Altering Natural Ecosystems Causes Negative Consequences on the Soil Physical Qualities: An Evidence-Based Study from Nilgiri Hill Region of Western Ghats, India
Abstract
1. Introduction
2. Materials and Methods
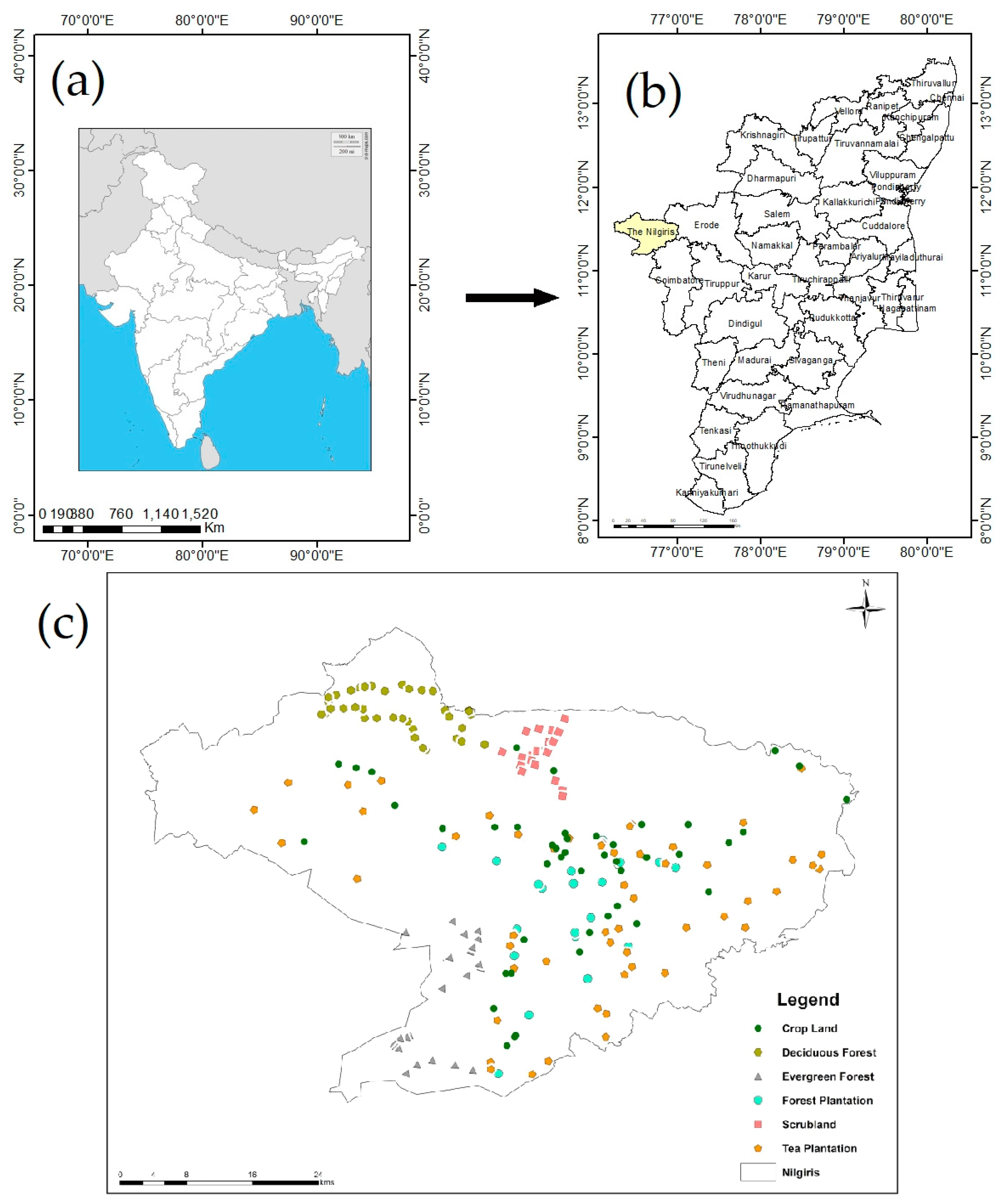
2.1. Study Area
2.2. Geomorphology and Soils
2.3. Field Description and Investigation
2.4. Soil Sampling and Analysis
2.5. Soil Physical Properties
2.6. Hydraulic Conductivity
2.7. Aggregate Analysis
2.8. Soil Organic Matter
2.9. Assessment of Soil Quality
2.10. Statistical Analyses
3. Results
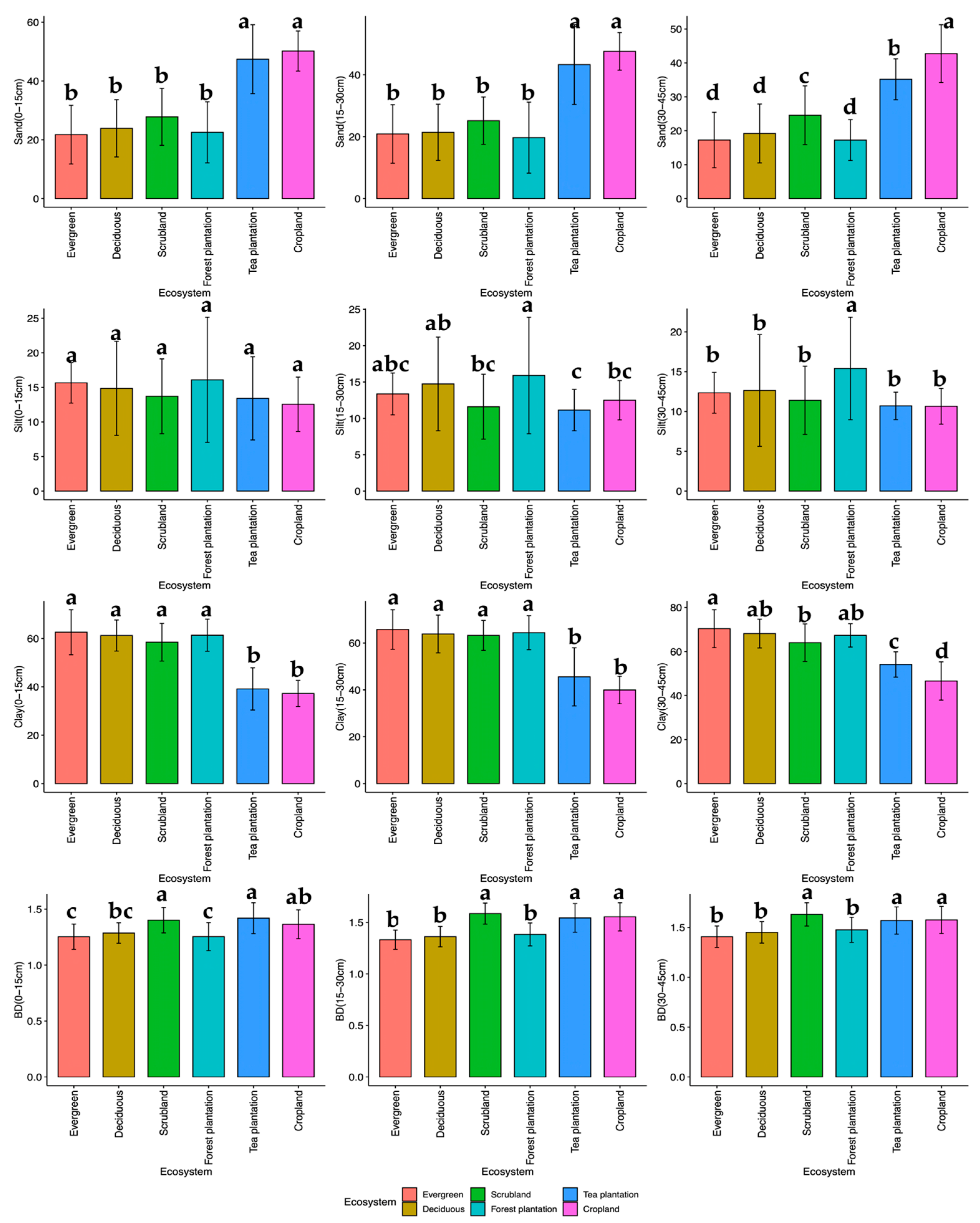
3.1. Particle Size Distribution (PSD)
3.1.1. Sand Content
3.1.2. Silt Content
3.1.3. Clay Content
3.2. Bulk Density
