Assessment of the Morphological Pattern of the Lebanon Cedar under Changing Climate: The Mediterranean Case
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Aarea and Target Species
2.2. Occurrence Data
2.3. Environmental Data
2.4. Ecological Niche Modeling
2.5. Environmental Data
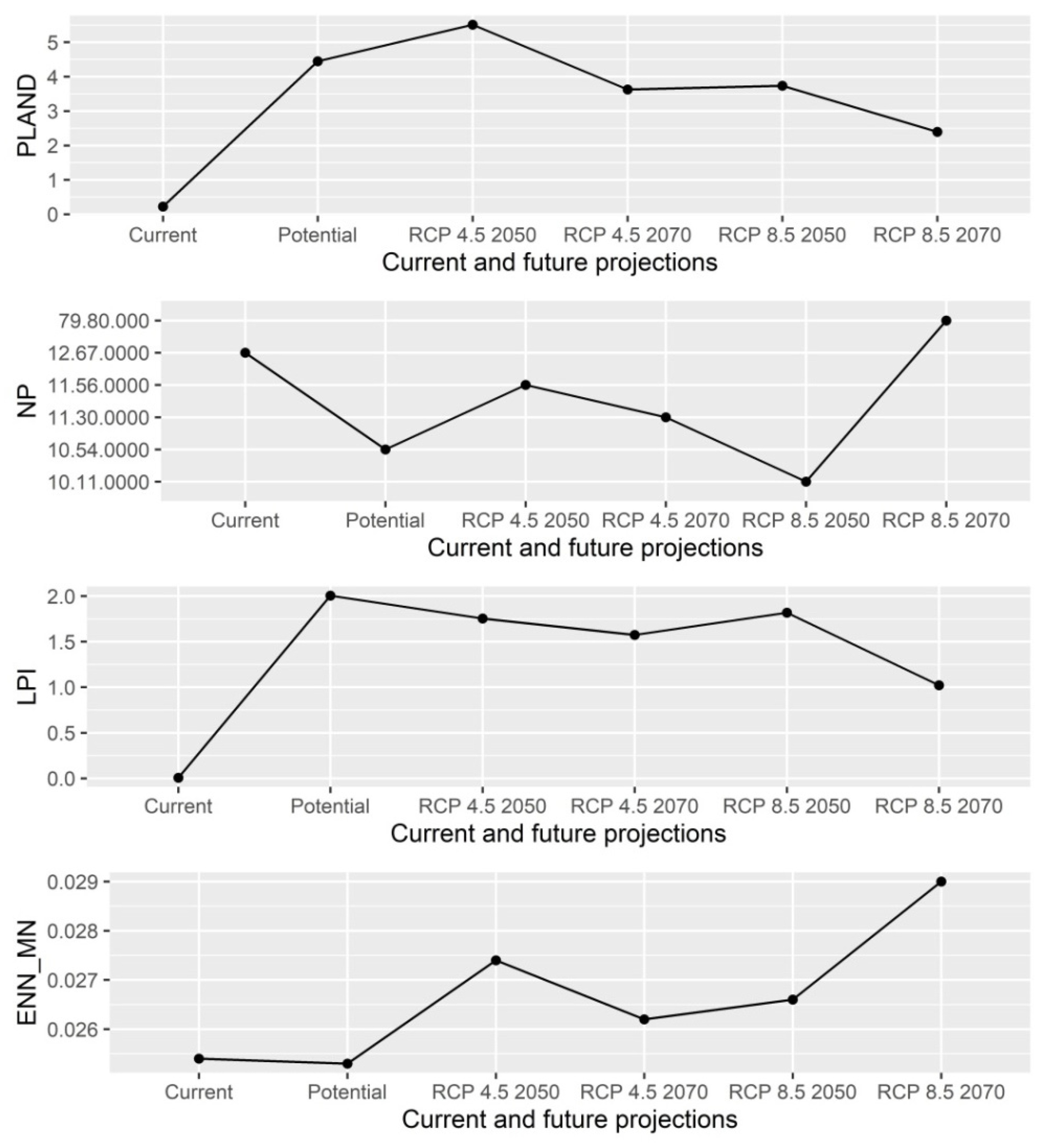
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Potential-RCP 4.5 2050 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Background | Core | Islet | Perforation | Edge | Loop | Bridge | Branch | |
| Background | 97.49 | 1.38 | 0.14 | 0.02 | 0.37 | 0.19 | 0.18 | 0.23 |
| Core | 14.87 | 57.48 | 1.45 | 0.33 | 13.53 | 4.93 | 4.24 | 3.16 |
| Islet | 60.27 | 13.95 | 10.86 | 0.09 | 6.64 | 2.46 | 1.75 | 3.97 |
| Perforation | 1.37 | 87.81 | 1.55 | 0.77 | 3.09 | 1.20 | 4.21 | 0.00 |
| Edge | 48.63 | 15.07 | 3.73 | 0.05 | 17.40 | 2.35 | 3.29 | 9.49 |
| Loop | 45.70 | 25.63 | 3.04 | 0.07 | 11.57 | 4.20 | 2.48 | 7.32 |
| Bridge | 32.16 | 32.61 | 3.41 | 0.47 | 15.44 | 4.71 | 4.08 | 7.13 |
| Branch | 70.54 | 9.29 | 4.59 | 0.05 | 4.29 | 1.44 | 1.29 | 8.51 |
| Potential-RCP 4.5 2070 | ||||||||
| Background | 98.38 | 0.96 | 0.09 | 0.01 | 0.21 | 0.11 | 0.14 | 0.10 |
| Core | 42.75 | 25.76 | 3.84 | 0.19 | 10.18 | 3.85 | 8.69 | 4.75 |
| Islet | 77.06 | 6.66 | 6.44 | 0.03 | 5.41 | 2.01 | 0.71 | 1.67 |
| Perforation | 9.61 | 65.32 | 0.26 | 2.83 | 8.76 | 1.72 | 10.99 | 0.52 |
| Edge | 69.10 | 10.17 | 4.21 | 0.04 | 6.70 | 1.33 | 3.08 | 5.38 |
| Loop | 63.91 | 18.61 | 3.67 | 0.20 | 4.54 | 1.96 | 3.18 | 3.95 |
| Bridge | 50.53 | 20.12 | 2.10 | 0.14 | 12.26 | 3.89 | 5.36 | 5.60 |
| Branch | 82.59 | 6.97 | 2.31 | 0.02 | 3.25 | 0.62 | 1.46 | 2.78 |
| Potential-RCP 8.5 2050 | ||||||||
| Background | Core | Islet | Perforation | Edge | Loop | Bridge | Branch | |
| Background | 98.10 | 1.20 | 0.07 | 0.02 | 0.24 | 0.11 | 0.14 | 0.13 |
| Core | 47.99 | 23.48 | 3.15 | 0.09 | 8.43 | 4.17 | 8.50 | 4.19 |
| Islet | 78.42 | 8.64 | 3.12 | 0.00 | 6.00 | 1.20 | 0.90 | 1.72 |
| Perforation | 16.31 | 56.65 | 0.17 | 0.86 | 5.84 | 0.86 | 18.28 | 1.03 |
| Edge | 71.82 | 9.94 | 3.46 | 0.01 | 5.72 | 1.14 | 3.37 | 4.53 |
| Loop | 66.61 | 18.30 | 3.48 | 0.13 | 4.49 | 0.79 | 3.26 | 2.93 |
| Bridge | 49.13 | 22.57 | 2.28 | 0.01 | 12.45 | 3.64 | 4.83 | 5.09 |
| Branch | 82.12 | 7.58 | 1.92 | 0.01 | 3.23 | 0.87 | 1.36 | 2.91 |
| Potential-RCP 8.5 2070 | ||||||||
| Background | Core | Islet | Perforation | Edge | Loop | Bridge | Branch | |
| Background | 98.51 | 0.87 | 0.10 | 0.01 | 0.21 | 0.07 | 0.11 | 0.12 |
| Core | 75.80 | 10.11 | 2.24 | 0.00 | 5.04 | 1.70 | 1.79 | 3.33 |
| Islet | 89.94 | 2.72 | 1.63 | 0.00 | 2.45 | 0.83 | 0.54 | 1.89 |
| Perforation | 38.28 | 40.09 | 1.03 | 0.00 | 8.84 | 1.37 | 8.24 | 2.15 |
| Edge | 85.31 | 6.09 | 1.23 | 0.00 | 3.83 | 0.86 | 0.88 | 1.80 |
| Loop | 77.41 | 12.64 | 1.33 | 0.00 | 4.62 | 1.20 | 1.66 | 1.13 |
| Bridge | 70.34 | 11.96 | 2.46 | 0.00 | 6.83 | 2.19 | 1.74 | 4.48 |
| Branch | 89.91 | 5.18 | 0.84 | 0.00 | 2.25 | 0.42 | 0.57 | 0.83 |
References
- Garcia, C.A.; Savilaakso, S.; Verburg, R.W.; Gutierrez, V.; Wilson, S.J.; Krug, C.B.; Sassen, M.; Robinson, B.E.; Moersberger, H.; Naimi, B.; et al. The Global Forest Transition as a Human Affair. One Earth 2020, 2, 417–428. [Google Scholar] [CrossRef]
