Response of an Invasive Plant Species (Cynanchum acutum L.) to Changing Climate Conditions and Its Impact on Agricultural Landscapes
Abstract
:1. Introduction
2. Material and Method
2.1. Species
2.2. Study Area
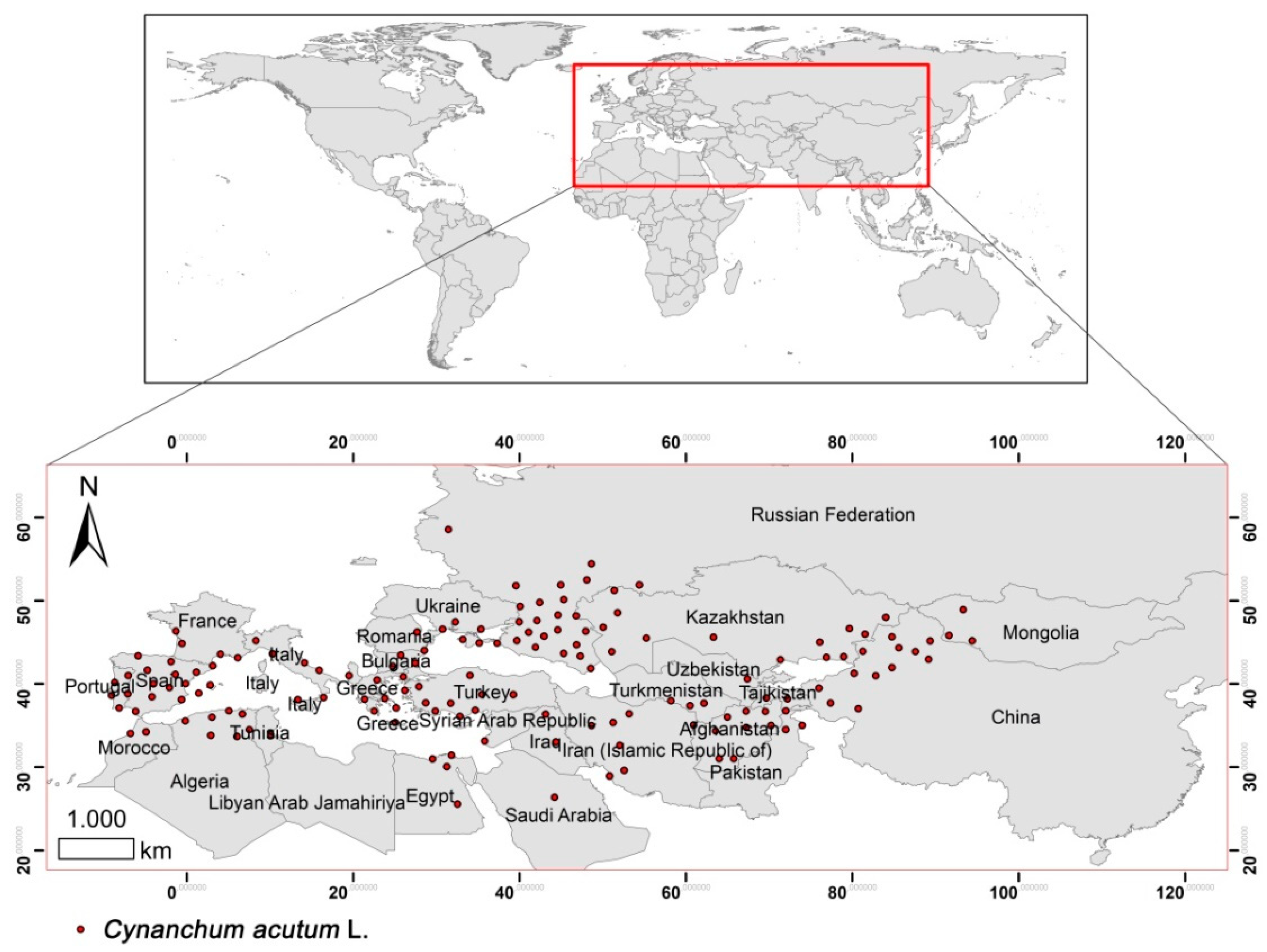
2.3. Occurrence Records and Environmental Variables
2.4. Ecological Niche Modelling
3. Results
3.1. Selection of the Model
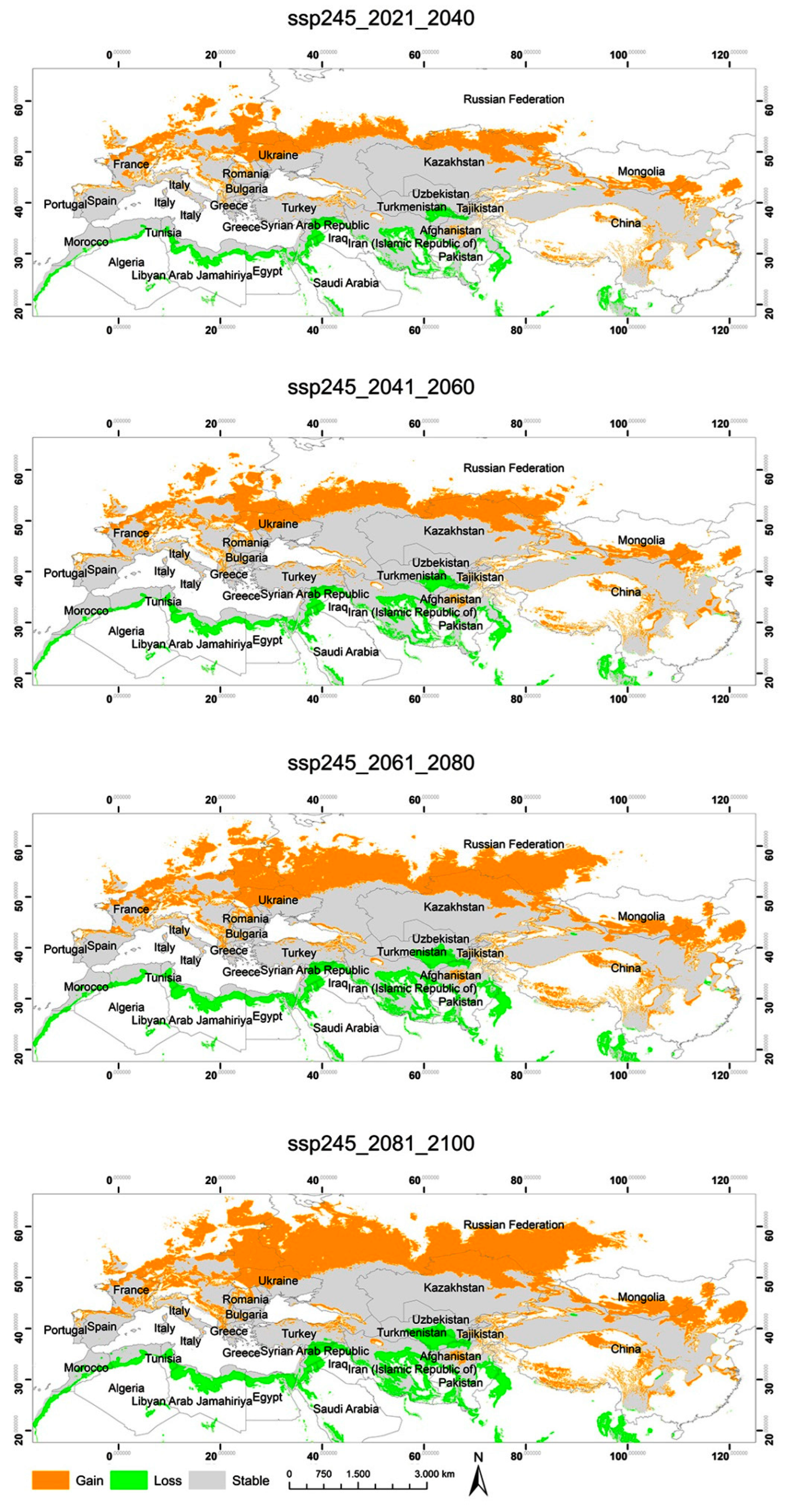
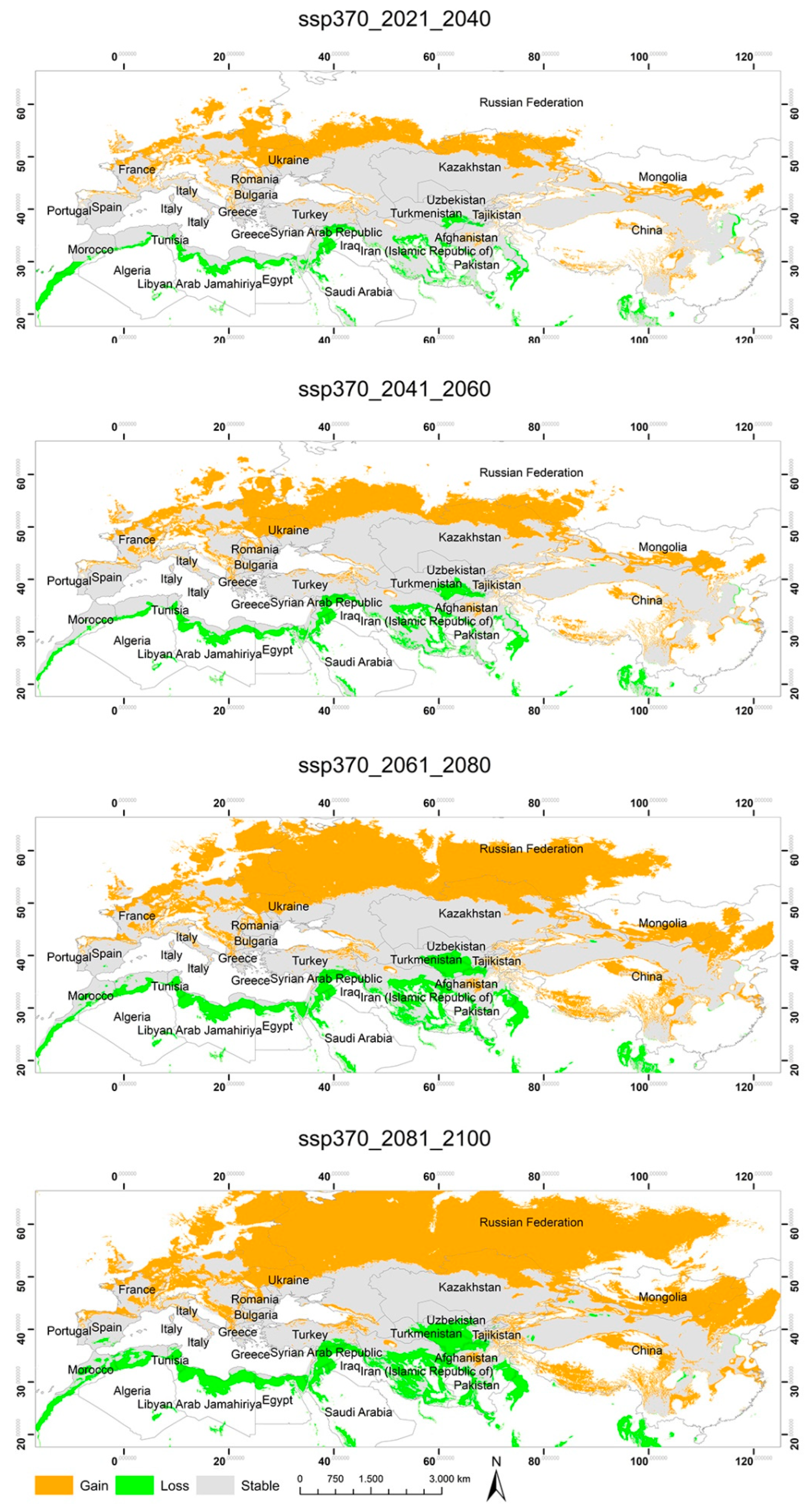
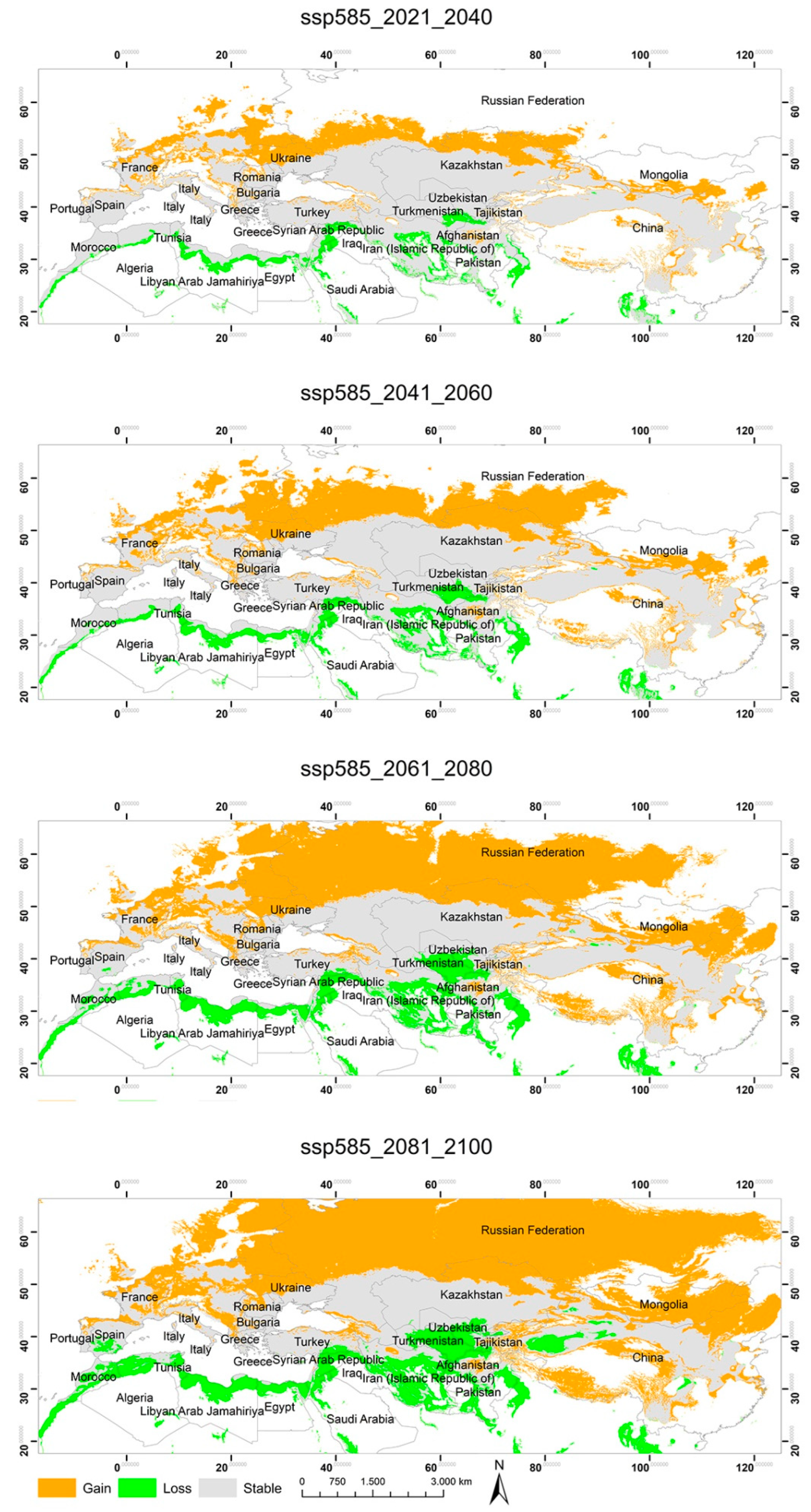
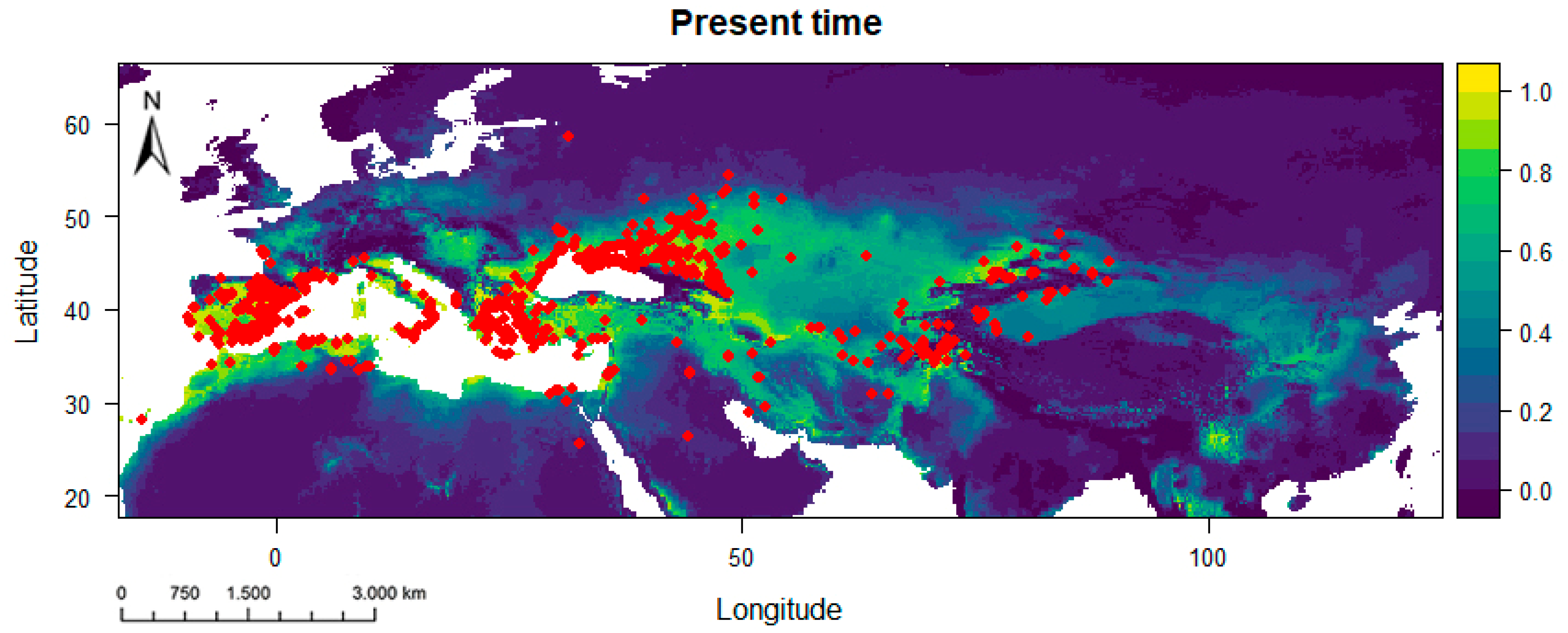
3.2. Potential Habitats—Current Situation and Predicted Geographical Changes under Three Climate Scenarios
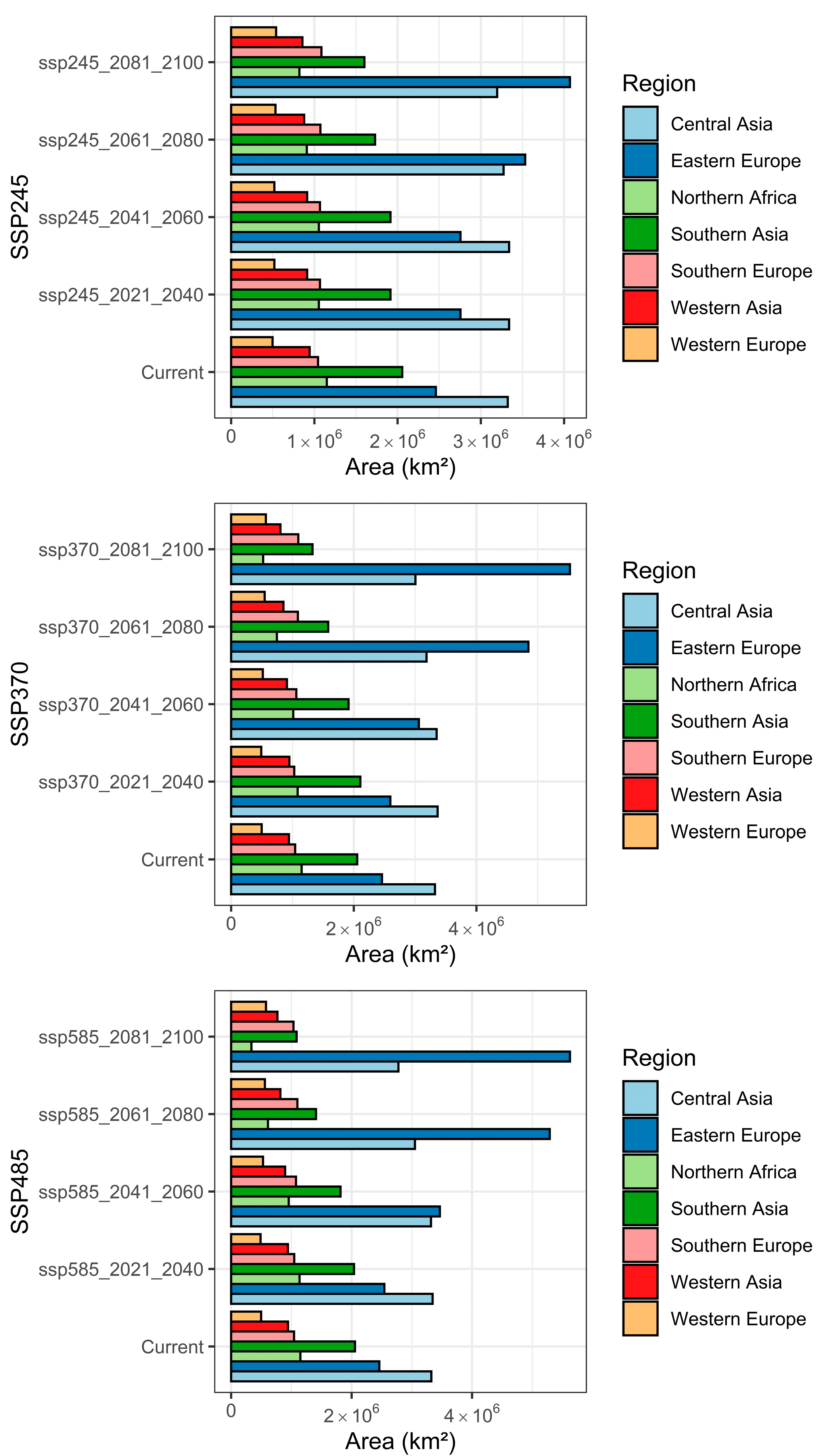
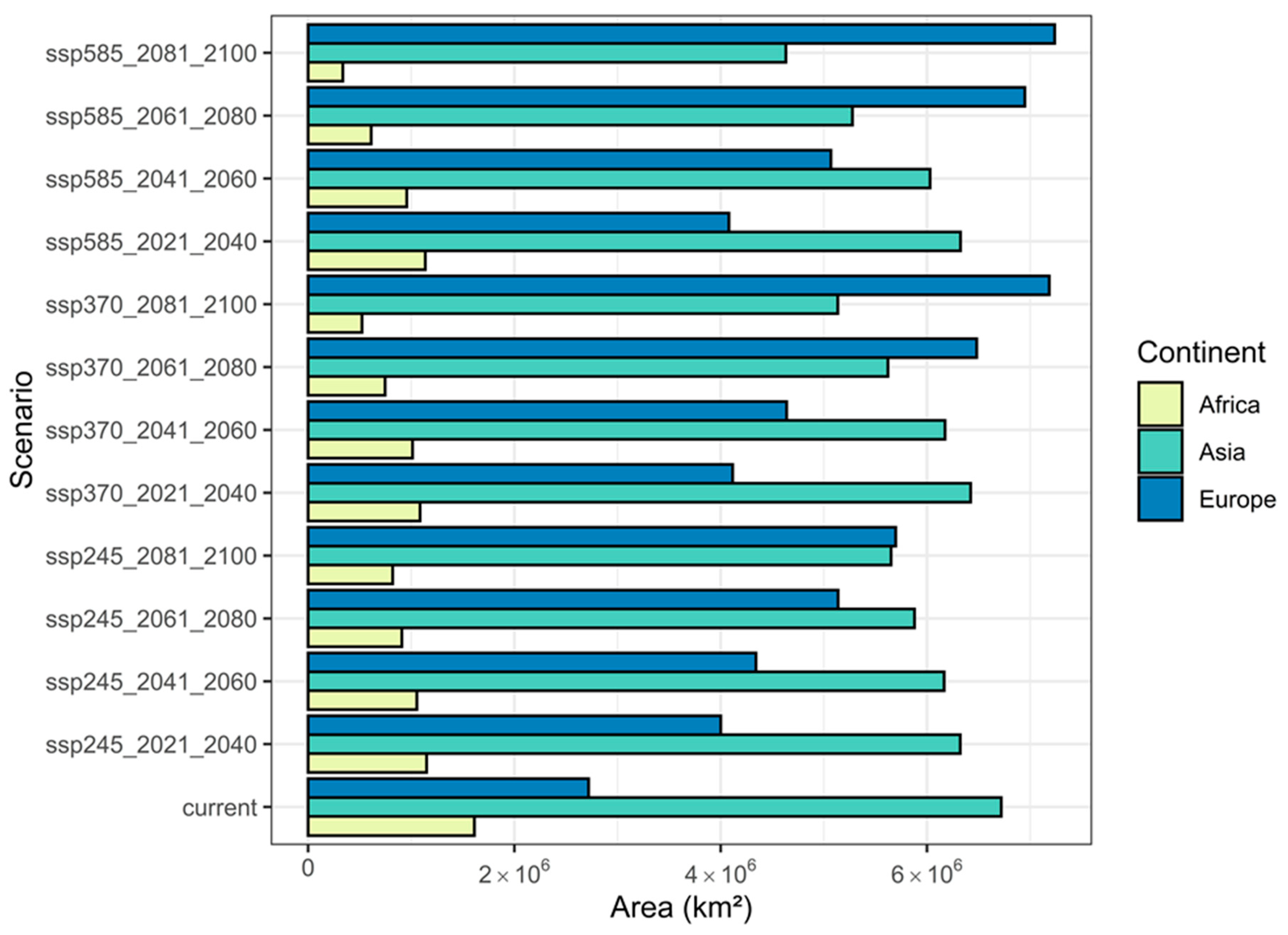
3.3. Changes in the Extent of the Suitable Areas for C. acutum under Future Climate Scenarios by Continent
3.4. Predicted Total Habitat Gains and Losses under Different Climate Scenarios
3.5. The Potential Spread of the Species to Agricultural Areas
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Model | Mean_ AUC_Ratio | Pval_ pROC | Omission_Rate_ at_5% | AICc | Delta_AICc | W_AICc | Num_ Parameters |
|---|---|---|---|---|---|---|---|
| M_0.1_F_lqp_ Set_26 | 1.361951528 | 0 | 0.065217391 | 4281.314 | 0 | 1 | 14 |
| M_0.1_F_lqp_ Set_39 | 1.355070872 | 0 | 0.065217391 | 4282.375 | 1.060487252 | 1 | 18 |


References