3.3. Particle Density
3.4. Pore Space
3.5. Available Soil Moisture
3.6. Hydraulic Conductivity
3.7. Soil Aggregate Index
3.7.1. Aggregate Stability
3.7.2. Mean Weight Diameter (MWD)
3.7.3. Water-Stable Aggregates (WSAs)
3.8. Soil Organic Matter (SOM)
3.9. Soil Quality Assessment
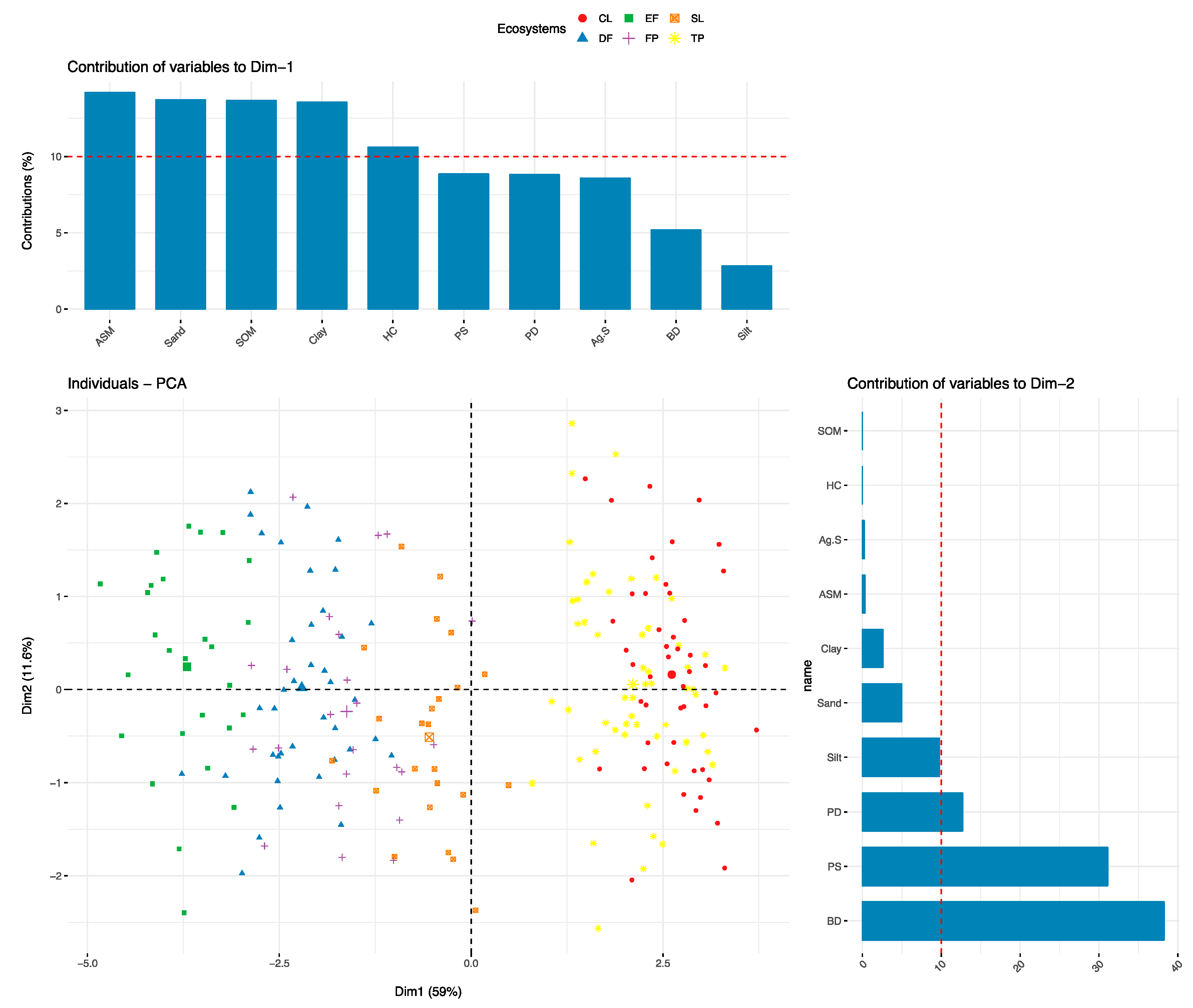
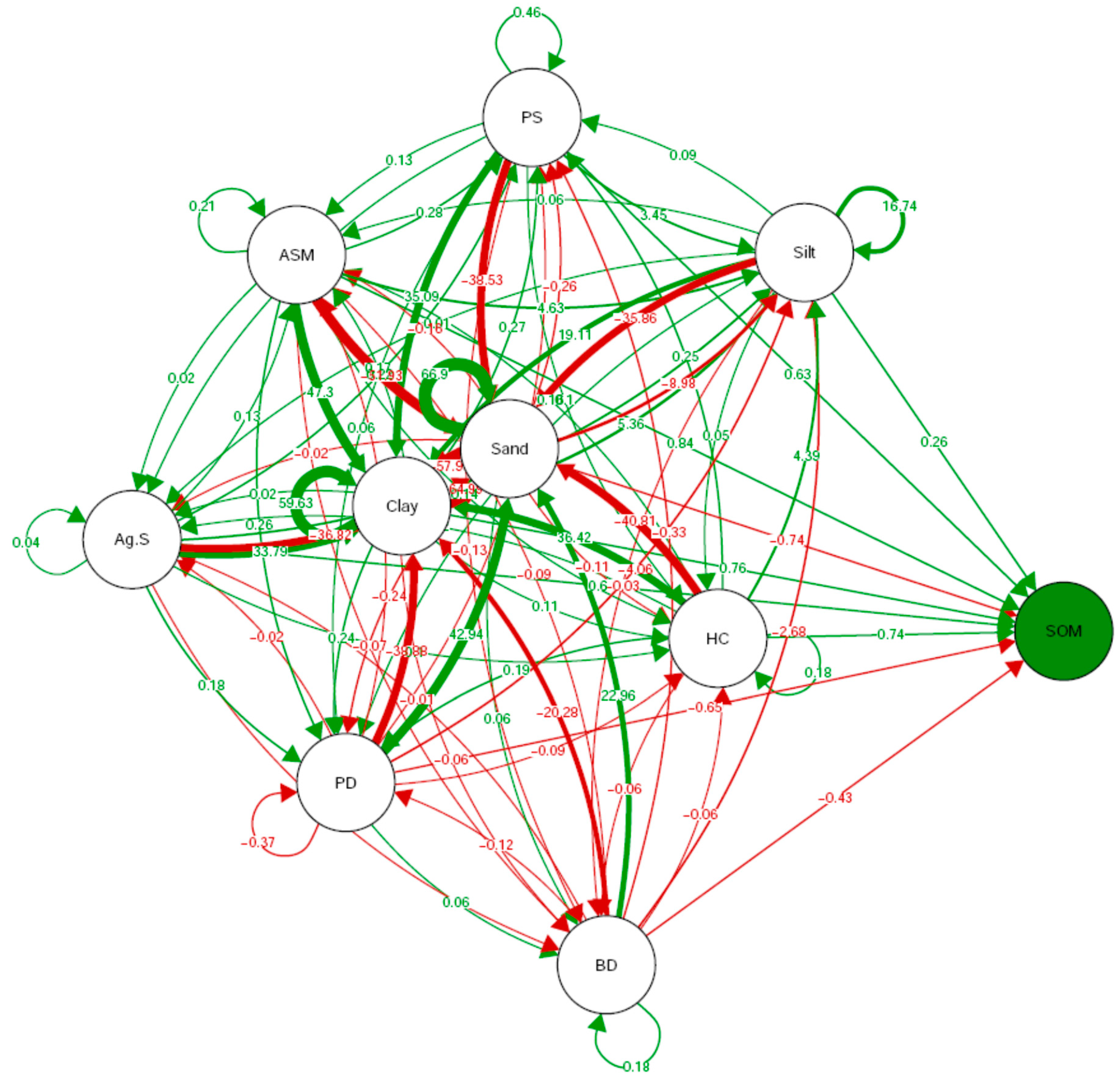
3.10. Relationship between Soil Physical Properties
4. Discussion
4.1. Particle Size Distribution (PSD)
4.2. Available Soil Moisture (ASM)
4.3. Hydraulic Conductivity (Ksat)
4.4. Soil Aggregate Stability (SAS)
- Implement erosion control measures such as contour plowing, terracing, and buffer strips to reduce runoff and soil loss.
