Abstract
(1) The tropical swallowtail moth Lyssa zampa received much public attention during its years of mass emergence in Singapore and Southeast Asia. However, despite its prominence, little is known about its population demographics and spatial ecology. This study aims to establish the annual abundance of L. zampa, determine its spatial patterns of occurrence, and examine morphological variation demonstrated by L. zampa with an emphasis on comparing urban vs. forest areas in Singapore. (2) Various sources (field surveys across 18 sites, citizen science datasets and expert knowledge) were used to catalogue L. zampa records from 2011 to 2020 and analyse its seasonal abundance. (3) We confirmed the seasonal peak of L. zampa emergence to be between May and July, with an unusually high mass emergence in 2014. The intensity of emergence was associated with the intensity of a dry spell in February of that year. The total number of L. zampa sighted in urban areas was higher despite the moth’s host plant being a tree that is restricted to mature forests and is absent from urban areas. This suggests that the occurrence of L. zampa in urban areas is likely due to the moth’s attraction to bright city lights. Our morphometric measurements further show that L. zampa individuals in urban areas have greater wing length and lighter body weights (smaller body widths) than their forest counterparts. (4) This implies that urban areas are not only drawing moths that are unable to find the host plants and, therefore, cannot produce offspring but are also attracting larger and better flyers out of forest areas. This situation is only likely to worsen as climate change intensifies and dry spells become longer and more intense.
1. Introduction
Moths are relatively less studied in the tropics, including in Singapore. Since Murphy’s seminal work on insects of Singapore in the 1980s [1], only a few ad hoc surveys have been conducted across this species-rich taxa (e.g., [2]). One exception is the tropical swallowtail moth, Lyssa zampa (Butler, 1869) (Uraniidae, Lepidoptera). This large, intermittently abundant species has received sporadic public attention during its years of abundant occurrence due to its size and presence in urban areas [3,4,5].
L. zampa [6,7] is distributed across Southeast Asia and Northeast Himalaya, and more recently, reports have extended the range to Taiwan and Japan [8,9]. It is the largest uraniid moth and second largest moth in Singapore after Attacus atlas (Family Saturniidae; [10]). The only host plant species recorded for the caterpillar has been Endospermum diadenum (Benn. & Muell. Arg) (syn. E. malaccense; family Euphorbiaceae)—itself a threatened species and limited to certain forest patches in Singapore [5,11,12].
The seasonal abundance of L. zampa has been documented in Singapore through recruitment of public sightings since 2005 [13,14,15,16,17], albeit in an ad hoc way. In May–June 2014, the largest emergence of L. zampa thus far known from Singapore was reported, and this resulted in numerous sightings around urban Singapore [17,18]. The emergence was also picked up by the national and international media [3,4], and a much larger emergence was reported from Malaysia, where it was reported to have disrupted a football match [19,20].
The life cycle of the L. zampa and mass herbivory of larval host plants during this mass emergence has been published by the lead author [5]. The 2014 study documented extensive presence (estimated to be a cluster of 15,000–20,000 individuals) of L.zampa caterpillars on several trees of E. diadenum within Singapore’s Central Catchment Nature Reserve. The trees were checked for several days when silk threads were observed hanging around the main trunk of trees, and larvae were hanging from them after several days. Subsequently, a few adult moths were observed at this forested site in future visits [5].
Despite the life history documentation of L. zampa during the mass emergence, the abundance of the species has not been mapped spatially, and the linkages between moths in the forest and urban habitats have not been directly made. This follow-up study was initiated to:
- (i)
- document the seasonal abundance of moths from 2011 to 2020 with an emphasis on the mass emergence in 2014.
- (ii)
- map the distribution of adult moths in forest and urban areas during the mass emergence in 2014.
- (iii)
- understand the movement of adult moths between forest and urban areas.
- (iv)
- demonstrate the morphological variation in L. zampa, by gender, location, and across emergence batches.
- (v)
- demonstrate the relationship between L. zampa abundance and rainfall patterns.
The distribution of L. zampa was mapped using data from public sightings and field surveys in forest and urban areas. A mark-recapture study was conducted to understand the movement between forest and urban areas. Understanding the patterns of mass emergence of insects and reasons behind them could help improve predictions of such events and, if needed, manage insect populations. This is important because insect populations are foundational to the populations of higher order organisms found in those habitats.
2. Materials and Methods
2.1. Distribution of Public Sightings from Habitatnews and NSS
Sightings of L. zampa were collected from the public between 2011 and 2020 through diverse sources: the National University of Singapore’s Habitatnews web portal (http://habitatnews.nus.edu.sg, accessed on 7 February 2023), iNaturalist (https://www.inaturalist.org/, accessed on 7 February 2023), and Nature Society (Singapore) or NSS’s members/supporters. Records from NSS members were obtained during the mass emergence of moths in 2014 using a customized Google Map (see https://goo.gl/maps/f4p5NkMhhB8QoZmTA, accessed on 7 February 2023) and were integrated with the larger database. All records were mapped and overlaid on a vegetation map of Singapore (courtesy: David Tan, National University of Singapore). Records with nonspecific location information, such as no GPS data or postal codes, were removed prior to analysis. The record locations were verified and further cleaned to exclude locations found in large water bodies, such as the Straits of Johor, or outside the borders of Singapore for analysis. Dates of sightings were categorized by month and plotted onto a graph to determine the seasonality of L. zampa observations.
