Landscape and Vegetation Patterns Zoning Is a Methodological Tool for Management Costs Implications Due to Xylella fastidiosa Invasion
Abstract
:1. Introduction
2. Materials and Methods
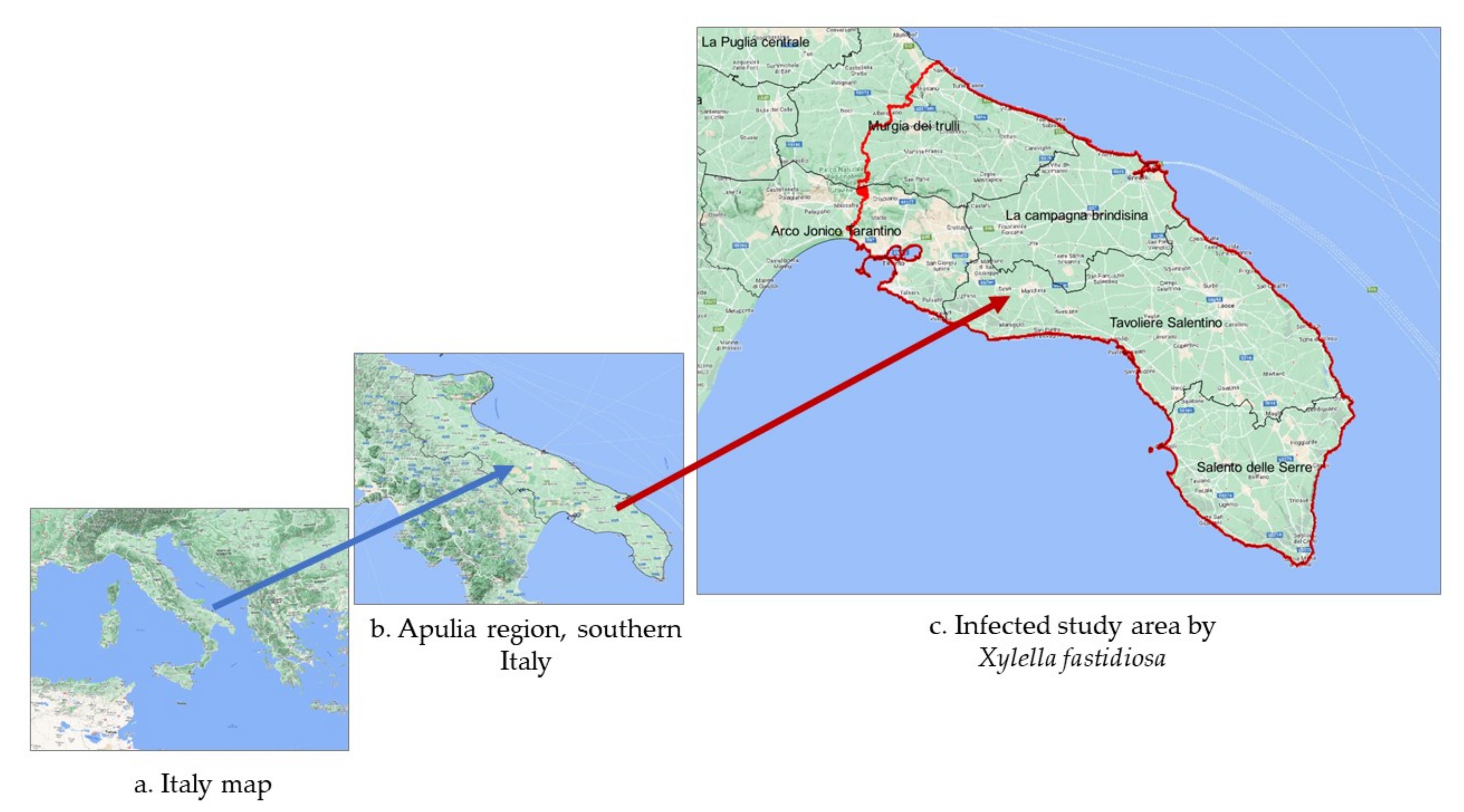
2.1. Agro-Ecological Overview of the Study Area
2.2. Conceptual Framework: Zoning and Mapping
2.3. Indicators Gathering and Calculation for Zoning
2.4. Indicators Zoning Implications for Management Costs
3. Results
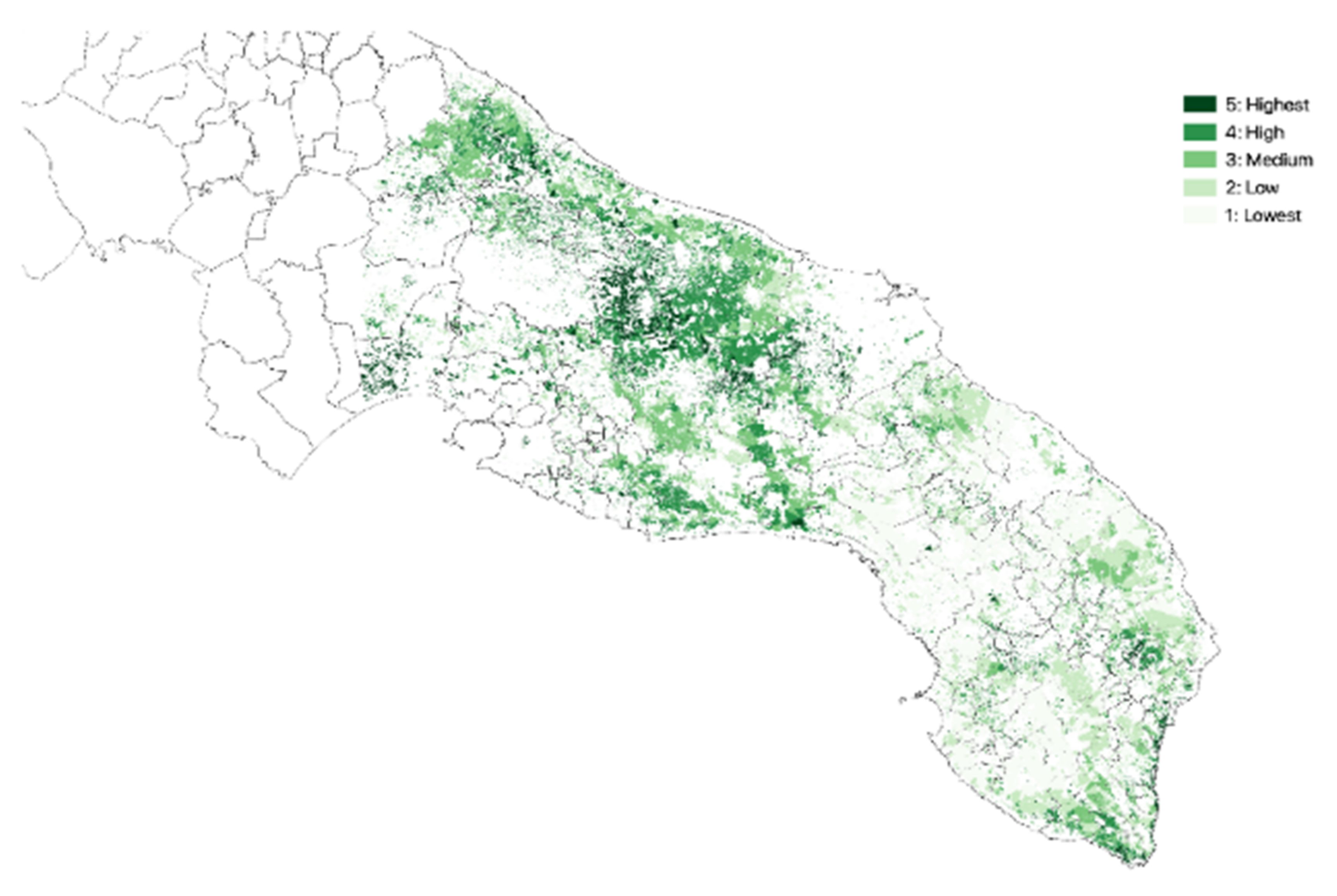
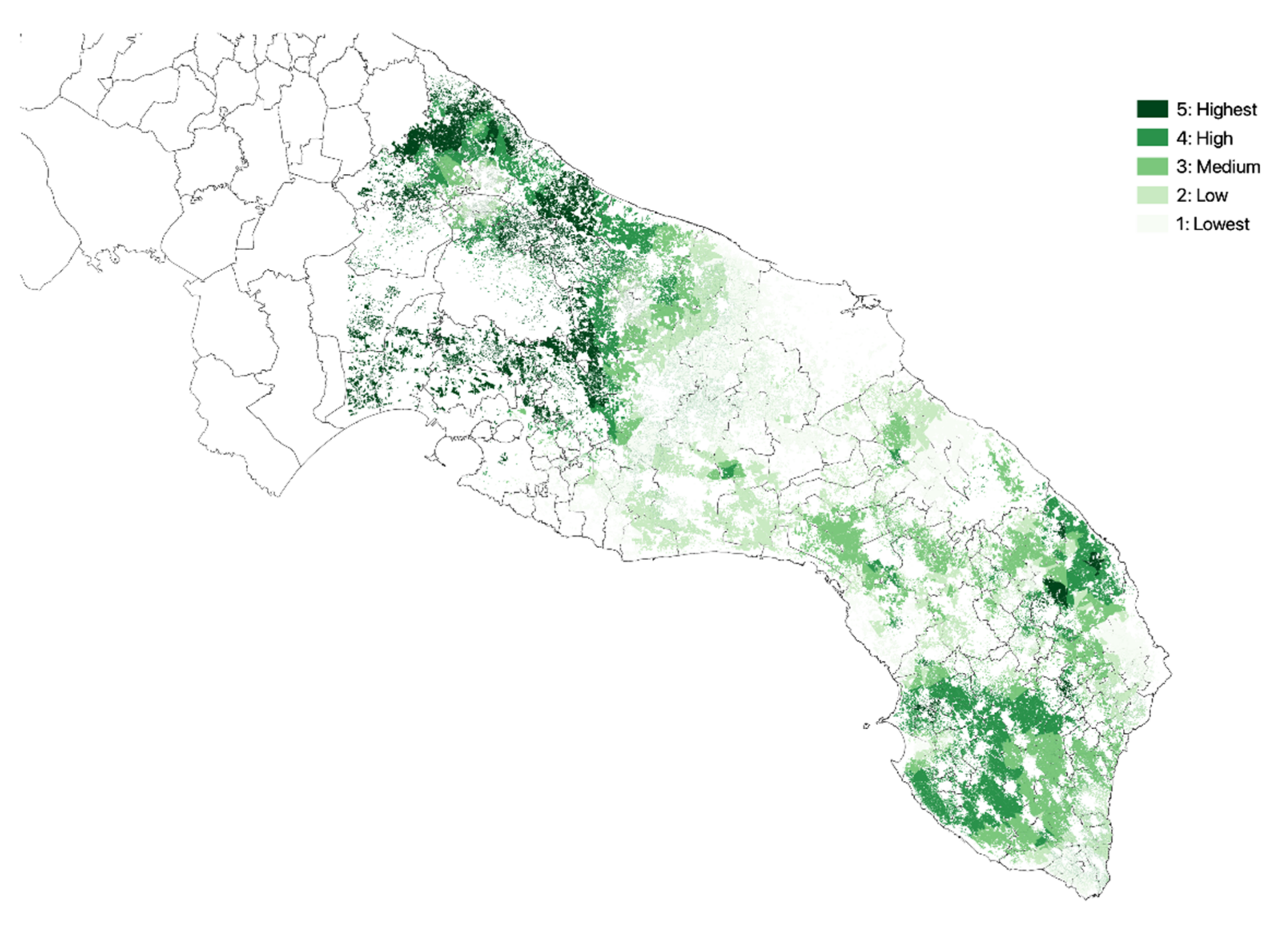
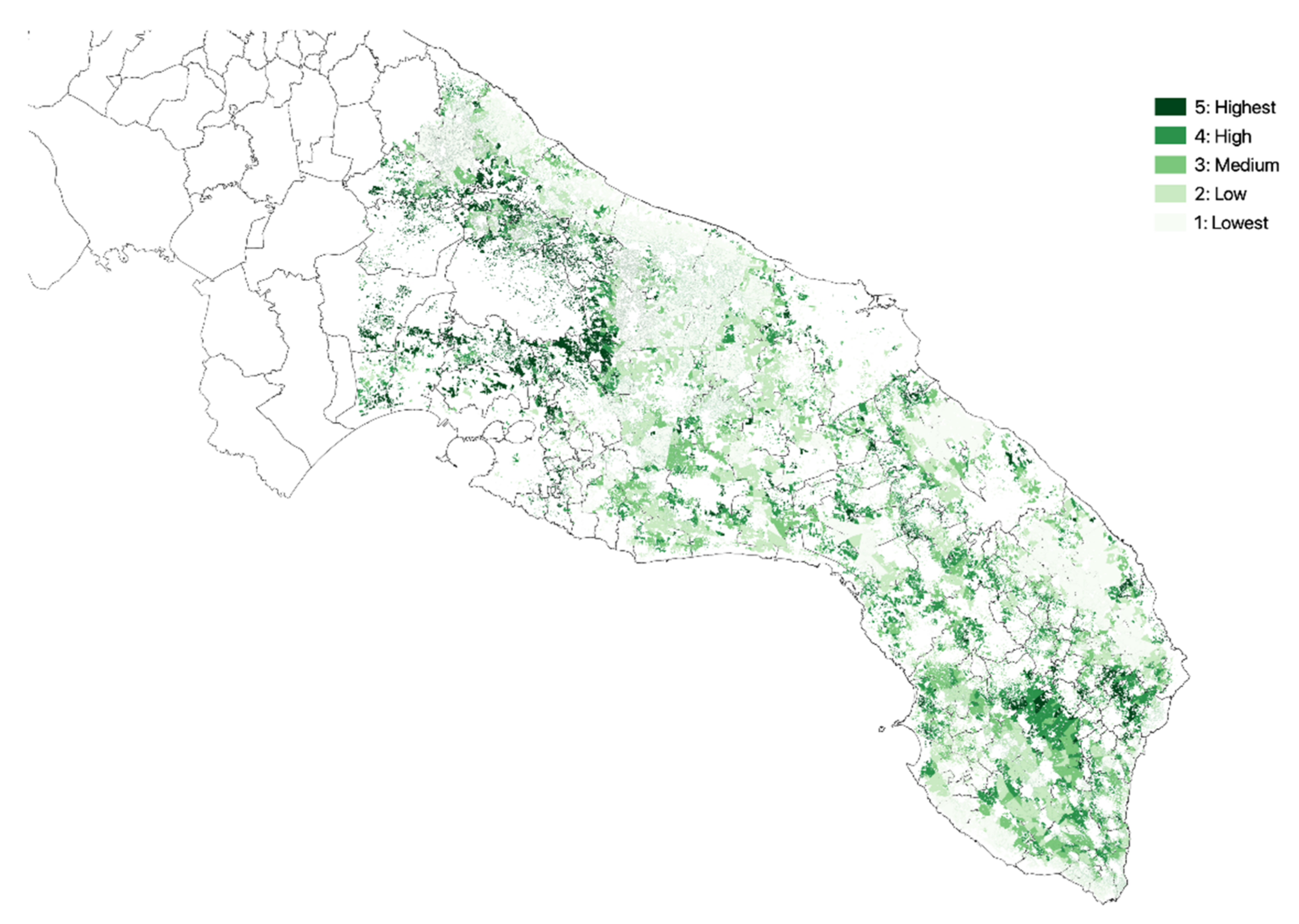
3.1. Olive Groves Indicators for Zoning
3.2. Zoning
3.3. Overall Impact of Zoning to Xf Vectors Management Costs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Indicator and Description | Source of Data and Mode of Calculation |
| Vegetation index: Presence of vegetation | Satellite images produced by sensors that acquire in the red (R: 0.7 µm) and near infrared (NIR: 0.9 µm). Vegetation index is calculated with the following formula: |
| Crop intensity: Level of agricultural use inputs (water, mechanization, fertilizers, and pesticides) | Georeferenced microdata by Farm Accountancy Data Network (FADN) aggregated at farm level and interpolated on a landscape scale according to Inverse Distance Weighting technique. Interpolated value u at a given point (x) is: Where: If d(x,xi) ≠ 0 for all i If d(x,xi) = 0 for some i Where: Is a weighting function in which: x denotes an interpolated (arbitrary) point, xi is an interpolating (known) point, d is a given distance from the known point xi to the unknown point x, N is the total number of known points used in interpolation and p is a positive real number, called the power parameter related to the aggregated and normalized value of agricultural inputs. |
| Crop diversity: Agricultural landscape structure | Shannon index calculated using crop typology classes incidence in each cell according to the following formula: where: C: constant equal to 1; Pj: percentage incidence of the surface of class J with respect to the total; s: number of classes of crop types J: J-th crop typology class |
| Density of agricultural landscape elements: Complexity of the agricultural landscape dry (stone walls, hedges, rows of tree) | Normalized relative value of the length of the elements of the agricultural landscape extracted from Regional Technical Map of Apulia and referred to the landscape reference unit |
| Altitude: Elevation from sea level | Digital Terrain Model of the Apulia region in meters above sea level on a raster file 20 × 20 m. |
References
