Selenium Nanoparticles Improve Physiological and Phytochemical Properties of Basil (Ocimum basilicum L.) under Drought Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Treatments
2.3. Measurements
2.3.1. Agronomic Traits and Dry Yield
2.3.2. Essential Oil Extraction and Analysis
2.3.3. Chlorophylls and Carotenoid
2.3.4. Proline
2.3.5. Total Soluble Sugars
2.4. Phenolic Acid
2.5. Statistical Analysis
3. Results
3.1. Agronomic Traits
3.2. Dry Yield
3.3. Essential Oil Content
3.4. Essential Oil Yield
3.5. Essential Oil Constituents
3.6. Phenolic Compounds
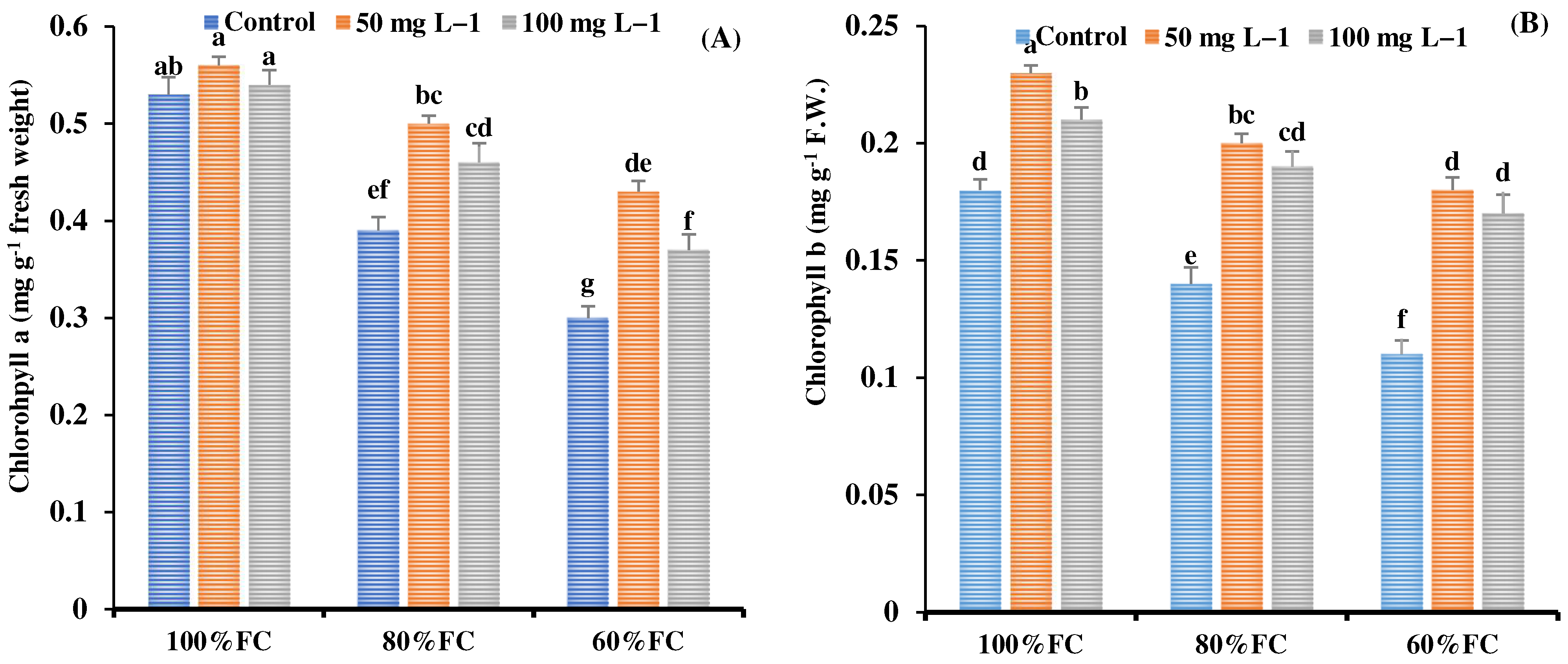
3.7. Chlorophyll Content
3.8. Carotenoid Content
3.9. Soluble Sugar Content
3.10. Proline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langworthy, A.D.; Rawnsley, R.P.; Freeman, M.J.; Pembleton, K.G.; Corkrey, R.; Harrison, M.T.; Lane, P.A.; Henry, D.A. Potential of summer-active temperate (C3) perennial forages to mitigate the detrimental effects of supraoptimal temperatures on summer home-grown feed production in south-eastern Australian dairying regions. Crop Pasture Sci. 2018, 69, 808–820. [Google Scholar] [CrossRef]
- Ibrahim, A.; Harrison, M.; Meinke, H.; Fan, Y.; Johnson, P.; Zhou, M. A regulator of early flowering in barley (Hordeum vulgare L.). PLoS ONE 2018, 13, e0200722. [Google Scholar] [CrossRef] [PubMed]
- Falster, D.; Gallagher, R.; Wenk, E.H.; Wright, I.J.; Indiarto, D.; Andrew, S.C.; Baxter, C.; Lawson, J.; Allen, S.; Fuchs, A.; et al. AusTraits, a curated plant trait database for the Australian flora. Sci. Data 2021, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Ostadi, A.; Javanmard, A.; Amani Machiani, M.; Sadeghpour, A.; Maggi, F.; Nouraein, M.; Morshedloo, M.R.; Hano, C.; Lorenzo, J.M. Co-Application of TiO2 Nanoparticles and Arbuscular Mycorrhizal Fungi Improves Essential Oil Quantity and Quality of Sage (Salvia officinalis L.) in Drought Stress Conditions. Plants 2022, 11, 1659. [Google Scholar] [CrossRef] [PubMed]
- Rawnsley, R.P.; Smith, A.P.; Christie, K.M.; Harrison, M.T.; Eckard, R.J. Current and future direction of nitrogen fertilizer use in Australian grazing systems. Crop Pasture Sci. 2019, 70, 1034–1043. [Google Scholar] [CrossRef]
- Christie, K.M.; Smith, A.P.; Rawnsley, R.P.; Harrison, M.T.; Eckard, R.J. Simulated seasonal responses of grazed dairy pastures to nitrogen fertilizer in SE Australia: N loss and recovery. Agric. Syst. 2020, 182, 102847. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Shabala, S.N.; Meinke, H.B.; Ahmed, I.; Zhang, Y.; Tian, X.; Zhou, M. The state of the art in modeling waterlogging impacts on plants: What do we know and what do we need to know. Earth’s Future 2020, 8, e2020EF001801. [Google Scholar] [CrossRef]
