Abstract
Tens of thousands of species are at risk of extinction globally. In many ecosystems, species declines are associated with deforestation. However, forest degradation also can profoundly affect biodiversity. I present a detailed case study of species declines associated with forest degradation in southeastern Australia’s montane ash (Eucalyptus spp.) forests. The case study is based on ~40 years of long-term monitoring focused on declines (and potential extinction trajectories) of arboreal marsupials and birds, with a particular emphasis on key drivers, especially logging, wildfire, habitat loss, climate change, and interactions among these drivers. I discuss policy failures contributing to species declines, including ongoing logging of high-conservation-value forests, poor regulation of forest management, and inadequate design of reserves. I conclude with general lessons for better conservation and forest management efforts aimed at reducing forest degradation and loss of ecosystem integrity. I contend that ongoing logging in already highly degraded montane ash forests is inconsistent with the Australian government’s commitment at the Glasgow COP26 meeting in 2021 on halting forest degradation. Similarly, the Australian Government has committed to preventing further extinctions in Australia, yet its current support for ongoing logging in montane ash forests through federal–state legislation will likely promote extinctions for some species. The inherent conflicts and contradictions between conservation and logging policies need to be addressed.
1. Introduction
Over 40,000 animal and plant species worldwide have been listed by the International Union for the Conservation of Nature (IUCN) as being threatened with extinction [1]. This comprises 28% of all species assessed, a very substantial increase from the 11,046 species reported as threatened in 2000 [1]. The average abundance of native species in most major terrestrial biomes has fallen by at least 20 percent, with human activities threatening more species with extinction now than ever before [2]. These sobering global patterns are reflected in the population trajectories of species in many nations around the world [3]. For example, at a continental level, Australia has lost almost 10% of its mammal fauna since European colonization [4] with three species of vertebrates (two mammals and one reptile) lost in the past decade alone [5]. Sometimes the direct cause of decline and/or extinction of a particular species is poorly known, which makes it difficult to identify and implement effective conservation measures. However, in other cases, long-term research and monitoring can uncover the key drivers of decline and extinction risk and in turn guide effective recovery actions [6,7].
In this paper, using insights from an array of observational studies and experiments [8] conducted over the past 40 years (see Table 1), I provide a detailed case study of species decline and erosion of ecosystem conditions in the tall, wet montane ash (Eucalyptus spp.) forests of the Central Highlands of Victoria. These forests support stands of the tallest flowering plants on earth, with old-growth trees reaching heights of ~90–100 m [9] (Figure 1). Given the majority of the forest on which I focus is subject to logging followed by stand regeneration, this case study relates to the drivers of species decline and the erosion of ecosystem conditions linked to forest degradation rather than deforestation. For the purposes of this paper, the term degradation is drawn from general definitions of the concept provided by Thompson et al. [10]. These definitions relate to altered forest stand structure, forest composition, ecological dynamics, and ecosystem function relative to a benchmark condition, with such changes arising primarily from human activities such as logging [11,12]. Notably, Australia is a signatory to the Glasgow Leaders Declaration on Forest and Land Use [13], which aims to halt the degradation of natural forests. I focus on the declines (and potential extinction trajectories) of arboreal marsupials and birds, with a particular emphasis on key drivers of those declines, especially logging, wildfire, habitat loss, climate change, and interactions among these drivers. I then discuss some of the policy failures contributing to species declines, including ongoing logging of high-conservation-value forests, poor regulation of forest management, and inadequate design of forest reserves. I provide recommendations for action to limit biodiversity decline in montane ash forests. I conclude with some general lessons for better conservation and forest management efforts aimed at reducing forest degradation linked to species decline and loss of ecosystem integrity.

Table 1.
Datasets collected or assembled and used in long-term studies in the montane ash forests of the Central Highlands of Victoria.

Figure 1.
Stand of old-growth mountain ash forest in the closed O’Shannassy Water Catchment in the Central Highlands of Victoria. The dominant overstory trees in this image are approximately 85 m tall. (Photo by Esther Beaton.)
2. Study Region and Disturbance Regimes
The Central Highlands region [35] is located approximately 100 km northeast of the city of Melbourne in the state of Victoria, southeastern Australia (Figure 2). The montane ash forests in this region are dominated by largely monotypic stands of one of three species of eucalypt trees—mountain ash (Eucalyptus regnans) (F. von Mueller), alpine ash (Eucalyptus delegatensis) (R.T. Baker), or shining gum (Eucalyptus nitens) (Deane and Maiden). The natural disturbance regime in these forests is comparatively rare, high-severity, stand-replacing wildfire, with the natural fire regime being a fire occurring once every ~75–150 years [36], which can occur over tens of thousands of hectares. However, major wildfires have become more frequent over the past century and have occurred in 1926, 1932, 1939, 1983, and 2009. For the purposes of various studies in montane ash forests, the fire severity is defined as the extent of loss or consumption of the vegetation and other biomass as a result of fire, and it can be determined from satellite data [23] or from on-the-ground surveys [37]. High-severity fires in montane ash forests are those conflagrations where there is a crown burn and much of the living vegetation is consumed [23].
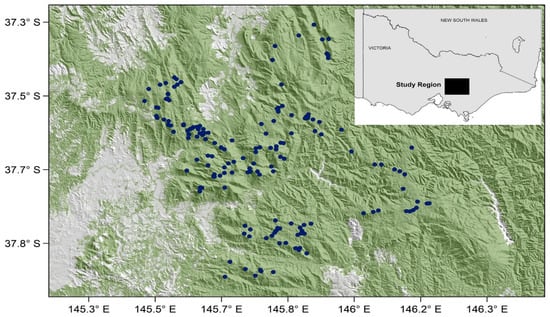
Figure 2.
The location of the montane ash forests in the Central Highlands of Victoria, showing long-term field sites in the region where arboreal marsupials, birds, and vegetation structure and composition have been monitored since mid-1983 [38] (see Table 1 for further description of relevant datasets).
The primary form of human disturbance in montane ash forests is high-intensity clear-cutting operations conducted on a 40–80 year rotation [39] (Figure 3). Cutblocks are typically 15–40 ha in size, with up to three contiguous adjacent logged areas able to be cut, summing to a maximum of 120 ha of harvested area [40]. Approximately 50% of the montane ash forest in the Central Highlands of Victoria has been clear-cut in the past 50 years [33]. In the event of wildfire, burnt stands of montane ash forest are typically subject to post-fire (“salvage”) logging [28]. Importantly, the forests in the Central Highlands of Victoria are owned largely by the Government of Victoria, with the majority of the forest estate (~80%) available for logging. Following logging, cutblocks are then subject to assisted regeneration practices (primarily through aerial seeding of overstory trees) [32].

Figure 3.
Clear-cut area in the montane ash forest of the Central Highlands of Victoria. (Photo by Chris Taylor.) This image shows an area that was formerly dominated by alpine ash forest.
2.1. Long-Term Biodiversity Monitoring in Montane Ash Forests
Montane ash forests have been the target of intensive animal and plant monitoring since July 1983 [41] (see Table 1). Much of this work is focused around monitoring on more than 180 long-term field sites where surveys of arboreal marsupials, birds, vegetation structure, and plant species composition are completed regularly (typically annually) [15,16,22]. These monitoring sites span forests in wood production forests and protected areas, enabling the analyses of the tenure differences in animal species occurrence, vegetation structure, and plant species composition.
