Abstract
As a principal part of the atmosphere–lithosphere interface, soil plays a key role in regulating the atmospheric CO2 concentration and global climate. Comprising two major pools (carbonate in soils and bicarbonate in groundwater), soil inorganic carbon (SIC) is deemed as the primary carbon (C) sink and source in areas with low mean annual rainfall. SIC may originate from soil parent material or from the formation of secondary carbonate when divalent cations from an extraneous source are supplied. The latter may result in pedogenic carbonate (PC) formation, increasing soil C content and sequestering atmospheric carbon. Since the sequestration of atmospheric CO2 through formation of pedogenic carbonate is gaining popularity as a method to support climate change mitigation efforts and to claim carbon credits, the mechanisms influencing the formation and migration of pedogenic carbonate need to be well understood. The present review provides an overview of the available literature on potential natural and anthropogenic factors influencing the pedogenic carbonate pool in soils. Firstly, the overall mechanisms of pedogenic carbonate formation, as well as the control factors, are described. Secondly, the impact of various land-use changes on pedogenic carbon pool modification is discussed. Then, the potential of stabilizing atmospheric CO2 through PC formation and the challenges and techniques of tracking the formation of PC through engineered pathways in soils are explored. Finally, isotopic signature as a technique for distinguishing neo-formed carbonate in soil is scrutinized.
1. Introduction
Containing more than 2500 PgC over the first meter of the vertical profile, soils are regarded as the largest terrestrial and the third-largest C reservoir on Earth, after fossil fuel reserves and the oceans [1,2]. As carbon storage in soil impacts diverse aspects of life on Earth, it has been subjected to scientific interest from actors working in various disciplines, such as earth scientists, agronomists, and even policymakers [3]. The dynamics of soil carbon are a function of several parameters that influence C flux exchange between the air and soil system, and thus contribute to moderating atmospheric CO2 levels [4,5,6].
Soil C comprises two discrete segments, soil organic carbon (SOC) and soil inorganic carbon (SIC), with the latter typically considered a more stable sink [2,5,7]. Decomposition of plant and animal residues, synthetic reactions and microbial activities are the main origins of the SOC pool [8,9]. On the contrary, SIC refers to mineral-based carbon, generated through the weathering of bedrock materials [8,10]. Turnover of SIC is believed to be 70–400 times longer compared to that of SOC [11]. While the SOC is estimated to be the major C pool in shallow (<1 m) soil globally [1], the SIC contribution has been found significant in arid and semi-arid areas, where the SIC/SOC ratio is in the range of 2~17 [12,13]. SIC is usually found in the form of calcite (CaCO3) [14], although a significant contribution of dolomite (CaMg(CO3)2) has been reported in a few studies [5,15,16]. Recently, there has been greater research interest in evaluating the SIC pool and its impact on the global C budget [10,17,18], and this trend overcomes the prior neglect of the SIC pool, in the context of climate change mitigation, as it had been traditionally viewed as being less dynamic compared to its organic counterpart [7,19].
The precipitation–dissolution–re-precipitation regime (CaCO3(s) + H2CO3(aq) ⇌ Ca2+ + 2HCO3–) is a primary factor in the distribution of the SIC pool over the vertical soil profile [20]. Monger et al. [17] described the SIC pool in humid and arid soil profiles using the terms ‘flushing’ and ‘non-flushing’, respectively. Flushing refers to a wet climatic condition favoring the downward migration of dissolved carbonate (Ca2+ + 2HCO3–, at near-neutral pH) to the groundwater system, whereas solid carbonate is more likely to accumulate in the non-flushing regime [17]. The global rate of sequestrated C influx (as HCO3–) to groundwater is approximately 0.2~0.36 Pg annually, with a residence time in the order of 100′s to 1000′s of years [10,14].
The SIC pool is categorized into two components, lithogenic carbonate (LC) and pedogenic carbonate (PC), with the former originating from the parent material of soil (e.g., limestone or carbonate-rich unconsolidated sediment) [10,18]. The dissolution–reprecipitation of LC, as well as the mechanism of solvation of CO2(g) in soil moisture (as CO32–/HCO3–) followed by its precipitation with Ca- and Mg-ions, lead to the formation of PC [17]. The formation of PC may modify soil characteristics, such as soil porosity, soil water content, and gaseous diffusion (e.g., of O2(g)) [4].
The carbon exchange between soil and air is influenced by PC dynamics and hence is connected with the global climate [21]. Photosynthesis and root respiration are the major pathways for moving CO2 from the atmosphere into the terrestrial ecosystem [10,12]. The main contributors to PC formation and accumulation include windblown dust [10,22], fertilizers [6], irrigation water [10,23], wet deposition [24,25], and bedrock weathering [10,22].
To accrue net SIC augmentation in the form of PC, the alkaline earth metal must be provided from an extraneous non-carbonate source; otherwise, the sequestered C equals the amount released to the atmosphere (e.g., during limestone calcination to produce quick lime) [17,26]. Hence, a narrower classification is needed to distinguish PC formation induced from an extraneous source and that resulting from recrystallization of pre-existing PC/LC; these PC sources are referred to as pedo-atmogenic and pedo-lithogenic carbonate, respectively [6,10,14]. A detailed description of the formation modes of both types can be found in Monger et al. [10].
The remainder of this paper reviews the mechanisms resulting in natural PC formation and the factors affecting its accumulation and migration. In addition, practices that modify carbonate pools (e.g., land-use change) and that improve PC formation (e.g., enhanced rock weathering) are discussed. Moreover, the application of isotopes as a tool for discriminating PC from LC is illustrated.
2. Pedogenic Carbonate Formation Mechanisms
Formation of PC involves a series of reactions: (1) dissolution of CO2 and an alkaline earth metal-bearing mineral in the soil–water system; (2) migration of dissolved ions with soil water flow; and (3) precipitation of carbonate under favorable geochemical conditions (i.e., one that results in carbonate saturation and that depends on chemical properties of the aqueous medium) [4]. Depending on the soil water movement direction, this process may occur through several mechanisms, namely perdescendum (downward movement of solution) [27], perascendum (upward movement of the solution) [28,29], in-situ (absence of significant movement), and biologically induced mechanisms [4,30]. These processes have been explained in detail in published studies, including Zamanian et al. [4] and Li et al. [31].
Li et al. [31] argued that mechanisms, such as perdescendum or perascendum, may not fully explain the formation of carbonate nodules in loess sedimentary deposits. Instead, these authors attributed the pedogenesis of this carbonate to an “evapotransporative” mechanism, comprising evaporation at the surface and transpiration in the subsurface, with the former as the dominant process [31]. Díaz-Hernández et al. [15] identified various physiochemical mechanisms for the formation of surficial calcite (dominant over 0–180 cm) and deep dolomite (dominant deeper than 180 cm) owing to the weathering of basaltic rocks in a volcanic deposit site. Accordingly, it was proposed that calcite forms in an evaporative medium, whereas dolomite evolves in equilibrium with the groundwater system [15]. Laudicina et al. [32] described two pathways of PC formation involving biological factors: (i) biogenic pathways, where there is organic carbon enriched with a supplement of extraneous Ca2+ through rainfall; and (ii) dissolution and recrystallization of LC with a lower biogenic contribution.