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of Global Forest Area: Results from the FAO Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Zhang, B.; Brack, C.L. Urban Forest Responses to Climate Change: A Case Study in Canberra. Urban For. Urban Green. 2021, 57, 126910. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Oppenheimer, M.; Warren, R.; Hallegatte, S.; Kopp, R.E.; Pörtner, H.O.; Scholes, R.; Birkmann, J.; Foden, W.; Licker, R.; et al. IPCC Reasons for Concern Regarding Climate Change Risks. Nat. Clim. Chang. 2017, 7, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S. Climate Change Adaptation in SIDS: A Systematic Review of the Literature Pre and Post the IPCC Fifth Assessment Report. WIREs Clim. Chang. 2020, 11, e653. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Estrada-Contreras, I.; Equihua, M.; Laborde, J.; Meyer, E.M.; Sánchez-Velásquez, L.R. Current and Future Distribution of the Tropical Tree Cedrela odorata L. in Mexico under Climate Change Scenarios Using MaxLike. PLoS ONE 2016, 11, e0164178. [Google Scholar] [CrossRef]
- Klein, T.; Cahanovitc, R.; Sprintsin, M.; Herr, N.; Schiller, G. A Nation-Wide Analysis of Tree Mortality under Climate Change: Forest Loss and Its Causes in Israel 1948–2017. For. Ecol. Manag. 2019, 432, 840–849. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, H.Y.H. Observations from Old Forests Underestimate Climate Change Effects on Tree Mortality. Nat. Commun. 2013, 4, 1655. [Google Scholar] [CrossRef]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate Change Effects on Plant Disease: Genomes to Ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef] [Green Version]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate Change and Forest Diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Bakkenes, M.; Alkemade, J.R.M.; Ihle, F.; Leemans, R.; Latour, J.B. Assessing Effects of Forecasted Climate Change on the Diversity and Distribution of European Higher Plants for 2050. Glob. Chang. Biol. 2002, 8, 390–407. [Google Scholar] [CrossRef]
- Thuiller, W. Climate Change and the Ecologist. Nature 2007, 448, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, J.; Jandova, V.; Macek, M.; Mudrak, O.; Altman, J.; Schweingruber, F.H.; Liancourt, P. Climate Warming Drives Himalayan Alpine Plant Growth and Recruitment Dynamics. J. Ecol. 2020, 109, 179–190. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Cao, G.; Ma, Z.; Li, Y.; Zhang, F.; Zhao, X.; Zhao, X.; Jiang, L.; Sanders, N.J.; et al. Alpine Grassland Plants Grow Earlier and Faster but Biomass Remains Unchanged over 35 Years of Climate Change. Ecol. Lett. 2020, 23, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Ammer, C. Diversity and Forest Productivity in a Changing Climate. New Phytol. 2018, 221, 50–66. [Google Scholar] [CrossRef] [Green Version]
- Boisvenue, C.; Running, S.W. Impacts of Climate Change on Natural Forest Productivity—Evidence since the Middle of the 20th Century. Glob. Chang. Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Jactel, H.; Scherer-Lorenzen, M.; García-Valdés, R.; Bugmann, H. Long-Term Response of Forest Productivity to Climate Change Is Mostly Driven by Change in Tree Species Composition. Sci. Rep. 2018, 8, 5627. [Google Scholar] [CrossRef] [Green Version]