- Wells, J.M.; Raju, B.C.; Hung, H.Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa gen. nov., sp. nov.: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. Bacteriol. 1987, 37, 136–143. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Plant Health. Scientific opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory, with the identification and evaluation of risk reduction options. EFSA J. 2015, 13, 3989. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Plant Health. Update of the scientific opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory. EFSA J. 2019, 17, 5665. [Google Scholar] [CrossRef]
- Bucci, E.M. Xylella fastidiosa, a new plant pathogen that threatens global farming: Ecology, molecular biology, search for remedies. Biochem. Biophys. Res. Commun. 2018, 502, 173–182. [Google Scholar] [CrossRef]
- Delbianco, A.; Gibin, D.; Pasinato, L.; Morelli, M. Update of the Xylella spp. host plant Database-Systematic literature search up to 31 December 2020. EFSA J. 2021, 19, 6674. [Google Scholar] [CrossRef]
- Morelli, M.; García-Madero, J.M.; Jos, Á.; Saldarelli, P.; Dongiovanni, C.; Kovacova, M.; Saponari, M.; Baños Arjona, A.; Hackl, E.; Webb, S. Xylella fastidiosa in olive: A review of control attempts and current management. Microorganisms 2021, 9, 1771. [Google Scholar] [CrossRef]
- Frem, M.; Chapman, D.; Fucilli, V.; Choueiri, E.; El Moujabber, M.; La Notte, P.; Nigro, F. Xylella fastidiosa invasion of new countries in Europe, the Middle East, and North Africa: Ranking the potential exposure scenarios. NeoBiota 2020, 59, 77–97. [Google Scholar] [CrossRef]
- Cardone, G.; Digiaro, M.; Djelouah, K.; El Bilali, H.; Frem, M.; Fucilli, V.; Ladisa, G.; Rota, C.; Yaseen, T. Potential socio-economic impact of Xylella fastidiosa in the Near East and North Africa (NENA): Risk of introduction and spread, risk perception and socio-economic effects. New Medit. 2021, 20, 27–51. [Google Scholar] [CrossRef]
- Frem, M.; Fucilli, V.; Nigro, F.; El Moujabber, M.; Abou Kubaa, R.; La Notte, P.; Bozzo, F.; Choueiri, E. The potential direct economic impact and private management costs of an invasive alien species: Xylella fastidiosa on Lebanese wine grapes. NeoBiota 2021, 70, 43–67. [Google Scholar] [CrossRef]
- Frem, M.; Santeramo, F.G.; Lamonaca, E.; El Moujabber, M.; Choueiri, E.; La Notte, P.; Nigro, F.; Bozzo, F.; Fucilli, V. Landscape restoration due to Xylella fastidiosa invasion in Italy: Assessing the hypothetical public’s preferences. NeoBiota 2021, 66, 31–54. [Google Scholar] [CrossRef]
- Cavalieri, V.; Dongiovanni, C.; Tauro, D.; Altamura, G.; Di Carolo, M.; Fumarola, G.L.; Saponari, M.; Bosco, D. Transmission of the Codiro strain of Xylella fastidiosa by different insect species. In Proceedings of the XI European Congress of Entomology, Naples, Italy, 2–6 July 2018. [Google Scholar] [CrossRef]