- Farina, R.; Sándor, R.; Abdalla, M.; Álvaro-Fuentes, J.; Bechini, L.; Bolinder, M.A.; Brilli, L.; Chenu, C.; Clivot, H.; De Antoni Migliorati, M.; et al. Ensemble modelling, uncertainty and robust predictions of organic carbon in long-term bare-fallow soils. Glob. Chang. Biol. 2021, 27, 904–928. [Google Scholar] [CrossRef]
- Chen, J.; Qi, T.; Hu, Z.; Fan, X.; Zhu, L.; Iqbal, M.F.; Yin, X.; Xu, G.; Fan, X. OsNAR2.1 positively regulates drought tolerance and grain yield under drought stress conditions in rice. Front. Plant Sci. 2019, 10, 197. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. A review on potential plant-based water stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Chen, H.Y.H.; Ruan, H. Response of plants to water stress: A meta-analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef]
- Ibrahim, A.; Harrison, M.T.; Meinke, H.; Zhou, M. Examining the yield potential of barley near-isogenic lines using a genotype by environment by management analysis. Eur. J. Agron. 2019, 105, 41–51. [Google Scholar] [CrossRef]
- Ostadi, A.; Javanmard, A.; Amani Machiani, M.; Kakaei, K. Optimizing Antioxidant Activity and Phytochemical Properties of Peppermint (Mentha piperita L.) by Integrative Application of Biofertilizer and Stress-Modulating Nanoparticles under Drought Stress Conditions. Plants 2023, 12, 151. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and Use of Selenium Nanoparticles as Fertilizers. ACS Omega 2020, 28, 17767–17774. [Google Scholar] [CrossRef]
- Bano, I.; Skalickova, S.; Sajjad, H.; Skladanka, J.; Horky, P. Uses of selenium nanoparticles in the plant production. Agronomy 2021, 11, 2229. [Google Scholar] [CrossRef]
- Zakeri, N.; kelishadi, M.R.; Asbaghi, O.; Naeini, F.; Afsharfar, M.; Mirzadeh, E.; Naserizadeh, S.K. Selenium supplementation and oxidative stress: A review. PharmaNutrition 2021, 17, 100263. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Habibi Machiani, R.; Sadeghpour, A. Arbuscular mycorrhizal fungi and changes in primary and secondary metabolites. Plants 2022, 11, 2183. [Google Scholar] [CrossRef]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive compounds and aroma profile of some Lamiaceae edible flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef]
- Egata, D. Benefit and use of Sweet Basil (Ocimum Basilicum L.) In Ethiopia: A Review. Nutr. Food Process. 2021, 4, 57–60. [Google Scholar] [CrossRef]
- Srivastava, S.; Lal, R.K.; Yadav, K.; Pant, Y.; Bawitlung, L.; Kumar, P.; Mishra, A.; Gupta, P.; Pal, A.; Rout, P.K.; et al. Chemical composition of phenylpropanoid rich chemotypes of Ocimum basilicum L. and their antimicrobial activities. Ind. Crops Prod. 2022, 183, 114978. [Google Scholar] [CrossRef]
