Biochar Application Increases Labile Carbon and Inorganic Nitrogen Supply in a Continuous Monocropping Soil
Abstract
:1. Introduction
2. Materials and Methods
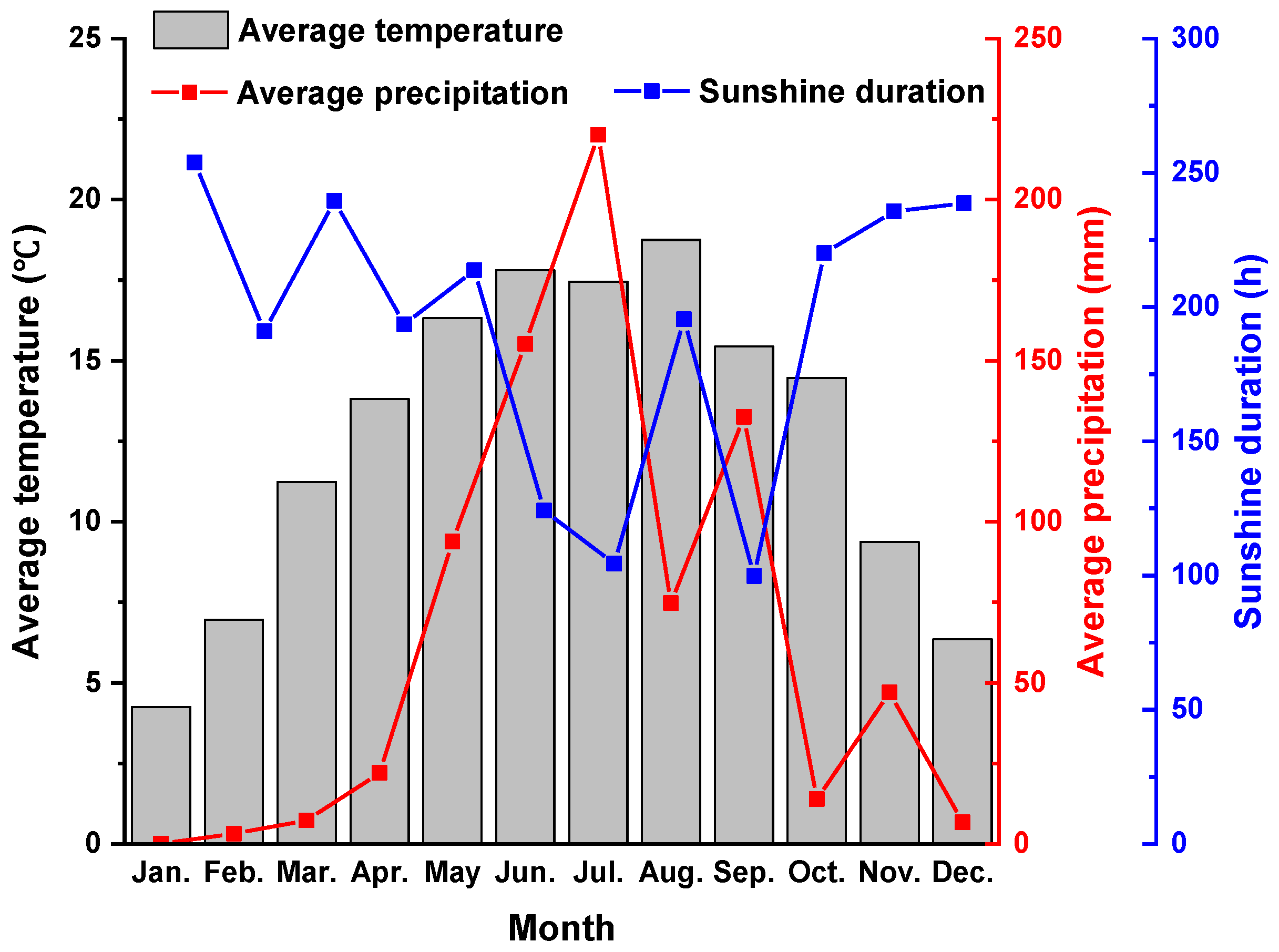
2.1. Study Site and Soil Characteristics
2.2. Experimental Materials
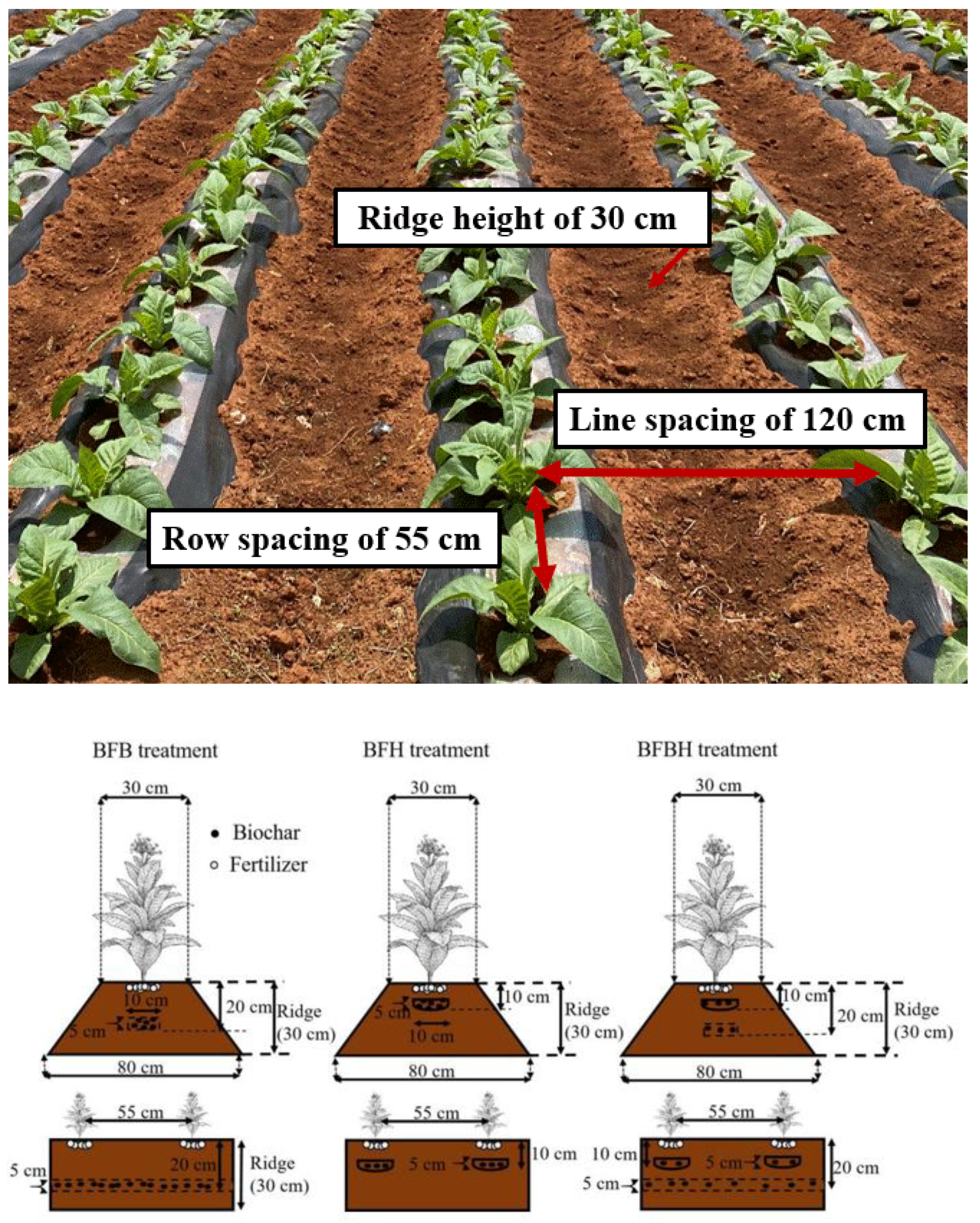
2.3. Experimental Design
2.4. Soil Sampling and Measurements
2.5. Calculations and Statistical Analyses
3. Results
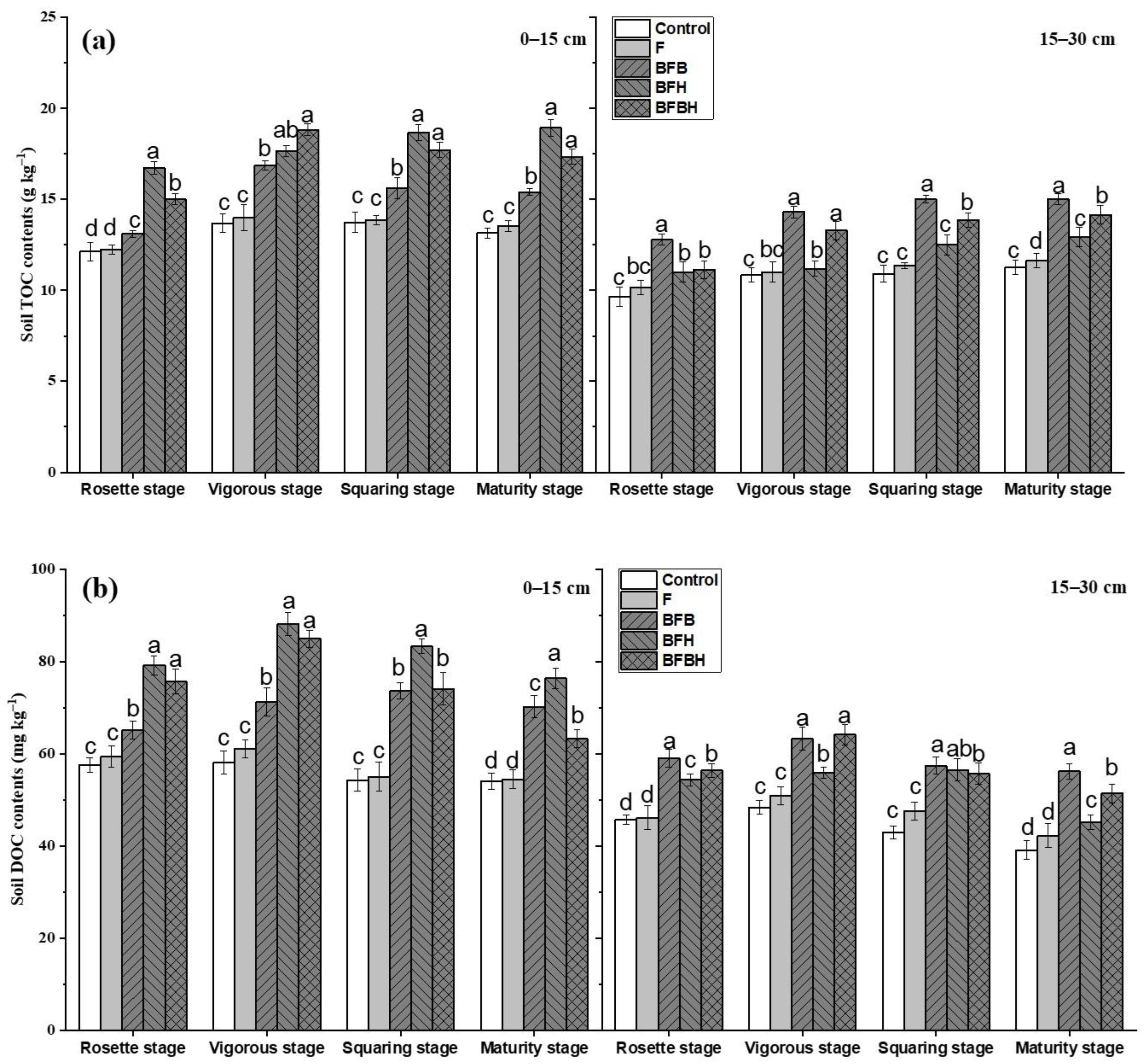
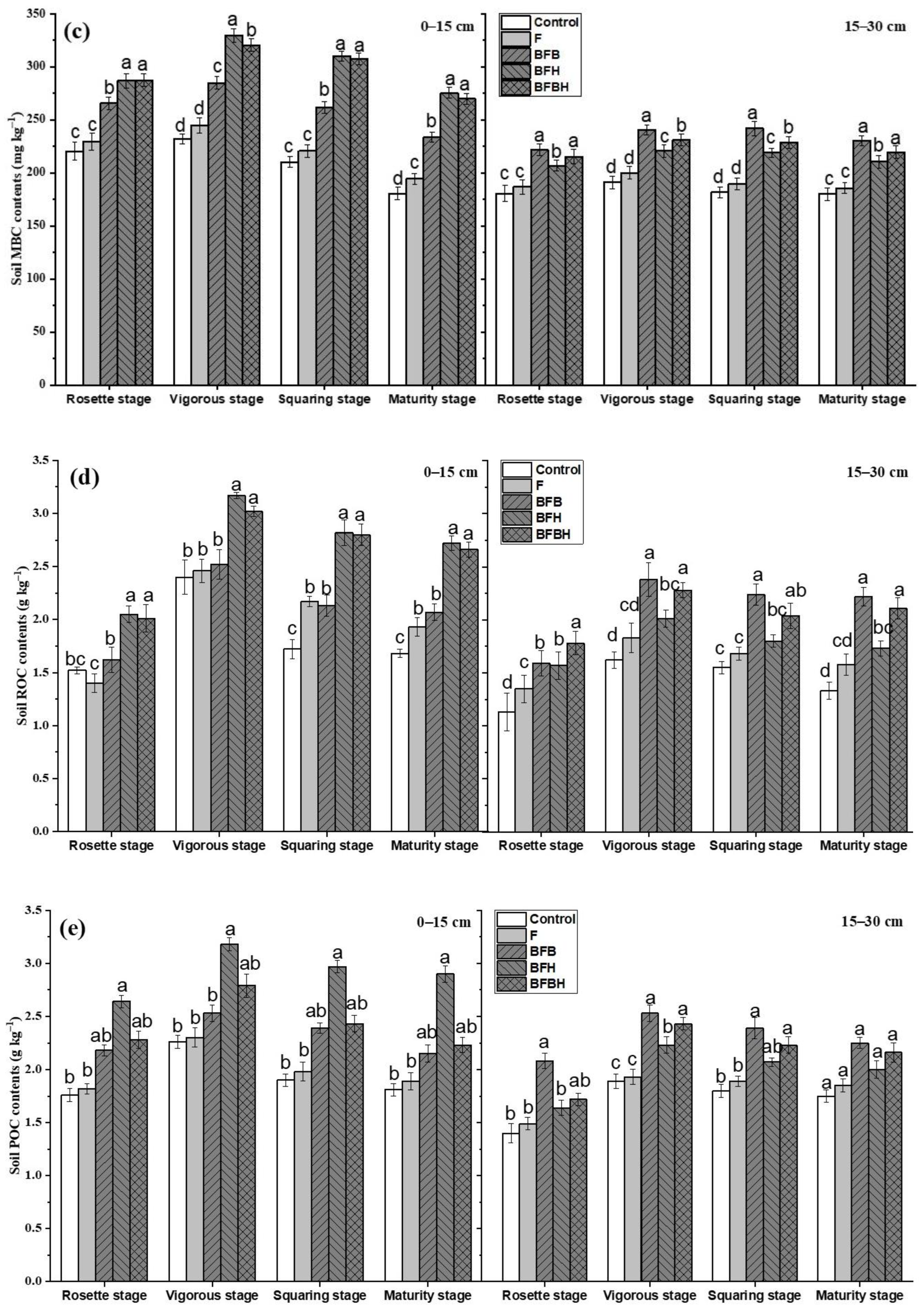
3.1. Soil TOC and LOC Fractions
3.2. Soil CPMI
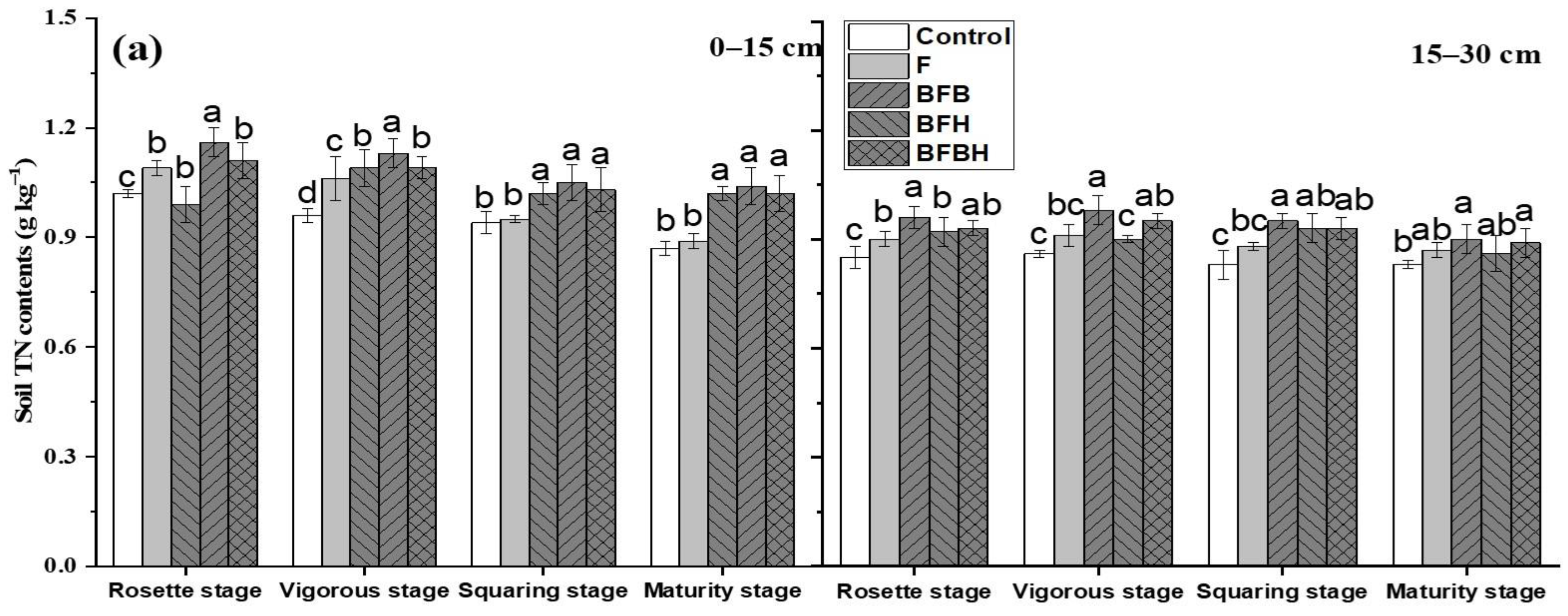
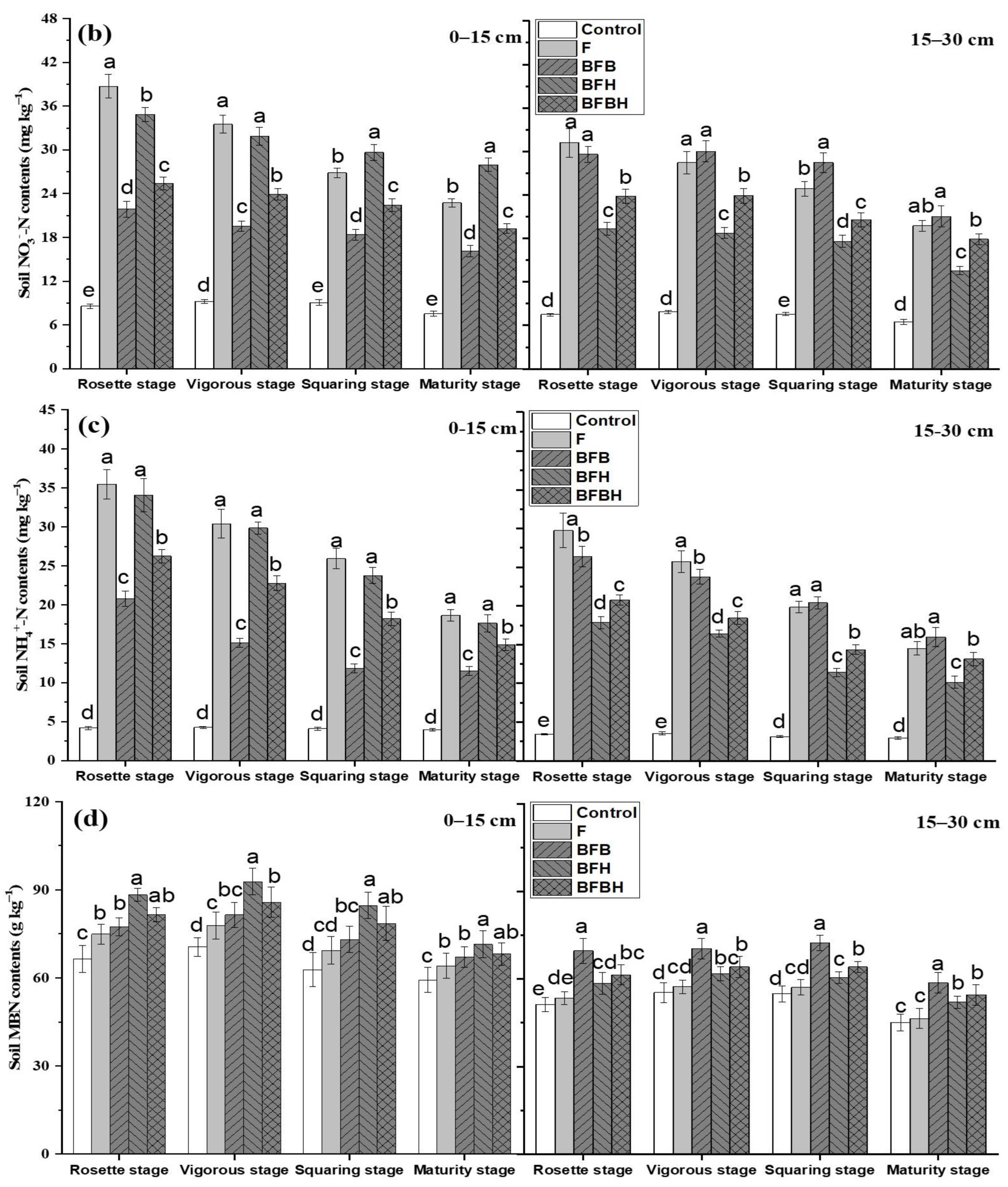
3.3. Soil N Fractions
3.4. Ratio of Soil C to N
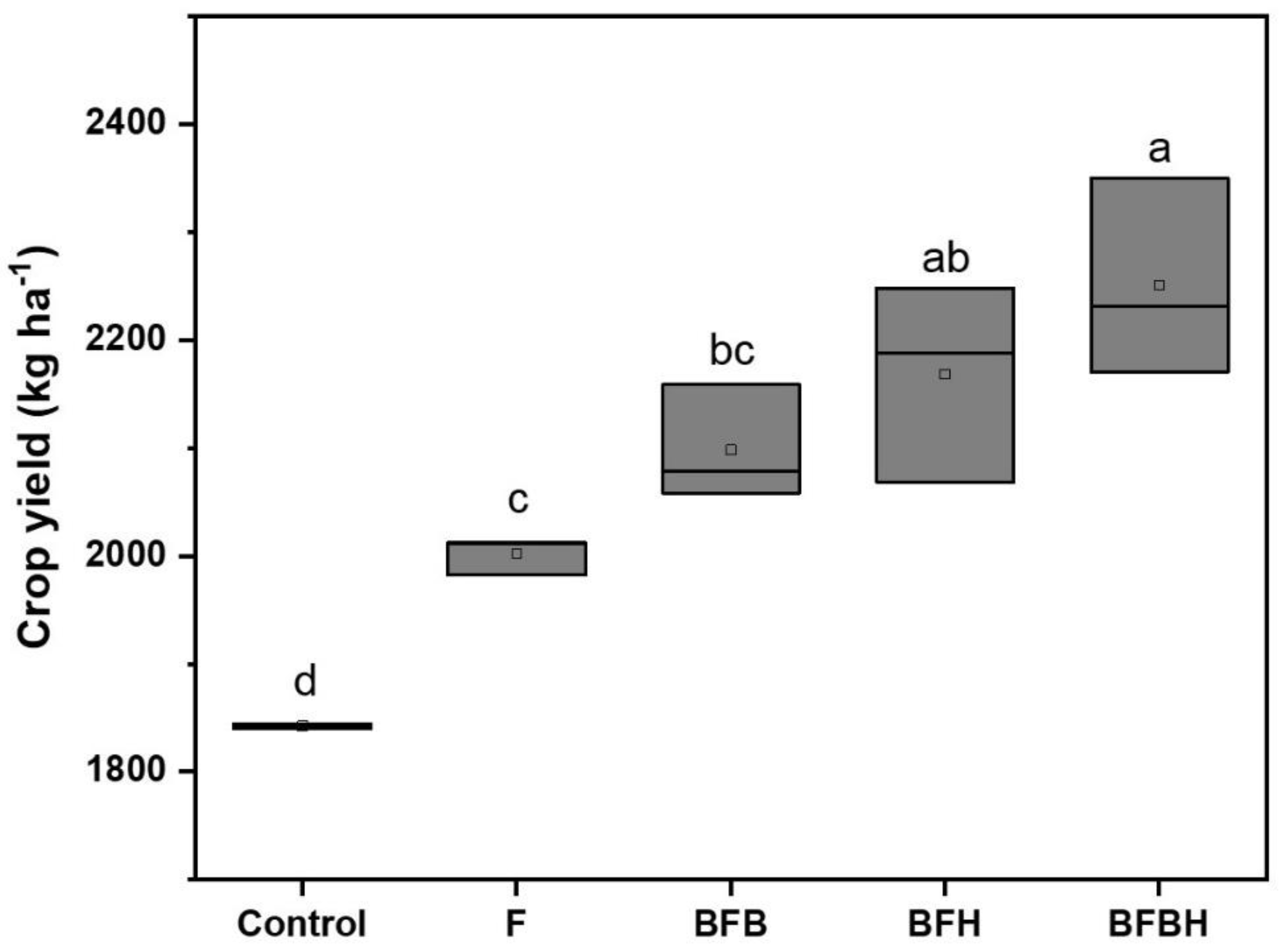
3.5. Crop Yield
4. Discussion
4.1. Effect of Biochar on the SOC Pool in a Tobacco Field
4.2. Effect of Biochar on the SOC Pool in a Tobacco Field
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Han, L.; Sun, K.; Yang, Y.; Xia, X.; Li, F.; Yang, Z.; Xing, B. Biochar’s stability and effect on the content, composition and turnover of soil organic carbon. Geoderma 2020, 364, 114184. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D.; Lan, Y.; Meng, J.; Jiang, L.; Sun, Q.; Cao, D.; Sun, Y.; Chen, W. Labile organic carbon fractions and carbon pool management index in a 3-year field study with biochar amendment. J. Soil. Sediment. 2018, 18, 1569–1578. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Murtaza, G.; Ditta, A.; Ullah, N.; Usman, M.; Ahmed, Z. Biochar for the management of nutrient impoverished and metal contaminated soils: Preparation, applications, and prospects. J. Soil Sci. Plant Nut. 2021, 212, 119–138. [Google Scholar] [CrossRef]
- Huang, R.; Tian, D.; Liu, J.; Lv, S.; He, X.; Gao, M. Responses of soil carbon pool and soil aggregates associated organic carbon to straw and straw-derived biochar addition in a dryland cropping mesocosm system. Agric. Ecosyst. Environ. 2018, 265, 576–586. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Y.; Liu, J.; Li, J.; Xu, G.; Luo, M.; Xu, C.; Ci, E.; Gao, M. Variation in N2O emission and N2O related microbial functional genes in straw- and biochar-amended and non-amended soils. Appl. Soil Ecol. 2019, 137, 57–68. [Google Scholar] [CrossRef]