- Aouissi, H.A.; Gasparini, J.; Belabed, A.I.; Bouslama, Z. Impact of greenspaces in city on avian species richness and abundance in Northern Africa. Comptes Rendus. Biol. 2017, 340, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.C.; Adams, H.; Hope, B.; Powell, M. Risk Assessment for Invasive Species. Risk Anal. 2004, 24, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef] [PubMed]
- Kulhanek, S.; Ricciardi, A.; Leung, B. Is Invasion History a Useful Tool for Predicting the Impacts of the World’s Worst Aquatic Invasive Species? Ecol. Appl. 2011, 21, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, Y.; Lee, G.; Lee, D.H.; Adhikari, P. Predicting Impacts of Climate Change on Northward Range Expansion of Invasive Weeds in South Korea. Plants 2021, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.L.; Burdett, C.L.; Farnsworth, M.L.; Sweeney, S.J.; Miller, R.S. A globally-distributed alien invasive species poses risks to United States imperiled species. Sci. Rep. 2018, 8, 5331. [Google Scholar] [CrossRef]
- Sürmen, M.; Kara, E. Effects of suppression applications of summer asphodel (Asphodelus aestivus Roth.) on yield, quality and vegetation change in Aegean rangelands. Turk. J. Field Crop. 2022, 27, 61–70. [Google Scholar] [CrossRef]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, S.S. Potential risks of invasive alien plant species on agriculture under climate change scenarios in Sri Lanka. Curr. Res. Environ. Sustain. 2021, 3, 100051. [Google Scholar] [CrossRef]