Surveys of arboreal marsupials have focused on a suite of species including the critically endangered Leadbeater’s possum (Gymnobelideus leadbeateri) (McCoy), the endangered southern greater glider (Petauroides volans) (McGregor), the yellow-bellied glider (Petaurus australis) (Shaw), Krefft’s glider (Petaurus notatus) (Cremona), the mountain brushtail possum (Trichosurus cunninghami) (Lindenmayer), the common ringtail possum (Pseudocheirus peregrinus) (Boddaert), and the feathertail glider (Acrobates pygmaeus) (Shaw). The majority of these species are cavity dependent, and their occurrence in montane ash forests is strongly associated with the abundance of large, old, hollow-bearing trees [15].
Surveys of birds on the long-term monitoring sites show that the bird assemblage in montane ash forests is comparatively species rich, comprising ~70 species from a wide range of guilds and functional groups [42]. The assemblage includes a number of species of conservation concern such as the crested shrike-tit (Falcunculus frontatus) (Latham), the flame robin (Petroica phoenicea) (Gould), and the gang-gang cockatoo (Callocephalon fimbriatum) (Lesson). Most species are strongly associated with old-growth stands [16], with the flame robin being the only early successional specialist in montane ash forests [43]. Notably, all of these bird species occur outside of montane ash forests and beyond the Central Highlands region.
Detailed vegetation surveys at the long-term sites in the montane ash forests include measurements of the condition of large, old, hollow-bearing trees (which are key nest sites for cavity-dependent fauna), the prevalence of understory trees (e.g., stocking rate and projective foliage cover) such as Acacia spp. trees, and elements of cool temperate rainforest such as Nothofagus cunninghamii (Hook.) [44], in addition to the abundance of an array of shrubs and other plants (e.g., tree ferns) [31]. There is a suite of rare and threatened plant species in montane ash forests, including shiny nematolepis (Nematolepis wilsonii) (Walsh & Albr.), tree geebung (Persoonia arborea) (F. von Mueller), and tall astelia (Astelia australiana) (Willis) [45].
2.2. Additional Supporting Datasets
A range of spatiotemporal datasets (see Table 1) have been assembled, such as those on the timing and extent of wildfires and logging, as well as datasets on environmental attributes such as slope, aspect, rainfall, and temperature. These data have been used primarily as covariates in statistical analyses of biodiversity responses. The logging and fire information are publicly available datasets extracted from satellite imagery, some of which have been ground-truthed with on-the-ground inventory surveys [33].
3. General Methodology
The general approach to the long-term studies in montane ash forests has been to use statistical modelling to relate a particular set of potential explanatory variables to response variables typically collected in detailed field surveys such as the presence/absence of a given species of arboreal marsupial, site occupancy by particular species of birds, or the occurrence or foliage cover of a particular plant species. The data on the explanatory variables were gathered in field surveys or extracted from government databases (e.g., slope, aspect, time since the last fire, and time since the last logging event).
All statistical analyses of the factors influencing key response variables were conducted under the supervision of, or in close collaboration with, a highly experienced professional statistical scientist. The results of the analyses have been written up in more than 150 peer-reviewed scientific articles, and they form the empirical basis for the summary presentation in this paper on species and ecosystem decline.
4. Evidence of Species and Ecosystem Decline
Time series data from long-term field sites (see Table 1) provide compelling evidence for the declines of a number of key species in montane ash forests [15]. For example, the levels of site occupancy of Leadbeater’s possum declined by ~50% between 1997 and 2020 [15]. The site occupancy of the southern greater glider has declined by ~80% over the same period [15]. The yellow-bellied glider has become too rare to conduct analyses of the species’ temporal trajectory.
There is evidence from the time series data that while some bird species are exhibiting recovery following the most recent major wildfires in 2009 [17], a number of species are undergoing a marked decline in site occupancy. Example species include the crested shrike-tit and the red-browed treecreeper (Climacteris erythrops). The early successional specialist, the flame robin, has become extremely abundant on sites burnt in the 2009 fires and continues to exhibit high levels of site occupancy in the 12+ years post-fire [17].
Beyond the marked declines in a number of mammal and bird species, a formal analysis using the IUCN Red Listed Ecosystem process revealed that the mountain ash ecosystem per se is at high risk of collapse [46] and has been classified as critically endangered [47]. The risk of ecosystem collapse is linked with regeneration failure associated with young eucalypt trees being burnt and killed by successive wildfires occurring in stands too young to be sexually mature [48,49,50]. Ecosystem collapse would manifest as the eucalypt-dominated forest being replaced by Acacia spp. woodland [32].
5. Drivers of Decline
Repeated analyses of our long-term datasets show there are a number of important drivers of species, habitat, and ecosystem decline, several of which act at multiple spatial scales (from the individual tree level to stand and landscape levels). These drivers are wildfire, logging, habitat fragmentation resulting from logging, loss of old-growth resulting from fire and past logging, loss of hollow-bearing trees through natural attrition and logging and wildfire, and climate change. I briefly outline the effects of these drivers of decline below. These drivers do not act in isolation but interact to further reinforce the loss of biodiversity, habitat degradation, and erosion of ecosystem integrity [51]. I have therefore also provided some commentary on the interactions among the drivers of species decline.
5.1. Fire
Fire is a natural ecological process in montane ash forests [48]. Indeed, mountain ash and alpine ash stands rely on periodic wildfires for natural regeneration [49,52]. However, repeated large-scale wildfires can have marked negative effects both on key elements of biodiversity and on ecosystem integrity. The effects of fire as a driver of species decline can manifest at several spatial scales. For example, at a stand level, fire can have direct impacts through killing animals on a burned site [53] or indirect impacts through reducing the abundance of habitat attributes such as large, old, hollow-bearing trees [54]. At a landscape scale, species such as Leadbeater’s possum, the southern greater glider, and the yellow-bellied glider are significantly less likely to occur where there has been widespread fire relative to areas where fire has been less extensive [15,37,55]. Importantly, there are high levels of spatial dependence in wildfire in montane ash forests; that is, areas next to highly flammable young forests are at risk of burning in the same fire event (Figure 4). This can affect, for example, remnant patches of old-growth forest that are adjacent to young forest (see Figure 4). Finally, at a whole-of-ecosystem level, repeated, high-severity fires with short inter-fire intervals may result in widespread regeneration failure and thereby trigger the collapse of the entire mountain ash ecosystem [50] and the alpine ash ecosystem [49]. Ecosystem collapse would have major implications for those species strongly associated with such environments (e.g., Leadbeater’s possum) [56].

Figure 4.
Burnt stand of old-growth mountain ash forest adjacent to burnt area of flammable young regrowth mountain ash forest. (Photo by David Lindenmayer.)
5.2. Logging
Logging can act as a driver of species decline at several spatial scales. At a site level, areas with a history of logging are characterized by fewer hollow-bearing trees [18], and this in turn leads to such places being unsuitable habitat for a range of cavity-dependent species [29]. Notably, areas of high conservation value for threatened biodiversity in montane ash forests are also those places often scheduled for logging under the Victorian Government’s Timber Release Plan [25]. Past logging operations dating back over 120 years have removed extensive areas of old-growth montane ash forest, and this has had negative impacts on a range of old-growth-associated taxa such as the southern greater glider [29] and the yellow-bellied glider [55,57]. At a landscape scale, the levels of site occupancy in species such as Leadbeater’s possum and the yellow-bellied glider are significantly lower with increasing amounts of logging in the landscape [15,55]. Similarly, increasing amounts of logging at a landscape scale results in increased rates of collapse of large, old, hollow-bearing trees, including in remaining areas of adjacent uncut forest [20].