- Adopt conservation tillage practices like no-till or minimum tillage, which help to preserve soil structure and reduce erosion.
- Maintain vegetative cover through cover cropping, crop rotation, or planting grass or trees to stabilize soil and reduce erosion.
- Use regenerative agriculture and agroforestry for diversified products and environment and soil amelioration.
- Avoid excessive machinery traffic or use controlled traffic farming systems to minimize soil compaction.
- Practice timely tillage or subsoiling to alleviate compaction and improve soil structure.
- Incorporate organic matter through composting or cover cropping to enhance soil aggregation and reduce compaction.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doran, J.W.; Parkin, T.B. Defining and Assessing Soil Quality. Defin. Soil. Qual. A Sustain. Environ. 1994, 35, 1–21. [Google Scholar]
- Doran, J.W.; Zeiss, M.R. Soil Health and Sustainability: Managing the Biotic Component of Soil Quality. Appl. Soil. Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Marzaioli, R.; D’Ascoli, R.; de Pascale, R.A.; Rutigliano, F.A. Soil Quality in a Mediterranean Area of Southern Italy as Related to Different Land Use Types. Appl. Soil. Ecol. 2010, 44, 205–212. [Google Scholar] [CrossRef]
- Takoutsing, B.; Weber, J.; Aynekulu, E.; Martín, J.A.R.; Shepherd, K.; Sila, A.; Tchoundjeu, Z.; Diby, L. Assessment of Soil Health Indicators for Sustainable Production of Maize in Smallholder Farming Systems in the Highlands of Cameroon. Geoderma 2016, 276, 64–73. [Google Scholar] [CrossRef]
- Guo, L.; Sun, Z.; Ouyang, Z.; Han, D.; Li, F. A Comparison of Soil Quality Evaluation Methods for Fluvisol along the Lower Yellow River. Catena 2017, 152, 135–143. [Google Scholar] [CrossRef]
- Roe, D.; Nelson, F.; Sandbrook, C. Community Management of Natural Resources in Africa: Impacts, Experiences and Future Directions; IIED: London, UK, 2009; ISBN 1843697556. [Google Scholar]
- Jagadesh, M.; Selvi, D.; Thiyageshwari, S.; Srinivasarao, C.; Kalaiselvi, T.; Lourdusamy, K.; Kumaraperumal, R.; Allan, V. Soil Carbon Dynamics Under Different Ecosystems of Ooty Region in the Western Ghats Biodiversity Hotspot of India. J. Soil. Sci. Plant Nutr. 2023, 23, 1374–1385. [Google Scholar] [CrossRef]
- Jagadesh, M.; Srinivasarao, C.; Selvi, D.; Thiyageshwari, S.; Kalaiselvi, T.; Kumari, A.; Singh, S.K.; Lourdusamy, K.; Kumaraperumal, R.; Allan, V. Quantifying the Unvoiced Carbon Pools of the Nilgiri Hill Region in the Western Ghats Global Biodiversity Hotspot—First Report. Sustainability 2023, 15, 5520. [Google Scholar] [CrossRef]
- Hoogsteen, M.J.J.; Lantinga, E.A.; Bakker, E.J.; Groot, J.C.J.; Tittonell, P.A. Estimating Soil Organic Carbon through Loss on Ignition: Effects of Ignition Conditions and Structural Water Loss. Eur. J. Soil. Sci. 2015, 66, 320–328. [Google Scholar] [CrossRef]
- Ojo, A.O.; Aliku, O.; Aladele, S.E.; Oshunsanya, S.O.; Olubiyi, M.R.; Olosunde, A.A.; Ayantayo-Ojo, V.I.; Alowonle, A.A. Impacts of Land-Use Types on Soil Physical Quality: A Case Study of the National Centre for Genetic Resources and Biotechnology (NACGRAB), Nigeria. Environ. Chall. 2022, 7, 100510. [Google Scholar] [CrossRef]
- Teferi, E.; Bewket, W.; Simane, B. Effects of Land Use and Land Cover on Selected Soil Quality Indicators in the Headwater Area of the Blue Nile Basin of Ethiopia. Environ. Monit. Assess. 2016, 188, 83. [Google Scholar] [CrossRef]
- Ogunwole, J. Land Use Impact on Soil Physical Quality and Soil Structure in Three Highland Watersheds of Ethiopia. Adv. Plants Agric. Res. 2014, 1, 111–119. [Google Scholar] [CrossRef][Green Version]
- Mueller, L.; Schindler, U.; Mirschel, W.; Shepherd, T.G.; Ball, B.C.; Helming, K.; Rogasik, J.; Eulenstein, F.; Wiggering, H. Assessing the Productivity Function of Soils. A Review. Agron. Sustain. Dev. 2010, 30, 601–614. [Google Scholar] [CrossRef]
- Reynolds, W.D.; Drury, C.F.; Yang, X.M.; Fox, C.A.; Tan, C.S.; Zhang, T.Q. Land Management Effects on the Near-Surface Physical Quality of a Clay Loam Soil. Soil. Tillage Res. 2007, 96, 316–330. [Google Scholar] [CrossRef]
- EC (European Commission) COM 2006/231 2006. Communication from the Commission to the Council, The European Economic and So- Cial Committee and the Committee of the Regions. Thematic Strategy for Soil Protection, Commission of the European Communities, Brussels, 2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2006:0231:FIN:EN:PDF (accessed on 29 August 2023).