PC occurs in several morphological forms, reflecting the dominance of certain abiotic/biotic processes [4,30]. Different morphological forms of carbonate may also be indicative of various sources (e.g., dissolution-recrystallization and atmospheric deposition), various PC formation mechanisms, as well as its emergence under certain humidity or vegetation regimes [16,29,30]. For instance, the dominance of biological activities may induce the formation of needle-fiber calcite crystals [33]. The distinctive morphological and physical characteristics of carbonate have been found useful for distinguishing several erosion-deposition sequences and soil type [34,35]. The formation of distinct types of PC may happen over different time scales, ranging between days and millennia [4].
3. Factors Affecting Pedogenic Carbonate Formation and Recrystallization
The formation of PC is a complex process, incorporating a wide variety of abiotic (e.g., climate, soil properties and characteristics, landscape) and biotic agents, as discussed and classified by Zamanian et al. [4] and illustrated in Figure 1. Each factor may affect morphology, leachability, and accumulation rate of PC as well as CO2 solubility and Ca2+ availability in soil; all are influential aspects of PC dynamics in soil. Ferdush and Paul [10] reviewed how different biotic and abiotic factors may modify the SIC pool under elevated atmospheric CO2 level conditions.
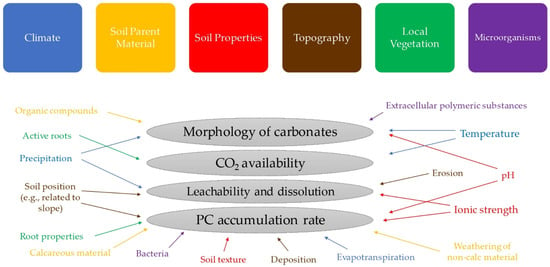
Figure 1.
Illustration of environmental factors affecting PC migration and accumulation in soil discussed in Zamanian et al. [4].
Optimized moisture content, suitable pH range (i.e., greater acidity is conducive to silicate mineral weathering, and near-neutral conditions are required for stability of solid carbonate), CO2 supply, and availability of divalent cations (particularly Ca2+) are four essential conditions for PC formation [17,36]. The recrystallization rate of PC increases with CO2 concentration elevation [37,38,39]. Zhao et al. [39] found that crystallization rate is a function of specific soil properties; the rate proportionally increases/decreases with salinity/pH, respectively. The latter effect is related to a negative correlation between CaCO3 solubility with pH, wherein lower pH eases the dissolution, and thus recrystallization, of CaCO3 [39]. They attributed the positive correlation between salinity and PC formation to a higher accumulation of exchangeable cations (e.g., Ca2+) in saline soil [39]. These authors also demonstrated that the increase of CO2 partial pressure from 0.04 vol% (atmospheric) to 4 vol% results in a 100-fold increase in the carbonate recrystallization rate. Although the same trend was observed in the experiment conducted by Gocke et al. [37], several controls (e.g., fluctuation of CO2 concentration in soil, temperature, root density, native crop regime, and soil texture) were also important as regulators of the rate of re-precipitation in natural soil systems. These factors, investigated in several studies as key aspects, are discussed next.
Hasinger et al. [36] demonstrated that the presence of a macroporous layer in soil facilitates CO2 gas diffusion over the vertical and lateral profile of soil, acting as feedstock for PC formation. Investigating inter-seasonal variability of PC formation and seasonality of rainfall, Gallagher et al. [40] determined hydrological parameters (rainfall, evaporation, and soil water content) as the primary factors, rather than the mean annual temperature that was believed to previously be the main control. However, the vital role of temperature in PC dynamics in forest-steppe soils (alongside the mentioned hydrological parameters and biological activities in the growing season) has also been documented [41].
Select studies have highlighted the role of fine particles (nominally, clay) in the formation and accumulation of secondary carbonate [15,42]. According to Díaz-Hernández et al. [42], clay particles take up water that has migrated to the subsoil and therefore are conducive to the precipitation of carbonate. In contrast, the presence of gravel and sand particles can influence the hydrological regime and PC dynamics by facilitating the transport of water to subsoil layers [42]. Li et al. [13] also observed the same trend of SIC accumulation (with a PC isotopic signature) in a clay layer in the Yellow River Delta.
Microbial and root respiration, including the mineralization of organic carbon in the rhizosphere, are the main factors in supplying CO2 in a subsoil medium [12,37]. Recent laboratory [43] and field-scale [44] studies showed that biotic processes (microbial activity) in deserts could induce biogenic PC formation through biomineralization with rates higher than abiotic pathways. Accordingly, the presence of bacteria may enhance the adsorption of Ca2+, by its attachment to the bacterial membrane, cell wall, and extracellular polymeric substances (EPS) layer, as highlighted by Liu et al. [43]. Furthermore, an increase in pH through metabolic activities (e.g., ureolysis, denitrification, ammonification, and sulfate reduction) can facilitate the precipitation of CaCO3 [44]. A genus of termites, macrotermes can contribute to secondary carbonate formation through supplying Ca2+ that originates from plant tissues and biomineralization [45,46]. On the other hand, in the absence of biotic agents, Fa et al. [47] showed that in semi-arid deserts carbon remains in the dissolved inorganic carbon (DIC) phase, and migration of CO2-originating carbon to groundwater is observed. Microorganisms have also been shown by Zhao et al. [38] to retard the recrystallization of PC due to partial surficial growth of microorganisms on CaCO3 crystals, stabilizing the formed PC.
4. Pedogenic Carbonate Pool Modification under Various Management Practices/Land-Use Change
As PC formation is one of the main factors modifying the SIC pool, it is essential to comprehend its dynamics under different land uses [48,49]. Land-use change may come with changes in soil thermal and moisture regimes, consequently influencing the pool of PC in soil [14,28]. More than 30% of the anthropogenic contributions to atmospheric CO2 concentration rise is due to land-use changes in the last 150 years [49]. A meta-analysis conducted by An et al. [49] denoted that various internal (e.g., pH, soil moisture, organic matter content) and environmental (e.g., mean annual temperature, rainfall) factors affect (positively or negatively) the SIC pool in the event of various land-use changes. In that study, SIC accumulation was observed in the conversion from grassland to farmland, and from sandy soil to forest soil, while conversion from farmland to grassland has reportedly led to an opposite correlation under certain scenarios [49]. Recently, Li et al. [50] assessed which of the key soil properties (e.g., bulk density, soil texture, pH, and nutrients availability) are influential on SIC and SOC dynamics through forestation and vegetation cover restoration, highlighting nutrients as the most relevant factor. It is important to note that the relationship between SIC and SOC is complex, and at times uncorrelated [49] (i.e., one can increase, while the other decreases); thus, land-use changes can have a different effect on total soil carbon than what changes in SIC or SOC alone imply. Practices at farmlands also vary substantially from crop to crop and region to region, and certain practices, such as nitrogen fertilization, have been shown to be measurably detrimental to the SIC pool [51].