5.3. Forest Fragmentation
Montane ash forests are highly fragmentated as a result of past logging and wildfires (see Figure 5). For example, the distance from a randomly selected point to a disturbance boundary (e.g., a road or logged cutblock) is just 71 m in wood production forests (versus > 1700 m in protected forests) [33]. Fragmentation has a number of important ramifications for biodiversity conservation and ecosystem conditions in montane ash forests. First, fragmentation is detrimental to area-sensitive taxa such as the yellow-bellied glider, which requires extensive areas of intact old-growth forest [57]. Second, fragmentation creates edge effects such as increased wind speeds that elevate the rates of collapse of large old trees [20] (which are in turn key elements of habitat for cavity-dependent species). Third, fragmented forests dominated by extensive areas of young, highly flammable forest are at risk of supporting high-severity fires that burn across large areas of the landscape [50], killing animals, damaging habitats, and reducing the extent of old-growth forest.
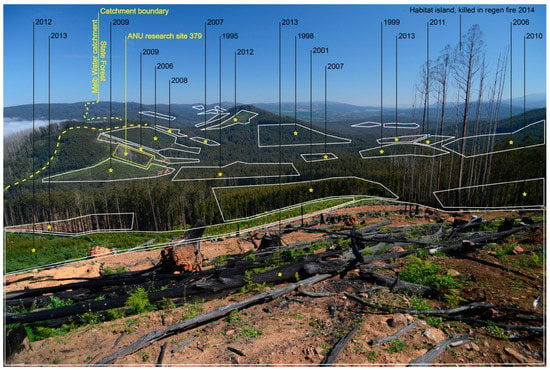
Figure 5.
The location of cutblocks (shown in white polygons and labelled by the year of cutting) highlighting the extent of fragmentation in a montane ash forest landscape in the Central Highlands of Victoria. One of the long-term sites (site #379) is shown by a yellow-bounded polygon in the central left of the image. (Photo by David Blair.)
5.4. Loss of Hollow-Bearing Trees and Old-Growth Forest
Large, old, hollow-bearing trees are critical habitat attributes for a wide range of cavity-dependent fauna, and their abundance is a significant explanatory variable in habitat suitability models for almost all species of arboreal marsupials in montane ash forests [15,58]. The abundance of these trees is declining rapidly, with ~50% of long-term sites that supported such trees in 1997 no longer having any hollow-bearing trees almost 25 years later [15]. Populations of hollow-bearing trees are forecast to decline in abundance by 90% by 2065 and before existing mature trees (that are currently ~80 years old) will reach an age where they first begin to develop cavities [56]. Many existing large, old, hollow-bearing trees were killed or scarred in major wildfires in 1939 but are now deteriorating rapidly in condition [19]. The marked decline in large, old, hollow-bearing trees in montane ash forests has been a result of recurrent wildfire, logging, and post-fire (salvage) logging over the past century [56].
Old-growth forest (where large, old, hollow-bearing trees are most abundant [59]) is an important habitat for some species such as the yellow-bellied glider and the southern greater glider [29,55]. In addition, the vast majority of birds in montane ash forests are strongly associated with old- growth forest [16]. Like hollow-bearing trees, there has been a marked decline in the amount of remaining old-growth forest relative to historical levels [30]. Only 1886 ha or ~1.2% of the ~171,000 ha of the mountain ash ecosystem in the Central Highlands is old-growth forest; it was formerly 30–60% more extensive than it is currently [60]. Old-growth alpine ash forest is even rarer; just 0.47% remains in the Central Highlands region. The major drivers of the decline of old-growth montane ash forest are the same as for large old trees—past extensive logging, wildfire, and post-fire salvage logging [30].
5.5. Climate Change
Climate change is having a range of direct and indirect impacts on biodiversity in montane ash forests. Bioclimatic modelling suggests that the environmental niche for species such as Leadbeater’s possum (and the montane ash forests on which the species relies) will shrink significantly as a result of climate change [61,62,63]. Climate change is also likely to have marked effects on other species such as the southern greater glider, which is known to be heat sensitive [64]. The southern greater glider is a foliage-feeding specialist [65], and high overnight temperatures in this ecosystem will likely impair the foraging ability of the species [66]. Notably, recent studies have shown that the southern greater glider is increasingly absent from lower-elevation sites in montane ash forests, with the occurrence of the species in these forest types now largely confined to areas 600–900 m above sea level [29].
Importantly, for some species such as the southern greater glider, there will be limited opportunity for uphill migration to higher elevations with increasing temperatures associated with climate change. This is because of elevation-related transitions from mountain ash to alpine ash forests, as the latter occur at higher and colder locations than the former [67]. However, stands of alpine ash appear to unsuitable habitat for the southern greater glider [29], possibly because they do not provide appropriate foraging leaf resources for the species.
Climate change is having a range of other effects in montane ash forests. Elevated temperatures appear to have contributed to the increased rates of mortality in (formerly) living large, old, hollow-bearing trees [54]. Climate change also may affect the regeneration niche of montane ash eucalypt tree species [68], potentially leading to ecosystem collapse [32]. The increased prevalence of high-severity wildfires is also strongly associated with climate change in southeastern Australia [69,70], including in montane ash forests. The impacts of fires on biodiversity in montane ash forests are outlined above.
6. Interactions among Drivers of Declines
The key drivers of decline outlined above can interact to further exacerbate species decline. For example, logging has been shown to elevate the risk of high-severity wildfire [23,24], with high-severity fire in turn having negative impacts on a wide range of species (e.g., [37,55]). When forests are burnt, they are subject to post-fire (“salvage”) logging (Figure 6), with major negative impacts on arboreal marsupials [71] and birds [28], plants [22,26], and soils [27]. There is also a likely interaction between fire dynamics and the loss of old-growth forest in montane ash forests [72], with the predominance of young, highly flammable forest having a major impact on spatial contagion in wildfires [50].

Figure 6.
Post-fire (“salvage”) logging operation following wildfires in 2009. (Photo by David Lindenmayer.)
Other interactions between drivers of decline were touched on in the preceding section, including the accelerated losses of hollow-bearing trees associated both with logging at a site level [18] and with increasing amounts of logging at a landscape scale [20]. Similarly, the effects of climate change on some elements of the biota are likely to be exacerbated by logging, as young regenerating stands experience significantly higher temperatures and greater fluctuations in temperatures than older forests [34]. An example is the heat-sensitive southern greater glider [66].
7. Ineffectiveness of Conservation Strategies
Management interventions such as the establishment of reserves have been shown to be important for conserving biodiversity in many parts of the world [73,74,75]. However, levels of reservation are inadequate in montane ash forests [76,77]. This is despite (1) clear evidence of marked declines in a range of species in montane ash forests, including iconic taxa such as Leadbeater’s possum, which is on a global extinction trajectory, and the southern greater glider, which is at risk of regional extinction (as has occurred elsewhere in its distribution [78]), and (2) compelling insights into the drivers of species decline.
A range of factors have contributed to the inadequate conservation outcomes to date in montane ash forests. First, analyses of the existing reserve system show that it does not support viable populations of many threatened forest-dependent species [25,76,77]. Indeed, in the case of the montane ash forests, independent assessments indicate that the entire ecosystem needs to be protected from logging to conserve species such as Leadbeater’s possum [79,80]. Conversely, ongoing logging remains a major threat to biodiversity and ecosystem integrity. Logging is taking place in areas of high conservation value for threatened biodiversity [25]. Notably, legal counsel for the Victorian government’s logging company VicForests admitted in court that his client must log threatened species habitat to remain economically viable [81] or at least make smaller financial losses than it already does. Moreover, areas targeted for logging are not adequately surveyed by VicForests staff prior to them being cut, resulting in losses of threatened species and their habitats [82].
