur Imminent Extirpation from an African Protected Area. Biol. Conserv. 2017, 216, 108–114. [Google Scholar] [CrossRef]
- Rottstock, T.; Göttert, T.; Zeller, U. Relatively Undisturbed African Savannas—An Important Reference for Assessing Wildlife Responses to Livestock Grazing Systems in European Rangelands. Glob. Ecol. Conserv. 2020, 23, e01124. [Google Scholar] [CrossRef]
- Holechek, J.; Valdez, R. Wildlife Conservation on the Rangelands of Eastern and Southern Africa: Past, Present, and Future. Rangel. Ecol. Manag. 2018, 71, 245–258. [Google Scholar] [CrossRef]
- Cox, R.L.; Underwood, E.C. The Importance of Conserving Biodiversity Outside of Protected Areas in Mediterranean Ecosystems. PLoS ONE 2011, 6, e14508. [Google Scholar] [CrossRef] [PubMed]
- TOIT, J.T. DU The Feeding Ecology of a Very Small Ruminant, the Steenbok (Raphicerus Campestris). Afr. J. Ecol. 1993, 31, 35–48. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral Decisions Made under the Risk of Predation: A Review and Prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Mayer, M.; Fog Bjerre, D.H.; Sunde, P. Better Safe than Sorry: The Response to a Simulated Predator and Unfamiliar Scent by the European Hare. Ethology 2020, 126, 704–715. [Google Scholar] [CrossRef]
- Young, T.P.; Porensky, L.M.; Riginos, C.; Veblen, K.E.; Odadi, W.O.; Kimuyu, D.M.; Charles, G.K.; Young, H.S. Relationships Between Cattle and Biodiversity in Multiuse Landscape Revealed by Kenya Long-Term Exclosure Experiment. Rangel. Ecol. Manag. 2018, 71, 281–291. [Google Scholar] [CrossRef]
- Drouilly, M.; Nattrass, N.; O’Riain, M.J. Dietary Niche Relationships among Predators on Farmland and a Protected Area. J. Wildl. Manag. 2018, 82, 507–518. [Google Scholar] [CrossRef]
- Beinart, W. The Rise of Conservation in South Africa: Settlers, Livestock, and the Environment; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- McCain, C.M.; Beck, J. Species Turnover in Vertebrate Communities along Elevational Gradients Is Idiosyncratic and Unrelated to Species Richness. Glob. Ecol. Biogeogr. 2016, 25, 299–310. [Google Scholar] [CrossRef]
- Berryman, E.M.; Barnard, H.R.; Adams, H.R.; Burns, M.A.; Gallo, E.; Brooks, P.D. Complex Terrain Alters Temperature and Moisture Limitations of Forest Soil Respiration across a Semiarid to Subalpine Gradient. J. Geophys. Res. Biogeosciences 2015, 120, 707–723. [Google Scholar] [CrossRef]
- Fritz, S.A.; Eronen, J.T.; Schnitzler, J.; Hof, C.; Janis, C.M.; Mulch, A.; Böhning-Gaese, K.; Graham, C.H. Twenty-Million-Year Relationship between Mammalian Diversity and Primary Productivity. Proc. Natl. Acad. Sci. USA 2016, 113, 10908–10913. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; RedfordD, K.H.; Wannebo, A.V.; Woolmer, G. The Human Footprint and the Last of the Wild. Bioscience 2002, 52, 891–904. [Google Scholar] [CrossRef]
- Gebert, F.; Njovu, H.K.; Treydte, A.C.; Steffan-Dewenter, I.; Peters, M.K. Primary Productivity and Habitat Protection Predict Elevational Species Richness and Community Biomass of Large Mammals on Mt. Kilimanjaro. J. Anim. Ecol. 2019, 88, 1860–1872. [Google Scholar] [CrossRef]
- Ferreira, G.B.; Collen, B.; Newbold, T.; Oliveira, M.J.R.; Pinheiro, M.S.; de Pinho, F.F.; Rowcliffe, M.; Carbone, C. Strict Protected Areas Are Essential for the Conservation of Larger and Threatened Mammals in a Priority Region of the Brazilian Cerrado. Biol. Conserv. 2020, 251, 108762. [Google Scholar] [CrossRef]
- Chen, C.; Brodie, J.F.; Kays, R.; Davies, T.J.; Liu, R.; Fisher, J.T.; Ahumada, J.; McShea, W.; Sheil, D.; Agwanda, B.; et al. Global Camera Trap Synthesis Highlights the Importance of Protected Areas in Maintaining Mammal Diversity. Conserv. Lett. 2022, 15, e12865. [Google Scholar] [CrossRef]
- Parker, K.; De Vos, A.; Clements, H.S.; Biggs, D.; Biggs, R. Impacts of a Trophy Hunting Ban on Private Land Conservation in South African Biodiversity Hotspots. Conserv. Sci. Pract. 2020, 2, e214. [Google Scholar] [CrossRef]
- Gallo, J.A.; Pasquini, L.; Reyers, B.; Cowling, R.M. The Role of Private Conservation Areas in Biodiversity Representation and Target Achievement within the Little Karoo Region, South Africa. Biol. Conserv. 2009, 142, 446–454. [Google Scholar] [CrossRef]
- Torres-Romero, E.J.; Giordano, A.J.; Ceballos, G.; López-Bao, J.V. Reducing the Sixth Mass Extinction: Understanding the Value of Human-Altered Landscapes to the Conservation of the World’s Largest Terrestrial Mammals. ABiol. Conserv. 2020, 249, 108706. [Google Scholar] [CrossRef]
- Clements, H.S.; Baum, J.; Cumming, G.S. Money and Motives: An Organizational Ecology Perspective on Private Land Conservation. Biol. Conserv. 2016, 197, 108–115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).