- Neris, J.; Jiménez, C.; Fuentes, J.; Morillas, G.; Tejedor, M. Vegetation and Land-Use Effects on Soil Properties and Water Infiltration of Andisols in Tenerife (Canary Islands, Spain). Catena 2012, 98, 55–62. [Google Scholar] [CrossRef]
- Gozukara, G.; Zhang, Y.; Hartemink, A.E.; Altunbas, S.; Mustafa, S. Soil Chronosequence and Biosequence on Old Lake Sediments of the Burdur Lake in Turkey. Pedosphere 2021, 31, 882–891. [Google Scholar] [CrossRef]
- Gebre, H.; Molla, D.; Jayne, T.S.; Shaffer, J. Designing strategies to support a transformation of agriculture in ethiopia. Food Secur. Y Rep. Report. 1997. [Google Scholar] [CrossRef]
- Chakraborty, D.; Garg, R.N.; Tomar, R.K.; Dwivedi, B.S.; Aggarwal, P.; Singh, R.; Behera, U.K.; Thangasamy, A.; Singh, D. Soil Physical Quality as Influenced by Long-Term Application of Fertilizers and Manure under Maize-Wheat System. Soil. Sci. 2010, 175, 128–136. [Google Scholar] [CrossRef]
- Ratta, R.; Lal, R. (Eds.) . Lal Rattan Soil Quality and Agricultural Sustainability; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Wiley: Hoboken, NJ, USA, 1986; Volume 5, pp. 635–662. [Google Scholar]
- van Genuchten, M.T. A Closed-form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil. Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Alem, S.; Pavlis, J. Conversion of Grazing Land into Grevillea Robusta Plantation and Exclosure: Impacts on Soil Nutrients and Soil Organic Carbon. Environ. Monit. Assess. 2014, 186, 4331–4341. [Google Scholar] [CrossRef]
- Zhang, J.J.; Fu, M.C.; Zeng, H.; Geng, Y.H.; Hassani, F.P. Variations in Ecosystem Service Values and Local Economy in Response to Land Use: A Case Study of Wu’an, China. Land. Degrad. Dev. 2013, 24, 236–249. [Google Scholar] [CrossRef]
- Nosrati, K. Assessing Soil Quality Indicator under Different Land Use and Soil Erosion Using Multivariate Statistical Techniques. Environ. Monit. Assess. 2013, 185, 2895–2907. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Bordoloi, L.J.; Kumar, M.; Hazarika, S.; Parmar, B. Land Use Impact on Soil Quality in Eastern Himalayan Region of India. Environ. Monit. Assess. 2014, 186, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Vasantha, K.S.; Bhagavanulu, D.V.S. Effect of Deforestation on Landslides in Nilgiris District—A Case Study. J. Indian. Soc. Remote Sens. 2008, 36, 105–108. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; de Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P. Soil Quality–A Critical Review. Soil. Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Gunnell, Y.; Louchet, A. The Influence of Rock Hardness and Divergent Weatherin g on the Interpretation of Apatite Fission-Track Denudation Rates. Evidence from Charnockites in South India and Sri Lanka. Z. Geomorphol. 2000, 44, 33–57. [Google Scholar] [CrossRef]
- Caner, L.; Bourgeon, G. Andisols of the Nilgiri Highlands: New Insight into Their Classification, Age and Genesis; Geological Society of India: Bangalore, India, 2001. [Google Scholar]
- Dash, M.; Thiyageshwari, S.; Selvi, D.; Anandham, R.; Rajan, K.; Maduraimuthu, D.; Singh, S.K.; Muthumani, J.; Singh, S.; Pramanick, B. Unravelling the Release Kinetics of Exchangeable Magnesium in Acid Soil of Nilgiris. Sustainability 2023, 15, 9848. [Google Scholar] [CrossRef]
- Gupta, R.P.; Dakshinamoorthy, C. Procedures for Physical Analysis of Soil and Collection of Agrometeorological Data; Indian Agricultural Research Institute: New Delhi, India, 1980; p. 293. [Google Scholar]
- Piper, C. International Pipette Method. In Soil and Plant Analysis; Scientific Publishers: New Delhi, India, 1966. [Google Scholar]
- Aitchison, G.D.; Richards, B.G. Broad-Scale Study of Moisture Conditions in Pavement Subgrades throughout Australia; Butterworths: Sydney, Australia, 1965. [Google Scholar]
- Yoder, R.E. A Direct Method of Aggregate Analysis of Soils and a Study of the Physical Nature of Erosion Losses; John and Wiley and Sons: Hoboken, NJ, USA, 1936. [Google Scholar]
- van Bavel, C.H.M. Mean Weight-Diameter of Soil Aggregates as a Statistical Index of Aggregation. Proc. Soil. Sci. Soc. Am. 1950, 14, 20–23. [Google Scholar] [CrossRef]
- Larson, W.E.; Pierce, F.J. The Dynamics of Soil Quality as a Measure of Sustainable Management. Defin. Soil. Qual. A Sustain. Environ. 1994, 35, 37–51. [Google Scholar]
- Singh, A.K. Evaluation of Soil Quality under Integrated Nutrient Management. J. Indian. Soc. Soil. Sci. 2007, 55, 58–61. [Google Scholar]
- Karlen, D.L.; Ditzler, C.A.; Andrews, S.S. Soil Quality: Why and How? Geoderma 2003, 114, 145–156. [Google Scholar] [CrossRef]
- Govaerts, B.; Sayre, K.D.; Deckers, J. A Minimum Data Set for Soil Quality Assessment of Wheat and Maize Cropping in the Highlands of Mexico. Soil. Tillage Res. 2006, 87, 163–174. [Google Scholar] [CrossRef]
- Qiu, Y.; Fu, B.; Wang, J.; Chen, L.; Meng, Q.; Zhang, Y. Spatial Prediction of Soil Moisture Content Using Multiple-Linear Regressions in a Gully Catchment of the Loess Plateau, China. J. Arid Environ. 2010, 74, 208–220. [Google Scholar] [CrossRef]
- Alabi, A.A.; Adewale, A.O.; Adebo, B.; Ogungbe, A.S.; Coker, J.O.; Akinboro, F.G.; Bolaji, G. Effects of Different Land Uses on Soil Physical and Chemical Properties in Odeda LGA, Ogun State, Nigeria. Environ. Earth Sci. 2019, 78, 207. [Google Scholar] [CrossRef]
- Diack, M.; Stott, D.E. Development of a Soil Quality Index for the Chalmers Silty Clay Loam from the Midwest USA. Purdue University: USDA-ARS National Soil Erosion Research Laboratory 2001, 550–555. Available online: https://www.researchgate.net/profile/Diane-Stott/publication/228806766_Development_of_a_soil_quality_index_for_the_Chalmers_silty_clay_loam_from_the_Midwest_USA/links/00463529ca9e211e54000000/Development-of-a-soil-quality-index-for-the-Chalmers-silty-clay-loam-from-the-Midwest-USA.pdf (accessed on 29 August 2023).