Second (and related to the first problem outlined above), logging operations are poorly regulated in montane ash forests [83]. Logging exclusion zones are routinely cut illegally, as are forests on steep slopes that are supposed to be exempt from logging [84]. The state government regulatory body, the Office of the Conservation Regulator, has been found to be ineffective in regulating the logging operations conducted by VicForests, lacking the resources, data, and monitoring capability to effectively do so [83].
Third, no provision is being made to adequately protect the existing areas of currently advanced regrowth forest to eventually be recruited to become the “next old growth” [85]. This is despite the amount of old-growth forest being at historically low levels [60]. Moreover, changes in the definition of old-growth forest have made it far harder for areas to be classified as old growth and therefore more difficult to protect them [30]. Old-growth mountain ash forest was formerly defined as stands of dominant overstory eucalypt trees exceeding 120 years old [86]. This is the age when large Eucalyptus trees first begin to develop cavities [87], becoming critical nesting and denning resources for an array of cavity-dependent species in these ecosystems [88]. However, the Government of Victoria redefined old-growth mountain ash forest as stands of overstory eucalypt trees exceeding 250 years [89], although the ecological basis for this change is flawed, not only for cavity-dependent animals but also because more than 75% of old-growth forest cover has been lost in Victoria in the past 25 years [30].
Fourth, some new activities, such as the creation of expansive fire breaks that result in the establishment of “laneways” of cut areas throughout the forest, are accelerating the rate of loss of hollow-bearing trees.
Finally, ineffective legislation at state and Australian government levels means that logging operations take precedence over threatened species protection, even when such operations have been found to routinely breach codes of forest practice [81]. Moreover, even when logging operations have been found to be illegal (e.g., because they have occurred on steep slopes or in harvesting exclusion areas) [84], rather than change practices, ineffective laws have been altered to make previously illegal logging legal [90].
8. What Is Needed to Prevent Extinctions?
The montane ash forests of the Central Highlands of Victoria are, arguably, among the best studied forest ecosystems globally [38,41]. The long-term work in these ecosystems is a rare example of a set of environments where declines have been well documented and the drivers of decline are well understood. Given such information, What is needed to prevent declines and even possible extinctions of biodiversity in these ecosystems? In Table 2 and then also further below, I outline some of the key approaches to tackle threats in montane ash forests and limit the risks of species declines and extinctions.
An obvious first step to improve conservation outcomes in montane ash forests, as well as limit species declines and potential future extinctions, must be to reduce the number of stressors in these ecosystems. Multiple interacting stressors can place species at elevated risk of decline and extinction. Indeed, the number of threats to which a species is exposed is a key factor driving the decline in populations of vertebrates globally [91]. I argue that the removal of native forest logging as a major stressor in montane ash forests should occur as quickly as possible. This could (and should) be done within the next 1–2 years—there is precedent for this in other Australian states [92] and internationally (e.g., in New Zealand) [93]. Sources of plantation timber to substitute for pulpwood and woodchips derived from montane ash forests are readily available (and are actually superior feedstock for activities such as paper manufacturing) [93]. Part of the rapid transition could be supported financially by having a price on carbon, whereby the emissions prevented by not logging forests [94] would attract investment from other industries seeking to offset their greenhouse gas emissions.
A second task to better conserve montane ash forests and their associated biota will be to commence active restoration programs. These programs would aim to expand the old-growth estate to be as extensive as possible given not only its potential role in limiting the spread and spatial contagion in fire [72] but also its value for biodiversity conservation [30,60]. Part of such efforts to expand the size of the old-growth estate would include investing in new technologies to better protect montane ash forests from wildfires. Such technologies include drones and sensor arrays to quickly detect ignitions when they occur and then dispense pilotless aerial vehicles with payloads of water or retardant to suppress fires as quickly as possible when it is easiest to extinguish them [95]. In addition, there are extensive areas of montane ash forest that have poor levels of natural regeneration (Taylor et al., unpublished data), and hence, the restoration of tree cover is urgently needed in these places.
A third task must be to maintain long-term monitoring programs to continue to quantify changes in species occurrence and ecosystem conditions (and links between them), as well as document how they respond to interventions such as the cessation of logging and increased forest protection.

Table 2.
Threats, threat effects, and ways to mitigate them in the montane ash forests of the Central Highlands of Victoria (see text for further details).
Table 2.
Threats, threat effects, and ways to mitigate them in the montane ash forests of the Central Highlands of Victoria (see text for further details).
| Threat | Threat Effects | Mitigation of Threat | Citation/s |
|---|---|---|---|
| Wildfire | Directly kills animals Alters habitat suitability (e.g., promotes the rate of loss of hollow-bearing trees) Removes old-growth forest Alters stand-level microclimate | Preclude fire wherever possible Use new technology to facilitate rapid detection and rapid suppression of wildfires Exclude logging that promotes severe fire | [23,77,95] |
| Logging | Directly kills animals Alters habitat suitability (e.g., loss of hollow-bearing trees) Sets back the rate of recruitment of suitable new habitat (e.g., old-growth forest) Increases rates of collapse of hollow-bearing trees Alters stand-level microclimate | Cease logging native forests Expand protected areas to better conserve threatened species | [18,53,54,56,60,76,77] |
| Fragmentation | Elevates windthrow of large old trees Compromises habitat suitability for cavity-dependent species Alters fire dynamics at stand and landscape levels May influence patterns of genetic variability within key populations | Cease logging native forests | [20,50,72,96,97] |
| Loss of hollow-bearing trees | Impairs habitat suitability for cavity-dependent fauna | Protect all existing hollow-bearing trees, even individual large old trees Ensure provision of sufficient recruit trees to replace existing old trees when they are lost Cease logging native forests Expand protected areas | [15,25,88] |
| Loss of old growth | Alters fire dynamics Reduces the extent of suitable habitat for old-growth-associated species | Cease logging native forests Bolster protection of areas in landscapes where old-growth forest is most likely to develop. Take stronger legal action to enhance old-growth protection. Redefine old-growth forest | [30,60,98] |
| Climate change | Reduces the spatial extent of species distributions Elevates mortality of large old trees Increases stressors on heat-sensitive species Increases the risk of wildfires | Cease logging native forests Reduce greenhouse gas emissions | [54,61,62,66,69] |
Delays in Cessation of Native Forest Logging in Montane Ash Forests
The Government of Victoria has indicated that in 2030 it plans to cease native forest logging not only in the Central Highlands of Victoria but also statewide [99]. While I welcome this decision, it nevertheless allows a further seven years of additional logging to take place, which is highly problematic given that 65% of all logging in Victoria is concentrated in the Central Highlands region. Failure to make a rapid transition out of logging in montane ash forests will continue to erode populations of key species, degrade their habitat, exacerbate the losses of large old trees, set back the recruitment of new cohorts of eventual old-growth forest, increase the fire proneness of the landscape (risking ecosystem collapse), and make the task of recovery and restoration more difficult. Indeed, prolonged and steep declines that have been documented in this study are likely to be indicative of extinction trajectories, at regional or even global scales. Continued declines such as those associated with ongoing logging risk creating the need for “crisis management” approaches to biodiversity conservation in montane ash forests. Crisis management can include expensive, high-risk strategies that often fail, such as captive breeding and reintroduction programs. Notably, such programs for the critically endangered Leadbeater’s possum have already failed repeatedly but are continuing even though the species’ habitat is being logged at the same time (D. Harley, personal communication). I believe that it makes little sense to embark on expensive reintroduction and translocation programs if the major drivers of decline (such as logging) are still in place.