Studies investigating PC pool modification in agricultural fields are abundant in the literature. Sanderman [14] reviewed three possible management practices influencing the SIC pool in the agricultural sector: irrigation, soil acidification, and liming. While the latter was identified as the undisputable C emission source (through dissolving in the soil–water system and efflux of CO2 to the atmosphere), it remained unclear if the first two activities can act as either atmospheric C source or sink [14]. Depending on the objective, the studies investigating neo-formed carbonate in agricultural fields can be classified into two categories: (i) alteration of native soil in land-use change to agriculture (e.g., [23,29,52]) and (ii) investigating the impact of various agricultural management practices on the PC pool (e.g., [51,53,54]).
When establishing new agricultural fields, Nyachoti et al. [23] indicated a higher accumulation rate of pedogenic carbonate in agricultural land compared to natural soil in an arid environment. These authors identified the abundance of Ca2+ in irrigated water, as well as the provision of more DIC, as the main factors for a higher accumulation rate of carbonate in the cropland [23]. This study highlights the role of cation supplement from an extraneous source as a contributing factor in pedogenic carbonate formation. Conversion of shrubland to cropland has contributed to 0.87 t C·ha−1·yr−1 uptake in semi-arid croplands of the Yanqi Basin as reported by Wang et al. [27]. Kim et al. [54] observed a loss in the SIC pool due to land-use change to farmland, likely because of the leaching of carbonate in rain-fed and irrigated agricultural lands.
Focusing on agricultural management practices, Wang et al. [53] noted enhancement of PC and SIC pools over the vertical soil profile of arid and semi-arid croplands under various fertilizer management practices, with higher accumulation in soil amended with considerable amounts of organic matter. According to Bughio et al. [11], in deep layers of soils, an increase in plant biomass due to irrigation and fertilization practices leads to an increase in soil CO2 levels (due to root and microbial respiration), providing an opportunity for PC formation in cases of Ca2+ and Mg2+ supplement. The biomass also contributes to evaporation, which is favorable for carbonate precipitation [11]. In another study [55], tillage and crop straw were effective for PC formation due to the rise in SOC level.
In addition to agriculture-related conversion/management, PC dynamics have been investigated in other forms of land-use change/rehabilitation, including forestation [56] and vegetation restoration [50]. In this respect, Gao et al. [56] noted that afforestation can enhance the accumulation of PC in arid soils over a 30-year period and within a 100 cm vertical soil profile.
SIC and SOC Correlation
There is a large body of studies reporting a correlation between SOC, SIC, and soil CO2 level (e.g., [27,48,56]). When SOC level is high in the soil, the CO2 concentration will increase due to organic matter decomposition, leading to the formation of HCO3– and H+ [57]. Accordingly, higher SOC stock leads to more PC formation, as was observed in arid lands of the Yanqi Basin [27], in northwest China [56], in arid cropland of China [55], in the Yellow River Delta [13], and in the Mu Us desert [50]. In contrast, progressive desertification and shifting to a less humid climate have been found to lead to a decrease in SOC and SIC in topsoil (e.g., 0–30 cm), contributing to the loss of PC stock [3,58].
While this SOC/SIC positive correlation has been largely detected in arid and semi-arid regions, the negative correlation of SOC and SIC (and PC) in regions with higher mean annual precipitation may be indicative of the leaching of carbonate to the subsoil and groundwater at depth [2,3]. Besides this, other environmental factors, such as soil properties and management practices, as well as native plant community, may lead to the negative correlation between SIC and SOC [9,59].
5. Sequestration of Atmospheric CO2 as Pedogenic Carbonate through Engineered Pathways
The availability of cations, particularly Ca2+ and Mg2+, is believed to be the main limiting factor for the PC formation process [53,55]. Hence, the addition of Ca- and Mg-rich silicate minerals to soils (a.k.a. ‘engineered soils’) is among the popular techniques proposed to enhance the PC pool in agricultural [60,61], artificial [62], and urban soils [63]. These methods generally seek to reduce the atmospheric CO2 level by enhancing the SIC pool, which under drier climates (and other amenable conditions) means augmenting the PC pool.
To accelerate the weathering of Ca- and Mg-rich silicate minerals in the soil-water system, the crushed form of these minerals is applied [60,64,65]. This process, known as enhanced rock weathering (ERW), consists of three steps: (1) atmospheric CO2 solvation (e.g., in rainwater); (2) mineral dissolution (releasing base cations in the soil–water system); and (3) precipitation of carbonate (under favorable geochemical conditions) [60,66]. However, when new water is introduced, the newly formed carbonate might redissolve leading to the liberation of cations that speciate with bicarbonate and carbonate anions in the soil–water system as DIC [64,66,67]. This may result in either re-precipitation of PC in deeper layers of the soil or migration of DIC downward to groundwater [64,66]. In the latter scenario, the cations and DIC find their way to greater depths in the subsurface, discharge into freshwater systems, and eventually find the oceans as the ultimate sink [64,65].
Basalt (characteristically labradorite-rich (Ca,Na)(Al,Si)4O8) [61], olivine (characteristically forsterite-rich (Mg2SiO4)) [68] and wollastonite (CaSiO3) [60,66] are Mg- and Ca-bearing minerals proposed for enhanced rock weathering in croplands. Several studies have highlighted the accumulation of SIC in field-scale experiments through the amendment of agricultural soils with these minerals [60,66]. In this respect, Haque et al. [60] demonstrated that the rate of SIC accumulation and sequestrated CO2 increases over time when higher doses of wollastonite are spread onto the soil of commercial farms. In a controlled environment, Kelland et al. [61] showed the possibility of carbonate formation in soil columns (up to 4 t CO2 after 5 years) due to the application of basaltic dust. ERW has also been found effective in enhancing plant growth apart from CO2 sequestration [61,64,69]. From an agricultural and environmental perspective, the practice of ERW can serve as an alternative for liming agents, which are conventionally applied as a Ca source for soil structural improvement [6,14,60] but is known to generate atmospheric CO2 emissions (0.273 PgC annually) globally [51,70].
Focusing on urban and artificial soils, Ca-mineral bearing materials, such as cement (bearing portlandite (Ca(OH)2)) and plaster (bearing gypsum (CaSO4·2H2O)) are the main sources of calcium in construction and demolition (C&D) waste [71]. Synthetic soils, a combination of composted waste material and quarry fines enriched in silicate minerals, were found to be effective for trapping atmospheric CO2 and enhancing the PC pool [62]. A summary of studies that have reported SIC increase due to ERW is shown in Table 1.

Table 1.
CO2 fixation uptakes (on areal and total basis) reported in ERW studies conducted at field-scale.
As is the case with natural PC formation, various climatic and environmental factors affect SIC formation in engineered soils [60,72]. This includes pH [64,67,72], fineness of particles [72,74], hydrological parameters (redistributing SIC content over the soil profile) [60,72], and additive mineral characteristics [62].