- Karlen, D.L.; Stott, D.E. A Framework for Evaluating Physical and Chemical Indicators of Soil Quality. Defin. Soil. Qual. A Sustain. Environ. 1994, 35, 53–72. [Google Scholar]
- Singh, M.J.; Khera, K.L. Physical Indicators of Soil Quality in Relation to Soil Erodibility under Different Land Uses. Arid. Land. Res. Manag. 2009, 23, 152–167. [Google Scholar] [CrossRef]
- Ray, S.K.; Bhattacharyya, T.; Reddy, K.R.; Pal, D.K.; Chandran, P.; Tiwary, P.; Mandal, D.K.; Mandal, C.; Prasad, J.; Sarkar, D. Soil and Land Quality Indicators of the Indo-Gangetic Plains of India. Curr. Sci. 2014, 1470–1486. [Google Scholar]
- Tollefson, M. Introduction: Plot (), Qplot (), and Ggplot (), Plus Some. In Visualizing Data in R 4; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–7. [Google Scholar]
- Mulat, Y.; Kibret, K.; Bedadi, B.; Mohammed, M. Soil Quality Evaluation under Different Land Use Types in Kersa Sub-Watershed, Eastern Ethiopia. Environ. Syst. Res. 2021, 10, 19. [Google Scholar] [CrossRef]
- Abad, J.R.S.; Khosravi, H.; Alamdarlou, E.H. Assessment the Effects of Land Use Changes on Soil Physicochemical Properties in Jafarabad of Golestan Province, Iran. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 296–300. [Google Scholar]
- Sheik, C.S.; Mitchell, T.W.; Rizvi, F.Z.; Rehman, Y.; Faisal, M.; Hasnain, S.; McInerney, M.J.; Krumholz, L.R. Exposure of Soil Microbial Communities to Chromium and Arsenic Alters Their Diversity and Structure. PLoS ONE 2012, 7, e40059. [Google Scholar] [CrossRef]
- Ayele, T.; Beyene, S.; Esayas, A. Changes in Land Use on Soil Physicochemical Properties: The Case of Smallholders Fruit-Based Land Use Systems in Arba Minch, Southern Ethiopia. Int. J. Curr. Res. 2013, 5, 3203–3210. [Google Scholar]
- Sonaimuthu, M.; Sivakumar, K.; Rajan, K.; Tilak, M.; Sudhagar, R.J.; Raja, P.; Dinesh, D. Effect of Land Uses on Soil Physical Qualities in Mountainous Ecosystem of Western Ghats, India. J. Indian. Soc. Soil. Sci. 2018, 66, 249–257. [Google Scholar] [CrossRef]
- Yeshaneh, G.T. Assessment of Soil Fertility Variation in Different Land Uses and Management Practices in Maybar Watershed, South Wollo Zone, North Ethiopia. Int. J. Environ. Bioremedi. Biodegrad. 2015, 3, 15–22. [Google Scholar]
- Li, Y.; Lindstrom, M.J. Evaluating Soil Quality–Soil Redistribution Relationship on Terraces and Steep Hillslope. Soil. Sci. Soc. Am. J. 2001, 65, 1500–1508. [Google Scholar] [CrossRef]
- Lobe, I.; Amelung, W.; du Preez, C.C. Losses of Carbon and Nitrogen with Prolonged Arable Cropping from Sandy Soils of the South African Highveld. Eur. J. Soil. Sci. 2001, 52, 93–101. [Google Scholar] [CrossRef]
- Padbhushan, R.; Kumar, U.; Sharma, S.; Rana, D.S.; Kumar, R.; Kohli, A.; Kumari, P.; Parmar, B.; Kaviraj, M.; Sinha, A.K. Impact of Land-Use Changes on Soil Properties and Carbon Pools in India: A Meta-Analysis. Front. Environ. Sci. 2022, 722, 794866. [Google Scholar] [CrossRef]
- Getachew, F.; Abdulkadir, A.; Lemenih, M.; Fetene, A. Effects of Different Land Uses on Soil Physical and Chemical Properties in Wondo Genet Area, Ethiopia. NY Sci. J. 2012, 5, 110–118. [Google Scholar]
- Chimdi, A.; Gebrekidan, H.; Kibret, K.; Tadesse, A. Status of Selected Physicochemical Properties of Soils under Different Land Use Systems of Western Oromia, Ethiopia. J. Biodivers. Environ. Sci. 2012, 2, 57–71. [Google Scholar]
- Zhang, Y.; Zhao, Y.C.; Shi, X.Z.; Lu, X.X.; Yu, D.S.; Wang, H.J.; Sun, W.X.; Darilek, J.L. Variation of Soil Organic Carbon Estimates in Mountain Regions: A Case Study from Southwest China. Geoderma 2008, 146, 449–456. [Google Scholar] [CrossRef]
- Kakaire, J.; Makokha, G.L.; Mwanjalolo, M.; Mensah, A.K.; Menya, E. Effects of Mulching on Soil Hydro-Physical Properties in Kibaale Sub-Catchment, South Central Uganda. Appl. Ecol. Environ. Sci. 2015, 3, 127–135. [Google Scholar]
- Ravina da Silva, M. Impact of Eucalyptus Plantations on Pasture Land on Soil Properties and Carbon Sequestration in Brazil. 2014. Available online: https://stud.epsilon.slu.se/6394/7/ravina_da_silva_m_140130.pdf (accessed on 29 August 2023).
- Moges, A.; Holden, N.M. Soil Fertility in Relation to Slope Position and Agricultural Land Use: A Case Study of Umbulo Catchment in Southern Ethiopia. Environ. Manag. 2008, 42, 753–763. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Harris, N.L.; Zutta, B.R.; Saatchi, S.S.; Brown, S.; Salas, W.; Buermann, W.; Silman, M.; Petrova, S.; Lewis, S.L. Benchmark Map of Forest Carbon Stocks in Tropical Regions across Three Continents. Proc. Natl. Acad. Sci. USA 2010, 108, 9899–9904. [Google Scholar]
- Kolay, A.K.; Kolay, A.K. Basic Concepts of Soil Science; New Age International (P) Limited, Publishers: Delhi, India, 2002. [Google Scholar]
- Blanco-Canqui, H.; Lal, R. Mechanisms of Carbon Sequestration in Soil Aggregates. CRC Crit. Rev. Plant Sci. 2004, 23, 481–504. [Google Scholar] [CrossRef]
- Sparling, G.P. Ratio of Microbial Biomass Carbon to Soil Organic Carbon as a Sensitive Indicator of Changes in Soil Organic Matter. Soil. Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Ayoubi, S.; Khormali, F.; Sahrawat, K.L.; de Lima, A.C.R. Assessing Impacts of Land Use Change on Soil Quality Indicators in a Loessial Soil in Golestan Province, Iran. J. Agr. Sci. Tech. 2011, 13, 727–742. [Google Scholar]
- Selassie, Y.G.; Ayanna, G. Effects of Different Land Use Systems on Selected Physico-Chemical Properties of Soils in Northwestern Ethiopia. J. Agric. Sci. 2013, 5, 112. [Google Scholar] [CrossRef]
- Santos-Francés, F.; Martínez-Graña, A.; Ávila-Zarza, C.; Criado, M.; Sánchez-Sánchez, Y. Soil Quality and Evaluation of Spatial Variability in a Semi-Arid Ecosystem in a Region of the Southeastern Iberian Peninsula (Spain). Land 2021, 11, 5. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of Organic Carbon in Deep Soil Layers Controlled by Fresh Carbon Supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- da Cunha, E.; Walter, F.; Smail, I.R.; Swinbank, A.M.; Simpson, J.M.; Decarli, R.; Hodge, J.A.; Weiss, A.; van der Werf, P.P.; Bertoldi, F. An ALMA Survey of Sub-Millimeter Galaxies in the Extended Chandra Deep Field South: Physical Properties Derived from Ultraviolet-to-Radio Modeling. Astrophys. J. 2015, 806, 110. [Google Scholar] [CrossRef]