9. General Lessons
This case study from the montane ash forests of the Central Highlands of Victoria has focused on circumstances where biodiversity loss and the erosion of ecosystem conditions are outcomes of the impacts of forest degradation—similar to the forest degradation problems in many natural forests globally [10,11,12]. There is now a global agreement to address forest degradation, and Australia is a signatory to that initiative [13]. On this basis, I argue there are some lessons from this case study that may have broader implications for biodiversity conservation and forest management in other jurisdictions.
First, quantifying declines and associated risks of extinction has been possible only through long-term monitoring. I argue that monitoring and assessment needs to be completed at multiple levels of biological organization—individual keystone structures, individual species, assemblages, habitat suitability, landscape patterns and heterogeneity, and overall ecosystem conditions. Such monitoring needs to document relationships among species, habitat suitability, and ecosystem decline, as well as the impacts of stressors (and their interaction). Monitoring programs are underpinned by an understanding that persistent and prolonged declines of species or attributes of habitat suitability (e.g., the abundance of large old trees) are a major warning sign that stronger and more effective management actions are needed [100]. Part of the focus on stressors should include an assessment of the implications of disruptions to key ecological processes such as, in the case of montane ash forests, changes in fire dynamics, reduced levels of hollow tree recruitment, and impaired regeneration success.
Second, the effectiveness of management interventions to mitigate the effects of stressors needs to be monitored and at different spatial scales (as done in this case study for old growth at individual tree, stand, and landscape levels). Of course, gathering high-quality data from monitoring programs (and the improved understanding that comes with it) of species population trajectories and ecological processes will amount to little if policy and management practices do not respond to this information [100]. Reactions to such information need to be timely to avoid the dire situation that species and ecosystems require crisis management strategies such as captive breeding programs.
A third key lesson from this case study is that simple conservation targets, even relatively ambitious ones such as 30% reservation levels, may be inadequate for species, habitats, and ecosystems that are highly threatened. This is an interesting point as the Australian Government has committed to conserving 30% of all of the nation’s ecosystems by 2030. However, in the case of the montane ash forests, the very high risks of ecosystem collapse and the marked declines in the populations of many species indicate that targets such as 30% reservation will be insufficient. Indeed, all current data indicate that all of the mountain ash and alpine ash forest estate needs to be protected from logging [79,80].
Finally, there is a critical need to determine whether management actions at a landscape and ecosystem level for a given ecosystem are consistent with national and international commitments and agreements. For example, ongoing logging in the already highly degraded montane ash forests (that will only further degrade this ecosystem) is inconsistent with the Australian Government’s commitment at the Glasgow COP26 meeting in 2021 on halting forest degradation [13]. Similarly, the Australian Government has committed to preventing further extinctions in Australia [101], yet their current support for ongoing logging in montane ash forests through federal–state legislation such as the Regional Forest Agreements [81] will likely lock in regional (and potentially global) extinctions for some species. The inherent conflicts and contradictions between conservation policies and policies that promote logging need to be urgently addressed.
10. Conclusions
The results from an extensive body of scientific research and monitoring conducted in the montane ash forests of the Central Highlands of Victoria over the past ~40 years show that some species are declining, and some are on a regional or even global extinction trajectory. There is also strong evidence of declines in the amount of suitable habitat for wildlife and evidence of the widespread erosion of ecosystem conditions in montane ash forests. Fire, logging, habitat loss, and climate change are the primary drivers of these declines and are major contributors to forest degradation. Current conservation policies are ineffective and have ignored, in part, much of the body of scientific evidence gathered in these forests. Indeed, policies to continue logging clash with those to protect biodiversity and are at odds with national and international commitments to protect biodiversity and prevent forest degradation. Removing logging immediately is critical to tackle the widespread ecological problems in montane ash forests if extinctions are to be avoided. Targeted restoration programs through the protection of high-conservation-value areas, revegetation on sites with failed natural regeneration, and efforts to limit extensive high-severity wildfire will be critical to recover montane ash forests and their associated biodiversity. Timely intervention is essential to prevent the situation becoming so dire that expensive, high-risk, crisis management strategies such as captive breeding and translocation programs will be required.
11. Recommendations for Policy-Makers and Forest Managers
The 40 years of work in montane ash forests have some important implications for policy-makers and forest managers. A small subset of these are briefly outlined below.
- Cease all logging operations in montane ash forests as soon as possible.
- Bolster legal and management protection of natural assets such as old-growth forest, large old trees, and forest habitats where key species of conservation concern have been recorded, including locations where detections of critically endangered and endangered species have been made.
- Strengthen fire protection measures in montane ash forests, including through the use of new technologies to quickly detect and rapidly suppress ignitions.
- Develop and implement restoration programs, including in places where natural regeneration has failed. This includes advance preparation for restoration in the event of further wildfires and where burnt young forest may not regenerate naturally and artificial stand regeneration measures (such as through additional seeding) might be required.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
I thank Dominick DellaSala, Tim Cadman, and Ed Morgan for encouraging me to write this paper. Tabitha Boyer and Luke Gordon assisted admirably with many key aspects of manuscript preparation. I thank the Editors and two anonymous referees for comments that improved an earlier version of the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- IUCN. The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2022. [Google Scholar]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES). IPBES Global Assessment Summary for Policymakers; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef] [PubMed]
- Woinarski, J.C.; Burbidge, A.A.; Harrison, P.L. Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proc. Natl. Acad. Sci. USA 2015, 112, 4531–4540. [Google Scholar] [CrossRef] [PubMed]
- Woinarski, J.C.Z.; Garnett, S.T.; Legge, S.M.; Lindenmayer, D.B. The contribution of policy, law, management, research and advocacy failings to the recent extinctions of three Australian vertebrate species. Conserv. Biol. 2017, 31, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Garnett, S.; Latch, P.; Lindenmayer, D.B.; Woinarski, J. Recovering Australian Threatened Species. A Book of Hope; CSIRO Publishing: Melbourne, Australia, 2018. [Google Scholar]
- Legge, S.M.; Lindenmayer, D.B.; Robinson, N.M.; Scheele, B.C.; Southwell, D.M.; Wintle, B.A. (Eds.) Monitoring Threatened Species and Ecological Communities; CSIRO Publishing: Melbourne, Australia, 2018; 480p. [Google Scholar]
- Cunningham, R.; Lindenmayer, D.B. Approaches to landscape scale inference and design issues. Curr. Landsc. Ecol. Rep. 2016, 2, 42–50. [Google Scholar] [CrossRef]
- Ashton, D.H. The seasonal growth of Eucalyptus regnans F. Muell. Aust. J. Bot. 1975, 23, 239–252. [Google Scholar] [CrossRef]
- Thompson, I.D.; Guariguata, M.R.; Okabe, K.; Bahamondez, C.; Nasi, R.; Heymell, V.; Sabogal, C. An operational framework for defining and monitoring forest degradation. Ecol. Soc. 2013, 18, 20. [Google Scholar] [CrossRef]
- Felton, A.M.; Engstrom, L.M.; Felton, A.; Knott, C.D. Orangutan population density, forest structure and fruit availability in hand-logged and unlogged peat swamp forests in West Kalimantan, Indonesia. Biol. Conserv. 2003, 114, 91–101. [Google Scholar] [CrossRef]
- Thorn, S.; Seibold, S.; Leverkus, A.B.; Michler, T.; Muller, J.; Noss, R.F.; Stork, N.; Vogel, S.; Lindenmayer, D.B. The living dead: Acknowledging life after tree death to stop forest degradation. Front. Ecol. Environ. 2020, 18, 505–512. [Google Scholar] [CrossRef]
- United Nations Climate Change Conference of the Parties. Glasgow Leaders’ Declaration on Forests and Land Use. Available online: https://ukcop26.org/glasgow-leaders-declaration-on-forests-and-land-use/ (accessed on 30 November 2022).