- Varol, T.; Cetin, M.; Ozel, H.B.; Sevik, H.; Zeren Cetin, I. The Effects of Climate Change Scenarios on Carpinus betulus and Carpinus orientalis in Europe. Water Air Soil Pollut. 2022, 233, 45. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How Much Does Climate Change Threaten European Forest Tree Species Distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Naudiyal, N.; Wang, J.; Ning, W.; Gaire, N.P.; Peili, S.; Yanqiang, W.; Jiali, H.; Ning, S. Potential Distribution of Abies, Picea, and Juniperus Species in the Sub-Alpine Forest of Minjiang Headwater Region under Current and Future Climate Scenarios and Its Implications on Ecosystem Services Supply. Ecol. Indic. 2021, 121, 107131. [Google Scholar] [CrossRef]
- Rashid, I.; Romshoo, S.A. Impact of Climate Change on Vegetation Distribution in the Kashmir Himalaya. In Biodiversity of the Himalaya: Jammu and Kashmir State; Dar, G.H., Khuroo, A.A., Eds.; Topics in Biodiversity and Conservation; Springer: Singapore, 2020; pp. 1029–1047. ISBN 978-981-329-174-4. [Google Scholar]
- Zhang, Y.; Liang, W.; Liao, Z.; Han, Z.; Xu, X.; Jiao, R.; Liu, H. Effects of Climate Change on Lake Area and Vegetation Cover over the Past 55 Years in Northeast Inner Mongolia Grassland, China. Theor. Appl. Climatol. 2019, 138, 13–25. [Google Scholar] [CrossRef]
- Fekete, I.; Lajtha, K.; Kotroczó, Z.; Várbíró, G.; Varga, C.; Tóth, J.A.; Demeter, I.; Veperdi, G.; Berki, I. Long-Term Effects of Climate Change on Carbon Storage and Tree Species Composition in a Dry Deciduous Forest. Glob. Chang. Biol. 2017, 23, 3154–3168. [Google Scholar] [CrossRef] [PubMed]
- Lenihan, J.M.; Drapek, R.; Bachelet, D.; Neilson, R.P. Climate Change Effects on Vegetation Distribution, Carbon, and Fire in California. Ecol. Appl. 2003, 13, 1667–1681. [Google Scholar] [CrossRef]
- Iverson, L.R.; Thompson, F.R.; Matthews, S.; Peters, M.; Prasad, A.; Dijak, W.D.; Fraser, J.; Wang, W.J.; Hanberry, B.; He, H.; et al. Multi-Model Comparison on the Effects of Climate Change on Tree Species in the Eastern U.S.: Results from an Enhanced Niche Model and Process-Based Ecosystem and Landscape Models. Landsc. Ecol. 2017, 32, 1327–1346. [Google Scholar] [CrossRef]
- Morin, X.; de Coligny, F.; Martin-StPaul, N.; Bugmann, H.; Cailleret, M.; Limousin, J.M.; Ourcival, J.M.; Prevosto, B.; Simioni, G.; Vennetier, M.; et al. Beyond Forest Succession: A Gap Model to Study Ecosystem Functioning and Tree Community Composition under Climate Change. Funct. Ecol. 2021, 35, 955–975. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Thompson, F.R.; Fraser, J.S.; Dijak, W.D. Changes in Forest Biomass and Tree Species Distribution under Climate Change in the Northeastern United States. Landsc. Ecol. 2017, 32, 1399–1413. [Google Scholar] [CrossRef]
- Alagador, D.; Cerdeira, J.O.; Araújo, M.B. Shifting Protected Areas: Scheduling Spatial Priorities under Climate Change. J. Appl. Ecol. 2014, 51, 703–713. [Google Scholar] [CrossRef]