- Zagoto, M.; Cardia, G.F.E.; Rocha, E.M.T.; da Mourão, K.S.M.; Janeiro, V.; Cuman, R.K.N.; Pinto, A.A.; Contiero, R.L.; Freitas, P.S.L. Biological activities of basil essential oil: A review of the current evidence. Res. Soc. Dev. 2021, 10, 1–8. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Amani Machiani, M.; Javanmard, A.; Mahdavikia, H.; Maggi, F.; Morshedloo, M.R. Vermicompost application in different intercropping patterns improves the mineral nutrient uptake and essential oil compositions of Sweet Basil (Ocimum basilicum L.). J. Soil Sci. Plant Nutr. 2021, 21, 450–466. [Google Scholar] [CrossRef]
- Perveen, K.; Bokhari, N.A.; Al-Rashid, S.A.I.; Al-Humaid, L.A. Chemical Composition of Essential Oil of Ocimum basilicum L. and Its Potential in Managing the Alternaria Rot of Tomato. J. Essent. Oil-Bearing Plants 2020, 23, 1428–1437. [Google Scholar] [CrossRef]
- Harrison, M.T. Climate change benefits negated by extreme heat. Nat. Food 2021, 2, 855–856. [Google Scholar] [CrossRef]
- Harrison, M.T.; Cullen, B.R.; Mayberry, D.E.; Cowie, A.L.; Bilotto, F.; Badgery, W.B.; Liu, K.; Davison, T.; Christie, K.M.; Muleke, A.; et al. Carbon myopia: The urgent need for integrated social, economic and environmental action in the livestock sector. Glob. Chang. Biol. 2021, 27, 5726–5761. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Mahdavikia, H.; Hadi, H.; Alipour, H.; Kulak, M.; Caruso, G.; Siddique, K.H.M. The effect of exogenously applied plant growth regulators and zinc on some physiological characteristics and essential oil constituents of moldavian balm (Dracocephalum moldavica L.) under water stress. Physiol. Mol. Biol. Plants 2021, 27, 2021–2214. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant 1992, 84, 67–72. [Google Scholar] [CrossRef]
- Namazi, Y.; Rezaei-Chiyaneh, E.; Moghaddam, S.S.; Battaglia, M.L. The effects of microbial inoculation and intercropping on yield and active ingredients of savory (Satureja hortensis L.) intercropped with common bean (Phaseolus vulgaris L.). Int. J. Environ. Sci. Technol. 2022, 19, 8273–8288. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Yu, S.; Huang, Y.; Liu, H.; Chen, W.; He, X. Effects of drought stress on growth, physiology and secondary metabolites of Two Adonis species in Northeast China. Sci. Hortic. 2020, 259, 108795. [Google Scholar] [CrossRef]
- Javanmard, A.; Ashrafi, M.; Morshedloo, M.R.; Machiani, M.A.; Rasouli, F.; Maggi, F. Optimizing Phytochemical and Physiological Characteristics of Balangu (Lallemantia iberica) by Foliar Application of Chitosan Nanoparticles and Myco-Root Inoculation under Water Supply Restrictions. Horticulturae 2022, 8, 695. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; De La Fuente, M.C.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.D.A.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147. [Google Scholar] [CrossRef]