- Knoblauch, C.; Maarifat, A.A.; Pfeiffer, E.M.; Haefele, S.M. Degradability of black carbon and its impact on trace gas fluxes and carbon turnover in paddy soils. Soil Biol. Biochem. 2011, 43, 1768–1778. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Manevski, K.; Neumann, M.; Li, Y. Biochar and alternate wetting-drying cycles improving rhizosphere soil nutrients availability and tobacco growth by altering root growth strategy in Ferralsol and Anthrosol. Sci. Total Environ. 2022, 806, 150513. [Google Scholar] [CrossRef]
- Majeed, A.; Muhmood, A.; Niaz, A.; Ditta, A.; Nawaz Rajpar, M. Comparative efficacy of different biochars and traditional manures in the attenuation of cadmium toxicity in rice (Oryza sativa L.). Arab. J. Geosci. 2022, 15, 209. [Google Scholar] [CrossRef]
- Zhu, L.X.; Xiao, Q.; Shen, Y.F.; Li, S.Q. Effects of biochar and maize straw on the short-term carbon and nitrogen dynamics in a cultivated silty loam in China. Environ. Sci. Pollut. Res. 2017, 24, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, B.; Shen, J.; Zhu, X.; Wu, J. Contrasting effects of straw and straw-derived biochar applications on soil carbon accumulation and nitrogen use efficiency in double-rice cropping systems. Agric. Ecosyst. Environ. 2021, 311, 107286. [Google Scholar] [CrossRef]
- Morrissey, E.M.; Berrier, D.J.; Neubauer, S.C.; Franklin, R.B. Using microbial communities and extracellular enzymes to link soil organic matter characteristics to greenhouse gas production in a tidal freshwater wetland. Biogeochemistry 2014, 117, 473–490. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefory, R.D.B.; Lise, L. Soil carbon fractions based on their degree of oxidation and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 393–406. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Zwieten, L.V.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- He, G.; Jiang, X.L.; Yao, L.G.; Liu, G.H.; Yang, Y.Y.; Jiang, Y.; Liu, W.Z. Effects of tetracycline on nitrogen and carbon cycling rates and microbial abundance in sediments with and without biochar amendment. Chemosphere 2021, 270, 129509. [Google Scholar] [CrossRef]