- Cerrejón, C.; Valeria, O.; Mansuy, N.; Barbé, M.; Fenton, N.J. Predictive Mapping of Bryophyte Richness Patterns in Boreal Forests Using Species Distribution Models and Remote Sensing Data. Ecol. Indic. 2020, 119, 106826. [Google Scholar] [CrossRef]
- Li, G.; Xiao, N.; Luo, Z.; Liu, D.; Zhao, Z.; Guan, X.; Zang, C.; Li, J.; Shen, Z. Identifying Conservation Priority Areas for Gymnosperm Species under Climate Changes in China. Biol. Conserv. 2021, 253, 108914. [Google Scholar] [CrossRef]
- Dinerstein, E.; Joshi, A.R.; Vynne, C.; Lee, A.T.L.; Pharand-Deschênes, F.; França, M.; Fernando, S.; Birch, T.; Burkart, K.; Asner, G.P.; et al. A “Global Safety Net” to Reverse Biodiversity Loss and Stabilize Earth’s Climate. Sci. Adv. 2020, 6, eabb2824. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, H.; Luo, W.; Wang, M.; Lu, X.; Huang, T.; Zhao, J.; Li, Q. Predicting the Potential Distribution of the Asian Citrus Psyllid, Diaphorina citri (Kuwayama), in China Using the MaxEnt Model. PeerJ 2019, 7, e7323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.; He, Y.; Wang, H.; Wang, C.; Duan, Y. Potential Geographical Distribution and Habitat Shift of the Genus Ammopiptanthus in China under Current and Future Climate Change Based on the MaxEnt Model. J. Arid Environ. 2021, 184, 104328. [Google Scholar] [CrossRef]
- Rana, S.K.; Rana, H.K.; Luo, D.; Sun, H. Estimating Climate-Induced ‘Nowhere to Go’ Range Shifts of the Himalayan Incarvillea Juss. Using Multi-Model Median Ensemble Species Distribution Models. Ecol. Indic. 2020, 121, 107127. [Google Scholar] [CrossRef]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Zhang, S.H. Maxent Modeling for Predicting the Potential Distribution of Endangered Medicinal Plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Yesuf, G.U.; Brown, K.A.; Walford, N.S.; Rakotoarisoa, S.E.; Rufino, M.C. Predicting Range Shifts for Critically Endangered Plants: Is Habitat Connectivity Irrelevant or Necessary? Biol. Conserv. 2021, 256, 109033. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Fischer, J. Tackling the Habitat Fragmentation Panchreston. Trends Ecol. Evol. 2007, 22, 127–132. [Google Scholar] [CrossRef]
- Kramer, R.D.; Ishii, H.R.; Carter, K.R.; Miyazaki, Y.; Cavaleri, M.A.; Araki, M.G.; Azuma, W.A.; Inoue, Y.; Hara, C. Predicting Effects of Climate Change on Productivity and Persistence of Forest Trees. Ecol. Res. 2020, 35, 562–574. [Google Scholar] [CrossRef]
- Taleshi, H.; Jalali, S.G.; Alavi, S.J.; Hosseini, S.M.; Naimi, B.; Zimmermann, N.E. Climate Change Impacts on the Distribution and Diversity of Major Tree Species in the Temperate Forests of Northern Iran. Reg. Environ. Chang. 2019, 19, 2711–2728. [Google Scholar] [CrossRef]
- Norberg, A.; Abrego, N.; Blanchet, F.G.; Adler, F.R.; Anderson, B.J.; Anttila, J.; Araújo, M.B.; Dallas, T.; Dunson, D.; Elith, J.; et al. A Comprehensive Evaluation of Predictive Performance of 33 Species Distribution Models at Species and Community Levels. Ecol. Monogr. 2019, 89, e01370. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011; ISBN 978-1-4008-4067-0. [Google Scholar]
- Prasad, A.; Pedlar, J.; Peters, M.; McKenney, D.; Iverson, L.; Matthews, S.; Adams, B. Combining US and Canadian Forest Inventories to Assess Habitat Suitability and Migration Potential of 25 Tree Species under Climate Change. Divers. Distrib. 2020, 26, 1142–1159. [Google Scholar] [CrossRef]