2.2. Field Surveys during Mass Emergence
The majority of citizen science sightings during the mass emergence of L. zampa in 2014 came from urban areas in Singapore despite the high visitation to nature reserves, parks and nature areas in Singapore. To supplement this citizen science data, a range of natural habitats were surveyed using Pollard walks [21] in 2014 to estimate the abundance of L. zampa in these habitats in comparison to the urban areas.
A total of six vegetated patches, six urban parks and park connectors, and six urban areas widely spread around Singapore were visited (Figure 1). Each survey site was visited four times and once every week—twice during the observed peak emergence period between 13 and 26 July 2014 and twice during the postemergence period from 20 September to 12 October 2014.
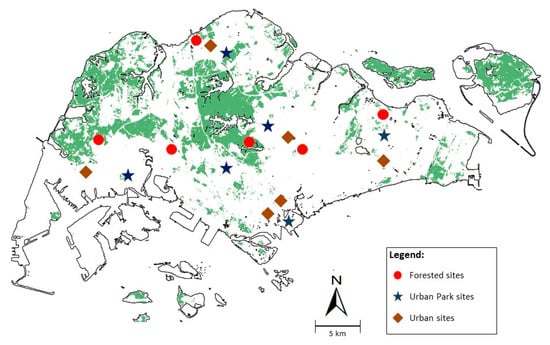
Figure 1.
Field survey locations across 6 forest, 6 urban park and 6 urban sites in 2014 spread across Singapore based around sites of high occurrence of L. zampa as recorded on Habitatnews and NSS. One 500 metres transect was established at each site. ( ) are forested survey sites at Admiralty Park forest trail, Bidadari Muslim section, Dairy Farm Pass, Jurong Eco Garden forest trail, Macritchie Nature Trail, and Pasir Ris Mangroves. (
) are forested survey sites at Admiralty Park forest trail, Bidadari Muslim section, Dairy Farm Pass, Jurong Eco Garden forest trail, Macritchie Nature Trail, and Pasir Ris Mangroves. ( ) are urban park survey sites including Bishan Park, Fort Canning Park, Gardens by the Bay, Jurong Central Park, Mandai-Tekong Park, and Tampines Eco Green. (
) are urban park survey sites including Bishan Park, Fort Canning Park, Gardens by the Bay, Jurong Central Park, Mandai-Tekong Park, and Tampines Eco Green. ( ) are urban survey sites including Arcade Mall, Bishan Stadium, Jurong West Stadium, Marina Bay Financial Centre, Raffles Place, Republic Polytechnic, and Tampines Swimming Complex.
) are urban survey sites including Arcade Mall, Bishan Stadium, Jurong West Stadium, Marina Bay Financial Centre, Raffles Place, Republic Polytechnic, and Tampines Swimming Complex.
 ) are forested survey sites at Admiralty Park forest trail, Bidadari Muslim section, Dairy Farm Pass, Jurong Eco Garden forest trail, Macritchie Nature Trail, and Pasir Ris Mangroves. (
) are forested survey sites at Admiralty Park forest trail, Bidadari Muslim section, Dairy Farm Pass, Jurong Eco Garden forest trail, Macritchie Nature Trail, and Pasir Ris Mangroves. ( ) are urban park survey sites including Bishan Park, Fort Canning Park, Gardens by the Bay, Jurong Central Park, Mandai-Tekong Park, and Tampines Eco Green. (
) are urban park survey sites including Bishan Park, Fort Canning Park, Gardens by the Bay, Jurong Central Park, Mandai-Tekong Park, and Tampines Eco Green. ( ) are urban survey sites including Arcade Mall, Bishan Stadium, Jurong West Stadium, Marina Bay Financial Centre, Raffles Place, Republic Polytechnic, and Tampines Swimming Complex.
) are urban survey sites including Arcade Mall, Bishan Stadium, Jurong West Stadium, Marina Bay Financial Centre, Raffles Place, Republic Polytechnic, and Tampines Swimming Complex.
2.3. Mark Recapture of L. zampa
Mark recapture was conducted on 59 live individuals of L. zampa between June and July 2014—31 individuals from Sime Forest (forested site), nine individuals from Bidadari (also a forested site), and 19 individuals from Raffles Place (urban site) were captured, marked, and released. The capture site was revisited for two consecutive days between 10:00 and 14:00.
L. zampa were captured using standard insect nets and marked on the underside and upper side of the wing using a fine point marker pen (STAEDTLER Lumocolor permanent pen 318); each individual was marked with a specific code and then released. This method is widely used in other lepidopteran studies and, when properly executed, does not damage individuals [22] or affect recapture rates. All marked individuals were photographed for future reference. Measurements of body length, body width, wing length, as well as date, time, and GPS locations, were recorded before the release of the moths. For recaptures, if the marked code could be easily read with the naked eye or using binoculars, the individual was not netted to avoid stressing the animal.
