Distribution of Soil Microbes in Urban Parks: An Effect of Under-Tree Crown and Hillside Position on Testate Amoeba Assemblages in Subtropics (Shenzhen, China)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description and Sampling
2.2. Testate Amoeba Analysis
2.3. Statistical Analysis
3. Results
3.1. Overall Characteristics of TA Communities and Environmental Variables
3.2. Variation in Abundance and Diversity of TA Communities
3.3. Variation in Species Composition of TA Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, A.L. Increasing the Utility of Urban Environmental Quality Information. Landsc. Urban Plan. 2003, 65, 85–93. [Google Scholar] [CrossRef]
- Caruso, T.; Migliorini, M.; Rota, E.; Bargagli, R. Highly Diverse Urban Soil Communities: Does Stochasticity Play a Major Role? Appl. Soil Ecol. 2017, 110, 73–78. [Google Scholar] [CrossRef]
- Milano, V.; Cortet, J.; Baldantoni, D.; Bellino, A.; Dubs, F.; Nahmani, J.; Strumia, S.; Maisto, G. Collembolan Biodiversity in Mediterranean Urban Parks: Impact of History, Urbanization, Management and Soil Characteristics. Appl. Soil Ecol. 2017, 119, 428–437. [Google Scholar] [CrossRef]
- Park, S.-J.; Taylor, R.A.J.; Grewal, P.S. Spatial Organization of Soil Nematode Communities in Urban Landscapes: Taylor’s Power Law Reveals Life Strategy Characteristics. Appl. Soil Ecol. 2013, 64, 214–222. [Google Scholar] [CrossRef]
- Salinitro, M.; Alessandrini, A.; Zappi, A.; Melucci, D.; Tassoni, A. Floristic Diversity in Different Urban Ecological Niches of a Southern European City. Sci. Rep. 2018, 8, 15110. [Google Scholar] [CrossRef]
- Fu, Q.; Shao, Y.; Wang, S.; Liu, F.; Tian, G.; Chen, Y.; Yuan, Z.; Ye, Y. Soil Microbial Distribution Depends on Different Types of Landscape Vegetation in Temperate Urban Forest Ecosystems. Front. Ecol. Evol. 2022, 10. [Google Scholar] [CrossRef]
- Korneykova, M.V.; Vasenev, V.I.; Nikitin, D.A.; Dolgikh, A.V.; Soshina, A.S.; Myazin, V.A.; Nakhaev, M.R. Soil Microbial Community of Urban Green Infrastructures in a Polar City. Urban Ecosyst. 2022, 25, 1399–1415. [Google Scholar] [CrossRef]
- Santos, M.N.; Delabie, J.H.C.; Queiroz, J.M. Biodiversity Conservation in Urban Parks: A Study of Ground-Dwelling Ants (Hymenoptera: Formicidae) in Rio de Janeiro City. Urban Ecosyst. 2019, 22, 927–942. [Google Scholar] [CrossRef]
- Yang, T.; Shi, Y.; Zhu, J.; Zhao, C.; Wang, J.; Liu, Z.; Fu, X.; Liu, X.; Yan, J.; Yuan, M.; et al. The Spatial Variation of Soil Bacterial Community Assembly Processes Affects the Accuracy of Source Tracking in Ten Major Chinese Cities. Sci. China Life Sci. 2021, 64, 1546–1559. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Borcard, D.; Buttler, A.J.; Grosvernier, P.; Gilbert, D.; Gobat, J.-M.; Ecol, M. Horizontal Distribution Patterns of Testate Amoebae (Protozoa) in a Sphagnum Magellanicum Carpet. Microb. Ecol. 2000, 39, 290–300. [Google Scholar] [CrossRef]
- Carballeira, R.; Pontevedra-Pombal, X. Diversity of Testate Amoebae as an Indicator of the Conservation Status of Peatlands in Southwest Europe. Diversity 2021, 13, 269. [Google Scholar] [CrossRef]
- Jassey, V.E.J.; Chiapusio, G.; Mitchell, E.A.D.; Binet, P.; Toussaint, M.-L.; Gilbert, D. Fine-Scale Horizontal and Vertical Micro-Distribution Patterns of Testate Amoebae Along a Narrow Fen/Bog Gradient. Microb. Ecol. 2011, 61, 374–385. [Google Scholar] [CrossRef]