- Tumas, H.R.; Shamblin, B.M.; Woodrey, M.; Nibbelink, N.P.; Chandler, R.; Nairn, C. Landscape Genetics of the Foundational Salt Marsh Plant Species Black Needlerush (Juncus roemerianus Scheele) across the Northeastern Gulf of Mexico. Landsc. Ecol. 2018, 33, 1585–1601. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira-Junior, N.D.; Heringer, G.; Bueno, M.L.; Pontara, V.; Meira-Neto, J.A.A. Prioritizing Landscape Connectivity of a Tropical Forest Biodiversity Hotspot in Global Change Scenario. For. Ecol. Manag. 2020, 472, 118247. [Google Scholar] [CrossRef]
- Günal, N. The Effects of the Climate on the Natural Vegetation in Turkey. Acta Turc. Çevrimiçi Temat. Türkol. Derg. 2013, 1, 1–22. [Google Scholar]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity Redistribution under Climate Change: Impacts on Ecosystems and Human Well-Being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Elsen, P.R.; Monahan, W.B.; Dougherty, E.R.; Merenlender, A.M. Keeping Pace with Climate Change in Global Terrestrial Protected Areas. Sci. Adv. 2020, 6, eaay0814. [Google Scholar] [CrossRef]
- Wan, J.Z.; Wang, C.J.; Yu, F.H. Spatial Conservation Prioritization for Dominant Tree Species of Chinese Forest Communities under Climate Change. Clim. Chang. 2017, 144, 303–316. [Google Scholar] [CrossRef]
- De Montis, A.; Caschili, S.; Mulas, M.; Modica, G.; Ganciu, A.; Bardi, A.; Ledda, A.; Dessena, L.; Laudari, L.; Fichera, C.R. Urban–Rural Ecological Networks for Landscape Planning. Land Use Policy 2016, 50, 312–327. [Google Scholar] [CrossRef]
- Hepcan, Ş.; Hepcan, Ç.C.; Bouwma, I.M.; Jongman, R.H.G.; Özkan, M.B. Ecological Networks as a New Approach for Nature Conservation in Turkey: A Case Study of İzmir Province. Landsc. Urban Plan. 2009, 90, 143–154. [Google Scholar] [CrossRef]
- Özcan, A.U.; Erzin, P.E. Assessment of GIS-Assisted Movement Patches Using LCP for Local Species: North Central Anatolia Region, Turkey. Cerne 2020, 26, 130–139. [Google Scholar] [CrossRef]
- McRae, B.H.; Hall, S.A.; Beier, P.; Theobald, D.M. Where to Restore Ecological Connectivity? Detecting Barriers and Quantifying Restoration Benefits. PLoS ONE 2012, 7, e52604. [Google Scholar] [CrossRef]
- Nor, A.N.M.; Corstanje, R.; Harris, J.A.; Grafius, D.R.; Siriwardena, G.M. Ecological Connectivity Networks in Rapidly Expanding Cities. Heliyon 2017, 3, e00325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boydak, M. Regeneration of Lebanon Cedar (Cedrus libani A. Rich.) on Karstic Lands in Turkey. For. Ecol. Manag. 2003, 178, 231–243. [Google Scholar] [CrossRef]
- Linares, J.C. Biogeography and Evolution of Abies (Pinaceae) in the Mediterranean Basin: The Roles of Long-Term Climatic Change and Glacial Refugia. J. Biogeogr. 2011, 38, 619–630. [Google Scholar] [CrossRef]
- Boydak, M. Ecology, Natural Regeneration and Karst Area Afforestation of Lebanon Cedar. 2. National Mediterranean Forest and En-vironment Symposium, Isparta, Turkey. 2014, p. 25. Available online: http://ormanweb.isparta.edu.tr/ormanvecevre/belgeler/bildiriler/AA-1.pdf (accessed on 20 April 2022).