- Hillel, D. Environmental Soil Physics; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Diaz-Ravina, M.; Carballas, T.; Acea, M.J. Microbial Biomass and Metabolic Activity in Four Acid Soils. Soil. Biol. Biochem. 1988, 20, 817–823. [Google Scholar] [CrossRef]
- Mustafa, A.; Minggang, X.; Shah, S.; Abrar, M.M.; Nan, S.; Baoren, W.; Núñez-Delgado, A. Soil aggregation and soil aggregate stability regulate organic carbon and nitrogen storage in a red soil of southern China. J. Environ. Manage. 2020, 270, 110894. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Han, X.; Li, Y.; Li, Y.; He, B.; Duan, X. Effects of Past Land Use on Soil Organic Carbon Changes after Dam Construction. Sci. Total Environ. 2019, 686, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Cengage Learning: Boston, MA, USA, 2013; ISBN 1285607198. [Google Scholar]
- Nimmo, J.R. Porosity and Pore Size Distribution. Encycl. Soils Environ. 2004, 3, 295–303. [Google Scholar]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Rao, C.S.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U. Soil Organic Carbon Dynamics: Impact of Land Use Changes and Management Practices: A Review. Adv. Agron. 2019, 156, 1–107. [Google Scholar]
- Wubie, M.A.; Assen, M. Effects of Land Cover Changes and Slope Gradient on Soil Quality in the Gumara Watershed, Lake Tana Basin of North–West Ethiopia. Model. Earth Syst. Environ. 2020, 6, 85–97. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Huang, C.; Liu, Y.; Bu, Z. Impact of Land Use Change on Profile Distributions of Organic Carbon Fractions in Peat and Mineral Soils in Northeast China. Catena 2017, 152, 1–8. [Google Scholar] [CrossRef]
- Zerga, B. Ecological Impacts of Eucalyptus Plantation in Eza Wereda, Ethiopia. Int. Inv. J. Agric. Soil. Sci. 2015, 3, 47–51. [Google Scholar]
- Pan, Y.; Wang, X. Factors Controlling the Spatial Variability of Surface Soil Moisture within Revegetated-stabilized Desert Ecosystems of the Tengger Desert, Northern China. Hydrol. Process. An. Int. J. 2009, 23, 1591–1601. [Google Scholar] [CrossRef]
- Chaves, J.; Neill, C.; Germer, S.; Neto, S.G.; Krusche, A.; Elsenbeer, H. Land Management Impacts on Runoff Sources in Small Amazon Watersheds. Hydrol. Process. An. Int. J. 2008, 22, 1766–1775. [Google Scholar] [CrossRef]
- Brevik, E.; Cerdà, A.; Mataix-S Olera., J.; Pereg, L.; Quinton, J.; Six, J.; Van Oost, K. The interdisciplinary nature of SOIL. Soil 2015, 1, 117–129. [Google Scholar] [CrossRef]
- Cerdà, A. Soil Aggregate Stability under Different Mediterranean Vegetation Types. Catena 1998, 32, 73–86. [Google Scholar] [CrossRef]
- Tellen, V.A.; Yerima, B.P.K. Effects of Land Use Change on Soil Physicochemical Properties in Selected Areas in the North West Region of Cameroon. Environ. Syst. Res. 2018, 7, 1–29. [Google Scholar]
- Cao, Y.; Fu, S.; Zou, X.; Cao, H.; Shao, Y.; Zhou, L. Soil Microbial Community Composition under Eucalyptus Plantations of Different Age in Subtropical China. Eur. J. Soil. Biol. 2010, 46, 128–135. [Google Scholar] [CrossRef]
- Gomez, A.A.; Kelly, D.E.S.; Syers, J.K.; Coughlan, K.J. Measuring Sustainability of Agricultural Systems at the Farm Level. Methods Assess. Soil. Qual. 1997, 49, 401–410. [Google Scholar]
- Gonzalez, R.F.; Cooperband, L.R. Compost Effects on Soil Physical Properties and Field Nursery Production. Compos. Compost. Sci. Util. 2002, 10, 226–237. [Google Scholar] [CrossRef]
- Hudson, B.D. Soil Organic Matter and Available Water Capacity. J. Soil. Water Conserv. 1994, 49, 189–194. [Google Scholar]
- Keller, T.; Sutter, J.A.; Nissen, K.; Rydberg, T. Using Field Measurement of Saturated Soil Hydraulic Conductivity to Detect Low-Yielding Zones in Three Swedish Fields. Soil. Tillage Res. 2012, 124, 68–77. [Google Scholar] [CrossRef]
- Bodner, G.; Scholl, P.; Loiskandl, W.; Kaul, H.-P. Environmental and Management Influences on Temporal Variability of near Saturated Soil Hydraulic Properties. Geoderma 2013, 204, 120–129. [Google Scholar] [CrossRef]
- Jarvis, N.; Koestel, J.; Messing, I.; Moeys, J.; Lindahl, A. Influence of Soil, Land Use and Climatic Factors on the Hydraulic Conductivity of Soil. Hydrol. Earth Syst. Sci. 2013, 17, 5185–5195. [Google Scholar] [CrossRef]
- Rasse, D.P.; Smucker, A.J.M.; Santos, D. Alfalfa Root and Shoot Mulching Effects on Soil Hydraulic Properties and Aggregation. Soil. Sci. Soc. Am. J. 2000, 64, 725–731. [Google Scholar] [CrossRef]
- Wilcox, B.P.; Breshears, D.D.; Turin, H.J. Hydraulic Conductivity in a Piñon-juniper Woodland: Influence of Vegetation. Soil. Sci. Soc. Am. J. 2003, 67, 1243–1249. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Bergeron, Y.; Paré, D. Differences in Fine Root Productivity between Mixed-and Single-species Stands. Funct. Ecol. 2011, 25, 238–246. [Google Scholar] [CrossRef]
- Breulmann, M.; Schulz, E.; Weißhuhn, K.; Buscot, F. Impact of the Plant Community Composition on Labile Soil Organic Carbon, Soil Microbial Activity and Community Structure in Semi-Natural Grassland Ecosystems of Different Productivity. Plant Soil. 2012, 352, 253–265. [Google Scholar] [CrossRef]
- Cookson, W.R.; Osman, M.; Marschner, P.; Abaye, D.A.; Clark, I.; Murphy, D.V.; Stockdale, E.A.; Watson, C.A. Controls on Soil Nitrogen Cycling and Microbial Community Composition across Land Use and Incubation Temperature. Soil. Biol. Biochem. 2007, 39, 744–756. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Gale, W.J.; Zhang, X.; Li, L. Dynamics of Aggregate-Associated Organic Carbon Following Conversion of Forest to Cropland. Soil. Biol. Biochem. 2013, 57, 876–883. [Google Scholar] [CrossRef]
- Vandevivere, P.; Baveye, P. Effect of Bacterial Extracellular Polymers on the Saturated Hydraulic Conductivity of Sand Columns. Appl. Environ. Microbiol. 1992, 58, 1690–1698. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, J.; Meng, M.; Chen, H.Y.H.; Guo, X.; Liu, S.; Ye, L. Impacts of Changes in Vegetation on Saturated Hydraulic Conductivity of Soil in Subtropical Forests. Sci. Rep. 2019, 9, 8372. [Google Scholar] [CrossRef]