- Jiang, C.; Zu, C.; Shen, J.; Shao, F.; Li, T. Effects of selenium on the growth and photosynthetic characteristics of flue-cured tobacco (Nicotiana tabacum L.). Acta Soc. Bot. Pol. 2015, 84, 71–77. [Google Scholar] [CrossRef]
- Kiumarzi, F.; Morshedloo, M.R.; Zahedi, S.M.; Mumivand, H.; Behtash, F.; Hano, C.; Chen, J.T.; Lorenzo, J.M. Selenium Nanoparticles (Se-NPs) Alleviates Salinity Damages and Improves Phytochemical Characteristics of Pineapple Mint (Mentha suaveolens Ehrh.). Plants 2022, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Aghaee, A.; Maggi, F. Funneliformis mosseae inoculation under water deficit stress improves the yield and phytochemical characteristics of thyme in intercropping with soybean. Sci. Rep. 2021, 11, 15279. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.; Oraei, M.; Gohari, G.; Panahirad, S.; Farmarzi, A. Chitosan-selenium nanoparticles (Cs–Se NPs) modulate the photosynthesis parameters, antioxidant enzymes activities and essential oils in Dracocephalum moldavica L. under cadmium toxicity stress. Plant Physiol. Biochem. 2021, 167, 257–268. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A.; Tokupova, E. Improvement of phenolic compounds, essential oil content and antioxidant properties of sweet basil (Ocimum basilicum L.) depending on type and concentration of selenium application. Plants 2019, 8, 458. [Google Scholar] [CrossRef]
- Li, D.; An, Q.; Wu, Y.; Li, J.Q.; Pan, C. Foliar application of selenium nanoparticles on celery stimulates several nutrient component levels by regulating the α-linolenic acid pathway. ACS Sustain. Chem. Eng. 2020, 8, 10502–10510. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Farag, R.E.; Shehata, S.A. Effect of putrescine application on some growth, biochemical and anatomical characteristics of Thymus vulgaris L. under drought stress. Ann. Agric. Sci. 2019, 64, 129–137. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Janmohammadi, M.; Maggi, F. Funneliformis mosseae application improves the oil quantity and quality and eco-physiological characteristics of Soybean (Glycine max L.) under water stress conditions. J. Soil Sci. Plant Nutr. 2021, 21, 3076–3090. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah, F.M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Seliem, M.K.; Hafez, Y.; El-Ramady, H. Using of Nano - Selenium in Reducing the Negative Effects of High Temperature Stress on Chrysanthemum morifolium Ramat. J. Sustain. Agric. Sci. 2020, 46, 47–59. [Google Scholar] [CrossRef]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef]
- Hussein, H.A.A.; Darwesh, O.M.; Mekki, B.B. Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101080. [Google Scholar] [CrossRef]



| Texture | pH | Electrical Conductivity (dS m−1) | Organic Matter (%) | Total N (%) | Phosphorus (%) | Potassium (%) |