- Hajar, L.; François, L.; Khater, C.; Jomaa, I.; Déqué, M.; Cheddadi, R. Cedrus libani (A. Rich) Distribution in Lebanon: Past, Present and Future. C. R. Biol. 2010, 333, 622–630. [Google Scholar] [CrossRef]
- López-Tirado, J.; Vessella, F.; Stephan, J.; Ayan, S.; Schirone, B.; Hidalgo, P.J. Effect of Climate Change on Potential Distribution of Cedrus libani A. Rich in the Twenty-First Century: An Ecological Niche Modeling Assessment. New For. 2021, 52, 363–376. [Google Scholar] [CrossRef]
- Arar, A.; Nouidjem, Y.; Bounar, R.; Tabet, S.; Kouba, Y. Modeling of the Current and Future Potential Distribution of Atlas Cedar (Cedrus atlantica) Forests Revealed Shifts in the Latitudinal, Longitudinal and Altitudinal Range towards More Humid Conditions. Ecol. Quest. 2020, 31, 49–62. [Google Scholar] [CrossRef]
- Sattout, E.J.; Nemer, N. Managing Climate Change Effects on Relic Forest Ecosystems: A Program for Lebanese Cedar. Biodiversity 2008, 9, 122–130. [Google Scholar] [CrossRef]
- Ibrahem, A.; Koubaily, E.; Thabeet, A. Assessment of Suitable Habitat of the Natural Regeneration C. libani A. Richard in Slenfeh (Syria). Egypt. J. Remote Sens. Space Sci. 2021, 24, 163–171. [Google Scholar] [CrossRef]
- GDF. State of Turkey’s Forest; General Directorate of Forestry: Ankara, Turkey, 2015. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Atalay, İ. General Ecological Properties of Natural Occurrence Areas of Cedar (Cedrus libani a. Rich) Forests and Regioning of Seed Transfer of Cedar in Turkey; Report of General Directorate of Forestry: Ankara, Turkey, 1987. [Google Scholar]
- Kantarcı, D. Cedars of Turkey (Cedrus libani A. Richard) and Some Ecological Relations in Its Natural. J. Fac. for Istanbul U. 1982, 32, 113–198. [Google Scholar]
- GDF. Turkey Forestry Statistics; General Directorate of Forestry: Ankara, Turkey, 2020. [Google Scholar]
- Beals, E.W. The Remnant Cedar Forests of Lebanon. J. Ecol. 1965, 53, 679–694. [Google Scholar] [CrossRef]
- Khuri, S.; Shmoury, M.R.; Baalbaki, R.; Maunder, M.; Talhouk, S.N. Conservation of the Cedrus libani Populations in Lebanon: History, Current Status and Experimental Application of Somatic Embryogenesis. Biodivers. Conserv. 2000, 9, 1261–1273. [Google Scholar] [CrossRef]
- Wazen, N.; Garavaglia, V.; Picard, N.; Besacier, C.; Fady, B. Distribution Maps of Twenty-Four Mediterranean and European Ecologically and Economically Important Forest Tree Species Compiled from Historical Data Collections. Ann. Silvic. Res. 2020, 44, 95–101. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial Filtering to Reduce Sampling Bias Can Improve the Performance of Ecological Niche Models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Fourcade, Y.; Engler, J.O.; Rödder, D.; Secondi, J. Mapping Species Distributions with MAXENT Using a Geographically Biased Sample of Presence Data: A Performance Assessment of Methods for Correcting Sampling Bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef] [Green Version]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. Kuenm: An R Package for Detailed Development of Ecological Niche Models Using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [Green Version]
- Soberón, J.; Nakamura, M. Niches and Distributional Areas: Concepts, Methods, and Assumptions. Proc. Natl. Acad. Sci. USA 2009, 106, 19644–19650. [Google Scholar] [CrossRef] [Green Version]
- Soberón, J.; Peterson, A.T. Interpretation of Models of Fundamental Ecological Niches and Species’ Distributional Areas. Biodivers. Inf. 2005, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gent, P.R.; Danabasoglu, G. Response to Increasing Southern Hemisphere Winds in CCSM4. J. Clim. 2011, 24, 4992–4998. [Google Scholar] [CrossRef]
- Voldoire, A.; Sanchez-Gomez, E.; Salas y Mélia, D.; Decharme, B.; Cassou, C.; Sénési, S.; Valcke, S.; Beau, I.; Alias, A.; Chevallier, M.; et al. The CNRM-CM5.1 Global Climate Model: Description and Basic Evaluation. Clim. Dyn. 2013, 40, 2091–2121. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.D.; Hughes, J.K.; Bellouin, N.; Hardiman, S.C.; Jones, G.S.; Knight, J.; Liddicoat, S.; O’Connor, F.M.; Andres, R.J.; Bell, C.; et al. The HadGEM2-ES Implementation of CMIP5 Centennial Simulations. Geosci. Model Dev. 2011, 4, 543–570. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Suzuki, T.; O’ishi, R.; Komuro, Y.; Watanabe, S.; Emori, S.; Takemura, T.; Chikira, M.; Ogura, T.; Sekiguchi, M.; et al. Improved Climate Simulation by MIROC5: Mean States, Variability, and Climate Sensitivity. J. Clim. 2010, 23, 6312–6335. [Google Scholar] [CrossRef]
- Sanderson, B.M.; Knutti, R.; Caldwell, P. A Representative Democracy to Reduce Interdependency in a Multimodel Ensemble. J. Clim. 2015, 28, 5171–5194. [Google Scholar] [CrossRef] [Green Version]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Heikkinen, R.K.; Luoto, M.; Araújo, M.B.; Virkkala, R.; Thuiller, W.; Sykes, M.T. Methods and Uncertainties in Bioclimatic Envelope Modelling under Climate Change. Prog. Phys. Geogr. Earth Environ. 2006, 30, 751–777. [Google Scholar] [CrossRef] [Green Version]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017; ISBN 978-0-521-76513-8. [Google Scholar]
- Naimi, B. usdm R library: Uncertainty analysis for species distribution models. In R Package Version; 2017; pp. 1–18. Available online: https://cran.r-project.org/web/packages/usdm (accessed on 20 April 2022).