- Lindenmayer, D.B.; Wood, J.T.; McBurney, L.; Michael, D.; Crane, M.; MacGregor, C.; Montague-Drake, R.; Gibbons, P.; Banks, S. Cross-sectional versus longitudinal research: A case study of trees with hollows and marsupials in Australian forests. Ecol. Monogr. 2011, 81, 557–580. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Blanchard, W.; Blair, D.; McBurney, L.; Taylor, C.; Scheele, B.C.; Westgate, M.J.; Robinson, N.; Foster, C. The response of arboreal marsupials to long-term changes in forest disturbance. Anim. Conserv. 2020, 24, 246–258. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; Blair, D.; Westgate, M.J.; Scheele, B.C. Spatio-temporal effects of logging and fire on forest birds. Ecol. Appl. 2019, 29, e01999. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Blanchard, W.; Bowd, E.; Scheele, B.; Foster, C.; Lavery, T.; McBurney, L.; Blair, D. Rapid bird species recovery following high-severity wildfire but in the absence of early successional specialists. Divers. Distrib. 2022, 28, 2110–2123. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; Blair, D.; McBurney, L.; Banks, S.C. Environmental and human drivers of large old tree abundance in Australian wet forests. For. Ecol. Manag. 2016, 372, 226–235. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; Blair, D.; McBurney, L. The road to oblivion—Quantifying pathways in the decline of large old trees. For. Ecol. Manag. 2018, 430, 259–264. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; Blair, D.; McBurney, L.; Stein, J.; Banks, S.C. Empirical relationships between tree fall and landscape-level amounts of logging and fire. PLoS ONE 2018, 13, e0193132. [Google Scholar] [CrossRef] [PubMed]
- Blair, D.; McBurney, L.; Blanchard, W.; Banks, S.; Lindenmayer, D.B. Disturbance gradient shows logging affects plant functional groups more than fire. Ecol. Appl. 2016, 26, 2280–2301. [Google Scholar] [CrossRef]
- Bowd, E.J.; Banks, S.C.; Bissett, A.; May, T.W.; Lindenmayer, D.B. Direct and indirect disturbance impacts in forests. Ecol. Lett. 2021, 24, 1225–1236. [Google Scholar] [CrossRef]
- Taylor, C.; McCarthy, M.A.; Lindenmayer, D.B. Non-linear effects of stand age on fire severity. Conserv. Lett. 2014, 7, 355–370. [Google Scholar] [CrossRef]
- Taylor, C.; Blanchard, W.; Lindenmayer, D.B. Does forest thinning reduce fire severity in Australian eucalypt forests? Conserv. Lett. 2020, 14, e12766. [Google Scholar] [CrossRef]
- Taylor, C.; Lindenmayer, D.B. The adequacy of Victoria’s protected areas for conserving its forest-dependent fauna. Austral Ecol. 2019, 44, 1076–1090. [Google Scholar] [CrossRef]
- Bowd, E.J.; Lindenmayer, D.B.; Banks, S.C.; Blair, D.P. Logging and fire regimes alter plant communities. Ecol. Appl. 2018, 28, 826–841. [Google Scholar] [CrossRef] [PubMed]
- Bowd, E.J.; Banks, S.C.; Strong, C.L.; Lindenmayer, D.B. Long-term impacts of wildfire and logging on forest soils. Nat. Geosci. 2019, 12, 113–118. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; McBurney, L.; Blair, D.; Wood, J.; Banks, S.C. From unburnt to salvage logged: Quantifying bird responses to different levels of disturbance severity. J. Appl. Ecol. 2018, 55, 1626–1636. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; McBurney, L.; Blanchard, W.; Marsh, K.; Bowd, E.; Watchorn, D.; Taylor, C.; Youngentob, K. Elevation, disturbance, and forest type drive the occurrence of a specialist arboreal folivore. PLoS ONE 2022, 17, e0265963. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Taylor, C. Extensive recent wildfires demand more stringent protection of critical old growth forest. Pac. Conserv. Biol. 2020, 26, 384. [Google Scholar] [CrossRef]
- Bowd, E.; Blair, D.P.; Lindenmayer, D.B. Prior disturbance legacy effects on plant recovery post-high severity wildfire. Ecosphere 2021, 12, e03480. [Google Scholar] [CrossRef]
- Bowd, E.; McBurney, L.; Lindenmayer, D.B. The characteristics of regeneration failure and their potential to shift wet temperate forests into alternate stable states. For. Ecol. Manag. 2022, 529, 120673. [Google Scholar] [CrossRef]
- Taylor, C.; Lindenmayer, D.B. Temporal fragmentation of a critically endangered ecosystem. Austral Ecol. 2020, 45, 340–354. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; McBurney, L.; Bowd, E.; Youngentob, K.; Marsh, K.; Taylor, C. Stand age related differences in forest microclimate. For. Ecol. Manag. 2022, 510, 120101. [Google Scholar] [CrossRef]
- Commonwealth of Australia and Department of Natural Resources and Environment. Comprehensive Regional Assessment—Biodiversity. Central Highlands of Victoria; The Commonwealth of Australia and Department of Natural Resources and Environment: Canberra, Australia, 1997.
- Cary, G.; Blanchard, W.; Foster, C.N.; Lindenmayer, D.B. Effects of altered fire regimes on critical timber production and conservation rotations. Int. J. Wildland Fire 2021, 30, 322–328. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; McBurney, L.; Blair, D.; Banks, S.; Driscoll, D.; Smith, A.; Gill, A.M. Fire severity and landscape context effects on arboreal marsupials. Biol. Conserv. 2013, 167, 137–148. [Google Scholar] [CrossRef]
- Lindenmayer, D.B. Forest Pattern and Ecological Process: A Synthesis of 25 Years of Research; CSIRO Publishing: Melbourne, Australia, 2009. [Google Scholar]
- Flint, A.; Fagg, P. Mountain Ash in Victoria’s State Forests; Department of Sustainability and Environment: Melbourne, Australia, 2007.
- DELWP. Code of Forest Practices for Timber Production 2014 (as Amended in 2021); Department of Environment, Land, Water and Planning: Melbourne, Australia, 2021.
- Lindenmayer, D.B. The Great Forest; Allen and Unwin: Sydney, Australia, 2022. [Google Scholar]
- Loyn, R.H. Bird populations in successional forests of Mountain Ash Eucalyptus regnans in Central Victoria. Emu 1985, 85, 213–230. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; McBurney, L.; Blair, D.; Banks, S.C.; Driscoll, D.A.; Smith, A.; Gill, A.M. Complex responses of birds to landscape-level fire extent, fire severity and environmental drivers. Divers. Distrib. 2014, 20, 467–477. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; Bowd, E.; McBurney, L.; Ashman, K.; Blair, D. What factors influence the extent and abundance of midstorey Acacia in Mountain Ash forests? Austral Ecol. 2021, 46, 532–544. [Google Scholar] [CrossRef]
- Mueck, S.G. The Floristic Composition of Mountain Ash and Alpine Ash Forests in Victoria: Silvicultural Systems Project No.4; Department of Conservation and Environment: Melbourne, Australia, 1990.