- Lopes, S.A.; Marcussi, S.; Torres, S.C.Z.; Souza, V.; Fagan, C.; França, S.C.; Fernandes, N.G.; Lopes, J.R.S. Weeds as alternative hosts of the citrus, coffee, and plum strains of Xylella fastidiosa in Brazil. Plant Dis. 2003, 87, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (hemiptera, aphrophoridae) to different host plants. J. Appl. Entomol. 2017, 141, 80–87. [Google Scholar] [CrossRef]
- López, M.M.; Narco-Noales, E.; Peñalver, J.; Morente, C.; Monterde, A. The world threat of Xylella fastidiosa. In Xylella fastidiosa & the Olive Quick Decline Syndrome (OQDS). A Serious Worldwide Challenge for the Safeguard of Olive Trees; D’Onghia, A.M., Brunel, S., Valentini, F., Eds.; Options Méditerranéennes; CIHEAM: Bari, Italy, 2017; 172p. [Google Scholar]
- Sun, Q.; Sun, Y.; Walker, M.A.; Labavitch, J.M. Vascular occlusions in grapevines with Pierce’s disease make disease symptom development worse. Plant Physiol. 2013, 161, 1529–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orusa, T.; Orusa, R.; Viani, A.; Carella, E.; Borgogno Mondino, E. Geomatics and EO Data to Support Wildlife Diseases Assessment at Landscape Level: A Pilot Experience to Map Infectious Keratoconjunctivitis in Chamois and Phenological Trends in Aosta Valley (NW Italy). Remote Sens. 2020, 12, 3542. [Google Scholar] [CrossRef]
- Metzger, J.P.; Muller, E. Characterizing the complexity of landscape boundaries by remote sensing. Landsc. Ecol. 1996, 11, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Frohn, R.C. Remote Sensing for Landscape Ecology: New Metric Indicators for Monitoring, Modeling, and Assessment of Ecosystems, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar] [CrossRef]
- Ding, Y.F.; Wang, G.Y.; Fu, E.P.; Shu, C. The role of miR398 in plant stress responses. Hereditas 2010, 32, 129–134. [Google Scholar] [CrossRef]
- Bozzo, F.; Fucilli, V.; Petrontino, A.; Girone, S. Identification of high nature value farmland: A methodological proposal. Ital. Rev. Agric. Econ. 2019, 74, 29–41. [Google Scholar]
- Petrontino, A.; Fucilli, V. High nature value forests identification: A case study in Apulia region—Italy. Aestimum 2013, 62, 67–88. [Google Scholar]
- Turner, B.L.; Doolittle, W.E. The concept and measure of agricultural intensity. Prof. Geogr. 1978, 30, 297–301. [Google Scholar] [CrossRef]
- Temme, A.J.A.M.; Verburg, P.H. Mapping and modelling of changes in agricultural intensity in Europe. Agric. Ecosyst. Environ. 2011, 140, 46–56. [Google Scholar] [CrossRef]
- Teillard, F.; Allaire, G.; Cahuzac, E.; Leger, F.; Maigne, E.; Tichit, M. A novel method for mapping agricultural intensity reveals its spatial aggregation: Implications for conservation policies. Agric. Ecosyst. Environ. 2012, 149, 135–143. [Google Scholar] [CrossRef]