- Weber, E.; Sun, S.G.; Li, B. Invasive alien plants in China: Diversity and ecological insights. Biol. Invasions 2008, 10, 1411–1429. [Google Scholar] [CrossRef]
- Kara, E.; Sürmen, M. The Effects of Secondary Metabolites of Rangeland and Pasture Plants on the Animal Health in Mediterranean Ecological Conditions. J. US-China Med. Sci. 2019, 16, 63–72. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.; Wang, T.; Jiang, X.; Hu, X.; Feng, J. Double-edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020, 740, 139933. [Google Scholar] [CrossRef] [PubMed]
- Fantle-Lepczyk, J.E.; Haubrock, P.J.; Kramer, A.M.; Cuthbert, R.N.; Turbelin, A.J.; Crystal-Ornelas, R.; Diagne, C.; Courchamp, F. Economic costs of biological invasions in the United States. Sci. Total Environ. 2021, 806, 151318. [Google Scholar] [CrossRef] [PubMed]
- Jimenezvalverde, A.; Peterson, A.T.; Soberon, J.; Overton, J.M.; Aragón, P.; Lobo, J.M. Use of niche models in invasive species risk assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Sutherst, R.W. Prediction of species geographical ranges. J. Biogeogr. 2003, 30, 805–816. [Google Scholar] [CrossRef]
- Mwangi, E.; Swallow, B. Invasion of Prosopis Juliflora and Local Livelihoods: Case Study from the Lake Baringo Area of Kenya; ICRAF Working Paper no. 3; World of Agroforestry Centre (ICRAF): Nairobi, Kenya, 2005. [Google Scholar] [CrossRef]