Several prospective studies have assessed how ERW could contribute to carbon sequestration and climate change mitigation, globally. Using reactive transport modeling, Beerling et al. [67] have estimated the long-term (up to the year 2050) potential capacity of CO2 removal in several countries through ERW of basalt in cropland areas, considering mineral demand and process costs. Recently, Haque et al. [75] propounded extending the ERW technique to urban roofs and balconies, labeled as ‘urban farming’. These authors estimated more than 13.6 Mt C could be sequestrated by applying this method to 1% of urban areas worldwide [75]. Manning et al. [76] proposed two techniques for enhancing PC formation at a global scale: (1) afforestation on lands with basaltic igneous rock subsurface structure; and (2) investment in the management of historically industrial lands that are enriched in materials whose composition is suitable for enhanced weathering. In addition to C&D waste, other industrial by-products, such as fly and bottom ash [71] and mining residues [77], can be suitable feedstocks for enhancing SIC stocks since they are rich in alkaline earth metals.
The practice of mining, crushing, and transporting minerals to the targeted land for performing ERW could be a source of CO2 emissions into the atmosphere [78]. The life cycle assessment (LCA) study conducted by Lefebvre et al. [78] denoted that the application of basalt from quarries to cropland of Sao Paulo, Brazil could release, on average, 110 kg of CO2(eq) per tonne of CO2(eq) removed from the atmosphere. The potential unintended impact of minerals on ecosystems (including native land and water) is another factor that should be considered when ERW is applied on a terrestrial environment [66,79].
The current challenge of ERW implementation is the uncertainty with respect to the fate of neo-formed PC and its permanence. This points to the importance of developing predictive tools, such as a reactive transport modeling framework [67,80], in order to predict the long-term fate of PC in soil over decades to millennial time scales. It is also recognized that such complex carbon removal approaches require the participation of transdisciplinary actors to enable wide dissemination of its potential and best practices, and equitable and sustainable implementation, which in turn is critical for the inclusion of this approach into relevant global climate policies [81].
The application of biochar is another engineered management practice recently found to be effective in increasing PC stocks by capturing atmospheric CO2 [82,83]. According to these studies, biochar can enhance PC formation through an increase in Ca2+ and pH as well as soil porosity (thus facilitating labile SOC decomposition and increasing soil CO2 concentration) [82,83]. In a 10-year field experiment by Shi et al. [83], biochar application was found to be effective in increasing SIC over the 0–15 cm layer of soil.
A summary of the impact of engineered management practices and land-use change on carbonate formation based on the results of the reviewed studies is illustrated in Figure 2.
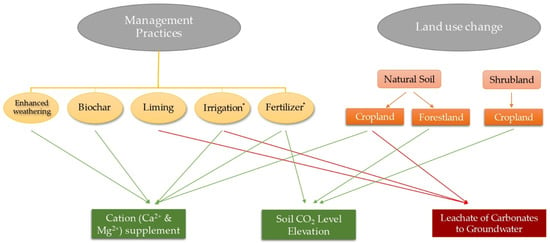
Figure 2.
A summary of management practices and land-use change influencing the soil PC pool as documented in reviewed studies, highlighting how each activity might modify the carbonate pool (green: accumulation; red: dissolution and downward migration). * Depending on fertilizer/irrigation water and soil properties, different results were reported in reviewed studies. As this is an emerging field of research, note that a lack of arrow does not necessarily mean an influence is not possible; it just has not been reported to date.
6. Isotopic Signatures: A Tool for Detecting and Quantifying Pedogenic Carbonate in Soil
There is a large body of literature demonstrating the success of the isotope technique in estimating PC for various objectives, such as paleoenvironmental reconstruction [84,85], neo-formed PC detection [62,86], land-use change [55,56], and labeling experiments [87]. The isotope signature of different types of carbonate varies in a soil horizon, reflecting the parent material, dissolution-reprecipitation cycle, and climatic factors [85,88,89]. Various isotope signatures including δ13C [62], 14C [29], δ18O [42,62], clumped isotopes [40], and U [23] have been used to study PC formation and accumulation. However, the focus of this section is on the first three signatures (δ13C, 14C, and δ18O) that are usually concerned with detecting and quantifying modern PC in the literature.
The δ13C signature of PC is influenced by processes occurring during the growing season in agricultural soil, including the decomposition of organic matter and the respiration of roots and microorganisms [4]. Accordingly, the δ13C value of PC is determined based on the contribution of C3 and C4 (photosynthesis carbon fixation classifications) plant endmembers, which have a mean value of –27‰ and –13‰, respectively [58]. Comparatively, the isotope value of the soil’s CO2 is 4.5‰ enriched relative to reported biomass values (–27‰ and –13‰) in the literature [15]. As the PC precipitated from soil solution will be in equilibrium with soil CO2 in terms of isotopic exchange, several studies have investigated the dynamics of PC in the atmosphere–soil–water system employing C-isotopic fractionation [15,36]. For instance, Hasinger et al. [36] described the evolution of atmospheric CO2 to PC (through photosynthesis → SOM decay → root respiration → dissolution in meteoric water → precipitation) using C-fractionation.
Due to different origins, LC and PC demonstrate higher (near zero) and lower (negative values reflecting soil respiration) values, respectively [58]. Given the distinct isotope values of C3 and C4 biomass, many efforts have been made to relate the contribution of secondary carbonate to different vegetation regimes as well as environmental factors (e.g., [53,56]). As reported by Gao et al. [56], forestation leads to a progressive reduction in δ13C values (towards the C3 and C4 signature, aforementioned) over a 30-year time scale, reflecting neo-carbonate accumulation in the profile of the soil.
14C can also serve as a good proxy for distinguishing carbonate formed over the vertical soil profile and under different formation mechanisms (e.g., per-ascendum/descendum, in-situ) [19,29]. Using a 14C labeling experiment, Gocke et al. [87] noted most carbonate is leached from the 0–15 cm layer of soil, whereas the highest accumulation was observed at a depth of 20–35 cm, corresponding to the zone dominated by root biomass.
The δ18O isotope signature of local meteoric water is another useful isotope signature that has been used in high evaporative systems [42,89]. The PC formed under such conditions is correlated with enriched δ18O values [42].
Usually, a combination of the above-mentioned isotopic techniques is employed to investigate processes influencing PC formation and accumulation. Some key examples follow. Using isotopic signatures of δ13C and δ18O, it has been estimated that approximately 40% of neo-formed PC is formed through a photosynthetic process in synthetic [62] and urban soils [86]. These findings underscore the usefulness of using isotopic signatures to describe processes (e.g., photosynthesis) that lead to uptake of atmospheric CO2 and their relative contributions to PC formation in soils amended with Ca and Mg bearing minerals. Mikhailova et al. [19] used both δ13C and 14C to quantify the PC fraction of total soil carbon and the percentage of modern carbon (pMC), respectively. They showed that pMC can vary between 73% at 30–40 cm and 25% for the 160–200 cm horizon of croplands in Russia [19]. Díaz-Hernández et al. [42] showed that tracking δ13C and δ18O is useful for describing pedogenesis of inherited and neo-formed dolomites formed under various geochemical conditions within the vertical soil profile. In another study, these authors explained the formation of deep dolomite vs. surficial calcite through distinguished δ18O values (with more negative values attributed to calcite) [15].