- Dietrich, J.P.; Schmitz, C.; Müller, C.; Fader, M.; Lotze-Campen, H.; Popp, A. Measuring agricultural land-use intensity–A global analysis using a model-assisted approach. Ecol. Modell. 2012, 232, 109–118. [Google Scholar] [CrossRef]
- Shannon, C.; Weaver, W. The Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Feil, H.; Purcell, A. Temperature-dependent growth and survival of Xylella fastidiosa in vitro and in potted grapevines. Plant Dis. 2001, 85, 1230–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- McCarigal, K.; Marks, B.J. Fragstats: Spatial Pattern Analysis Program for Quantifying Landscape Structure; Gen. Tech. Rep. PNW-GTR-351; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 1995; p. 122. [Google Scholar]
- Turner, M.G. Landscape ecology: The effect of pattern on process. Annu. Rev. Ecol. Syst. 1989, 20, 171–197. [Google Scholar] [CrossRef]
- Turner, M.G. Landscape ecology: What is the state of the science? Annu. Rev. Ecol. Evol. Syst. 2005, 36, 319–344. [Google Scholar] [CrossRef]
- Marini, L.; Fontana, P.; Battisti, A.; Gaston, K. Agricultural management, vegetation traits and landscape drive orthopteran and butterfly diversity in a grassland-forest mosaic: A multi-scale approach. Insect Conserv. Divers. 2009, 2, 213–220. [Google Scholar] [CrossRef]
- Raffini, F.; Bertorelle, G.; Biello, R.; D’Urso, G.; Russo, D.; Bosso, L. From nucleotides to satellite imagery: Approaches to identify and manage the invasive pathogen Xylella fastidiosa and its insect vectors in Europe. Sustainability 2020, 12, 1450. [Google Scholar] [CrossRef]
- Baguette, M.; Dyck, H. Landscape connectivity and animal behavior: Functional grain as a key determinant for dispersal. Landsc. Ecol. 2007, 22, 1117–1129. [Google Scholar] [CrossRef]
- Castrignanò, A.; Belmonte, A.; Antelmi, I.; Quarto, R.; Quarto, F.; Shaddad, S.; Sion, V.; Muolo, M.R.; Ranieri, N.A.; Gadaleta, G.; et al. A geostatistical fusion approach using UAV data for probabilistic estimation of Xylella fastidiosa subsp. pauca infection in olive trees. Sci. Total Environ. 2021, 752, 141814. [Google Scholar] [CrossRef]
- Castrignanò, A.; Belmonte, A.; Antelmi, I.; Quarto, R.; Quarto, F.; Shaddad, S.; Sion, V.; Muolo, M.R.; Ranieri, N.A.; Gadaleta, G.; et al. Semi-automatic method for early detection of Xylella fastidiosa in olive trees using UAV multispectral imagery and geostatistical-discriminant analysis. Remote Sens. 2021, 13, 14. [Google Scholar] [CrossRef]