- Qin, Y.; Xie, S.; Smith, H.G.; Swindles, G.T.; Gu, Y. Diversity, Distribution and Biogeography of Testate Amoebae in China: Implications for Ecological Studies in Asia. Eur. J. Protistol. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Qin, Y.; Xie, S.; Gu, Y.; Wang, J.; Zhou, X. An Excellent Indicator for Quaternary Paleoenvironmental Reconstructions - Advances in the Study of Testate Amoebae (Thecamoebians, Arcellacean). Adv. Earth Sci. 2008, 23, 803–812, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Ettema, C.H.; Wardle, D.A. Spatial Soil Ecology. Trends Ecol. Evol. 2002, 17, 177–183. [Google Scholar] [CrossRef]
- Wang, Y.; Long, Y.; Li, Y.; Wang, Y.; Lu, G.; Yang, J.; Gao, X.; Chang, S.; Yang, X. Relationships of Soil Physicochemical Properties to the Distribution and the Composition of Microbial Community under Populus Euphratica’s Crown. Acta Ecol. Sin. 2021, 41, 5669–5684, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Hybholt, T.K.; Aamand, J.; Johnsen, A.R. Quantification of Centimeter-Scale Spatial Variation in PAH, Glucose and Benzoic Acid Mineralization and Soil Organic Matter in Road-Side Soil. Environ. Pollut. 2011, 159, 1085–1091. [Google Scholar] [CrossRef]
- Michelland, R.; Thioulouse, J.; Kyselková, M.; Grundmann, G.L. Bacterial Community Structure at the Microscale in Two Different Soils. Microb. Ecol. 2016, 72, 717–724. [Google Scholar] [CrossRef]
- Booth, R.K.; Sullivan, M.E.; Sousa, V.A. Ecology of Testate Amoebae in a North Carolina Pocosin and Their Potential Use as Environmental and Paleoenvironmental Indicators. Ecoscience 2008, 15, 277–289. [Google Scholar] [CrossRef]
- Escobar, J.; Brenner, M.; Whitmore, T.J.; Kenney, W.F.; Curtis, J.H. Ecology of Testate Amoebae (Thecamoebians) in Subtropical Florida Lakes. J. Paleolimnol. 2008, 40, 715–731. [Google Scholar] [CrossRef]
- Klerk, P.; Bobrov, A.; Theuerkauf, M.; Joosten, H. Short-Distance Distribution Patterns of Testate Amoebae in an Arctic Ice-Wedge Polygon Mire (Berelekh-Indigirka Lowlands, NE Siberia). Polar Biol. 2020, 43, 1321–1340. [Google Scholar] [CrossRef]
- Sim, T.G.; Swindles, G.T.; Morris, P.J.; Baird, A.J.; Charman, D.J.; Amesbury, M.J.; Beilman, D.; Channon, A.; Gallego-Sala, A.V. Ecology of Peatland Testate Amoebae in Svalbard and the Development of Transfer Functions for Reconstructing Past Water-Table Depth and PH. Ecol. Indic. 2021, 131, 108122. [Google Scholar] [CrossRef]
- Tsyganov, A.; Mazei, Y. Distribution of Soil Testate Amoeba Assemblages Along Catenas in the Northern Taiga Zone (Karelia, Russia). Protistology 2012, 7, 71–78. [Google Scholar]
- Tsyganov, A.N.; Temmerman, S.; Ledeganck, P.; Beyens, L. The Distribution of Soil Testate Amoebae under Winter Snow Cover at the Plot-Scale Level in Arctic Tundra (Qeqertarsuaq/Disko Island, West Greenland). Acta Protozool. 2012, 51, 155–167. [Google Scholar] [CrossRef]
- Heger, T.J.; Derungs, N.; Theurillat, J.P.; Mitchell, E.A.D. Testate Amoebae Like It Hot: Species Richness Decreases Along a Subalpine-Alpine Altitudinal Gradient in Both Natural Calluna Vulgaris Litter and Transplanted Minuartia Sedoides Cushions. Microb. Ecol. 2016, 71, 725–734. [Google Scholar] [CrossRef]
- Tsyganov, A.; Bobrov, A.; Shimano, S.; Mitchell, E.; Hagiwara, Y.; Wall, A.; Mazei, N.; Chernyshov, V.; Wanner, M.; Zhong, Y.; et al. Distribution of Soil Testate Amoeba Assemblages along an Elevation Gradient on Mount Fuji (Japan). Eur. J. Protistol. 2022, 84, 125894. [Google Scholar] [CrossRef]
- Vogt, J.C.; Olefeld, J.L.; Bock, C.; Boenigk, J.; Albach, D.C. Patterns of Protist Distribution and Diversification in Alpine Lakes across Europe. Microbiologyopen 2021, 10, e1216. [Google Scholar] [CrossRef]