2.4. Specimens Collection and Measurement
Fifty-nine dead specimens and sixty-one live specimens of L. zampa were collected and measured from both urban and forested areas with the help of volunteers across Singapore between May 2014 and July 2014. A vernier calliper was used to measure body length, body width, and wing length of all specimens (Figure 2) using standard lepidopteran measurement protocols [6,23]. The specimens were divided into two batches depending on the month of data collection. Batch 1 spanned from 23 May to 9 June 2014, whereas Batch 2 spanned from 23 June to 31 July 2014.

Figure 2.
Lyssa zampa moths in forested and urban areas photographed during the mass emergence in 2014 across Singapore (photos by Anuj Jain). Several individual moths were seen attracted to city lights and eventually trapped in glass panels or light bulbs.
All dead specimens were photographed, numbered, and deposited in the Zoological Reference Collection at the Lee Kong Chian National History Museum, National University of Singapore.
2.5. Statistics
Significant differences in wing length, body length, and body width between sexes, batches, and locations were determined using the t-test or Wilcoxon signed rank test, depending on the normality of the datasets. The datasets were tested for normality using the Shapiro–Wilk test. The analysis was conducted using R Studio V1.2.1335.
3. Results
3.1. Seasonal Abundance of L. zampa
A total of 2547 L. zampa sightings were reported on Habitatnews, NSS and iNaturalist between January 2011 and December 2020. Of these, 2416 sightings (94.9%) were in 2014, and 2078 sightings (81.6%) were between April and July 2014 (Table 1). The peak emergence of L. zampa was identified to be in May 2014 (Table 2).

Table 1.
L. zampa sightings from 2011 to 2020 for the entire year (January–December) and during the peak emergence season (April–July).

Table 2.
The total number of sightings of L. zampa classified by months in 2014.
The field surveys in July 2014 were conducted during the end of the peak emergence period of L. zampa. Surveys in September and October 2014 were conducted during the post-peak emergence period of L. zampa. These surveys across 18 sites showed a total of 11 individuals of L. zampa (an initial high of nine L. zampa individuals in forested areas in week 1, i.e., 13–20 July 2014, and two individuals in the following week; Table 3). Sightings in urban areas totaled 10 individuals with an increase from 4 to 6 individuals in two weeks. Only one individual was seen in urban areas during the post-peak emergence period in September 2014 (Table 3).

Table 3.
Total number of L. zampa recorded through trail surveys across forested areas, urban parks, and urban areas.
3.2. Spatial Distribution of L. zampa in Singapore
We recorded the highest number of sightings in areas of high human density in the central business district and southeastern regions of Singapore, namely Tampines and Bedok (Figure 2 and Figure 3). Forested areas, such as the Central and Western Catchments, reflected few adult records, although mass emergence was documented here [5] (Figure 3). The majority of other green spaces also reported fewer than 30 sightings (Figure 3).
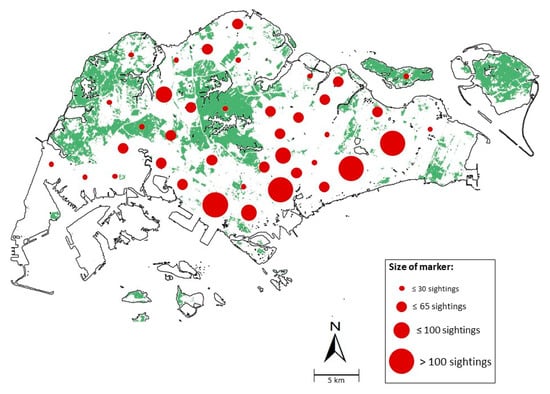
Figure 3.
Number of sightings of L. zampa recorded in Habitatnews, iNaturalist and NSS across Singapore from 2011 to 2020 with a map scale of 1:50,000. Sightings within the Urban Redevelopment Authority Planning Zones were clustered and represented as a circle. Zones of relatively high sightings include Tampines (105 sightings) and Bedok (107 sightings) in the east, Queenstown (165 sightings) in the central west and the central business district in southern Singapore (>300 sightings). The base vegetation map was obtained from David Tan and used with permission.
3.3. Morphological Variation between Sexes, Batches, and Locations
While the results from the mark-recapture study were inconclusive, as only one of 59 marked L. zampa was recaptured, the dead and live specimens captured during the study and submitted by other volunteers helped us do a morphometric analysis between wing length, body length, and body width (measured in cm).
Measurements showed significant differences in wing length and body width between L. zampa males (n = 86) and females (n = 34; Table 4). Females have significantly longer wing lengths (p < 0.001, W = 757) and wider body widths (p = 0.002, W = 295) than male L. zampa (Table 4). Body lengths between male and female L. zampa were not significantly different. Similarly, urban moths (n = 76) have significantly longer wing lengths (p < 0.001, t = 2.47) and smaller body widths (p < 0.001, W = 517) than forest moths (n = 42).

Table 4.