Nevertheless, estimating the PC pool using isotope techniques usually comes with a degree of uncertainty, arising from the decoupling between CO2 production and carbonate precipitation spatially and temporally [53]. For instance, an increase in aridity leads to a decrease in the respiration rate of roots, which in turn results in the dominance of atmospheric CO2 in soil pores [52,88]. In such a case, intrusion of atmospheric CO2 (with a discreet isotopic signature) into soil pores is predictable, modifying the isotopic signature of soil CO2 and soil carbonate (shifting to more positive values), particularly in topsoil [27,52,88]. Da et al. [88] noted that the contribution of atmospheric CO2 in soil CO2 could be up to 60% in a desert area.
7. Conclusions and Prospect
Detecting and quantifying PC is of significance as it is a dynamic pool in soils and could contribute to sequestrating atmospheric carbon and mitigating global warming. As discussed in this review, the occurrence and migration of PC in soil is a function of several hydrological and climatic factors. Moreover, soil physiochemical properties, microorganisms, and vegetation cover are the other factors that have been the focus of researchers investigating PC formation in the soil medium. All the above-mentioned factors control the morphological structure of secondary carbonate as well as the timing of PC formation.
Exploring the impact of land-use change on the PC pool has been a matter of interest to several researchers. This reflects the importance of tracking the PC reservoir as a part of the SIC pool particularly in arid and semi-arid environments where SIC is the dominant form of soil carbon. A change in the cation budget of soil and the presence of SOC, which affects soil CO2 concentration, are the main driving factors governing PC modification in the case of land-use change practices.
As a technique to address the limitation of Ca2+ and Mg2+ cations in PC formation, enhanced rock weathering has been highlighted as an engineered pathway to increase the pool of secondary carbonate in agricultural and urban soils. Such a passive method has demonstrated great reliability in converting atmospheric carbon into secondary minerals, and it is recognized as a negative emissions technology so long as the manufacturing and transport of the comminuted rock supply has a low carbon footprint. There are still many unanswered questions about this technique, so it might gain more popularity as more large-scale studies are conducted in the field and a better understanding of the co-benefits and potential negative impacts emerge.
Verifying the atmospheric origin of neo-formed soil carbonate is one of the main challenges in evaluating the efficiency and feasibility of engineered carbon dioxide removal technologies. Although tracking isotopic signatures over time and space provides an opportunity to enhance comprehension of the mechanisms governing PC formation and transport over a certain period, the other concern is to ensure this neo-formed carbonate is stable for a long time after accumulation. Developing predictive tools for investigating the long-term fate of carbonate in soil and subsoil systems will be critical in this context.
Author Contributions
Conceptualization, R.K. and R.M.S.; writing—original draft preparation, R.K.; writing—review and editing, E.A. and R.M.S.; supervision, R.M.S.; funding acquisition, R.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support provided by the Ontario Agri-Food Innovation Alliance (Gryphon’s LAAIR Product Development grant UG-GLPD-2021-101200), the Natural Sciences and Engineering Research Council of Canada (Discovery Grant 401497), and the Canada First Research Excellence Fund (Food from Thought Product Development grant 499149).
Acknowledgments
This publication is dedicated to the memory of Klaas Baan (1981–2022), who was a pioneering enabler of enhanced rock weathering and soil remineralization in Canada and a close collaborator of the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, W.; Wang, X.; Lu, T.; Shi, H.; Zhao, Y. Influences of soil properties and hydrological processes on soil carbon dynamics in the cropland of North China Plain. Agric. Ecosyst. Environ. 2020, 295, 106886. [Google Scholar] [CrossRef]
- Jin, Z.; Dong, Y.; Wang, Y.; Wei, X.; Wang, Y.; Cui, B.; Zhou, W. Natural vegetation restoration is more beneficial to soil surface organic and inorganic carbon sequestration than tree plantation on the Loess Plateau of China. Sci. Total Environ. 2014, 485–486, 615–623. [Google Scholar] [CrossRef]
- Raheb, A.; Heidari, A.; Mahmoodi, S. Organic and inorganic carbon storage in soils along an arid to dry sub-humid climosequence in northwest of Iran. Catena 2017, 153, 66–74. [Google Scholar] [CrossRef]
- Zamanian, K.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic carbonates: Forms and formation processes. Earth-Sci. Rev. 2016, 157, 1–17. [Google Scholar] [CrossRef]
- Díaz-Hernández, J.L. Is soil carbon storage underestimated? Chemosphere 2010, 80, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Singh, B.; Dalal, R.; Dijkstra, F.A. Carbon dynamics from carbonate dissolution in Australian agricultural soils. Soil Res. 2015, 53, 144–153. [Google Scholar] [CrossRef]
- You, M.; Han, X.; Hu, N.; Du, S.; Doane, T.A.; Li, L.-J. Profile storage and vertical distribution (0–150 cm) of soil inorganic carbon in croplands in northeast China. Catena 2019, 185, 104302. [Google Scholar] [CrossRef]
- Lal, R. Carbon Management in Agricultural Soils. Mitig. Adapt. Strat. Glob. Chang. 2006, 12, 303–322. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Shi, H.; Guo, Y. Carbon Sequestration in Arid Lands: A Mini Review; Springer: Singapore, 2018; ISBN 9789811070228. [Google Scholar]
- Monger, H.C.; Kraimer, R.A.; Khresat, S.; Cole, D.R.; Wang, X.; Wang, J. Sequestration of inorganic carbon in soil and groundwater. Geology 2015, 43, 375–378. [Google Scholar] [CrossRef]