- Lamentowicz, M.; Mitchell, E.; Lamentowicz, M.; Bragazza, L.; Buttler, A.; Jassey, V.E.J.; Mitchell, E.A.D. Seasonal Patterns of Testate Amoeba Diversity, Community Structure and Species-Environment Relationships in Four Sphagnum-Dominated Peatlands along a 1300 m Altitudinal Gradient in Switzerland. Soil Biol. Biochem. 2013, 67, 1–11. [Google Scholar] [CrossRef]
- Bobrov, A.A. Ecological and Geographic Patterns of Distribution and Community Structure of Testate Amoebae (Protozoa: Testacea). Ph.D. Thesis, Lomonosov Moscow State University, Moscow, Russia, 1999. (In Russian). [Google Scholar]
- Tsyganov, A.N.; Komarov, A.A.; Mitchell, E.A.D.; Shimano, S.; Smirnova, O.V.; Aleynikov, A.A.; Mazei, Y.A. Additive Partitioning of Testate Amoeba Species Diversity across Habitat Hierarchy within the Pristine Southern Taiga Landscape (Pechora-Ilych Biosphere Reserve, Russia). Eur. J. Protistol. 2015, 51, 42–54. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Cai, J.; Wang, H.; Dai, Y.; Shi, Z.; Feng, S. Study on Soil Physical and Chemical Properties of Shenzhen Park Greenland. J. Anhui Agric. Sci. 2019, 47, 52–55, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Liu, J.; Guo, W.; Luo, L.; Wu, G.; Liao, W.; Zhu, B. Vegetation Types and Their Ecological Landscape Planning in Nanshan Park, Shenzhen. Sun Yatsen Univ. Forum 2007, 27, 238–243. (In Chinese) [Google Scholar]
- Wei, H. Study on the Plant Species Disposing in Bao’an Park, Shenzhen City. For. Environ. Sci. 2009, 25, 31–38. (In Chinese) [Google Scholar]
- Zhang, J. Exploration on the Plant Configuration of Shenzhen Parks. Mod. Hortic. 2012, 71–73. (In Chinese) [Google Scholar] [CrossRef]
- Feng, X. Major Soil Types and Their Utilization in Ecological Landscape Forests in Shenzhen City. J. For. Eng. 2001, 15, 16–18. (In Chinese) [Google Scholar]
- Mazei, Y.A.; Chernyshov, V.A. Testate Amoebae Communities in the Southern Tundra and Forest-Tundra of Western Siberia. Biol. Bull. 2011, 38, 789–796. [Google Scholar] [CrossRef]
- Mazei, Y.A.; Tsyganov, A.N. Freshwater Testate Amoebae; KMK: Moscow, Russia, 2006. [Google Scholar]
- Mazei, Y.; Warren, A. A Survey of the Testate Amoeba Genus Difflugia Leclerc, 1815 Based on Specimens in the E. Penard and CG Ogden Collections of the Natural History Museum, London. Part 1: Species with Shells That Are Pointed Aborally and/or Have Aboral Protuberances. Protistology 2012, 7, 121–171. [Google Scholar]
- Mazei, Y.; Warren, A. A Survey of the Testate Amoeba Genus Difflugia Leclerc, 1815 Based on Specimens in the E. Penard and CG Ogden Collections of the Natural History Museum, London. Part 2: Species with Shells That Are Pyriform or Elongate. Protistology 2014, 8, 133–171. [Google Scholar]
- Mazei, Y.; Warren, A. A Survey of the Testate Amoeba Genus Difflugia Leclerc, 1815 Based on Specimens in the E. Penard and CG Ogden Collections of the Natural History Museum, London. Part 3: Species with Shells That Are Spherical or Ovoid. Protistology 2015, 9, 3–49. [Google Scholar]
- Ogden, G.G.; Hedley, R.H. An Atlas of Freshwater Testate Amoebae. Soil Sci. 1980, 130, 176. [Google Scholar] [CrossRef]
- Siemensma, F.J. Microworld, World of Amoeboid Organisms; World-Wide Electronic Publication: Kortenhoef, The Netherlands, 2019. [Google Scholar]
- Todorov, M.; Bankov, N. An Atlas of Sphagnum-Dwelling Testate Amoebae in Bulgaria; Pensoft Publishers: Sophia, Bulgaria, 2019; ISBN 978-954-642-973-5. [Google Scholar]