- Celik, I.; Ortas, I.; Kilic, S. Effects of Compost, Mycorrhiza, Manure and Fertilizer on Some Physical Properties of a Chromoxerert Soil. Soil. Tillage Res. 2004, 78, 59–67. [Google Scholar] [CrossRef]
- Fu, T.; Chen, H.; Zhang, W.; Nie, Y.; Wang, K. Vertical Distribution of Soil Saturated Hydraulic Conductivity and Its Influencing Factors in a Small Karst Catchment in Southwest China. Environ. Monit. Assess. 2015, 187, 92. [Google Scholar] [CrossRef]
- Van Lier, Q.J.; Wendroth, O.; van Dam, J.C. Prediction of Winter Wheat Yield with the SWAP Model Using Pedotransfer Functions: An Evaluation of Sensitivity, Parameterization and Prediction Accuracy. Agric. Water Manag. 2015, 154, 29–42. [Google Scholar] [CrossRef]
- Chapuis, R.P. Predicting the Saturated Hydraulic Conductivity of Soils: A Review. Bull. Eng. Geol. Environ. 2012, 71, 401–434. [Google Scholar] [CrossRef]
- Ben-Hur, M.; Yolcu, G.; Uysal, H.; Lado, M.; Paz, A. Soil Structure Changes: Aggregate Size and Soil Texture Effects on Hydraulic Conductivity under Different Saline and Sodic Conditions. Soil. Res. 2009, 47, 688–696. [Google Scholar] [CrossRef]
- Aggelides, S.M.; Londra, P.A. Effects of Compost Produced from Town Wastes and Sewage Sludge on the Physical Properties of a Loamy and a Clay Soil. Bioresour. Technol. 2000, 71, 253–259. [Google Scholar] [CrossRef]
- Benjamin, J.G.; Mikha, M.M.; Vigil, M.F. Organic Carbon Effects on Soil Physical and Hydraulic Properties in a Semiarid Climate. Soil. Sci. Soc. Am. J. 2008, 72, 1357–1362. [Google Scholar] [CrossRef]
- Lawrence, C.R.; Harden, J.W.; Xu, X.; Schulz, M.S.; Trumbore, S.E. Long-term controls on soil organic carbon with depth and time: A case study from the Cowlitz River Chronosequence, WA USA. Geoderma. 2015, 247, 73–87. [Google Scholar] [CrossRef]
- Abiven, S.; Menasseri, S.; Chenu, C. The Effects of Organic Inputs over Time on Soil Aggregate Stability–A Literature Analysis. Soil. Biol. Biochem. 2009, 41, 1–12. [Google Scholar] [CrossRef]
- Amézketa, E. Soil Aggregate Stability: A Review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil Structure and Management: A Review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Kay, B.D. Soil Structure and Organic Carbon: A Review. Soil Structure and Organic Carbon: A Review; CRC Press: Boca Raton, FL, USA, 2018; pp. 169–197. [Google Scholar]
- Angers, D.A.; Eriksen-Hamel, N.S. Full-inversion Tillage and Organic Carbon Distribution in Soil Profiles: A Meta-analysis. Soil. Sci. Soc. Am. J. 2008, 72, 1370–1374. [Google Scholar] [CrossRef]
- Field, D.J.; Sullivan, L.A.; Cattle, S.R.; Koppi, A.J. Comparison of Four Methods for Liberating Various Aggregate Fractions in Vertosols to Study Their Morphology. Soil. Res. 2004, 42, 29–37. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil Macroaggregate Turnover and Microaggregate Formation: A Mechanism for C Sequestration under No-Tillage Agriculture. Soil. Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Minasny, B.; Field, D.J. Measurement of Aggregate Bond Energy Using Ultrasonic Dispersion. Eur. J. Soil. Sci. 2009, 60, 695–705. [Google Scholar] [CrossRef]
- Arshad, M.A.; Coen, G.M. Characterization of Soil Quality: Physical and Chemical Criteria. Am. J. Altern. Agric. 1992, 7, 25–31. [Google Scholar] [CrossRef]
- Hortensius, D.; Welling, R. International Standardization of Soil Quality Measurements. Commun. Soil. Sci. Plant Anal. 1996, 27, 387–402. [Google Scholar] [CrossRef]
- Duiker, S.W.; Rhoton, F.E.; Torrent, J.; Smeck, N.E.; Lal, R. Iron (Hydr) Oxide Crystallinity Effects on Soil Aggregation. Soil. Sci. Soc. Am. J. 2003, 67, 606–611. [Google Scholar] [CrossRef]
- Malamoud, K.; McBratney, A.B.; Minasny, B.; Field, D.J. Modelling How Carbon Affects Soil Structure. Geoderma 2009, 149, 19–26. [Google Scholar] [CrossRef]
- Onweremadu, E.U.; Onyia, V.N.; Anikwe, M.A.N. Carbon and Nitrogen Distribution in Water-Stable Aggregates under Two Tillage Techniques in Fluvisols of Owerri Area, Southeastern Nigeria. Soil. Tillage Res. 2007, 97, 195–206. [Google Scholar] [CrossRef]
- Daynes, C.N.; Field, D.J.; Saleeba, J.A.; Cole, M.A.; McGee, P.A. Development and Stabilisation of Soil Structure via Interactions between Organic Matter, Arbuscular Mycorrhizal Fungi and Plant Roots. Soil. Biol. Biochem. 2013, 57, 683–694. [Google Scholar] [CrossRef]
- Six, J.; Callewaert, P.; Lenders, S.; de Gryze, S.; Morris, S.J.; Gregorich, E.G.; Paul, E.A.; Paustian, K. Measuring and Understanding Carbon Storage in Afforested Soils by Physical Fractionation. Soil. Sci. Soc. Am. J. 2002, 66, 1981–1987. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; de Courcelles, V.R.; Singh, K. The Knowns, Known Unknowns and Unknowns of Sequestration of Soil Organic Carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Singh, B.R.; Sitaula, B.K.; Lal, R.; Bajracharya, R.M. Soil Aggregate-and Particle-associated Organic Carbon under Different Land Uses in Nepal. Soil. Sci. Soc. Am. J. 2007, 71, 1194–1203. [Google Scholar] [CrossRef]
- Dorji, T.; Field, D.J.; Odeh, I.O.A. Soil Aggregate Stability and Aggregate-associated Organic Carbon under Different Land Use or Land Cover Types. Soil. Use Manag. 2020, 36, 308–319. [Google Scholar] [CrossRef]
- García-Orenes, F.; Guerrero, C.; Mataix-Solera, J.; Navarro-Pedreño, J.; Gómez, I.; Mataix-Beneyto, J. Factors Controlling the Aggregate Stability and Bulk Density in Two Different Degraded Soils Amended with Biosolids. Soil. Tillage Res. 2005, 82, 65–76. [Google Scholar] [CrossRef]
- Buczko, U.; Bens, O.; Hüttl, R.F. Water Infiltration and Hydrophobicity in Forest Soils of a Pine–Beech Transformation Chronosequence. J. Hydrol. 2006, 331, 383–395. [Google Scholar] [CrossRef]
- Piccolo, A.; Mbagwu, J.S.C. Role of Hydrophobic Components of Soil Organic Matter in Soil Aggregate Stability. Soil. Sci. Soc. Am. J. 1999, 63, 1801–1810. [Google Scholar] [CrossRef]
- Goebel, M.-O.; Bachmann, J.; Woche, S.K.; Fischer, W.R. Soil Wettability, Aggregate Stability, and the Decomposition of Soil Organic Matter. Geoderma 2005, 128, 80–93. [Google Scholar] [CrossRef]