Wing length, body length, and body width variation between sexes, batches and habitats (forest, urban areas) in L. zampa. Body length was measured from the head to the end of the abdomen. Body width was measured at the thickest point of the specimen’s abdomen. Wing length was measured from the midpoint of the head to the tip of the most intact wing. ‘t’ value refers to t-test and was used when the datasets were normally distributed. ‘W’ value refers to Wilcoxon signed rank test and was used when the datasets were not normally distributed. Level of significance is marked with asterisk: *** p < 0.001, ** p < 0.01, * p < 0.05.
When comparing males only, urban males (n = 47) had marginally longer wing lengths (p =0.045, t = 2.05) and wider body widths (p < 0.001, W = 225) than forest males (n = 37). Despite the small sample size, when comparing females only, urban females (n = 29) had longer wing lengths (p = 0.008, W = 128) than forest females (n = 5).
Across batches, males of batch 1 (n = 27) had longer wing lengths (p = 0.006, W = 1092) than males of batch 2 (n = 59). Females of batch 1 (n= 21) also had longer wing lengths (p = 0.021, t = 2.47) than females of batch 2 (n = 13).
3.4. Relationship between L. zampa Abundance and Rainfall
A weak relationship was observed between L. zampa’s annual total abundance between 2011 and 2020 and rainfall (mm) in February (Pearson’s R = −0.27, p = 0.41, t = −0.86; Appendix A), as well as between L. zampa’s annual total abundance and number of rainy days in February (Pearson’s R = −0.43, p = 0.177, t = −1.47, Appendix A).
4. Discussion
Using field surveys, citizen science and expert datasets, we show the once-in-a-decade mass emergence of Lyssa zampa in Singapore. We also show that the highest number of L. zampa sightings were in areas of high human density, thus suggesting that this may have to do with moths being attracted to city lights.
4.1. Seasonal Abundance of L. zampa and Reasons behind Mass Emergence
Our observation of L. zampa’s peak emergence from May to August and that of the L. zampa’s annual total abundance between 2011 and 2020 was weakly related to rainfall (mm) in February, and the number of rainy days in February corroborates with the tropical literature in which insect populations tend to peak following dry spells [24,25,26]. February is usually the driest month in Singapore [27]. In 2014, the mass emergence of L. zampa in Singapore followed a record-long dry period with only 0.2 mm of rainfall in February—the driest month on record in Singapore since 1869 [27,28]. A minor peak emergence was observed in 2021 (estimated 200 records), which was preceded by a dry spell of only 1 mm of rainfall in February —the second lowest month on record since 1869 [29]. Back in 2010, there were anecdotal records of a minor peak emergence of L. zampa (but only eight records submitted by citizen scientists), and this was preceded by a dry spell in February 2010 with 6.3 mm of rainfall received in that month [30]. Beyond rainfall and number of rainy days in February, there may also be other factors that determine L. zampa abundance and which need further investigation.
Peak abundance in tropical areas contrasts with observed insect seasonality in temperate regions ([25,26,31,32,33], where insects tend to be more abundant during wet seasons [25,33].
Dry spells have, in part, been observed to induce mass flowering events [34], which is true of 2005, 2010, and 2014 [13,35]. Furthermore, studies by [26] suggest that fresher green leaves are produced following the falling of older leaves during the dry season. Fresh green leaves are more nutritious for larvae [26,33], and hence their presence would provide an ideal environment for the reproduction of L. zampa. The dry spells may thus have triggered the mass emergence of L. zampa.
We also hypothesize that L. zampa may be exploiting a window of opportunity to feed on its toxic larval host plant, E. diadenum, because plant defenses are lower during the dry spells [36]. However, a longer and more rigorous study may be needed to disentangle the effect of host plant toxicity and relate it to dry spells that prompted mass emergence.
4.2. Forests as Source, Urban Areas as Sinks
The only known host plant of L. zampa, E. diadenum, is a nationally threatened tree species limited to mature forests in Singapore (A. J. pers. comm. Ali Ibrahim; [37]). Our field surveys from July to October 2014 and subsequent consultation with botanists also confirmed the lack of host plant E. diadenum in urban areas and parks. During the mass emergence in 2014, the mass eclosion of thousands of L. zampa caterpillars from a cluster of host plant trees from the Sime Forest in central Singapore was documented [5]. This location is, by far, the only known breeding source of L. zampa in Singapore. The finding implied that all L. zampa originate from Singapore’s mature forests, where the host plants are present.
L. zampa is known to be nomadic and may migrate ‘out of forest’ in search of potential host plant clusters. Indeed, L. zampa host plants in Sime Forest were recorded to be nearly completely eaten away during the mass emergence in 2014 [5]. The many records of L. zampa in densely populated urban zones suggests that adult L. zampa was attracted out of the forest areas and into the brightly lit urban environment potentially in search of the larval host and/or nectar plants. Because urban areas do not have any L. zampa host plants, it is clear that these areas would act as population sinks, as the moths would not be able to find host plants to lay eggs and sustain their offspring.