- Pan, S.Y.; Dong, C.D.; Su, J.F.; Wang, P.Y.; Chen, C.W.; Chang, J.S.; Hyunook, K.; Huang, C.P.; Hung, C.M. The role of biochar in regulating the carbon, phosphorus, and nitrogen cycles exemplified by soil systems. Sustainability 2021, 13, 5612. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Ali, M.; Ahmed, I.; Wang, X.; Al-Ashkar, I.; Qamar, R.; Habib-Ur-Rahman, M.; Ditta, A.; EL Sabagh, A. Biochar enriched with buffalo slurry improved soil nitrogen and carbon dynamics, nutrient uptake and growth attributes of wheat by reducing leaching losses of nutrients. Land 2021, 212, 1392. [Google Scholar] [CrossRef]
- Castaldi, S.; Riondino, M.; Baronti, S.; Esposito, F.R.; Marzaioli, R.; Rutigliano, F.A.; Vaccari, F.P.; Miglietta, F. Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 2011, 85, 1464–1471. [Google Scholar] [CrossRef]
- Chen, S.; Qi, G.; Luo, T.; Zhang, H.; Jiang, Q.; Wang, R.; Zhao, X. Continuous-cropping tobacco caused variance of chemical properties and structure of bacterial network in soils. Land Degrad. Dev. 2018, 29, 4106–4120. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Cai, K.; Cai, L.; Lu, N.; Shi, J. Identification of benzoic acid and 3-phenylpropanoic acid in tobacco root exudates and their role in the growth of rhizosphere micro-organisms. Appl. Soil Ecol. 2015, 93, 78–87. [Google Scholar] [CrossRef]
- Shen, H.; Yan, W.; Yang, X.; He, X.; Wang, X.; Zhang, Y.; Wang, B.; Xia, Q. Co-occurrence network analyses of rhizosphere soil microbial PLFAs and metabolites over continuous cropping seasons in tobacco. Plant Soil. 2020, 452, 119–135. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Wang, M.; Wu, S.; Wang, H.; Niazi, N.K.; Man, Y.B.; Christie, P.; Shan, S.; Wong, M.H. Effect of tobacco stem-derived biochar on soil metal immobilization and the cultivation of tobacco plant. J. Soil. Sediment. 2019, 19, 2313–2321. [Google Scholar] [CrossRef]
- Chavez, M.D.; Berentsen, P.B.M.; Oude Lansink, A.G.J.M. Analyzing diversification possibilities on specialized tobacco farms in Argentina using a bio-economic farm model. Agrl. Sys. 2014, 128, 35–43. [Google Scholar] [CrossRef]
- Giménez Monge, J.L.; Mercado Cárdenas, G.; Lacci, F.; Ortega, A.; Arzeno, J.L. Un enfoque integral del amarillamiento en el tabaco. INTA-EEA Salta. Panor. Agropecu. 2009, 52, 3–7. (In Spanish) [Google Scholar]