- Masters, G.; Norgrove, L. Climate Change and Invasive Alien Species; Cabi Working Paper; CABI: Delémont, Switzerland, 2010. [Google Scholar]
- Kazmi, J.H.; Haase, D.; Shahzad, A.; Shaikh, S.; Zaidi, S.M.; Qureshi, S. Mapping spatial distribution of invasive alien species through satellite remote sensing in Karachi, Pakistan: An urban ecological perspective. Int. J. Environ. Sci. Technol. 2021, 19, 3637–3654. [Google Scholar] [CrossRef]
- Belabed, A.; Aouissi, H.A.; Zediri, H.; Djemadi, I.; Driss, K.; Houhamdi, M.; Eraud, C.; Bouslama, Z. The effect of urbanization on the phenotype of the Collared Dove (Streptopelia decaocto) in northeastern Algeria. Bull. Inst. Sci. Rabat. Sect. Sci. Vie 2014, 35, 155–164. [Google Scholar]
- Storkey, J.; Mead, A.; Addy, J.; MacDonald, A.J. Agricultural intensification and climate change have increased the threat from weeds. Glob. Chang. Biol. 2021, 27, 2416–2425. [Google Scholar] [CrossRef]
- Downey, P.O.; Williams, M.C.; Whiffen, L.K.; Turner, P.J.; Burley, A.L.; Hamilton, M.A. Weeds and biodiversity conservation: A review of managing weeds under the New South Wales Threatened Species Conservation Act 1995. Ecol. Manag. Restor. 2009, 10, S53–S58. [Google Scholar] [CrossRef]