- Santoiemma, G.; Tamburini, G.; Sanna, F.; Mori, N.; Marini, L. Landscape composition predicts the distribution of Philaenus spumarius, vector of Xylella fastidiosa, in olive groves. J. Pest Sci. 2019, 92, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- White, S.M.; Bullock, J.M.; Hooftman, D.A.P.; Chapman, D.S. Modelling the spread of Xylella fastidiosa in the early stages of invasion in Apulia, Italy. Biol. Invasions 2017, 19, 1825–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornara, D.; Marra, M.; Tedone, B.; Cavalieri, V.; Porcelli, F.; Fereres, A.; Purcell, A.; Saponari, M. No evidence for cicadas’ implication in Xylella fastidiosa epidemiology. Entomologia 2020, 20, 125–132. [Google Scholar] [CrossRef]
- Strona, G.; Carstens, C.J.; Beck, P.S.A. Network analysis reveals why Xylella fastidiosa will persist in Europe. Sci. Rep. 2017, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Soria, A.; Picciotti, U.; Lopez-Moya, F.; Lopez-Cepero, J.; Porcelli, F.; Lopez-Llorca, L.V. Volatile organic compounds from Entomopathogenic and Nematophagous fungi, repel Banana Black Weevil (Cosmopolites sordidus). Insects 2020, 11, 509. [Google Scholar] [CrossRef]
- Dalbon, V.A.; Acevedo, J.P.M.; Ribeiro Junior, K.A.L.; Ribeiro, T.F.L.; da Silva, J.M.; Fonseca, H.G.; Santana, A.E.G.; Porcelli, F. Perspectives for Synergic Blends of Attractive Sources in South American Palm Weevil Mass Trapping: Waiting for the Red Palm Weevil Brazil Invasion. Insects 2021, 12, 828. [Google Scholar] [CrossRef] [PubMed]
- Sardaro, R.; Roseli, L.; Grittani, R.; Scrascia, M.; Pazzani, I.C.; Russo, V.; Garganese, F.; Porfido, C.; Diana, L.; Porcelli, F. Community preferences for the preservation of Canary Palm from Red Palm Weevil in the city of Bari. Arab. J. Plant Prot. 2019, 37, 206–211. [Google Scholar] [CrossRef]
- Scrascia, M.; D’Addabbo, P.; Roberto, R.; Porcelli, F.; Oliva, M.; Calia, C.; Dionisi, A.M.; Pazzani, C. Characterization of CRISPR-cas systems in Serratia marcescens isolated from Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Curculionidae). Microorganisms 2019, 7, 368. [Google Scholar] [CrossRef] [Green Version]
- Mifsud, D.; Porcelli, F. The psyllid Macrohomotoma gladiata Kuwayama, 1908 (Hemiptera: Psylloidea: Homotomidae): A Ficus pest recently introduced in the EPPO region. EPPO Bull. 2012, 42, 161–164. [Google Scholar] [CrossRef]
- Nugnes, F.; Laudonia, S.; Jesu, G.; Jansen, M.G.M.; Bernardo, U.; Porcelli, F. Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in some European countries: Diffusion, hosts, molecular characterization, and natural enemies. Insects 2020, 11, 4241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellizzari, G.; Porcelli, F.; Convertini, S.; Marotta, S. Description of nymphal instars and adult female of Kermes vermilio Planchon (Hemiptera, Coccoidea, Kermesidae), with a synopsis of the European and Mediterranean species. Zootaxa 2012, 3336, 36–50. [Google Scholar] [CrossRef]
- Cioffi, M.; Cornara, D.; Corrado, I.; Jansen, M.G.M.; Porcelli, F. The status of Aleurocanthus spiniferus from its unwanted introduction in Italy to date. Bull. Insectol. 2013, 66, 273–281. [Google Scholar]