- Chen, Z.; Wang, H.; Liu, X.; Lu, D.; Zhou, J. The fates of 15 N-labeled fertilizer in a wheat-soil system as influenced by fertilization practice in a loamy soil. Sci. Rep. 2016, 6, 34754. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Wang, H.; Liu, X.; Liu, Y.; Gao, S.; Zhou, J. The effect of N fertilizer placement on the fate of urea-15N and yield of winter wheat in Southeast China. PLoS ONE 2016, 11, e0153701. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Ma, L.; Chadwick, D.; Chen, X. Combined applications of organic and synthetic nitrogen fertilizers for improving crop yield and reducing reactive nitrogen losses from china’s vegetable systems: A meta-analysis. Environ. Pollut. 2021, 269, 116143. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Wang, S.; Zhang, Y.; Wang, S.; Shangguan, Z. Effects of application patterns and amount of biochar on water infiltration and evaporation. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2016, 32, 135–144. (In Chinese) [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yan, W.; Shangguan, Z. Effect of biochar application method on nitrogen leaching and hydraulic conductivity in a silty clay soil. Soil Till. Res. 2018, 183, 100–108. [Google Scholar] [CrossRef]
- Fallah, N.; Tayyab, M.; Yang, Z.; Zhang, C.; Abubakar, A.; Lin, Z.; Pang, Z.; Allison, A.; Zhang, H. Depth-dependent Influence of Biochar Application on the Abundance and Community Structure of Diazotrophic Under Sugarcane Growth. PLoS ONE 2021, 16, e0253970. [Google Scholar] [CrossRef] [PubMed]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. Microbial biomass measurements in forest soils: The use of the chloroform fumigation-incubation method in strongly acid soils. Soil Biol. Biochem. 1987, 19, 697–702. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Carbon and nitrogen distribution in aggregates from cultivated and native grassland soils. Soil Sci. Soc. Am. J. 1993, 57, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Walkley, A.; Black, L.A. An examination of the method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Kirkegaard, J.A.; Richardson, A.E.; Wade, L.J.; Blanchard, C.; Baten, G. Stable soil organic matter: A comparison of C/N/P:S ratios in Australian and other world soils. Geoderma 2011, 162, 197–208. [Google Scholar] [CrossRef]