- Kumari, M.; Chakraborty, D.; Gathala, M.K.; Pathak, H.; Dwivedi, B.S.; Tomar, R.K.; Garg, R.N.; Singh, R.; Ladha, J.K. Soil Aggregation and Associated Organic Carbon Fractions as Affected by Tillage in a Rice–Wheat Rotation in North India. Soil. Sci. Soc. Am. J. 2011, 75, 560–567. [Google Scholar] [CrossRef]
- Ayoubi, S.; Karchegani, P.M.; Mosaddeghi, M.R.; Honarjoo, N. Soil Aggregation and Organic Carbon as Affected by Topography and Land Use Change in Western Iran. Soil. Tillage Res. 2012, 121, 18–26. [Google Scholar] [CrossRef]
- Liu, M.Y.; Chang, Q.R.; Qi, Y.B.; Liu, J.; Chen, T. Aggregation and soil organic carbon fractions under different land uses on the tableland of the Loess Plateau of China. Catena 2014, 115, 19–28. [Google Scholar]
- An, S.; Mentler, A.; Mayer, H.; Blum, W.E.H. Soil Aggregation, Aggregate Stability, Organic Carbon and Nitrogen in Different Soil Aggregate Fractions under Forest and Shrub Vegetation on the Loess Plateau, China. Catena 2010, 81, 226–233. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K.; Elliott, E.T.; Combrink, C. Soil Structure and Organic Matter I. Distribution of Aggregate-size Classes and Aggregate-associated Carbon. Soil. Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Wei, G.; Zhou, Z.; Guo, Y.; Dong, Y.; Dang, H.; Wang, Y.; Ma, J. Long-Term Effects of Tillage on Soil Aggregates and the Distribution of Soil Organic Carbon, Total Nitrogen, and Other Nutrients in Aggregates on the Semi-Arid Loess Plateau, China. Arid. Land. Res. Manag. 2014, 28, 291–310. [Google Scholar] [CrossRef]
- Dai, J.; Hu, J.; Zhu, A.; Bai, J.; Wang, J.; Lin, X. No Tillage Enhances Arbuscular Mycorrhizal Fungal Population, Glomalin-Related Soil Protein Content, and Organic Carbon Accumulation in Soil Macroaggregates. J. Soils Sediments 2015, 15, 1055–1062. [Google Scholar] [CrossRef]
- Du, Z.; Ren, T.; Hu, C.; Zhang, Q. Transition from Intensive Tillage to No-till Enhances Carbon Sequestration in Microaggregates of Surface Soil in the North China Plain. Soil. Tillage Res. 2015, 146, 26–31. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Srivastava, A.K.; Cao, M.-Q.; Wang, J. Mycorrhizal Function on Soil Aggregate Stability in Root Zone and Root-Free Hyphae Zone of Trifoliate Orange. Arch. Agron. Soil. Sci. 2015, 61, 813–825. [Google Scholar] [CrossRef]
- Mekuria, W.; Aynekulu, E. Exclosure Land Management for Restoration of the Soils in Degraded Communal Grazing Lands in Northern Ethiopia. Land. Degrad. Dev. 2013, 24, 528–538. [Google Scholar] [CrossRef]
- Damene, S.; Tamene, L.; Vlek, P.L.G. Performance of Exclosure in Restoring Soil Fertility: A Case of Gubalafto District in North Wello Zone, Northern Highlands of Ethiopia. Catena 2013, 101, 136–142. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Carbon Balance in Terrestrial Detritus. Annu. Rev. Ecol. Syst. 1977, 8, 51–81. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, H.; Zhang, T.; Zhao, X.; Drake, S. Community Succession along a Chronosequence of Vegetation Restoration on Sand Dunes in Horqin Sandy Land. J. Arid. Environ. 2005, 62, 555–566. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Soil Carbon Content Drives the Biogeographical Distribution of Fungal Communities in the Black Soil Zone of Northeast China. Soil. Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Houben, D.; Faucon, M.-P.; Mercadal, A.-M. Response of Organic Matter Decomposition to No-Tillage Adoption Evaluated by the Tea Bag Technique. Soil. Syst. 2018, 2, 42. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994; ISBN 0471594741. [Google Scholar]
- Six, J.; Ogle, S.M.; Jay Breidt, F.; Conant, R.T.; Mosier, A.R.; Paustian, K. The Potential to Mitigate Global Warming with No-tillage Management Is Only Realized When Practised in the Long Term. Glob. Chang. Biol. 2004, 10, 155–160. [Google Scholar] [CrossRef]



| Sl. No | Soil Properties | Methodology | Reference |
|---|---|---|---|
| 1 | Bulk density | The cylinder method | [32] |
| 2 | Particle density | The cylinder method | [32] |
| 3 | Pore space | The cylinder method | [32] |
| 4 | Soil texture | International pipette method | [33] |
| 5 | Available soil moisture | Gravimetric method | [34] |
| Class | Value Range | Grade |
|---|---|---|
| I | 90–100 | Best |
| II | 80–90 | Very good |
| III | 70–80 | Good |
| IV | 60–70 | Average |
| V | <60 | Poor |
| S. No | Ecosystem | Additive Index (AI) | RSQI (AI) (%) | Weighted Index (WI) | RSQI (WI) (%) |
|---|---|---|---|---|---|
| 1. | Evergreen | 7.32 | 100.00 | 0.53 | 100.00 |
| 2. | Deciduous | 6.75 | 92.16 | 0.51 | 95.69 |
| 3. | Scrubland | 6.04 | 82.52 | 0.44 | 81.92 |
| 4. | Forest plantation | 6.54 | 89.33 | 0.52 | 97.49 |
| 5. | Tea plantation | 5.04 | 68.92 | 0.33 | 62.39 |
| 6. | Cropland | 4.87 | 66.57 | 0.31 | 58.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagadesh, M.; Selvi, D.; Thiyageshwari, S.; Srinivasarao, C.; Raja, P.; Surendran, U.P.; Al-Ansari, N.; Mattar, M.A. Altering Natural Ecosystems Causes Negative Consequences on the Soil Physical Qualities: An Evidence-Based Study from Nilgiri Hill Region of Western Ghats, India. Land 2023, 12, 1869. https://doi.org/10.3390/land12101869
Jagadesh M, Selvi D, Thiyageshwari S, Srinivasarao C, Raja P, Surendran UP, Al-Ansari N, Mattar MA. Altering Natural Ecosystems Causes Negative Consequences on the Soil Physical Qualities: An Evidence-Based Study from Nilgiri Hill Region of Western Ghats, India. Land. 2023; 12(10):1869. https://doi.org/10.3390/land12101869
Chicago/Turabian StyleJagadesh, M., Duraisamy Selvi, Subramanium Thiyageshwari, Cherukumalli Srinivasarao, Pushpanathan Raja, Udayar Pillai Surendran, Nadhir Al-Ansari, and Mohamed A. Mattar. 2023. "Altering Natural Ecosystems Causes Negative Consequences on the Soil Physical Qualities: An Evidence-Based Study from Nilgiri Hill Region of Western Ghats, India" Land 12, no. 10: 1869. https://doi.org/10.3390/land12101869
APA StyleJagadesh, M., Selvi, D., Thiyageshwari, S., Srinivasarao, C., Raja, P., Surendran, U. P., Al-Ansari, N., & Mattar, M. A. (2023). Altering Natural Ecosystems Causes Negative Consequences on the Soil Physical Qualities: An Evidence-Based Study from Nilgiri Hill Region of Western Ghats, India. Land, 12(10), 1869. https://doi.org/10.3390/land12101869