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-Source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making Better Maxent Models of Species Distributions: Complexity, Overfitting and Evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking Receiver Operating Characteristic Analysis Applications in Ecological Niche Modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Anderson, R.P.; Lew, D.; Peterson, A.T. Evaluating Predictive Models of Species’ Distributions: Criteria for Selecting Optimal Models. Ecol. Model. 2003, 162, 211–232. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological Niche Modeling in Maxent: The Importance of Model Complexity and the Performance of Model Selection Criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting Thresholds of Occurrence in the Prediction of Species Distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Vogt, P.; Riitters, K. GuidosToolbox: Universal Digital Image Object Analysis. Eur. J. Remote Sens. 2017, 50, 352–361. [Google Scholar] [CrossRef]
- Soille, P.; Vogt, P. Morphological Segmentation of Binary Patterns. Pattern Recognit. Lett. 2009, 30, 456–459. [Google Scholar] [CrossRef]
- Ameztegui, A.; Morán-Ordóñez, A.; Márquez, A.; Blázquez-Casado, Á.; Pla, M.; Villero, D.; García, M.B.; Errea, M.P.; Coll, L. Forest Expansion in Mountain Protected Areas: Trends and Consequences for the Landscape. Landsc. Urban Plan. 2021, 216, 104240. [Google Scholar] [CrossRef]
- Wang, Y.; Brandt, M.; Zhao, M.; Xing, K.; Wang, L.; Tong, X.; Xue, F.; Kang, M.; Jiang, Y.; Fensholt, R. Do Afforestation Projects Increase Core Forests? Evidence from the Chinese Loess Plateau. Ecol. Indic. 2020, 117, 106558. [Google Scholar] [CrossRef]
- Ossola, A.; Locke, D.; Lin, B.; Minor, E. Yards Increase Forest Connectivity in Urban Landscapes. Landsc. Ecol. 2019, 34, 2935–2948. [Google Scholar] [CrossRef]
- Moulds, S.; Buytaert, W.; Mijic, A. An Open and Extensible Framework for Spatially Explicit Land Use Change Modelling: The Lulcc R Package. Geosci. Model Dev. 2015, 8, 3215–3229. [Google Scholar] [CrossRef] [Green Version]
- McGarigal, K.; Cushman, S.A.; Neel, M.C.; Ene, E. FRAGSTATS: Spatial Pattern Analysis Program for Categorical Maps. Computer Software Program Produced by the Authors at the University of Massachusetts, Amherst. 2002. Available online: www.umass.edu/landeco/research/fragstats/fragstats.html (accessed on 10 February 2022).
- Zwiener, V.P.; Padial, A.A.; Marques, M.C.M.; Faleiro, F.V.; Loyola, R.; Peterson, A.T. Planning for Conservation and Restoration under Climate and Land Use Change in the Brazilian Atlantic Forest. Divers. Distrib. 2017, 23, 955–966. [Google Scholar] [CrossRef]
- Baykal, N.U. Determining Potential Niche Competition Regions between Kazdagi Fir (Abies nordmanniana Subsp. Equi-Trojani) & Anatolian Black Pine (Pinus nigra Subsp. Pallasiana) and Conservation Priority Areas under Climate Change by Using Maxent Algorithm. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2019. [Google Scholar]
- Bede-Fazekas, Á. Modeling the Future Distribution of Mediterranean Pinus Species; Neményi, M., Varga, L., Facskó, F., Lőrincz, I., Eds.; Nyugat-Magyarországi Egyetem Kiadó: Sopron, Hungary, 2013; pp. 149–154. ISBN 978-963-334-103-2. [Google Scholar]
- Bede-Fazekas, Á.; Horváth, L.; Kocsis, M. Impact of Climate Change on the Potential Distribution of Mediterranean Pines. Időjárás/Q. J. Hung. Meteorol. Serv. 2014, 118, 41–52. [Google Scholar]
- Marchi, M.; Nocentini, S.; Ducci, F. Future Scenarios and Conservation Strategies for a Rear-Edge Marginal Population of Pinus nigra Arnold in Italian Central Apennines. For. Syst. 2016, 25, 1–12. [Google Scholar] [CrossRef]
- Colwell, R.K.; Brehm, G.; Cardelús, C.L.; Gilman, A.C.; Longino, J.T. Global Warming, Elevational Range Shifts, and Lowland Biotic Attrition in the Wet Tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogués-Bravo, D.; Araújo, M.B.; Errea, M.P.; Martínez-Rica, J.P. Exposure of Global Mountain Systems to Climate Warming during the 21st Century. Glob. Environ. Chang. 2007, 17, 420–428. [Google Scholar] [CrossRef]
- Krosby, M.; Tewksbury, J.; Haddad, N.M.; Hoekstra, J. Ecological Connectivity for a Changing Climate. Conserv. Biol. 2010, 24, 1686–1689. [Google Scholar] [CrossRef]
- Velázquez, J.; Gutiérrez, J.; García-Abril, A.; Hernando, A.; Aparicio, M.; Sánchez, B. Structural Connectivity as an Indicator of Species Richness and Landscape Diversity in Castilla y León (Spain). For. Ecol. Manag. 2019, 432, 286–297. [Google Scholar] [CrossRef]