- Niu, Y.L.; Chen, X.; Wu, Y.; Jiang, H.Q.; Zhang, X.L.; Li, E.T.; Li, Y.Y.; Zhou, H.L.; Liu, J.G.; Wang, D.Y. Chemical constituents from Cynanchum paniculatum (Bunge) Kitag. Biochem. Syst. Ecol. 2015, 61, 139–142. [Google Scholar] [CrossRef]
- Douglass, C.H.; Weston, L.A.; DiTommaso, A. Black and Pale Swallow-Wort (Vincetoxicum nigrum and V. rossicum): The Biology and Ecology of Two Perennial, Exotic and Invasive Vines. In Management of Invasive Weeds; Springer: Dordrecht, The Netherlands, 2009; pp. 261–277. [Google Scholar] [CrossRef]
- Fawzy, G.A.; Abdallah, H.M.; Marzouk, M.S.A.; Soliman, F.M.; Sleem, A. Antidiabetic and Antioxidant Activities of Major Flavonoids of Cynanchum acutum L. (Asclepiadaceae) Growing in Egypt. Z. Nat. C 2008, 63, 658–662. [Google Scholar] [CrossRef]
- Meighani, F.; Karaminejad, M.R.; Farrokhi, Z. Invasive weed swallow-wort (Cynanchum acutum L.) response to chemical and mechanical practices. Weed Biol. Manag. 2021, 21, 124–132. [Google Scholar] [CrossRef]
- El-Katony, T.M.; Khedr, A.-H.A.-F.; Mergeb, S.O. Drought stress affects gas exchange and uptake and partitioning of minerals in swallowwort (Cynanchum acutum L.). Rendiconti Lince 2017, 29, 23–34. [Google Scholar] [CrossRef]
- Meighani, F.; Mirvakili, M.; Shimi, P.; Baghestani, M.A. Integrated Management Study (Chemical and Mechanical) of Swallow-Wort (Cynanchum Acutum) in Qazvin Province. Iran. Plants Prot. Res. 2014, 28, 387–392. [Google Scholar] [CrossRef]