- Picciotti, U.; Lahbib, N.; Sefa, V.; Porcelli, F.; Garganese, F. Aphrophoridae Role in Xylella fastidiosa subsp. pauca ST53 Invasion in Southern Italy. Pathogens 2021, 10, 1035. [Google Scholar] [CrossRef]
- Scrascia, M.; Pazzani, C.; Valentini, F.; Oliva, M.; Russo, V.; D’Addabbo, P.; Porcelli, F. Identification of pigmented Serratia marcescens symbiotically associated with Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae). Microbiol. Open 2016, 5, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Troccoli, A.; Oreste, M.; Tarasco, E.; Fanelli, E.; De Luca, F. Mononchoides macrospiculum n. sp. (Nematoda: Neodiplogastridae) and Teratorhabditis synpapillata Sudhaus, 1985 (Nematoda: Rhabditidae): Nematode associates of Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae) in Italy. Nematology 2015, 17, 953–966. [Google Scholar] [CrossRef]
- Petr, K.; Torsten, V.D.H. Zelus renardii (Hemiptera: Heteroptera: Reduviidae): First records from Croatia, Montenegro, and an accidental introduction to the Czech Republic. Heteroptera Pol. 2022, 6, 7–14. [Google Scholar] [CrossRef]
- Lahbib, N.; Picciotti, U.; Sefa, V.; Boukhris-Bouhachem, S.; Porcelli, F.; Garganese, F. Zelus renardii Roaming in Southern Italy. Insects 2022, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, A.; Fierro, A.; Garganese, F.; Picciotti, U.; Porcelli, F. A biological control model to manage the vector and the infection of Xylella fastidiosa on olive trees. PLoS ONE 2020, 15, 4. [Google Scholar] [CrossRef]
- Bubici, G.; Prigigallo, M.I.; Garganese, F.; Nugnes, F.; Jansen, M.; Porcelli, F. First Report of Aleurocanthus spiniferus on Ailanthus altissima: Profiling of the Insect Microbiome and MicroRNAs. Insects 2020, 11, 161. [Google Scholar] [CrossRef] [Green Version]
- Sardaro, R.; Grittani, R.; Scrascia, M.; Pazzani, C.; Russo, V.; Garganese, F.; Porfido, C.; Diana, L.; Porcelli, F. The Red Palm Weevil in the City of Bari: A First Damage Assessment. Forests 2018, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Salerno, M.; Mazzeo, G.; Suma, P.; Russo, A.; Diana, L.; Pellizzari, G.; Porcelli, F. Aspidiella hartii (Cockerell 1895) (Hemiptera: Diaspididae) on yam (Dioscorea spp.) tubers: A new pest regularly entering the European part of the EPPO region. EPPO Bull. 2018, 48, 287–292. [Google Scholar] [CrossRef]
- Tamburini, G.; Santoiemma, G.; O’Rourke, M.E.; Bommarco, R.; Chaplin-Kramer, R.; Dainese, M.; Marini, L. Species traits elucidate crop pest response to landscape composition: A global analysis. Proc. R. Soc. B 2020, 287, 1937. [Google Scholar] [CrossRef] [PubMed]