- Elsen, P.R.; Tingley, M.W. Global Mountain Topography and the Fate of Montane Species under Climate Change. Nat. Clim. Change 2015, 5, 772–776. [Google Scholar] [CrossRef]
- King, A.W.; With, K.A. Dispersal Success on Spatially Structured Landscapes: When Do Spatial Pattern and Dispersal Behavior Really Matter? Ecol. Model. 2002, 147, 23–39. [Google Scholar] [CrossRef]
- Remmel, T.K.; Mitchell, S.W. Landscape Pattern Analysis. In The Routledge Handbook of Landscape Ecology; Routledge: London, UK, 2021; pp. 283–311. [Google Scholar]
- Jackson, S.T.; Overpeck, J.T. Responses of Plant Populations and Communities to Environmental Changes of the Late Quaternary. Paleobiology 2000, 26, 194–220. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and Eltonian Niches and Geographic Distributions of Species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef]
- Cox, C.B.; Moore, P.D.; Ladle, R.J. Biogeography: An Ecological and Evolutionary Approach; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-118-96858-1. [Google Scholar]
- Hernando, A.; Velázquez, J.; Valbuena, R.; Legrand, M.; García-Abril, A. Influence of the Resolution of Forest Cover Maps in Evaluating Fragmentation and Connectivity to Assess Habitat Conservation Status. Ecol. Indic. 2017, 79, 295–302. [Google Scholar] [CrossRef]
- Davis, P.H. Distribution Patterns in Anatolia with Particular Reference to Endemism. Plant Life South West Asia 1971, 15–27. [Google Scholar]
- Gür, H. The Anatolian Diagonal Revisited: Testing the Ecological Basis of a Biogeographic Boundary. Zool. Middle East 2016, 62, 189–199. [Google Scholar] [CrossRef]
- Loarie, S.R.; Carter, B.E.; Hayhoe, K.; McMahon, S.; Moe, R.; Knight, C.A.; Ackerly, D.D. Climate Change and the Future of California’s Endemic Flora. PLoS ONE 2008, 3, e2502. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Jackson, H.B.; Fahrig, L. Habitat Loss and Fragmentation. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 50–58. ISBN 978-0-12-384720-1. [Google Scholar]
- Costanza, J.K.; Terando, A.J. Landscape Connectivity Planning for Adaptation to Future Climate and Land-Use Change. Curr. Landsc. Ecol. Rep. 2019, 4, 1–13. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate Change Impacts, Adaptive Capacity, and Vulnerability of European Forest Ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Mayer, H.; Sevim, M. Die Libanonzeder: Ihre Ausrottung Im Libanon Während Der Vergangenen 5000 Jahre, Das Heutige Areal in Anatolien Und Überlegungen Zur Wiedereinbürgerung in Den Alpen. Jahrb. d. Vereins z. Schutze der Alpenpflanzen und -tiere. 1958, 23, 86–105. [Google Scholar]
- Boydak, M. Ecology and Silviculture of Cedar of Lebanon (Cedrus libani A. Rich.) and Conservation of Its Natural Forests; Ministry of Forestry Publication Department: Ankara, Turkey, 1996. [Google Scholar]
- Battisti, C. Unifying the Trans-Disciplinary Arsenal of Project Management Tools in a Single Logical Framework: Further Suggestion for IUCN Project Cycle Development. J. Nat. Conserv. 2018, 41, 63–72. [Google Scholar] [CrossRef]




| MSPA Classes (%) | |||||||
|---|---|---|---|---|---|---|---|
| Core | Islet | Perforation | Edge | Loop | Bridge | Branch | |
| Current | 3.85 | 67.66 | 0.00 | 9.2 | 3.55 | 4.38 | 11.36 |
| Potential | 54.68 | 4.38 | 1.27 | 25.18 | 2.78 | 4.62 | 7.09 |
| RCP4.5-2050 | 56.9 | 4,67 | 0.8 | 24.13 | 2.48 | 2.84 | 8.18 |
| RCP4.5-2070 | 50.12 | 7.03 | 0.67 | 25.48 | 2.68 | 5.69 | 8.33 |
| RCP8.5-2050 | 53.59 | 5.31 | 0.63 | 23.44 | 2.68 | 6.36 | 7.99 |
| RCP8.5-2070 | 52.3 | 7.59 | 0.28 | 24.01 | 1.91 | 3.83 | 10.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özcan, A.U.; Velázquez, J.; Rincón, V.; Gülçin, D.; Çiçek, K. Assessment of the Morphological Pattern of the Lebanon Cedar under Changing Climate: The Mediterranean Case. Land 2022, 11, 802. https://doi.org/10.3390/land11060802
Özcan AU, Velázquez J, Rincón V, Gülçin D, Çiçek K. Assessment of the Morphological Pattern of the Lebanon Cedar under Changing Climate: The Mediterranean Case. Land. 2022; 11(6):802. https://doi.org/10.3390/land11060802
Chicago/Turabian StyleÖzcan, Ali Uğur, Javier Velázquez, Víctor Rincón, Derya Gülçin, and Kerim Çiçek. 2022. "Assessment of the Morphological Pattern of the Lebanon Cedar under Changing Climate: The Mediterranean Case" Land 11, no. 6: 802. https://doi.org/10.3390/land11060802
APA StyleÖzcan, A. U., Velázquez, J., Rincón, V., Gülçin, D., & Çiçek, K. (2022). Assessment of the Morphological Pattern of the Lebanon Cedar under Changing Climate: The Mediterranean Case. Land, 11(6), 802. https://doi.org/10.3390/land11060802