- Töngel, M.Ö.; Ayan, İ. Samsun İli Çayır ve Meralarında Yetişen Bazı Zararlı Bitkiler ve Hayvanlar Üzerindeki Etkileri. Anadolu Tarım Bilimleri Dergisi 2005, 20, 84–93. [Google Scholar]
- Kara, A.; Ata, E. Tekirdağ İli Bağ Alanlarında Görülen Önemli Yabancı Ot Türleri, Yoğunlukları ve Rastlanma Sıklıklarının Belirlenmesi. Tekirdağ Ziraat Fakültesi Dergisi 2021, 18, 333–343. [Google Scholar] [CrossRef]
- Yılar, M.; Bayar, Y.; Akan, K. Kırşehir İli Nohut Üretim Alanlarında Görülen Yabancı Otların Yaygınlık ve Yoğunluklarının Belirlenmesi. Turk. J. Weed Sci. 2021, 24, 83–90. [Google Scholar]
- Sokat, Y. Denizli ve Manisa İli Kekik (Origanum onites L.) Alanlarında Sorun Olan Yabancı Ot Türlerinin Vegetasyon Dönemindeki Değişimi. Turk. J. Weed Sci. 2020, 23, 34–43. [Google Scholar]
- Wang, C.J.; Wan, J.Z. Assessing the habitat suitability of 10 serious weed species in global croplands. Glob. Ecol. Conserv. 2020, 23, e01142. [Google Scholar] [CrossRef]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, S.S. Invasive Plant Species Establishment and Range Dynamics in Sri Lanka under Climate Change. Entropy 2019, 21, 571. [Google Scholar] [CrossRef]
- Soberon, J.; Peterson, A.T. Interpretation of Models of Fundamental Ecological Niches and Species’ Distributional Areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- GBIF. Available online: https://doi.org/10.15468/dl.zhsyyf (accessed on 5 July 2022).