- Tsyganov, A.N.; Babeshko, K.V.; Mazei, Y.A. A Guide to Testate Amoebae with the Keys to Genera; Publishing House PGU: Penza, Russia, 2016. [Google Scholar]
- Cavalier-Smith, T.; Chao, E.E.-Y.; Oates, B. Molecular Phylogeny of Amoebozoa and the Evolutionary Significance of the Unikont Phalansterium. Eur. J. Protistol. 2004, 40, 21–48. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. The Phagotrophic Origin of Eukaryotes and Phylogenetic Classification of Protozoa. Int. J. Syst. Evol. Microbiol. 2002, 52, 297–354. [Google Scholar] [CrossRef]
- Meisterfeld, R. Arcellinida. In The Illustrated Guide to the Protozoa; Society of Protozoologists: Lawrence, KS, USA, 2000; Volume 2, pp. 827–859. [Google Scholar]
- Meisterfeld, R.; Lee, J.J.; Leedale, G.F.; Bradbury, P. Testate Amoebae with Filopodia. In An Illustrated Guide to the Protozoa; Society of Protozoologists: Lawrence, KS, USA, 2002; pp. 1054–1084. [Google Scholar]
- Marcisz, K.; Jassey, V.; Kosakyan, A.; Krashevska, V.; Fournier, B. Testate Amoeba Functional Traits and Their Use in Paleoecology. Front. Ecol. Evol. 2020, 8, 1–28. [Google Scholar] [CrossRef]
- Payne, R.J.; Mitchell, E.A.D. How Many Is Enough? Determining Optimal Count Totals for Ecological and Palaeoecological Studies of Testate Amoebae. J. Paleolimnol. 2009, 42, 483–495. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 2021; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package 2020. Available online: https://github.com/vegandevs/vegan (accessed on 6 December 2022).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; ISBN 978-1-5443-3647-3. [Google Scholar]
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arachchige, C. DescTools: Tools for Descriptive Statistics 2022, R package version 0.99.47. Available online: https://cran.r-project.org/package=DescTools (accessed on 6 December 2022).
- Legendre, P.; Gallagher, E.D. Ecologically Meaningful Transformations for Ordination of Species Data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. Ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Zhou, X.; Zuo, X.; Zhao, X.; Wang, S.; Liu, C.; Zhang, J.; Lv, P.; Zhang, J. Comparison Analyses of DCA, CCA and DCCA on Relationships Between Plant Community Distribution and Soil Properties of Horqin Sandy Land. Chin. J. Ecol. 2015, 34, 947–954, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Arbizu, P.M. pairwiseAdonis. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 6 December 2022).
- de Cáceres, M.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Cáceres, M.; Legendre, P.; Wiser, S.K.; Brotons, L. Using Species Combinations in Indicator Value Analyses. Methods Ecol. Evol. 2012, 3, 973–982. [Google Scholar] [CrossRef]
- Gaston, K.J. Global Patterns in Biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2010; ISBN 978-0-87893-494-2. [Google Scholar]
- Fierer, N.; Mccain, C.M.; Meir, P.; Zimmermann, M.; Rapp, J.M.; Silman, M.R.; Knight, R. Microbes Do Not Follow the Elevational Diversity Patterns of Plants and Animals. Ecology 2011, 92, 797–804. [Google Scholar] [CrossRef]
- Todorov, M.T. Observation on the Soil and Moss Testate Amoebae (Protozoa: Rhizopoda) from Pirin Mountain (Bulgaria). Acta Zool. Bulg. 1998, 50, 19–29. [Google Scholar]
- Mazei, Y.A.; Marfina, O.V.; Chernyshov, V.A. Distribution of Soil-Inhabiting Testate Amoebae along a Mountain Slope (Baikal Lake Region, Khamar-Daban Ridge, Cherskii Peak). Biol. Bull. 2012, 39, 800–804. [Google Scholar] [CrossRef]