|---|---|---|---|---|---|---|
| Silty | 8.25 | 0.88 | 1.30 | 0.13 | 10.33 | 211.6 |
| Canopy Diameter (cm) | Number of Leaves | Number of Lateral Branches | Dry Yield (g m−2) | Essential Oil Content (%) | Essential Oil Yield (g m−2) | |
|---|---|---|---|---|---|---|
| Irrigation levels (I) | ||||||
| FC100 | 54.22 a | 717.56 a | 20.55 a | 256.61 a | 0.68 c | 1.74 c |
| FC80 | 47.78 b | 663.78 b | 18.22 b | 218.58 b | 0.84 b | 1.84 b |
| FC60 | 44.11 c | 633.11 c | 15.11 c | 184.62 c | 1.03 a | 1.90 a |
| Se nanoparticles (S) | ||||||
| Control (0 mg L−1) | 46.22 b | 649.44 b | 16.67 b | 201.74 b | 0.67 b | 1.35 b |
| 50 mg L−1 | 50.22 a | 696.22 a | 19.11 a | 230.77 a | 0.89 a | 2.05 a |
| 100 mg L−1 | 49.67 a | 668.78 b | 18.11 a | 227.31 a | 0.91 a | 2.07 a |
| Source of variations | Significance | |||||
| I | ** | ** | ** | ** | ** | ** |
| S | * | * | ** | ** | ** | ** |
| I × S | NS | NS | NS | NS | NS | NS |
| Treatments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Components | RI a | FC100+ Control | FC100+ 50 mg L−1 Se | FC100+ 100 mg L−1 Se | FC80+ Control | FC100+ 50 mg L−1 Se | FC100+ 100 mg L−1 Se | FC60+ Control | FC100+ 50 mg L−1 Se | FC100+ 100 mg L−1 Se |
| 1 | α-Pinene | 925 | 0.21 | 0.18 | 0.18 | 0.21 | 0.2 | 0.19 | 0.17 | 0.22 | 0.17 |
| 2 | Sabinene | 964 | 0.12 | 0.1 | 0.09 | 0.12 | 0.11 | - | 0.09 | 0.13 | 0.09 |
| 3 | β-Pinene | 968 | 0.26 | 0.23 | 0.21 | 0.27 | 0.25 | 0.24 | 0.22 | 0.28 | 0.21 |
| 4 | Myrcene | 981 | 0.08 | 0.1 | 0.06 | 0.09 | 0.08 | 0.08 | 0.07 | 0.09 | 0.06 |
| 5 | 1,8-Cineole | 1024 | 4.83 | 5.43 | 4.91 | 5.87 | 5.81 | 5.54 | 5.61 | 6.15 | 5.83 |
| 6 | Linalool | 1097 | 37.78 | 38.89 | 38.09 | 39.09 | 41.51 | 41.09 | 38.12 | 39.23 | 40.06 |
| 7 | Terpinen-4-ol | 1177 | 1.18 | 0.83 | 0.91 | 0.55 | 0.61 | 0.34 | 0.56 | 0.19 | 0.57 |
| 8 | Methyl chavicol | 1195 | 39.74 | 42.45 | 42.62 | 42.80 | 43.61 | 43.08 | 41.93 | 42.35 | 41.42 |
| 9 | Neral | 1234 | 0.38 | 0.5 | - | - | 0.29 | - | 0.59 | 0.53 | 0.11 |
| 10 | Geraniol | 1249 | 0.72 | 0.92 | 0.1 | 0.21 | 0.1 | - | 0.55 | - | 0.05 |
| 11 | α-Copaene | 1369 | 0.07 | 0.09 | 0.08 | 0.07 | 0.07 | - | 0.09 | - | 0.08 |
| 12 | β-Cubebene | 1383 | 0.39 | 0.21 | 0.16 | 0.52 | 0.14 | 0.19 | 0.21 | 0.23 | 0.14 |
| 13 | Methyl eugenol | 1395 | 3.02 | 1.65 | 2.96 | 2.63 | 2.23 | 1.91 | 2.17 | 1.36 | 2.98 |
| 14 | trans-Caryophyllene | 1414 | 2.16 | 1.64 | 1.53 | 1.58 | 1.24 | 1.52 | 1.55 | 1.87 | 1.43 |
| 15 | (E)-β-Farnesene | 1433 | 0.05 | 0.06 | 0.05 | 0.07 | 0.07 | 0.06 | 0.06 | 0.08 | 0.06 |
| 16 | α-Humulene | 1447 | 0.84 | 0.67 | 0.64 | 0.7 | 0.58 | 0.62 | 0.67 | 0.8 | 0.63 |
| 17 | Germacrene D | 1475 | 1.46 | 0.96 | 1.02 | 0.88 | 0.79 | 0.98 | 0.23 | 0.82 | 0.99 |
| 18 | α-Bisabolene | 1498 | 0.08 | 0.09 | 0.09 | 0.08 | 0.08 | 0.09 | 1.12 | 0.11 | 0.08 |