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Campbell, L.P.; Luther, C.; Moo-Llanes, D.; Ramsey, J.M.; Danis-Lozano, R.; Peterson, A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140135. [Google Scholar] [CrossRef]
- Heikkinen, R.K.; Luoto, M.; Araújo, M.B.; Virkkala, R.; Thuiller, W.; Sykes, M.T. Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog. Phys. Geogr. Earth Environ. 2006, 30, 751–777. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2013, 37, 191–203. [Google Scholar] [CrossRef]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Kissling, W.D.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Sanderson, B.; Knutti, R.; Caldwell, P. A Representative Democracy to Reduce Interdependency in a Multimodel Ensemble. J. Clim. 2015, 28, 5171–5194. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Voldoire, A.; Saint-Martin, D.; Sénési, S.; Decharme, B.; Alias, A.; Chevallier, M.; Colin, J.; Guérémy, J.; Michou, M.; Moine, M.; et al. Evaluation of CMIP6 DECK Experiments With CNRM-CM6-1. J. Adv. Model. Earth Syst. 2019, 11, 2177–2213. [Google Scholar] [CrossRef]
- Séférian, R.; Nabat, P.; Michou, M.; Saint-Martin, D.; Voldoire, A.; Colin, J.; Decharme, B.; Delire, C.; Berthet, S.; Chevallier, M.; et al. Evaluation of CNRM Earth System Model, CNRM-ESM2-1: Role of Earth System Processes in Present-Day and Future Climate. J. Adv. Model. Earth Syst. 2019, 11, 4182–4227. [Google Scholar] [CrossRef]
- Swart, N.C.; Cole, J.N.S.; Kharin, V.V.; Lazare, M.; Scinocca, J.F.; Gillett, N.P.; Anstey, J.; Arora, V.; Christian, J.R.; Hanna, S.; et al. The Canadian Earth System Model version 5 (CanESM5.0.3). Geosci. Model Dev. 2019, 12, 4823–4873. [Google Scholar] [CrossRef] [Green Version]
- Shiogama, H.; Hirata, R.; Hasegawa, T.; Fujimori, S.; Ishizaki, N.; Chatani, S.; Watanabe, M.; Mitchell, D.; Lo, Y.T.E. Historical and Future Anthropogenic Warming Effects on the Year 2015 Droughts, Fires and Fire Emissions of CO2 and PM2.5 in Equatorial Asia. Earth system change: Climate prediction. Earth Syst. Dyn. Discuss. 2019, 11, 435–445. [Google Scholar] [CrossRef]
- Cobos, M.E.; Townsend Peterson, A.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2013, 41, 629–643. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Anderson, R.P.; Lew, D.; Peterson, A. Evaluating predictive models of species’ distributions: Criteria for selecting optimal models. Ecol. Model. 2003, 162, 211–232. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Lamigueiro, O.P.; Hijmans, R.; Lamigueiro, M.O.P. Package ‘RasterVis’. 2021. [Google Scholar]
- Potapov, P.; Turubanova, S.; Hansen, M.C.; Tyukavina, A.; Zalles, V.; Khan, A.; Song, X.-P.; Pickens, A.; Shen, Q.; Cortez, J. Global maps of cropland extent and change show accelerated cropland expansion in the twenty-first century. Nat. Food 2021, 3, 19–28. [Google Scholar] [CrossRef]
- Sürmen, M.; Yavuz, T.; Sürmen, B.; Kutbay, G. Samsun İli Çayır ve Mera Alanlarında İstilacı Türlerin Tespiti ve Yoğunluklarının Belirlenmesi. Turk. J. Weed Sci. 2015, 18, 1–5. [Google Scholar]
- Satil, F.; Selvi, S.; Tumen, G. Balıkesir Florasında İstilacı Karaktere Sahip Yerli Bitki Taksonları Üzerine Bir Araştırma. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Dergisi 2020, 23, 928–946. [Google Scholar] [CrossRef]
- Farooq, S.; Önen, H.; Akyol, N. Gözlerden Irak Bir İstilaci: Commelina Communis L.; Orta Karadeniz Geçit Kuşağı Tarımsal Araştırma İstasyonu Müdürlüğü: Tokat, Turkey, 2015.
- Hausfather, Z. CMIP6: The Next Generation of Climate Models Explained—Carbon Brief. Available online: https://www.carbonbrief.org/cmip6-the-next-generation-of-climate-models-explained/ (accessed on 30 June 2022).