- Bergstrom, D.M.; Wienecke, B.C.; van den Hoff, J.; Hughes, L.; Lindenmayer, D.B.; Ainsworth, T.D.; Baker, C.M.; Bland, L.; Bowman, D.M.J.S.; Brooks, S.T.; et al. Combating ecosystem collapse from the tropics to the Antarctic. Glob. Change Biol. 2021, 27, 1692–1703. [Google Scholar] [CrossRef]
- Burns, E.L.; Lindenmayer, D.B.; Stein, J.; Blanchard, W.; McBurney, L.; Blair, D.; Banks, S.C. Ecosystem assessment of mountain ash forest in the Central Highlands of Victoria, south-eastern Australia. Austral Ecol. 2015, 40, 386–399. [Google Scholar] [CrossRef]
- Ashton, D.H. Fire in tall open forests (wet sclerophyll forests). In Fire and the Australian Biota; Gill, A.M., Groves, R.H., Noble, I.R., Eds.; Australian Academy of Science: Canberra, Australia, 1981; pp. 339–366. [Google Scholar]
- Enright, N.J.; Fontaine, J.B.; Bowman, D.M.; Bradstock, R.A.; Williams, R.J. Interval squeeze: Altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes. Front. Ecol. Environ. 2015, 13, 265–272. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Bowd, E.; Taylor, C.; Likens, G.E. Interacting fire, logging and climate change has sprung a landscape trap in Victoria’s montane ash forests. Plant Ecol. 2022, 223, 733–749. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Foster, C.; Westgate, M.; Scheele, B.C.; Blanchard, W. Managing interacting disturbances: Lessons from a case study in Australian forests. J. Appl. Ecol. 2020, 57, 1711–1716. [Google Scholar] [CrossRef]
- Smith, A.L.; Blanchard, W.; Blair, D.; McBurney, L.; Banks, S.C.; Driscoll, D.A.; Lindenmayer, D.B. The dynamic regeneration niche of a forest following a rare disturbance event. Divers. Distrib. 2016, 22, 457–467. [Google Scholar] [CrossRef]
- Ward, M.; Tulloch, A.I.T.; Radford, J.Q.; Williams, B.A.; Reside, A.E.; Macdonald, S.L.; Mayfield, H.J.; Maron, M.; Possingham, H.P.; Vine, S.J.; et al. Impact of 2019-2020 mega-fires on Australian fauna habitat. Nat. Ecol. Evol. 2020, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Blanchard, W.; McBurney, L.; Blair, D.; Banks, S.; Likens, G.E.; Franklin, J.F.; Stein, J.; Gibbons, P. Interacting factors driving a major loss of large trees with cavities in a forest ecosystem. PLoS ONE 2012, 7, e41864. [Google Scholar] [CrossRef] [PubMed]
- Lefoe, M.; Rendall, A.R.; McKinnon, F.; Whisson, D.A. Logging and wildfire limit the distribution of a vulnerable arboreal mammal. For. Ecol. Manag. 2021, 503, 119773. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Sato, C. Hidden collapse is driven by fire and logging in a socioecological forest ecosystem. Proc. Natl. Acad. Sci. USA 2018, 115, 5181–5186. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Cunningham, R.B.; McCarthy, M.A. The conservation of arboreal marsupials in the montane ash forests of the Central Highlands of Victoria, south-eastern Australia: VIII. Landscape analysis of the occurrence of arboreal marsupials. Biol. Conserv. 1999, 89, 83–92. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Barton, P.S.; Lane, P.W.; Westgate, M.J.; McBurney, L.; Blair, D.; Gibbons, P.; Likens, G.E. An empirical assessment and comparison of species-based and habitat-based surrogates: A case study of forest vertebrates and large old trees. PLoS ONE 2014, 9, e89807. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Cunningham, R.B.; Donnelly, C.F.; Franklin, J.F. Structural features of old growth Australian montane ash forests. For. Ecol. Manag. 2000, 134, 189–204. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Bowd, E. Critical ecological roles, structural attributes and conservation of old growth forest: Insights from long-term research in Mountain Ash forest in south-eastern Australia. Front. For. Glob. Change 2022, 5, 878570. [Google Scholar] [CrossRef]
- Brereton, R.; Bennett, S.; Mansergh, I. Enhanced greenhouse climate change and its potential effect on selected fauna of south-eastern Australia: A trend analysis. Biol. Conserv. 1995, 72, 339–354. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Nix, H.A.; McMahon, J.P.; Hutchinson, M.F.; Tanton, M.T. The conservation of Leadbeater’s Possum, Gymnobelideus leadbeateri (McCoy): A case study of the use of bioclimatic modelling. J. Biogeogr. 1991, 18, 371–383. [Google Scholar] [CrossRef]
- Steffen, W.; Burbidge, A.; Hughes, L.; Kitching, R.; Lindenmayer, D.B.; Musgrave, W.; Stafford-Smith, M.; Werner, P. Australia’s Biodiversity and Climate Change; CSIRO Publishing: Melbourne, Australia, 2009. [Google Scholar]
- Rubsamen, K.; Hume, I.D.; Foley, W.J.; Rubsamen, U. Implications of the large surface area to body mass ratio on the heat balance of the greater glider (Petaroides volans: Marsupialia). J. Comp. Physiol. B 1984, 154, 105–111. [Google Scholar] [CrossRef]
- Hume, I. Marsupial Nutrition; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Youngentob, K.; Ford, K.; Lindenmayer, D.B.; Foley, W. An overlooked driver of climate change casualties. Trends Ecol. Evol. 2021, 36, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Mackey, B.; Nix, H.A. Climatic analyses of the distribution of four commercially-important wood production eucalypt trees from south-eastern Australia. Aust. For. 1996, 59, 11–26. [Google Scholar] [CrossRef]
- Singh, A.; Baker, P.; Kasel, S.; Trouve, R.; Stewart, S.B.; Nitschke, C. The role of climatic variability on Eucalyptus regeneration in south-eastern Australia. Glob. Ecol. Conserv. 2021, 32, e01929. [Google Scholar] [CrossRef]
- Canadell, J.G.; Meyer, C.P.; Cook, G.D.; Dowdy, A.; Briggs, P.R.; Knauer, J.; Pepler, A.; Haverd, V. Multi-decadal increase of forest burned area in Australia is linked to climate change. Nat. Commun. 2021, 12, 6921. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Clarke, H.; Clarke, M.F.; McColl-Gausden, S.C.; Nolan, R.H.; Penman, T.; Bradstock, R. Warmer and drier conditions have increased the potential for large and severe fire seasons across south-eastern Australia. Glob. Ecol. Biogeogr. 2022, 31, 1933–1948. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Ough, K. Salvage logging in the montane ash eucalypt forests of the Central Highlands of Victoria and its potential impacts on biodiversity. Conserv. Biol. 2006, 20, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Hobbs, R.J.; Likens, G.E.; Krebs, C.; Banks, S. Newly discovered landscape traps produce regime shifts in wet forests. Proc. Natl. Acad. Sci. USA 2011, 108, 15887–15891. [Google Scholar] [CrossRef]
- Bolam, F.C.; Mair, L.; Angelico, M.; Brooks, T.M.; Burgman, M.; Hermes, C.; Hoffmann, M.; Martin, R.W.; McGowan, P.J.; Rodrigues, A.S.; et al. How many bird and mammal extinctions has recent conservation action prevented? Conserv. Lett. 2020, 14, e12762. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Andelman, S.J.; Bakarr, M.I.; Boitani, L.; Brooks, T.M.; Cowling, R.M.; Fishpool, L.D.C.; da Fonseca, G.A.B.; Gaston, K.J.; Hoffmann, M.; et al. Effectiveness of the global protected area network in representing species diversity. Nature 2004, 428, 640–643. [Google Scholar] [CrossRef]
- Visconti, P.; Butchart, S.; Brooks, T.M.; Langhammer, P.F.; Marnewick, D.; Vergara, S.; Yanosky, A.; Watson, J.E. Protected area targets post-2020. Science 2019, 364, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Cadenhead, N.; Lindenmayer, D.B.; Wintle, B.A. Improving the design of a conservation reserve for a critically endangered species. PLoS ONE 2017, 12, e0169629. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.R.; Lindenmayer, D.B.; Stamation, K.; Acevedo-Catteneo, S.; Smith, S.; Lumsden, L.F. Assessing reserve effectiveness: Application to a threatened species in a dynamic fire prone forest landscape. Ecol. Model. 2016, 338, 90–100. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Wood, J.; MacGregor, C.; Foster, C.; Scheele, B.; Tulloch, A.; Barton, P.; Banks, S.; Robinson, N.; Dexter, N.; et al. Conservation conundrums and the challenges of managing unexplained declines of multiple species. Biol. Conserv. 2018, 221, 279–292. [Google Scholar] [CrossRef]
- Threatened Species Scientific Committee (TSSC). Approved Conservation Advice Gymnobelideus leadbeateri Leadbeater’s Possum; Department of the Environment: Canberra, Australia, 2015. Available online: http://www.environment.gov.au/biodiversity/threatened/species/pubs/273-conservation-advice.pdf (accessed on 14 February 2023).