- Huang, R.; Lan, T.; Song, X.; Li, J.; Ling, J.; Deng, O.; Wang, C.; Gao, X.; Li, Q.; Tang, X.; et al. Soil labile organic carbon impacts C:N:P stoichiometry in urban park green spaces depending on vegetation types and time after planting. Appl. Soil Ecol. 2021, 163, 103926. [Google Scholar] [CrossRef]
- Ahmed, A.; Kurian, J.; Raghavan, V. Biochar influences on agricultural soils, crop production, and the environment: A review. Environ. Rev. 2016, 4, 495–502. [Google Scholar] [CrossRef]
- Gao, L.; Wang, R.; Shen, G.; Zhang, J.; Meng, G.; Zhang, J. Effects of biochar on nutrients and the microbial community structure of tobacco-planting soils. J. Soil Sci. Plant Nut. 2017, 17, 884–896. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Xi, X.; Zheng, Q.; Liang, G.; Zhou, W.; Wang, X. Soil nutrient and microbial activity responses to two years after maize straw biochar application in a calcareous soil. Ecotox. Environ. Safe 2019, 180, 348–356. [Google Scholar] [CrossRef]
- Jones, D.L.; Murphy, D.V.; Khalid, M.; Ahmad, W.; Edwards-Jones, G.; DeLuca, T.H. Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol. Biochem. 2011, 43, 1723–1731. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Maestrini, B.; Nannipieri, P.; Abiven, S. A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy 2014, 7, 577–590. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Rudrappa, L.; Singh, D.; Swarup, A.; Bhadraray, S. Long-term impact of fertilizers on soil organic carbon pools and sequestration rates in maize-wheat-cowpea cropping system. Geoderma 2008, 144, 370–378. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long–term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Petersen, J. Recovery of N-15-ammonium-N-15-nitrate in spring wheat as affected by placement geometry of the fertilizer band. Nutr. Cycl. Agroecosyst. 2001, 61, 215–221. [Google Scholar] [CrossRef]
- Mahmud, A.F.; Camps-Arbestain, M.; Hedley, M. Investigating the influence of biochar particle size and depth of placement on nitrous oxide (N2O) emissions from simulated urine patches. Agriculture 2018, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef]