- Bughio, M.A.; Wang, P.; Meng, F.; Qing, C.; Kuzyakov, Y.; Wang, X.; Junejo, S.A. Neoformation of pedogenic carbonates by irrigation and fertilization and their contribution to carbon sequestration in soil. Geoderma 2016, 262, 12–19. [Google Scholar] [CrossRef]
- Ferdush, J.; Paul, V. A review on the possible factors influencing soil inorganic carbon under elevated CO2. Catena 2021, 204, 105434. [Google Scholar] [CrossRef]
- Li, Y.; Fu, C.; Zeng, L.; Zhou, Q.; Zhang, H.; Tu, C.; Wei, J.; Li, L.; Luo, Y. Carbon accumulation in the red clay layer of the subsoil in a major river delta: Contribution of secondary carbonate. Catena 2019, 186, 104391. [Google Scholar] [CrossRef]
- Sanderman, J. Can management induced changes in the carbonate system drive soil carbon sequestration? A review with particular focus on Australia. Agric. Ecosyst. Environ. 2012, 155, 70–77. [Google Scholar] [CrossRef]
- Diaz-Hernandez, J.; Navas, A.S.; Delgado, A.; Yepes, J.; Garcia-Casco, A. Textural and isotopic evidence for Ca-Mg carbonate pedogenesis. Geochim. Cosmochim. Acta 2017, 222, 485–507. [Google Scholar] [CrossRef]
- Prokof’Eva, T.; Shishkov, V.; Kiriushin, A. Calcium carbonate accumulations in Technosols of Moscow city. J. Soils Sediments 2020, 21, 2049–2058. [Google Scholar] [CrossRef]
- Monger, H.C. Soils as Generators and Sinks of Inorganic Carbon in Geologic Time. In Soil Carbon; Hartemink, A.E., McSweeney, K., Eds.; Springer: Cham, Switzerland, 2014; pp. 27–36. [Google Scholar]
- Leogrande, R.; Vitti, C.; Castellini, M.; Mastrangelo, M.; Pedrero, F.; Vivaldi, G.; Stellacci, A. Comparison of Two Methods for Total Inorganic Carbon Estimation in Three Soil Types in Mediterranean Area. Land 2021, 10, 409. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Bryant, R.B.; Galbraith, J.M.; Wang, Y.; Post, C.J.; Khokhlova, O.S.; Schlautman, M.A.; Cope, M.P.; Shen, Z. Pedogenic Carbonates and Radiocarbon Isotopes of Organic Carbon at Depth in the Russian Chernozem. Geosciences 2018, 8, 458. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ye, X.; Chu, Y.; Wang, X. Profile storage of organic/inorganic carbon in soil: From forest to desert. Sci. Total Environ. 2010, 408, 1925–1931. [Google Scholar] [CrossRef]
- Golubtsov, V.A.; Cherkashina, A.A.; Khokhlova, O.S. Carbonate Profile of Soils in the Baikal Region: Structure, Age, and Formation Conditions. Eurasian Soil Sci. 2019, 52, 1515–1532. [Google Scholar] [CrossRef]
- Dietrich, F.; Diaz, N.; Deschamps, P.; Ngatcha, B.N.; Sebag, D.; Verrecchia, E.P. Origin of calcium in pedogenic carbonate nodules from silicate watersheds in the Far North Region of Cameroon: Respective contribution of in situ weathering source and dust input. Chem. Geol. 2017, 460, 54–69. [Google Scholar] [CrossRef] [Green Version]
- Nyachoti, S.; Jin, L.; Tweedie, C.E.; Ma, L. Insight into factors controlling formation rates of pedogenic carbonates: A combined geochemical and isotopic approach in dryland soils of the US Southwest. Chem. Geol. 2019, 527, 118503. [Google Scholar] [CrossRef]
- Mikhailova, E.; Goddard, M.; Post, C.; Schlautman, M.; Galbraith, J. Potential Contribution of Combined Atmospheric Ca2+ and Mg2+ Wet Deposition within the Continental U.S. to Soil Inorganic Carbon Sequestration. Pedosphere 2013, 23, 808–814. [Google Scholar] [CrossRef]
- Da, J.; Zhang, Y.G.; Wang, H.; Balsam, W.; Ji, J. An Early Pleistocene atmospheric CO2 record based on pedogenic carbonate from the Chinese loess deposits. Earth Planet. Sci. Lett. 2015, 426, 69–75. [Google Scholar] [CrossRef]
- Carmi, I.; Kronfeld, J.; Moinester, M. Sequestration of atmospheric carbon dioxide as inorganic carbon in the unsaturated zone under semi-arid forests. Catena 2018, 173, 93–98. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Xu, M.; Zhang, W.; Fan, T.; Zhang, J. Carbon accumulation in arid croplands of northwest China: Pedogenic carbonate exceeding organic carbon. Sci. Rep. 2015, 5, 11439. [Google Scholar] [CrossRef]
- Chendev, Y.G.; Novykh, L.L.; Sauer, T.J.; Petin, A.N.; Zazdravnykh, E.A.; Burras, C.L. Evolution of Soil Carbon Storage and Morphometric Properties of Afforested Soils in the US. Great Plains. In Soil Carbon; Springer: Cham, Switzerland, 2014; pp. 475–482. [Google Scholar] [CrossRef]
- Khokhlova, O.; Myakshina, T.; Kuznetsova, A. Origins of hard carbonate nodules in arable Chernozems in the Central Russian Upland. Eur. J. Soil Sci. 2020, 72, 326–342. [Google Scholar] [CrossRef]
- Durand, N.; Monger, H.C.; Canti, M.G.; Verrecchia, E.P. Calcium Carbonate Features; Elsevier B.V.: Amsterdam, The Netherlands, 2018; ISBN 9780444635228. [Google Scholar]
- Li, Y.; Zhang, W.; Aydin, A.; Deng, X. Formation of calcareous nodules in loess–paleosol sequences: Reviews of existing models with a proposed new “per evapotranspiration model”. J. Southeast Asian Earth Sci. 2018, 154, 8–16. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Scalenghe, R.; Pisciotta, A.; Parello, F.; Dazzi, C. Pedogenic carbonates and carbon pools in gypsiferous soils of a semiarid Mediterranean environment in south Italy. Geoderma 2013, 192, 31–38. [Google Scholar] [CrossRef]
- Millière, L.; Gussone, N.; Moritz, T.; Bindschedler, S.; Verrecchia, E. Origin of strontium and calcium in pedogenic needle fibre calcite (NFC). Chem. Geol. 2019, 524, 329–344. [Google Scholar] [CrossRef]
- Gile, L.H. Eolian and Associated Pedogenic Features of the Jornada Basin Floor, Southern New Mexico. Soil Sci. Soc. Am. J. 1999, 63, 151–163. [Google Scholar] [CrossRef]
- Jiménez-Ballesta, R.; Bravo, S.; Amorós, J.A.; Pérez-De-Los-Reyes, C.; García-Pradas, J.; Sanchez, M.; García-Navarro, F.J. A morphological approach to evaluating the nature of vineyard soils in semiarid Mediterranean environment. Eur. J. Soil Sci. 2021, 73, 13201. [Google Scholar] [CrossRef]
- Hasinger, O.; Spangenberg, J.E.; Millière, L.; Bindschedler, S.; Cailleau, G.; Verrecchia, E.P. Carbon dioxide in scree slope deposits: A pathway from atmosphere to pedogenic carbonate. Geoderma 2015, 247–248, 129–139. [Google Scholar] [CrossRef]