- Youngquist, M.B.; Boone, M.D. Making the connection: Combining habitat suitability and landscape connectivity to understand species distribution in an agricultural landscape. Landsc. Ecol. 2014, 36, 2795–2809. [Google Scholar] [CrossRef]
- Avosani, S.; Tattoni, C.; Mazzoni, V.; Ciolli, M. Occupancy and detection of agricultural threats: The case of Philaenus spumarius, European vector of Xylella fastidiosa. Agric. Ecosyst. Environ. 2021, 324, 107707. [Google Scholar] [CrossRef]
- Bodino, N.; Demichelis, S.; Simonetto, A.; Volani, S.; Saladini, M.A.; Gilioli, G.; Bosco, D. Phenology, seasonal abundance, and host-plant association of spittlebugs (Hemiptera: Aphrophoridae) in vineyards of northwestern Italy. Insects 2021, 12, 1012. [Google Scholar] [CrossRef]
- Brunetti, M.; Capasso, V.; Montagna, M.; Venturino, E. A mathematical model for Xylella fastidiosa epidemics in the Mediterranean regions. Promoting good agronomic practices for their effective control. Ecol. Modell. 2020, 432, 109204. [Google Scholar] [CrossRef]
- Hornero, A.; Hernández-Clemente, R.; North, P.R.J.; Beck, P.S.A.; Boscia, D.; Navas-Cortes, J.A.; Zarco-Tejada, P.J. Monitoring the incidence of Xylella fastidiosa infection in olive orchards using ground-based evaluations, airborne imaging spectroscopy and Sentinel-2 time series through 3-D radiative transfer modelling. Remote Sens. Environ. 2020, 36, 111480. [Google Scholar] [CrossRef]
- Fierro, A.; Liccardo, A.; Porcelli, F. A lattice model to manage the vector and the infection of the Xylella fastidiosa on olive trees. Sci. Rep. 2019, 9, 8723. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Sanna, F.; Mori, N.; Santoiemma, G.; D’Ascenzo, D.; Scotillo, M.A.; Marini, L. Ground cover management in olive groves reduces populations of Philaenus spumarius (Hemiptera: Aphrophoridae), vector of Xylella fastidiosa. J. Econ. Entomol. 2021, 114, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M. Predisposing factors for “olive quick decline syndrome” in Salento (Apulia, Italy). Agronomy 2020, 10, 1445. [Google Scholar] [CrossRef]
- Weiers, S.; Bock, M.; Wissen, M.; Rossner, G. Mapping and indicator approaches for the assessment of habitats at different scales using remote sensing and GIS methods. Landsc. Urban Plan. 2004, 67, 43–65. [Google Scholar] [CrossRef]
- Chapman, D.; Purse, B.; Roy, H.; Bullock, J. Global trade networks determine the distribution of invasive non-native species. Glob. Ecol. Biogeogr. 2017, 26, 907–917. [Google Scholar] [CrossRef]
- Kottelenberg, D.; Hemerik, L.; Saponari, M.; van der Werf, W. Shape and rate of movement of the invasion front of Xylella fastidiosa spp. pauca in Puglia. Sci. Rep. 2021, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- El Handi, K.; Hafidi, M.; Sabri, M.; Frem, M.; El Moujabber, M.; Habbadi, K.; Haddad, N.; Benbouazza, A.; Abou Kubaa, R.; Achbani, E.H. Continuous pest surveillance and monitoring constitute a tool for sustainable agriculture: Case of Xylella fastidiosa in Morocco. Sustainability 2022, 14, 1485. [Google Scholar] [CrossRef]