- Cross, A.; Sohi, S.P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Petersen, J.; Mortensen, J.V. Dry matter production and 15N recovery in spring wheat as affected by placement geometry of the fertilizer band. Commun. Soil Sci. Plant Anal. 2002, 33, 163–178. [Google Scholar] [CrossRef]
- Ju, X.T.; Liu, X.J.; Pan, J.R.; Zhang, F.S. Fate of 15N-labeled urea under a winter wheat-summer maize rotation on the North China Plain. Pedosphere 2007, 17, 52–61. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Awad, Y.M.; Xiao, Y. Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 2018, 332, 100–108. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Ma, B.; Chang, S.X.; Gong, J. Biochar addition affected the dynamics of ammonia oxidizers and nitrification in microcosms of a coastal alkaline soil. Biol. Fertil. Soils 2013, 50, 321–332. [Google Scholar] [CrossRef]
- Oladele, S.O.; Adeyemo, A.J.; Awodun, M.A. Influence of rice husk biochar and inorganic fertilizer on soil nutrients availability and rain-fed rice yield in two contrasting soils. Geoderma 2019, 336, 1–11. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, E.; Mishra, R.; Kumar, S. Biochar as environmental armour and its diverse role towards protecting soil, water and air. Sci. Total Environ. 2022, 806, 150444. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.W.; Zheng, X.H.; Wang, Y.S.; Xu, X.K. Nitrous oxide emissions as influenced by amendment of plant residue with different C:N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar-root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Ventura, M.; Zhang, C.; Bald, E.; Fornasier, F.; Sorrenti, G.; Panzacchi, P.; Tonon, G. Effect of biochar addition on soil respiration partitioning and root dynamics in an apple orchard. Eur. J. Soil Sci. 2014, 65, 186–195. [Google Scholar] [CrossRef]
- Prommer, J.; Wanek, W.; Hofhans, F.; Trojan, D.; Offre, P.; Urich, T.; Schleper, C.; Sassmann, S.; Kitzler, B.; Soja, G.; et al. Biochar decelerates soil organic nitrogen cycling but stimulates soil nitrification in a temperate arable field trial. PLoS ONE 2014, 9, e86388. [Google Scholar] [CrossRef] [PubMed]