Because light intensity and population density are shown to be positively correlated [38,39], this implies that the brightest zones in Singapore recorded the maximum number of L. zampa moths. However, we cannot discount that the higher reporting rates from areas of high human density may be somewhat influenced by the larger population of interested individuals there. A massive survey effort across the island would be needed to correct for potential observer bias in such citizen science-based datasets.
L. zampa have been recorded being attracted to light traps during nocturnal field surveys in Singapore [2]. L. zampa has also been recorded entering lit houses at night and getting trapped in building facades or bulbs. Other Uranid moths, such as Urania fulgens (a species phylogenetically related to L. zampa), are also known to be attracted to light [40].
4.3. Morphological Variations
A closer comparison of the distribution data and morphometric measurements of L. zampa underscores variation between wing lengths of moths found between sexes, habitats, and batches.
4.3.1. Females Larger and Heavier Than Males
Our observation of L. zampa females being larger (longer wing length) and heavier (wider bodies but similar body length) than males corroborates with the literature in which larger females are observed in many lepidoptera [41,42,43,44,45]. These morphological differences can be attributed to the sexual selection theory in which larger females have extra energy for egg production, thereby offering them a fecundity advantage [41,43,46].
4.3.2. Urban Moths Are Longer and Lighter Than Forest Counterparts
Urban L. zampa individuals have significantly longer wing lengths and lighter bodies (smaller body widths) than forest individuals, suggesting a greater power of flight in urban individuals. This is also true for male L. zampa when compared between urban and forest areas. Moths with longer and larger wings tend to be better and stronger fliers [47]. This finding suggests that larger and stronger flying L. zampa migrate ‘out of forest’ and become trapped in urban areas, leaving smaller-sized and presumably less fecund individuals in forests. This could result in population level implications for the moths in just a few generations.
4.3.3. Smaller Moths over Time
Our observation that wing length reduced with time (i.e., first batch > second batch) can be attributed to an increase in host plant defense following the emergence of the first batch of L. zampa in early June 2014. Plants are known to protect themselves against larvae through chemical defenses (i.e., secretion of deterrent secondary plant compounds) against predation [48]. Alkaloids, cyanogenic glycosides, terpenoids, and phenolics employed in chemical defense have been shown to increase mortality rate and decrease fecundity of insects [49,50,51,52]. In another study, the larvae of Urania fulgens (a species related to L. zampa), when fed with leaves (formerly grazed by livestock), had a significantly slower growth rate and higher mortality [49]. Due to the high costs of toxin production, such defenses against predators are generally not long running and are triggered only by herbivory [53]. It is likely that chemical defenses by E. diadenum were triggered after the emergence of the first batch of moths, eventually resulting in smaller moths in subsequent batches.
Another explanation for the difference in moth size between the two batches may be due to food availability. The reduced availability of fresh leaves on the host plant after the emergence of the first batch of individuals may have resulted in smaller L. zampa during the second batch of emergence. The authors of [54,55] reported a decrease in wing length of monarch butterflies, Danaus plexippus, when larvae were starved of food.
4.4. Conservation Implications
It is clear that urban areas in Singapore are acting as sinks for L. zampa and drawing populations of the fitter moths (i.e., larger and better-flying individuals) from the forests. The majority of L. zampa individuals that are attracted to urban areas would not be able to find the host plants and lay eggs and complete their life cycle. The impacts of climate change on L. zampa populations seem like a two-fold problem. As dry spells are likely to become longer and more intense due to climate change in the future [56], it may mean more frequent mass emergences leading to more mortality of L. zampa in urban areas. With fitter individuals drawn to urban areas during mass emergence events, it may mean a degraded gene pool in a possibly declining population with every such event. This is over and above the fact that insects are prone to higher extinction risk as a result of climate-mediated shifts in temperature fluctuations [56].
Urgent steps may be needed to mitigate the impact of urban population sinks for this charismatic species. An immediate first step could be to plant the host plant of L. zampa in urban parks adjoining forested habitats and selected streetscapes of Singapore to provide adequate host plant resources at the time of the next mass emergence. Such larval host plantings in urban parks and gardens have been shown to increase populations for another charismatic and threatened Lepidopteran species, Pachliopta aristolochiae, in Singapore [57]. Another useful area of research could be to examine the larval host plant preference of L. zampa under laboratory conditions and identify if it may use other plant species as hosts that could be planted more widely across landscapes in Singapore.
Past records show flowers of the Tembusu tree (Cyrtophyllum fragrans) as the only recorded nectar source for L. zampa in Singapore. More research should be done to document the diversity of nectar plant preferences for L. zampa and to determine if other nectar plant species could be planted for L. zampa in urban areas. Whilst our mark-recapture study failed to get enough recaptures, a longer and more intensive study may be needed to document the trajectory of the movement of L. zampa individuals from ‘out of forest’ to urban areas. Reducing urban lights by introducing appropriate lighting solutions should also be considered along migratory/flight paths of moths to mitigate the impact of urban areas as sinks.
In parallel, awareness-raising efforts about this species’ unique ecology and interaction with urban areas should continue as these efforts form the bedrock of citizen science monitoring and research. Such initiatives will ensure that members of the public and experts continue to be interested and share L. zampa sightings across Singapore and take adequate conservation actions.