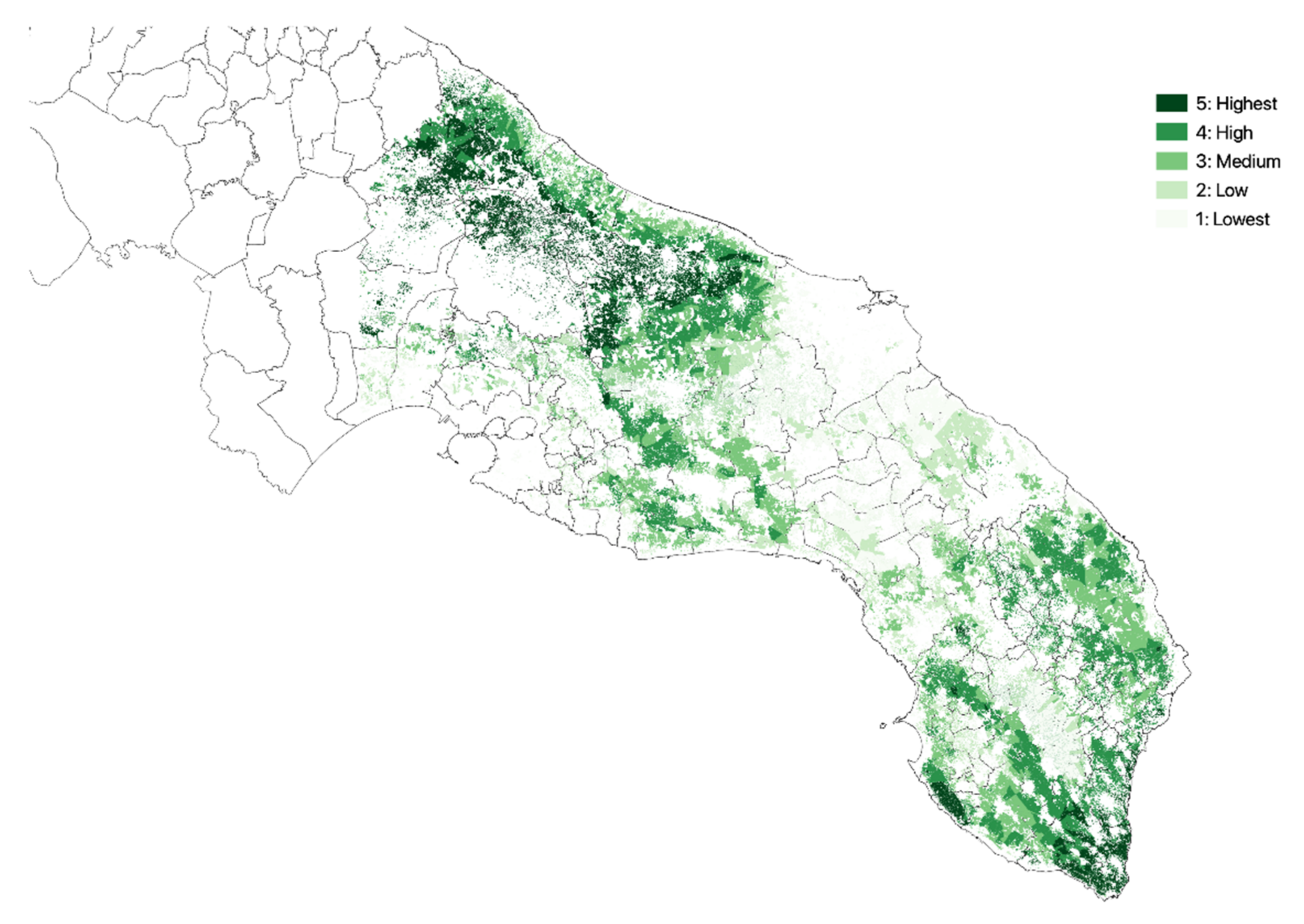
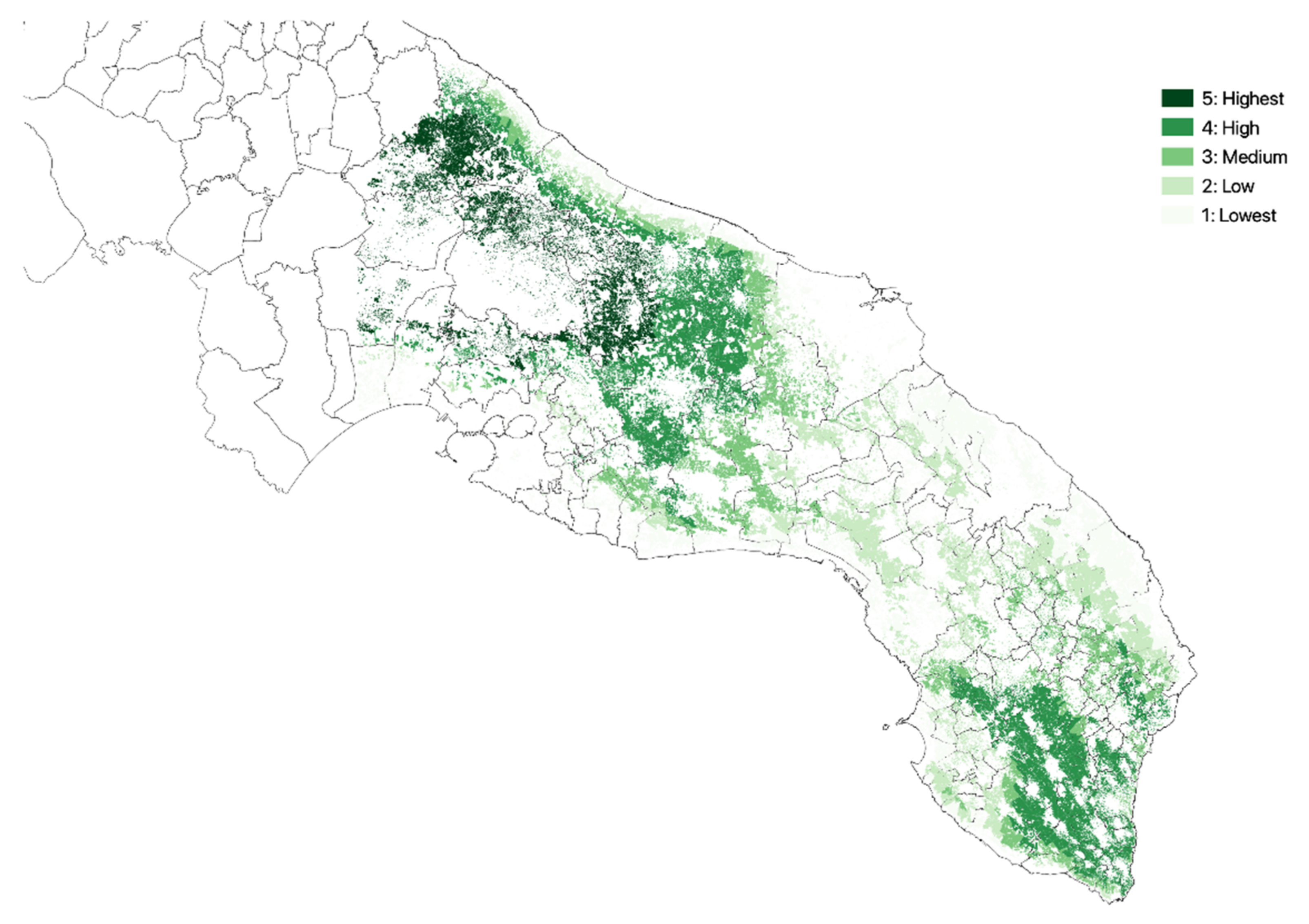

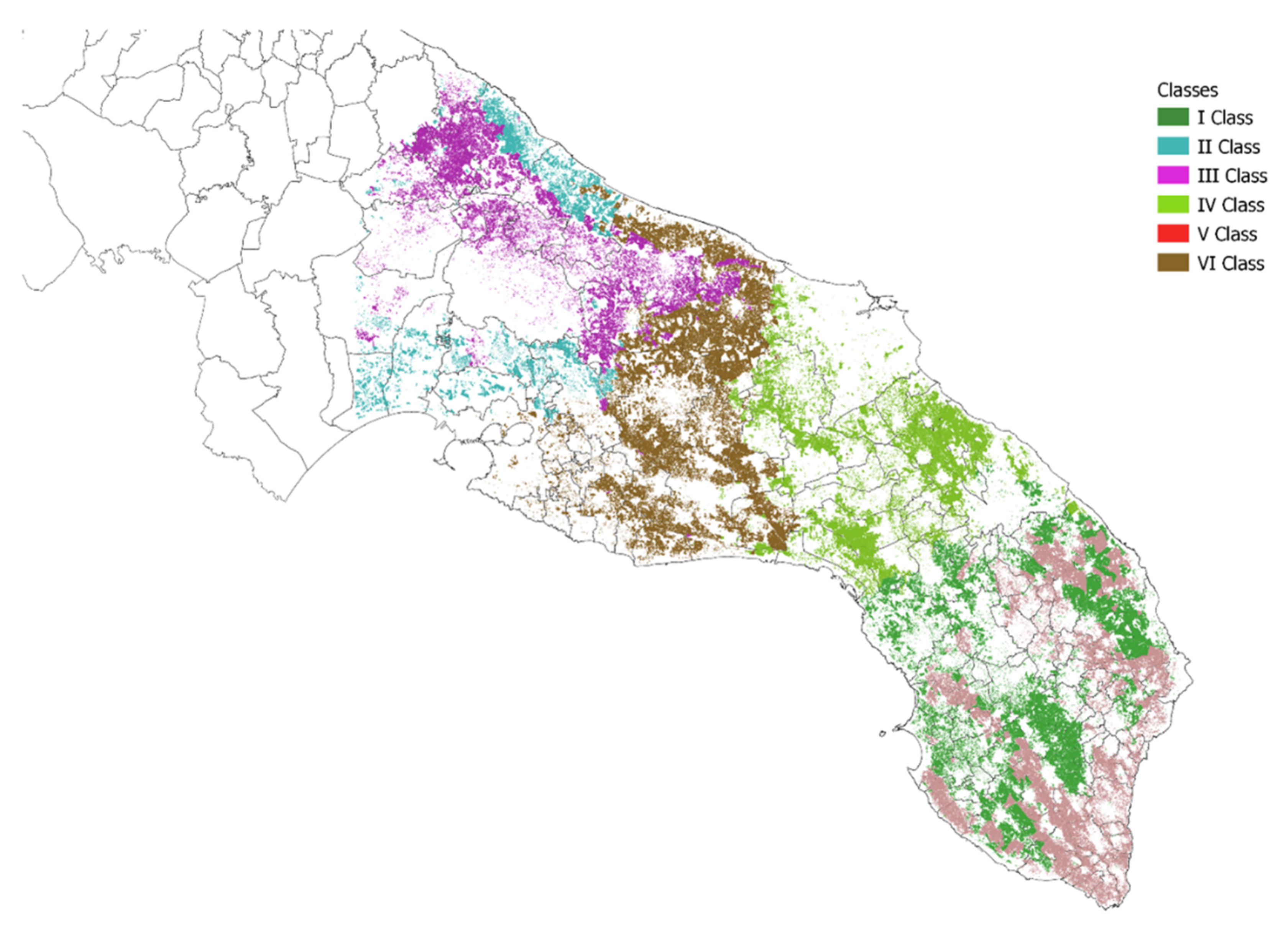
| Class | NDVI | Crop Intensity | Crop Diversity | Density of Agricultural Landscape Elements | Altitude |
|---|---|---|---|---|---|
| I | 0.286 | 0.371 | 0.574 | 0.259 | 63.391 |
| II | 0.341 | 0.917 | 0.664 | 0.308 | 147.110 |
| III | 0.344 | 0.583 | 0.664 | 0.897 | 322.897 |
| IV | 0.300 | 0.267 | 0.594 | 0.074 | 45.242 |
| V | 0.295 | 0.333 | 0.560 | 0.692 | 90.775 |
| VI | 0.325 | 0.286 | 0.537 | 0.262 | 85.650 |
| Class | NDVI | Crop Intensity | Crop Diversity | Density of Agricultural Landscape Elements | Altitude |
|---|---|---|---|---|---|
| I | − | = | − | = | − |
| II | + + | + + | + + | = | + |
| III | + + | + | + + | + + | + + |
| IV | − | − − | = | − − | − − |
| V | − | − | − | + | = |
| VI | + | − | − | = | = |
| Class | Number of Polygons | Total Area (Ha) | Average (Ha) |
|---|---|---|---|
| I | 6049.00 | 48,253.47 | 7.98 |
| II | 2675.00 | 16,503.12 | 6.17 |
| III | 5757.00 | 33,833.38 | 5.88 |
| IV | 5581.00 | 39,791.25 | 7.13 |
| V | 4509.00 | 42,205.80 | 9.36 |
| VI | 4403.00 | 55,352.73 | 12.57 |
| Total | 28,974 | 235,940 | 8.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozzo, F.; Frem, M.; Fucilli, V.; Cardone, G.; Garofoli, P.F.; Geronimo, S.; Petrontino, A. Landscape and Vegetation Patterns Zoning Is a Methodological Tool for Management Costs Implications Due to Xylella fastidiosa Invasion. Land 2022, 11, 1105. https://doi.org/10.3390/land11071105
Bozzo F, Frem M, Fucilli V, Cardone G, Garofoli PF, Geronimo S, Petrontino A. Landscape and Vegetation Patterns Zoning Is a Methodological Tool for Management Costs Implications Due to Xylella fastidiosa Invasion. Land. 2022; 11(7):1105. https://doi.org/10.3390/land11071105
Chicago/Turabian StyleBozzo, Francesco, Michel Frem, Vincenzo Fucilli, Gianluigi Cardone, Paolo Francesco Garofoli, Stefania Geronimo, and Alessandro Petrontino. 2022. "Landscape and Vegetation Patterns Zoning Is a Methodological Tool for Management Costs Implications Due to Xylella fastidiosa Invasion" Land 11, no. 7: 1105. https://doi.org/10.3390/land11071105
APA StyleBozzo, F., Frem, M., Fucilli, V., Cardone, G., Garofoli, P. F., Geronimo, S., & Petrontino, A. (2022). Landscape and Vegetation Patterns Zoning Is a Methodological Tool for Management Costs Implications Due to Xylella fastidiosa Invasion. Land, 11(7), 1105. https://doi.org/10.3390/land11071105