- Threatened Species Scientific Committee (TSSC). Approved Conservation Advice Gymnobelideus leadbeateri Leadbeater’s Possum; Department of the Environment: Canberra, Australia, 2019. Available online: https://www.environment.gov.au/biodiversity/threatened/species/pubs/273-conservation-advice-22062019.pdf (accessed on 14 February 2023).
- Lindenmayer, D.B.; Burnett, P. Biodiversity in court: Will the Regional Forest Agreements (RFAs) make the EPBC Act irrelevant? Pac. Conserv. Biol. 2021, 28, 393–397. [Google Scholar] [CrossRef]
- Wardell-Johnson, G.W.; Robinson, T.P. Considerations in the protection of marsupial gliders and other mature-forest dependent fauna in areas of intensive logging in the tall forests of Victoria, Australia. Pac. Conserv. Biol. 2022. [Google Scholar] [CrossRef]
- VAGO. Regulating Victoria’s Native Forests; Victorian Auditor Generals Office: Melbourne, Australia, 2022.
- Taylor, C.; Lindenmayer, D.B. Stakeholder engagement in a Forest Stewardship Council Controlled Wood assessment. Environ. Sci. Policy 2021, 120, 204–212. [Google Scholar] [CrossRef]
- Blair, D.; McBurney, L.; Lindenmayer, D.B. Failing to conserve Leadbeater’s Possum and its Mountain Ash forest habitat. Aust. Zool. 2018, 39, 443–448. [Google Scholar] [CrossRef]
- Macfarlane, M.A.; Seebeck, J.H. Draft Management Strategies for the Conservation of Leadbeater’s Possum, Gymnobelideus leadbeateri, in Victoria; Department of Conservation and Environment: Melbourne, Australia, 1991.
- Ambrose, G.J. An Ecological and Behavioural Study of Vertebrates Using Hollows in Eucalypt Branches. Ph.D. Thesis, La Trobe University, Melbourne, Australia, 1982. [Google Scholar]
- Lindenmayer, D.B. Conserving large old trees as small natural features. Biol. Conserv. 2017, 211, 51–59. [Google Scholar] [CrossRef]
- Office of the Conservation Regulator. Old Growth Forest Field Identification Procedure; Department of Environment, Land, Water and Planning: Melbourne, Australia, 2019.
- Lindenmayer, D.B.; Taylor, C. Australia threatens to weaken forest laws. Science 2021, 373, 752. [Google Scholar] [CrossRef]
- Capdevilia, P.; Noviello, N.; McRae, L.; Freeman, R.; Clements, C.F. Global patterns of resilience decline in vertebrate populations. Ecol. Lett. 2022, 25, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Government of Western Australia. Forest Management Plan 2024–2033; Conservation Parks Commission and Department of Biodiversity, Conservation and Attractions: Kensington, Australia, 2022.
- Lindenmayer, D.; Taylor, C. Diversifying forest landscape management—A case study of a shift from native forest logging to plantations in Australian wet forests. Land 2022, 11, 407. [Google Scholar] [CrossRef]
- Keith, H.; Lindenmayer, D.B.; Mackey, B.G.; Blair, D.; Carter, L.; McBurney, L.; Okada, S.; Konishi-Nagano, T. Managing temperate forests for carbon storage: Impacts of logging versus forest protection on carbon stocks. Ecosphere 2014, 5, 75. [Google Scholar] [CrossRef]
- Yebra, M.; Barnes, N.; Colleen Bryant, C.; Cary, G.J.; Durrani, S.; Mahony, R.; Palethorpe, E.; Stocks, M.; Stocks, R.; Tridgell, A.; et al. Towards an integrated hi-tech solution to detect small fires. Aust. J. Emerg. Manag. 2022, 37, 44–47. [Google Scholar]
- Banks, S.C.; McBurney, L.; Blair, D.; Davies, I.D.; Lindenmayer, D.B. Where do animals come from during post-fire population recovery? Implications for ecological and genetic patterns in post-fire landscapes. Ecography 2017, 40, 1325–1338. [Google Scholar] [CrossRef]
- Banks, S.C.; Cary, G.J.; Smith, A.L.; Davies, I.D.; Driscoll, D.A.; Gill, A.M.; Lindenmayer, D.B.; Peakall, R. How does ecological disturbance influence genetic diversity? Trends Ecol. Evol. 2013, 28, 670–679. [Google Scholar] [CrossRef]
- Mackey, B.; Lindenmayer, D.B.; Gill, A.M.; McCarthy, M.A.; Lindesay, J.A. Wildlife, Fire and Future Climate: A Forest Ecosystem Analysis; CSIRO Publishing: Melbourne, Australia, 2002. [Google Scholar]
- Government of Victoria. Victorian Government’s Action for Long-Term Sustainability of Victoria’s Native TIMBER Forests; Commissioner for Environmental Sustainability Victoria: Melbourne, Australia, 2019.
- Lindenmayer, D.B.; Piggott, M.; Wintle, B. Counting the books while the library burns: Why conservation monitoring programs need a plan for action. Front. Ecol. Environ. 2013, 11, 549–555. [Google Scholar] [CrossRef]
- Department of Climate Change, Energy, the Environment and Water. Minister launches Threatened Species Action Plan: Toward Zero Extinctions. Available online: https://minister.dcceew.gov.au/plibersek/media-releases/minister-launches-threatened-species-action-plan-toward-zero-extinctions (accessed on 30 November 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).