Author Contributions
Conceptualization, A.J. and N.S.; methodology, A.J. and L.Y.N.; software, A.J.; validation, A.J., L.Y.N. and N.S.; formal analysis, A.J. and L.Y.N.; investigation, A.J., L.Y.N. and N.S.; resources, A.J., and N.S.; data curation, A.J. and L.Y.N.; writing—original draft preparation, A.J. and L.Y.N.; writing—review and editing, A.J. and L.Y.N.; visualization, A.J. and L.Y.N.; supervision, A.J. and N.S.; project administration, A.J. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank members of public and contributors to Habitatnews, Nature Society (Singapore)’s Google Map database. Special thanks to Gan Cheong Weei with data collection efforts. This research was carried out under the NParks permit number NP/RP14-067 and Singapore Land Authority’s permit SLA/PPD/263.3.10-V5.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
The amount of rainfall and the number of rainy days in February across Singapore from 2011 to 2020. Source: Available online: www.data.govt.sg (accessed on 7 February 2023).
| Year | Feb_Rainfall_mm | Feb_Number_Rain_Days |
|---|---|---|
| 2011 | 23 | 8 |
| 2012 | 83.6 | 11 |
| 2013 | 395.2 | 22 |
| 2014 | 0.2 | 1 |
| 2015 | 18.8 | 6 |
| 2016 | 186 | 10 |
| 2017 | 158.4 | 15 |
| 2018 | 14.8 | 5 |
| 2019 | 31.6 | 5 |
| 2020 | 65 | 8 |
References
- Murphy, D.H. The natural history of insect herbivory on mangroves trees in and near Singapore. Raffles Bull. Zool. 1990, 38, 119–203. [Google Scholar]
- Karam, R.; Chong, J.H. Moths of Bukit Timah Nature Reserve, Singapore. Gard. Bull. Singap. 2019, 71 (Suppl. 1), 317–330. [Google Scholar] [CrossRef]
- Ee, D. Five Things You Should Know about the Lyssa zampa Moth. The Straits Times. 21 May 2014. Available online: http://www.straitstimes.com/news/singapore/more-singapore-stories/story/five-things-you-should-know-about-the-lyssa-zampa-moth-2 (accessed on 7 February 2023).
- Kwara, M. Moth Invasion in Singapore. Yahoo News. Available online: https://sg.news.yahoo.com/blogs/what-is-buzzing/moth-invasion-in-singapore-051923140.html (accessed on 7 February 2023).
- Jain, A.; Tea, Y.-K. Mass emergence of the tropical swallowtail moth Lyssa zampa (Lepidoptera: Uraniidae: Uraniinae) in Singapore, with notes on its partial life history. Trop. Lepid. Res. 2020, 30, 20–27. [Google Scholar]
- van Regteren Altena, C.O. A Revision of the Genus Nyctalemon Dalman (Lepidoptera: Uraniidae) with Notes on the Biology, Distribution, and Evolution of Its Species; Brill: Leiden, The Netherlands, 1953. [Google Scholar]
- Holloway, J.D. The biogeographical analysis of a transect sample of the moth fauna of Mt. Kinabalu, Sabah, using numerical methods. Biol. J. Linn. Soc. 1970, 2, 259–286. [Google Scholar] [CrossRef]
- Heppner, J.B.; Wang, H.Y. Lyssa zampa in Taiwan. Trop. Lepid. 1996, 7, 146. [Google Scholar]
- Tokeshi, M.; Yoko-o, M. New record of the tropical swallowtail moth Lyssa zampa (Butler)(Lepidoptera: Uraniidae) from mainland Japan. Entomol. Sci. 2007, 10, 103–106. [Google Scholar] [CrossRef]
- Wee, B. The Atlas Moth (Attacus atlas). WETlands 6(1). 1999. Available online: http://www.sbwr.org.sg/wetlands/text/99-6-1-7.htm (accessed on 7 February 2023).
- Yen, S.H.; Yu, H.S.; Mu, J.H.; Tan, H.J. On Lyssa zampa (Butler, 1867) (Uraniidae) from Taiwan. Jpn. Heterocerists J. 1995, 186, 173–175. [Google Scholar]
- Leong, T.M. Metamorphosis of the Swallowtail Moth Lyssa zampa; Singapore Biodiversity Records: Singapore, 2014; pp. 158–159. [Google Scholar]
- Sivasothi, N. It’s the Season for Lyssa zampa, the Large, Nocturnal, White-Striped Moth. Habitatnews. 27 May 2005. Available online: http://habitatnews.nus.edu.sg/index.php?entry=/nature/20050527-lyssa_zampa.txt (accessed on 7 February 2023).
- Sivasothi, N. Have you Seen Lyssa zampa Recently? Otterman Speaks. 21 May 2009. Available online: https://otterman.wordpress.com/2009/05/21/have-you-seen-lyzza-zampa-recently/ (accessed on 7 February 2023).