- Ulyett, J.; Sakrabani, R.; Kibblewwhite, M.; Hann, M. Impact of biochar addition on water retention, nitrification and carbon dioxide evolution from two sandy loam soils. Eur. J. Soil Sci. 2014, 65, 96–104. [Google Scholar] [CrossRef]
| Treatment | Basal Fertilizer (kg ha−1) | Topdressing (kg ha−1) | |||
|---|---|---|---|---|---|
| Biochar | Compound Fertilizer | Compound Fertilizer | Kalium Nitrate Fertilizer | Potassium Sulfate Fertilizer | |
| Control | 0 | 0 | 0 | 0 | 0 |
| F | 0 | 405 | 270 | 225 | 225 |
| BFB | 7600 | 405 | 270 | 225 | 225 |
| BFH | 7600 | 405 | 270 | 225 | 225 |
| BFBH | 7600 | 405 | 270 | 225 | 225 |
| TOC | DOC | MBC | ROC | POC | TN | NO3−-N | NH4+-N | MBN | TOC:TN | MBC:N | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | 16,287 * | 191.8 * | 684.1 * | 113.7 * | 17.6 * | 78.0 * | 580.5 * | 850.3 * | 83.7 * | 115.2 * | 61.5 * |
| Soil layer (S) | 7108 * | 53.0 * | 171.3 * | 157.9 * | 13.4 * | 51.8 * | 108.7 * | 291.5 * | 94.0 * | 300.1 * | 82.4 * |
| Growth stage (G) | 82,789 * | 921.5 * | 2387.9 * | 314.7 * | 36.2 * | 597.6 * | 73.5 * | 157.3 * | 733.0 * | 359.0 * | 68.3 * |
| T × S | 350 * | 4.4 * | 3.3 * | 1.5 | 0.2 | 0.9 | 10.0 * | 21.5 * | 0.3 | 6.4 * | 3.8 * |
| T × G | 6129 * | 40.7 * | 169.4 * | 36.2 * | 7.5 * | 8.8 * | 152.7 * | 161.5 * | 29.7 * | 65.2 * | 42.8 * |
| S × G | 754 * | 0.5 | 67.9 * | 14.4 * | 1.0 | 18.6 * | 3.0 * | 4.5 * | 10.9 * | 16.0 * | 47.1 * |
| T × S × G | 79 * | 2.6 * | 1.6 | 2.5 * | 0.1 | 1.7 | 1.6 | 3.2 * | 0.8 | 2.7 * | 3.6 * |
| Soil Layer | Treatment † | NLC (g kg−1) | L | LI | CPI | CPMI |
|---|---|---|---|---|---|---|
| 0–15 cm | Control | 11.35Ac | 0.16Aa | |||
| F | 11.43Ac | 0.17Aa | 1.08Aa | 1.02Ac | 110.30Ab | |
| BFB | 13.17Ab | 0.16Aa | 0.99Aa | 1.16Bb | 114.77Bb | |
| BFH | 15.31Aa | 0.18Aa | 1.10Aa | 1.37Ba | 150.12Aa | |
| BFBH | 14.61Aa | 0.18Aa | 1.13Aa | 1.31Aa | 147.70Aa | |
| 15–30 cm | Control | 9.26Bc | 0.15Ab | |||
| F | 9.44Bc | 0.17Aab | 1.13Aa | 1.04Ad | 116.82Ab | |
| BFB | 12.18Aa | 0.17Aab | 1.14Aa | 1.34Aa | 152.64Aa | |
| BFH | 10.14Bbc | 0.18Aab | 1.17Aa | 1.12Ac | 130.37Ab | |
| BFBH | 11.07Bab | 0.19Aa | 1.24Aa | 1.23Ad | 152.21Aa |