- Gocke, M.; Pustovoytov, K.; Kuzyakov, Y. Effect of CO2 concentration on the initial recrystallization rate of pedogenic carbonate-Revealed by 14C and 13C labeling. Geoderma 2010, 155, 351–358. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, C.; Stahr, K.; Kuzyakov, Y.; Wei, X. The effect of microorganisms on soil carbonate recrystallization and abiotic CO2 uptake of soil. Catena 2020, 192, 104592. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, C.; Wang, J.; Stahr, K.; Kuzyakov, Y. CaCO3 recrystallization in saline and alkaline soils. Geoderma 2016, 282, 1–8. [Google Scholar] [CrossRef]
- Gallagher, T.M.; Sheldon, N.D. Combining soil water balance and clumped isotopes to understand the nature and timing of pedogenic carbonate formation. Chem. Geol. 2016, 435, 79–91. [Google Scholar] [CrossRef]
- Golubtsov, V.; Bronnikova, M.; Khokhlova, O.; Cherkashina, A.; Turchinskaia, S. Morphological and isotopic study of pedogenic carbonate coatings from steppe and forest-steppe areas of Baikal region, South-Eastern Siberia. Catena 2020, 196, 104817. [Google Scholar] [CrossRef]
- Díaz-Hernández, J.; Sánchez-Navas, A.; Reyes, E. Isotopic evidence for dolomite formation in soils. Chem. Geol. 2013, 347, 20–33. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Fa, K.; Zhao, H.; Qin, S.; Yan, R.; Wu, B. Desert soil bacteria deposit atmospheric carbon dioxide in carbonate precipitates. Catena 2018, 170, 64–72. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Zhang, Y.; Qin, S.; Sun, Y.; Mao, H.; Miao, L. Desert soil sequesters atmospheric CO2 by microbial mineral formation. Geoderma 2019, 361, 114104. [Google Scholar] [CrossRef]
- Liu, X.; Monger, H.C.; Whitford, W.G. Calcium carbonate in termite galleries–biomineralization or upward transport? Biogeochemistry 2006, 82, 241–250. [Google Scholar] [CrossRef]
- Mujinya, B.; Mees, F.; Boeckx, P.; Bodé, S.; Baert, G.; Erens, H.; Delefortrie, S.; Verdoodt, A.; Ngongo, M.; Van Ranst, E. The origin of carbonates in termite mounds of the Lubumbashi area, D.R. Congo. Geoderma 2011, 165, 95–105. [Google Scholar] [CrossRef]
- Fa, K.; Liu, Z.; Zhang, Y.; Qin, S.; Wu, B.; Liu, J. Abiotic carbonate dissolution traps carbon in a semiarid desert. Sci. Rep. 2016, 6, 23570. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Wang, X.; Xu, M.; Yu, Z.; Luo, Y.; Smith, P. Dynamics of pedogenic carbonate in the cropland of the North China Plain: Influences of intensive cropping and salinization. Agric. Ecosyst. Environ. 2020, 292, 106820. [Google Scholar] [CrossRef]
- An, H.; Wu, X.; Zhang, Y.; Tang, Z. Effects of land-use change on soil inorganic carbon: A meta-analysis. Geoderma 2019, 353, 273–282. [Google Scholar] [CrossRef]
- Li, J.; Awasthi, M.K.; Zhu, Q.; Chen, X.; Wu, F.; Wu, F.; Tong, X. Modified soil physicochemical properties promoted sequestration of organic and inorganic carbon synergistically during revegetation in desertified land. J. Environ. Chem. Eng. 2021, 9, 106331. [Google Scholar] [CrossRef]
- Zamanian, K.; Zarebanadkouki, M.; Kuzyakov, Y. Nitrogen fertilization raises CO2 efflux from inorganic carbon: A global assessment. Glob. Chang. Biol. 2018, 24, 2810–2817. [Google Scholar] [CrossRef]
- Ortiz, A.C.; Jin, L.; Ogrinc, N.; Kaye, J.; Krajnc, B.; Ma, L. Dryland irrigation increases accumulation rates of pedogenic carbonate and releases soil abiotic CO2. Sci. Rep. 2022, 12, 464. [Google Scholar] [CrossRef]
- Wang, X.J.; Xu, M.G.; Wang, J.P.; Zhang, W.J.; Yang, X.Y.; Huang, S.M.; Liu, H. Fertilization enhancing carbon sequestration as carbonate in arid cropland: Assessments of long-term experiments in northern China. Plant Soil 2014, 380, 89–100. [Google Scholar] [CrossRef]
- Kim, J.H.; Jobbágy, E.G.; Richter, D.D.; Trumbore, S.E.; Jackson, R.B. Agricultural acceleration of soil carbonate weathering. Glob. Change Biol. 2020, 26, 5988–6002. [Google Scholar] [CrossRef]
- Bughio, M.A.; Wang, P.; Meng, F.; Chen, Q.; Li, J.; Shaikh, T.A. Neoformation of pedogenic carbonate and conservation of lithogenic carbonate by farming practices and their contribution to carbon sequestration in soil. Z. Pflanzenernahr. Bodenkd 2017, 180, 454–463. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, J.; Pang, Y.; Liu, J. Soil Inorganic Carbon Sequestration Following Afforestation Is Probably Induced by Pedogenic Carbonate Formation in Northwest China. Front. Plant Sci. 2017, 8, 1282. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dang, P.; Zhao, Q.; Liu, J.; Liu, J. Effects of vegetation rehabilitation on soil organic and inorganic carbon stocks in the Mu Us Desert, northwest China. Land Degrad. Dev. 2017, 29, 1031–1040. [Google Scholar] [CrossRef]
- An, H.; Li, Q.-L.; Yan, X.; Wu, X.-Z.; Liu, R.-T.; Fang, Y. Desertification control on soil inorganic and organic carbon accumulation in the topsoil of desert grassland in Ningxia, northwest China. Ecol. Eng. 2019, 127, 348–355. [Google Scholar] [CrossRef]
- Huber, D.P.; Lohse, K.A.; Commendador, A.; Joy, S.; Aho, K.; Finney, B.; Germino, M.J. Vegetation and precipitation shifts interact to alter organic and inorganic carbon storage in cold desert soils. Ecosphere 2019, 10, e02655. [Google Scholar] [CrossRef]
- Haque, F.; Santos, R.M.; Chiang, Y.W. CO2 sequestration by wollastonite-amended agricultural soils—An Ontario field study. Int. J. Greenh. Gas Control 2020, 97, 103017. [Google Scholar] [CrossRef]
- Kelland, M.E.; Wade, P.W.; Lewis, A.L.; Taylor, L.L.; Sarkar, B.; Andrews, M.G.; Lomas, M.R.; Cotton, T.E.A.; Kemp, S.J.; James, R.H.; et al. Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Glob. Change Biol. 2020, 26, 3658–3676. [Google Scholar] [CrossRef]
- Manning, D.; Renforth, P.; Lopez-Capel, E.; Robertson, S.; Ghazireh, N. Carbonate precipitation in artificial soils produced from basaltic quarry fines and composts: An opportunity for passive carbon sequestration. Int. J. Greenh. Gas Control 2013, 17, 309–317. [Google Scholar] [CrossRef]