- Sivasothi, N. The Hunt for Lyssa zampa. Otterman Speaks. 10 June 2010. Available online: https://otterman.wordpress.com/2010/06/10/the-hunt-for-lyssa-zampa/ (accessed on 7 February 2023).
- Sivasothi, N. Is Lyssa zampa Back? Send Me Your Records! Otterman Speaks. 1 May 2013. Available online: https://otterman.wordpress.com/2013/05/01/is-lyssa-zampa-back-send-me-your-records/ (accessed on 7 February 2023).
- Sivasothi, N. Will Lyssa zampa, the Tropical Swallowtail Moth, Make a Big Appearance This Year? Otterman Speaks. 14 April 2014. Available online: https://otterman.wordpress.com/2014/04/14/will-lyssa-zampa-the-tropical-swallowtail-moth-make-a-big-appearance-this-year/ (accessed on 7 February 2023).
- Mrbrown. The Myth of the Moth…Dude. 2014. Available online: http://www.mrbrown.com/blog/2014/05/the-myth-of-the-mothdude.html (accessed on 7 February 2023).
- Chen, H. Malaysia Swarmed by Giant Moths. BBC News. 11 June 2014. Available online: https://www.bbc.com/news/world-asia-27758640 (accessed on 7 February 2023).
- Wiener-Bronner, D. Giant Moths Are Swarming All Over Malaysia. The Wire. 11 June 2014. Available online: http://www.thewire.com/global/2014/06/giant-moths-malaysia/372557/ (accessed on 7 February 2023).
- Pollard, E.; Yates, T.J. Monitoring Butterflies for Ecology and Conservation: The British Butterfly Monitoring Scheme; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Ehrlich, P.R.; Davidson, S.E. Techniques for Capture-Recapture Studies of Lepidoptera Populations. J. Lepid. Soc. 1960, 14, 227–229. [Google Scholar]
- van Hook, T.; Williams, E.H.; Brower, L.P.; Borkin, S.; Hein, J. A Standardized Protocol for Ruler-based Measurement of Wing Length in Monarch Butterflies Danaus plexippus L. (Nymphalidae, Danainae). Trop. Lepid. Res. 2012, 22, 42–52. [Google Scholar]
- Gibbs, D.G.; Leston, D. Insect phenology in a forest cocoa-farm locality in West Africa. J. Appl. Ecol. 1970, 7, 519–548. [Google Scholar] [CrossRef]
- Wolda, H. Fluctuations in Abundance of Tropical Insects. Am. Nat. 1978, 112, 1017–1045. [Google Scholar] [CrossRef]
- Wolda, H. Seasonal Fluctuations in Rainfall, Food and Abundance of Tropical Insects. J. Anim. Ecol. 1978, 47, 369–381. [Google Scholar] [CrossRef]
- National Environment Agency. Advisories: Dry Spell Advisory (4 Mar); National Environment Agency: Singapore, 2014. Available online: http://app2.nea.gov.sg/corporate-functions/newsroom/advisories/dry-spell-advisory-(4-mar) (accessed on 7 February 2023).
- Ee, D. February Was the Driest Month in Singapore Since 1869, Says NEA. Straits Times. 4 March 2014. Available online: https://www.straitstimes.com/singapore/february-was-the-driest-month-in-singapore-since-1869-says-nea (accessed on 7 February 2023).
- Samanta, D.; Horton, B.P. Commentary: 2021 Has Already Seen the Wettest and Driest Months in Decades: Is Singapore Prepared for More? Channel News Asia. 15 March 2021. Available online: https://www.channelnewsasia.com/news/commentary/weather-january-february-monsoon-rain-dry-climate-change-mss-nea-14391482 (accessed on 7 February 2023).
- National Environment Agency. Annual Weather Review 2010; National Environment Agency: Singapore, 2011. [Google Scholar]
- Owen, D.F. Species diversity and seasonal abundance in tropical Sphingidae (Lepidoptera). Proc. R. Entomol. Soc. London. Ser. A Gen. Entomol. 1969, 44, 162–168. [Google Scholar] [CrossRef]
- Wolda, H. Insect Seasonality: Why? Ann. Rev. Ecol. Syst. 1988, 19, 1–18. [Google Scholar] [CrossRef]
- Tanaka, L.K.; Tanaka, S.K. Rainfall and Seasonal Changes in Arthropod Abundance on a Tropical Oceanic Island. Biotropica 1982, 14, 114–123. [Google Scholar] [CrossRef]
- Sakai, S.; Harrison, R.D.; Momose, K.; Kuraji, K.; Nagamasu, H.; Yasunari, T.; Chong, L.; Nakashizuka, T. Irregular droughts trigger mass flowering in aseasonal tropical forests in Asia. Am. J. Bot. 2006, 93, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Today. Dry Spell, Rain Could Have Led to Mass Flowering. 2014. Available online: http://www.todayonline.com/singapore/dry-spell-rain-could-have-led-mass-flowering (accessed on 7 February 2023).