| 19 | cis-α-Bisabolene | 1532 | 1.83 | 0.91 | 1.47 | 1.66 | 0.83 | 1.67 | 1.59 | 0.77 | 1.51 |
| 20 | Caryophyllene oxide | 1578 | 0.12 | 0.13 | 0.11 | 0.12 | 0.07 | 0.14 | 0.09 | - | |
| 21 | α-Bisabolol | 1674 | 0.05 | - | 0.05 | - | 0.13 | 0.05 | 0.07 | 0.06 | |
| Total identified (%) | 95.37 | 96.44 | 95.33 | 97.4 | 98.85 | 97.72 | 95.74 | 95.37 | 96.53 | ||
| ppm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Components | FC100+ Control | FC100+ 50 mg L−1 Se | FC100+ 100 mg L−1 Se | FC80+ Control | FC80+ 50 mg L−1 Se | FC80+ 100 mg L−1 Se | FC60+ Control | FC60+ 50 mg L−1 Se | FC60+ 100 mg L−1 Se |
| 1 | Gallic acid | 110.18 | 122.34 | 119.75 | 118.20 | 178.12 | 159.63 | 147.43 | 167.09 | 149.93 |
| 2 | Caffeic acid | 219.56 | 212.41 | 242.25 | 230.75 | 282.49 | 262.46 | 251.63 | 274.11 | 243.82 |
| 3 | Chlorogenic acid | 310.68 | 341.91 | 360.55 | 322.60 | 342.91 | 393.54 | 390.28 | 395.83 | 384.31 |
| 4 | Rutin | 42.72 | 43.36 | 49.78 | 42.23 | 53.38 | 59.16 | 43.38 | 44.29 | 50.20 |
| 5 | Comaric | 522.07 | 597.11 | 578.21 | 632.29 | 687.97 | 639.87 | 533.39 | 540.95 | 593.23 |
| 6 | Rosmaric acid | 31.01 | 33.58 | 32.79 | 35.56 | 38.78 | 39.59 | 37.90 | 46.56 | 43.76 |
| 7 | Quercetin | 51.67 | 57.11 | 67.56 | 69.97 | 59.81 | 69.16 | 79.75 | 89.17 | 86.10 |
| 8 | Cinnamic acid | 4.50 | 5.65 | 5.39 | 6.72 | 7.45 | 7.98 | 8.12 | 8.34 | 8.01 |
| 9 | Apigenin | 19.75 | 22.30 | 23.37 | 21.29 | 23.11 | 24.68 | 21.39 | 27.61 | 26.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghari, J.; Mahdavikia, H.; Rezaei-Chiyaneh, E.; Banaei-Asl, F.; Amani Machiani, M.; Harrison, M.T. Selenium Nanoparticles Improve Physiological and Phytochemical Properties of Basil (Ocimum basilicum L.) under Drought Stress Conditions. Land 2023, 12, 164. https://doi.org/10.3390/land12010164
Asghari J, Mahdavikia H, Rezaei-Chiyaneh E, Banaei-Asl F, Amani Machiani M, Harrison MT. Selenium Nanoparticles Improve Physiological and Phytochemical Properties of Basil (Ocimum basilicum L.) under Drought Stress Conditions. Land. 2023; 12(1):164. https://doi.org/10.3390/land12010164
Chicago/Turabian StyleAsghari, Javad, Hassan Mahdavikia, Esmaeil Rezaei-Chiyaneh, Farzad Banaei-Asl, Mostafa Amani Machiani, and Matthew Tom Harrison. 2023. "Selenium Nanoparticles Improve Physiological and Phytochemical Properties of Basil (Ocimum basilicum L.) under Drought Stress Conditions" Land 12, no. 1: 164. https://doi.org/10.3390/land12010164
APA StyleAsghari, J., Mahdavikia, H., Rezaei-Chiyaneh, E., Banaei-Asl, F., Amani Machiani, M., & Harrison, M. T. (2023). Selenium Nanoparticles Improve Physiological and Phytochemical Properties of Basil (Ocimum basilicum L.) under Drought Stress Conditions. Land, 12(1), 164. https://doi.org/10.3390/land12010164