- Washbourne, C.-L.; Lopez-Capel, E.; Renforth, P.; Ascough, P.L.; Manning, D.A.C. Rapid Removal of Atmospheric CO2 by Urban Soils. Environ. Sci. Technol. 2015, 49, 5434–5440. [Google Scholar] [CrossRef]
- Beerling, D.J.; Leake, J.R.; Long, S.P.; Scholes, J.; Ton, J.; Nelson, P.N.; Bird, M.; Kantzas, E.; Taylor, L.L.; Sarkar, B.; et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 2018, 4, 138–147. [Google Scholar] [CrossRef]
- Edwards, D.P.; Lim, F.; James, R.; Pearce, C.R.; Scholes, J.; Freckleton, R.; Beerling, D. Climate change mitigation: Potential benefits and pitfalls of enhanced rock weathering in tropical agriculture. Biol. Lett. 2017, 13, 20160715. [Google Scholar] [CrossRef] [PubMed]
- Khalidy, R.; Haque, F.; Chiang, Y.W.; Santos, R.M. Monitoring Pedogenic Inorganic Carbon Accumulation Due to Weathering of Amended Silicate Minerals in Agricultural Soils. J. Vis. Exp. 2021, 172, e61996. [Google Scholar] [CrossRef] [PubMed]
- Beerling, D.J.; Kantzas, E.P.; Lomas, M.R.; Wade, P.; Eufrasio, R.M.; Renforth, P.; Sarkar, B.; Andrews, M.G.; James, R.; Pearce, C.R.; et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 2020, 583, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Amann, T.; Hartmann, J.; Struyf, E.; Garcia, W.D.O.; Fischer, E.K.; Janssens, I.; Meire, P.; Schoelynck, J. Enhanced Weathering and related element fluxes—A cropland mesocosm approach. Biogeosciences 2020, 17, 103–119. [Google Scholar] [CrossRef]
- Haque, F.; Santos, R.M.; Chiang, Y.W. Optimizing Inorganic Carbon Sequestration and Crop Yield with Wollastonite Soil Amendment in a Microplot Study. Front. Plant Sci. 2020, 11, 1012. [Google Scholar] [CrossRef]
- Zamanian, K.; Zhou, J.; Kuzyakov, Y. Soil carbonates: The unaccounted, irrecoverable carbon source. Geoderma 2020, 384, 114817. [Google Scholar] [CrossRef]
- Jorat, M.E.; Aziz, M.A.; Marto, A.; Zaini, N.; Jusoh, S.N.; Manning, D.A. Sequestering Atmospheric CO2 Inorganically: A Solution for Malaysia’s CO2 Emission. Geosciences 2018, 8, 483. [Google Scholar] [CrossRef]
- Jorat, M.E.; Goddard, M.A.; Manning, P.; Lau, H.K.; Ngeow, S.; Sohi, S.P.; Manning, D.A. Passive CO2 removal in urban soils: Evidence from brownfield sites. Sci. Total Environ. 2019, 703, 135573. [Google Scholar] [CrossRef]
- Jorat, M.E.; Kraavi, K.E.; Manning, D.A. Removal of atmospheric CO2 by engineered soils in infrastructure projects. J. Environ. Manag. 2022, 314, 115016. [Google Scholar] [CrossRef]
- Dudhaiya, A.; Haque, F.; Fantucci, H.; Santos, R.M. Characterization of Physically Fractionated Wollastonite-Amended Agricultural Soils. Minerals 2019, 9, 635. [Google Scholar] [CrossRef]
- Haque, F.; Santos, R.M.; Chiang, Y.W. Urban Farming with Enhanced Rock Weathering As a Prospective Climate Stabilization Wedge. Environ. Sci. Technol. 2021, 55, 13575–13578. [Google Scholar] [CrossRef] [PubMed]
- Manning, D.A.C.; Renforth, P. Passive Sequestration of Atmospheric CO2 through Coupled Plant-Mineral Reactions in Urban soils. Environ. Sci. Technol. 2012, 47, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Khalidy, R.; Santos, R.M. The fate of atmospheric carbon sequestrated through weathering in mine tailings. Miner. Eng. 2021, 163, 106767. [Google Scholar] [CrossRef]
- Lefebvre, D.; Goglio, P.; Williams, A.; Manning, D.A.; de Azevedo, A.C.; Bergmann, M.; Meersmans, J.; Smith, P. Assessing the potential of soil carbonation and enhanced weathering through Life Cycle Assessment: A case study for Sao Paulo State, Brazil. J. Clean. Prod. 2019, 233, 468–481. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; Rosa, L.; Mazzotti, M. Life cycle assessment of carbon dioxide removal technologies: A critical review. Energy Environ. Sci. 2021, 14, 1701–1721. [Google Scholar] [CrossRef]
- Khalidy, R.; Santos, R.M. Assessment of geochemical modeling applications and research hot spots—a year in review. Environ. Geochem. Health 2021, 43, 3351–3374. [Google Scholar] [CrossRef]
- Zelikova, T.J. The future of carbon dioxide removal must be transdisciplinary. Interface Focus 2020, 10, 20200038. [Google Scholar] [CrossRef]
- Dong, X.; Singh, B.P.; Li, G.; Lin, Q.; Zhao, X. Biochar increased field soil inorganic carbon content five years after application. Soil Tillage Res. 2018, 186, 36–41. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Q.; Lou, Y.; Du, Z.; Wang, Q.; Hu, N.; Wang, Y.; Gunina, A.; Song, J. Soil organic and inorganic carbon sequestration by consecutive biochar application: Results from a decade field experiment. Soil Use Manag. 2020, 37, 95–103. [Google Scholar] [CrossRef]
- Luo, X.; Wang, H.; An, Z.; Zhang, Z.; Liu, W. Carbon and oxygen isotopes of calcified root cells, carbonate nodules and total inorganic carbon in the Chinese loess–paleosol sequence: The application of paleoenvironmental studies. J. Southeast Asian Earth Sci. 2020, 201, 104515. [Google Scholar] [CrossRef]
- Khormali, F.; Shahriari, A.; Ghafarpour, A.; Kehl, M.; Lehndorff, E.; Frechen, M. Pedogenic carbonates archive modern and past precipitation change—A transect study from soils and loess-paleosol sequences from northern Iran. Quat. Int. 2019, 552, 79–90. [Google Scholar] [CrossRef]
- Washbourne, C.-L.; Renforth, P.; Manning, D. Investigating carbonate formation in urban soils as a method for capture and storage of atmospheric carbon. Sci. Total Environ. 2012, 431, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Gocke, M.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic carbonate formation: Recrystallization versus migration-Process rates and periods assessed by 14C labeling. Glob. Biogeochem. Cycles 2012, 26, 1–12. [Google Scholar] [CrossRef]
- Da, J.; Zhang, Y.G.; Li, G.; Ji, J. Aridity-driven decoupling of δ13C between pedogenic carbonate and soil organic matter. Geology 2020, 48, 981–985. [Google Scholar] [CrossRef]
- Oerter, E.J.; Amundson, R. Climate controls on spatial and temporal variations in the formation of pedogenic carbonate in the western Great Basin of North America. GSA Bull. 2016, 128, 1095–1104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).