- Walter, J.; Hein, R.; Auge, H.; Beierkuhnlein, C.; Loffler, S.; Reifenrath, K.; Schadler, M.; Weber, M.; Jentsch, A. How do extreme drought and plant community composition affect host plant metabolites and herbivore performance? Arthropod-Plant Interact. 2011, 6, 15–25. [Google Scholar] [CrossRef]
- Chong, K.Y.; Tan, H.T.W.; Corlett, R.T. A Checklist of the Total Vascular Plant Flora of Singapore; Raffles Museum of Biodiversity Research, National University of Singapore: Singapore, 2009. [Google Scholar]
- Department of Statistics Singapore. Geographic Distribution of the Singapore Resident Population; Statistics Singapore Newsletter: Singapore, 2010. [Google Scholar]
- Liu, Q.; Sutton, P.C.; Elvidge, C.D. Relationships between Nighttime Imagery and PopulationDensity for Hong Kong. Proc. Asia-Pac. Adv. Netw. 2011, 31, 79–90. [Google Scholar]
- Calhoun, J.V. Massing of Urania fulgens at Lights in Belize (Lepidoptera: Uraniidae). Trop. Lepid. 2004, 12, 43–44. [Google Scholar]
- Begum, S.; Tsukuda, R.; Fujisaki, K.; Nakasuji, F. The Effects of Wild Cruciferous Host Plants on Morphology, Reproductive Performance and Flight Activity in the Diamondback Moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Res. Popul. Ecol. 1996, 38, 257–263. [Google Scholar] [CrossRef]
- Tammaru, T.; Esperk, T.; Castellanos, I. No evidence for costs of being large in females of Orgyia spp. (Lepidoptera, Lymantriidae): Larger is always better. Oecologia 2002, 133, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Teder, T.; Tammaru, T. Sexual size dimorphism within species increases with body size in insects. OIKOS 2005, 108, 321–334. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Blanckenhorn, W.U.; Teder, T.; Davidowitz, G.; Fox, C.W. Sex Differences in Phenotypic Plasticity Affect Variation in Sexual Size Dimorphism in Insects: From Physiology to Evolution. Annu. Rev. Entomol. 2009, 55, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.E.; Zwaan, B.J.; Brakefield, P.M. Evolution of Sexual Dimorphism in the Lepidoptera. Annu. Rev. Entomol. 2011, 56, 445–464. [Google Scholar] [CrossRef]
- Preziosi, R.F.; Fairbairn, D.J. Lifetime Selection on Adult Body Size and Components of Body Size in a Waterstrider: Opposing Selection and Maintenance of Sexual Size Dimorphism. Evolution 2000, 54, 558–566. [Google Scholar]
- Pinheiro, C.E.G. Palatablility and escaping ability in Neotropical butterflies: Tests with wild kingbirds (Tyrannus melancholicus, Tyrannidae). Biol. J. Linn. Soc. 1996, 59, 351–365. [Google Scholar] [CrossRef]
- Young, M. Plant defence against Larvae. In The Natural History of Moths; T&AD Poyser Ltd.: London, UK, 1997; pp. 133–149. [Google Scholar]
- Smith, N.G. Host plant toxicity and migration in the dayflying moth, Urania fulgens. Fla. Entomol. 1983, 66, 76–85. [Google Scholar] [CrossRef]
- Kite, G.C.; Fellows, L.E.; Lees, D.C.; Kitchen, D.; Monteith, G.B. Alkaloidal Glycosidase Inhibitors in Nocturnal and Diurnal Uraniine Moths and their Respective Foodplant Genera, Endospermum and Omphalea. Biochem. Syst. Ecol. 1991, 19, 441–445. [Google Scholar] [CrossRef]
- Scoble, M.J. Environmental and Ecological Importance of Lepidoptera. In The Lepidoptera: Form, Function, and Diversity; Oxford University Press: Oxford, UK, 1992; pp. 170–184. [Google Scholar]
- Awmack, C.S.; Leather, S.R. Host Plant Quality and Fecundity in Herbivorous Insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Agrawal, A.A. Induced Responses to Herbivory and Increased Plant Performance. Science 1998, 279, 1201–1202. [Google Scholar] [CrossRef]
- Johnson, H.; Solensky, M.J.; Satterfield, D.A.; Davis, A.K. Does Skipping a Meal Matter to a Butterfly’s Appearance? Effects of Larval Food Stress on Wing Morphology and Color in Monarch Butterflies. PLoS ONE 2014, 9, e93492. [Google Scholar] [CrossRef] [PubMed]
- Becker, C. A Possible Size-Determined Directional Selection in Danaus plexippus (Lepidoptera: Danaidae) with Evidence from Stable Isotope Analysis; University of Kansas: Lawrence, KS, USA, 2008. [Google Scholar]
- Duffy, K.; Gouhier, T.C.; Ganguly, A.R. Climate-mediated shifts in temperature fluctuations promote extinction risk. Nat. Clim. Chang. 2022, 12, 1037–1044. [Google Scholar] [CrossRef]
- Jain, A.; Zeng, Y.; Webb, E.L. Critical dependence of butterflies on a non-native host plant in the urban tropics. Front. Ecol. Evol. 2021, 9, 655012. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).