- Peters, K.; Breitsameter, L.; Gerowitt, B. Impact of climate change on weeds in agriculture: A review. Agron. Sustain. Dev. 2014, 34, 707–721. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D.; McNair, S.; Janecka, J.; Wightman, J.; Simmonds, C.; O’Connell, C.; Wong, E.; Russel, L.; Zern, J.; Aquino, T.; et al. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric. Ecosyst. Environ. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- Booth, B.D.; Murphy, S.D.; Swanton, C.J. Invasive Ecology of Weeds in Agricultural Systems. In Weed Biology and Management; Inderjit, Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 29–45. [Google Scholar] [CrossRef]
- Culliney, T.W. Benefits of Classical Biological Control for Managing Invasive Plants. Crit. Rev. Plant Sci. 2005, 24, 131–150. [Google Scholar] [CrossRef]
- Nasim, G.; Shabbir, A. Invasive Weed Species—A Threat to Sustainable Agriculture. In Crop Production for Agricultural Improvement; Ashraf, M., Öztürk, M., Ahmad, M.S.A., Aksoy, A., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 523–556. ISBN 978-94-007-4116-4. [Google Scholar]
- Bowmer, K.H. Ecosystem Effects from Nutrient and Pesticide Pollutants: Catchment Care as a Solution. Resources 2013, 2, 439–456. [Google Scholar] [CrossRef]
- Thapa, S.; Chitale, V.; Rijal, S.J.; Bisht, N.; Shrestha, B.B. Understanding the dynamics in distribution of invasive alien plant species under predicted climate change in Western Himalaya. PLoS ONE 2018, 13, e0195752. [Google Scholar] [CrossRef]
- Calinger, K.; Calhoon, E.; Chang, H.-C.; Whitacre, J.; Wenzel, J.; Comita, L.; Queenborough, S. Historic Mining and Agriculture as Indicators of Occurrence and Abundance of Widespread Invasive Plant Species. PLoS ONE 2015, 10, e0128161. [Google Scholar] [CrossRef]
- Önen, H. İstilacı Yabancı Türler ve İstila Süreçleri. In Türkiye İstilacı Bitkiler Katalogu; T. C. Gıda, Tarım ve Hayvancılık Bakanlığı, Tarımsal Araştırmalar ve Politikalar Genel Müdürlüğü Bitki Sağlığı Araştırmaları Daire Başkanlığı: Ankara, Turkey, 2015; p. 13. [Google Scholar]
- Farooq, S. Experimental and Ecological Niche Modelling Approaches to Predict Potential Distribution Areas of Some Invasive Weeds in Turkey. Ph.D. Thesis, Gaziosmanpaşa Üniversitesi, Tokat, Turkey, 2018. [Google Scholar]
- Zenni, R.D. Analysis of introduction history of invasive plants in Brazil reveals patterns of association between biogeographical origin and reason for introduction. Austral Ecol. 2013, 39, 401–407. [Google Scholar] [CrossRef]

| Scenario | Gain (km2) | Loss (km2) | Stable (km2) |
|---|---|---|---|
| ssp245 2021–2040 | 2,634,605.35 | 1,490,184.82 | 10,985,402.89 |
| ssp245 2041–2060 | 3,123,948.08 | 1,868,191.87 | 10,607,395.83 |
| ssp245 2061–2080 | 4,222,180.29 | 2,396,831.93 | 10,078,755.77 |
| ssp245 2081–2100 | 4,935,024.08 | 2,744,423.34 | 9,731,164.36 |
| ssp370 2021–2040 | 2,696,045.60 | 1,593,444.20 | 10,882,143.50 |
| ssp370 2041–2060 | 3,507,823.75 | 1,920,419.49 | 10,555,168.21 |
| ssp370 2061–2080 | 5,865,243.74 | 2,868,879.52 | 9,606,708.18 |
| ssp370 2081–2100 | 6,826,764.28 | 3,680,999.46 | 8,794,588.25 |
| ssp585 2021–2040 | 2,664,147.07 | 1,522,821.45 | 10,952,766.24 |
| ssp585 2041–2060 | 4,074,629.67 | 2,159,356.30 | 10,316,231.39 |
| ssp585 2061–2080 | 6,468,715.67 | 3,410,208.87 | 9,065,378.84 |
| ssp585 2081–2100 | 7,111,382.35 | 4,504,907.16 | 7,970,680.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ar, B.; Tuttu, G.; Gülçin, D.; Özcan, A.U.; Kara, E.; Sürmen, M.; Çiçek, K.; Velázquez, J. Response of an Invasive Plant Species (Cynanchum acutum L.) to Changing Climate Conditions and Its Impact on Agricultural Landscapes. Land 2022, 11, 1438. https://doi.org/10.3390/land11091438
Ar B, Tuttu G, Gülçin D, Özcan AU, Kara E, Sürmen M, Çiçek K, Velázquez J. Response of an Invasive Plant Species (Cynanchum acutum L.) to Changing Climate Conditions and Its Impact on Agricultural Landscapes. Land. 2022; 11(9):1438. https://doi.org/10.3390/land11091438
Chicago/Turabian StyleAr, Buse, Gamze Tuttu, Derya Gülçin, Ali Uğur Özcan, Emre Kara, Mustafa Sürmen, Kerim Çiçek, and Javier Velázquez. 2022. "Response of an Invasive Plant Species (Cynanchum acutum L.) to Changing Climate Conditions and Its Impact on Agricultural Landscapes" Land 11, no. 9: 1438. https://doi.org/10.3390/land11091438
APA StyleAr, B., Tuttu, G., Gülçin, D., Özcan, A. U., Kara, E., Sürmen, M., Çiçek, K., & Velázquez, J. (2022). Response of an Invasive Plant Species (Cynanchum acutum L.) to Changing Climate Conditions and Its Impact on Agricultural Landscapes. Land, 11(9), 1438. https://doi.org/10.3390/land11091438