| Treatment † at Different Soil Layers | TOC:TN | MBC:N | ||||||
|---|---|---|---|---|---|---|---|---|
| Rosette Stage | Vigorous Stage | Squaring Stage | Maturity | Rosette Stage | Vigorous Stage | Squaring Stage | Maturity | |
| 0–15 cm | ||||||||
| Control | 11.89Ac | 14.27Ac | 14.61Ac | 15.13Ac | 3.31Ba | 3.28Bb | 3.35Ac | 3.04Bd |
| F | 11.25Ac | 13.20Ad | 14.61Ac | 15.22Ac | 3.06Bb | 3.14Bc | 3.18Ad | 3.03Bd |
| BFB | 13.23Ab | 15.49Ab | 15.32Ab | 15.11Bc | 3.43Aa | 3.49Aab | 3.58Abc | 3.48Bc |
| BFH | 14.42Aa | 15.63Ab | 17.78Aa | 18.20Aa | 3.25Bab | 3.55Aa | 3.66Ab | 3.85Ab |
| BFBH | 13.54Ab | 17.28Aa | 17.19Aa | 17.00Ab | 3.52Aa | 3.73Aa | 3.91Aa | 3.96Aa |
| 15–30 cm | ||||||||
| Control | 11.34Bb | 12.63Bc | 13.14Bc | 13.58Bc | 3.52Aa | 3.45Ab | 3.30Ab | 3.98Aa |
| F | 11.29Ab | 12.10Bc | 12.91Bc | 13.39Bc | 3.49Aa | 3.48Ab | 3.32Ab | 3.99Aa |
| BFB | 13.32Aa | 14.61Ba | 15.81Aa | 16.68Aa | 3.19Bb | 3.41Ab | 3.34Bb | 3.92Aa |
| BFH | 11.97Bb | 12.44Bc | 13.44Bc | 15.06Bb | 3.53Aa | 3.57Aa | 3.62Aa | 4.04Aa |
| BFBH | 11.99Bb | 13.99Bb | 14.92Bb | 15.91Ab | 3.50Aa | 3.60Aa | 3.56Ba | 4.02Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Li, B.; Chen, Y.; Tao, Q.; Xu, Q.; Wen, D.; Gao, X.; Li, Q.; Tang, X.; Wang, C. Biochar Application Increases Labile Carbon and Inorganic Nitrogen Supply in a Continuous Monocropping Soil. Land 2022, 11, 473. https://doi.org/10.3390/land11040473
Huang R, Li B, Chen Y, Tao Q, Xu Q, Wen D, Gao X, Li Q, Tang X, Wang C. Biochar Application Increases Labile Carbon and Inorganic Nitrogen Supply in a Continuous Monocropping Soil. Land. 2022; 11(4):473. https://doi.org/10.3390/land11040473
Chicago/Turabian StyleHuang, Rong, Bing Li, Yulan Chen, Qi Tao, Qiang Xu, Denghong Wen, Xuesong Gao, Qiquan Li, Xiaoyan Tang, and Changquan Wang. 2022. "Biochar Application Increases Labile Carbon and Inorganic Nitrogen Supply in a Continuous Monocropping Soil" Land 11, no. 4: 473. https://doi.org/10.3390/land11040473
APA StyleHuang, R., Li, B., Chen, Y., Tao, Q., Xu, Q., Wen, D., Gao, X., Li, Q., Tang, X., & Wang, C. (2022). Biochar Application Increases Labile Carbon and Inorganic Nitrogen Supply in a Continuous Monocropping Soil. Land, 11(4), 473. https://doi.org/10.3390/land11040473