- Krashevska, V.; Bonkowski, M.; Maraun, M.; Scheu, S. Testate Amoebae (Protista) of an Elevational Gradient in the Tropical Mountain Rain Forest of Ecuador. Pedobiologia 2007, 51, 319–331. [Google Scholar] [CrossRef]
- Mazei, Y.A.; Trulova, A.; Mazei, N.G.; Payne, R.J.; Tsyganov, A.N. Contributions of Temporal and Spatial Variation to the Diversity of Soil-Dwelling Testate Amoeba Assemblages in a Swampy Forest. Pedobiologia 2020, 81–82, 150660. [Google Scholar] [CrossRef]
- Mazei, Y.A.; Blinokhvatova, Y.V.; Embulaeva, E.A. Specific Features of the Microspatial Distribution of Soil Testate Amoebae in the Forests of the Middle Volga Region. Arid. Ecosyst. 2011, 1, 46–52. [Google Scholar] [CrossRef]
- Payne, R.J.; Belyakova, O.; Mazei, Y. Diversity and Community Ecology of Forest Epiphyte Testate Amoebae from European Russia. Eur. J. Protistol. 2015, 51, 450–459. [Google Scholar] [CrossRef]
- Marcisz, K.; Colombaroli, D.; Jassey, V.E.J.; Tinner, W.; Kołaczek, P.; Gałka, M.; Karpińska-Kołaczek, M.; Słowiński, M.; Lamentowicz, M. A Novel Testate Amoebae Trait-Based Approach to Infer Environmental Disturbance in Sphagnum Peatlands. Sci. Rep. 2016, 6, 33907. [Google Scholar] [CrossRef]
- Krashevska, V.; Sandmann, D.; Marian, F.; Maraun, M.; Scheu, S. Leaf Litter Chemistry Drives the Structure and Composition of Soil Testate Amoeba Communities in a Tropical Montane Rainforest of the Ecuadorian Andes. Microb. Ecol. 2017, 74, 681–690. [Google Scholar] [CrossRef]
- Balik, V. Testate Amoebae Community (Protozoa, Rhizopoda) in a Meadow-Spruce Forest Mesoecotone. Biol. Bratisl. 1996, 51, 117–124. [Google Scholar]
- Balik, V. Testate Amoebae Communities (Protozoa, Rhizopoda) in Two Moss-Soil Microecotones. Biol. Bratisl. 1996, 51, 125–133. [Google Scholar]
- Mieczan, T.; Tarkowska-Kukuryk, M.; Bielańska-Grajner, I. Hydrochemical and Microbiological Distinction and Function of Ombrotrophic Peatland Lagg as Ecotone between Sphagnum Peatland and Forest Catchment (Poleski National Park, Eastern Poland). Ann. De Limnol.—Int. J. Limnol. 2012, 48, 323–336. [Google Scholar] [CrossRef][Green Version]
- Berg, M.P.; Bengtsson, J. Temporal and Spatial Variability in Soil Food Web Structure. Oikos 2007, 116, 1789–1804. [Google Scholar] [CrossRef]
- Potapov, A.M.; Rozanova, O.L.; Semenina, E.E.; Leonov, V.D.; Belyakova, O.I.; Bogatyreva, V.Y.; Degtyarev, M.I.; Esaulov, A.S.; Korotkevich, A.Y.; Kudrin, A.A.; et al. Size Compartmentalization of Energy Channeling in Terrestrial Belowground Food Webs. Ecology 2021, 102, e03421. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Z.; Chen, Q.L.; Zhang, Y.J.; He, J.Z.; Hu, H.W. Industrial Development as a Key Factor Explaining Variances in Soil and Grass Phyllosphere Microbiomes in Urban Green Spaces. Environ. Pollut. 2020, 261, 114201. [Google Scholar] [CrossRef]
- Yuan, Z.S.; Liu, F.; He, S.B.; Zhou, L.L.; Pan, H. Community Structure and Diversity Characteristics of Rhizosphere and Root Endophytic Bacterial Community in Different Acacia Species. PLoS ONE 2022, 17, e0262909. [Google Scholar] [CrossRef]
- Zhang, M.H.; Huang, B.L.; Cheng, L.; Lv, C.Q.; Li, Z.X.; Wei, L.X. Features of Soil Microorganism in Mixed Forest of Eucalyptus Spp and Acacia Confusa. Guangxi Agric. Sci. 2009, 40, 681–685, (In Chinese with English abstract). [Google Scholar]
- Krashevska, V.; Maraun, M.; Scheu, S. How Does Litter Quality Affect the Community of Soil Protists (Testate Amoebae) of Tropical Montane Rainforests? FEMS Microbiol. Ecol. 2012, 80, 603–607. [Google Scholar] [CrossRef][Green Version]


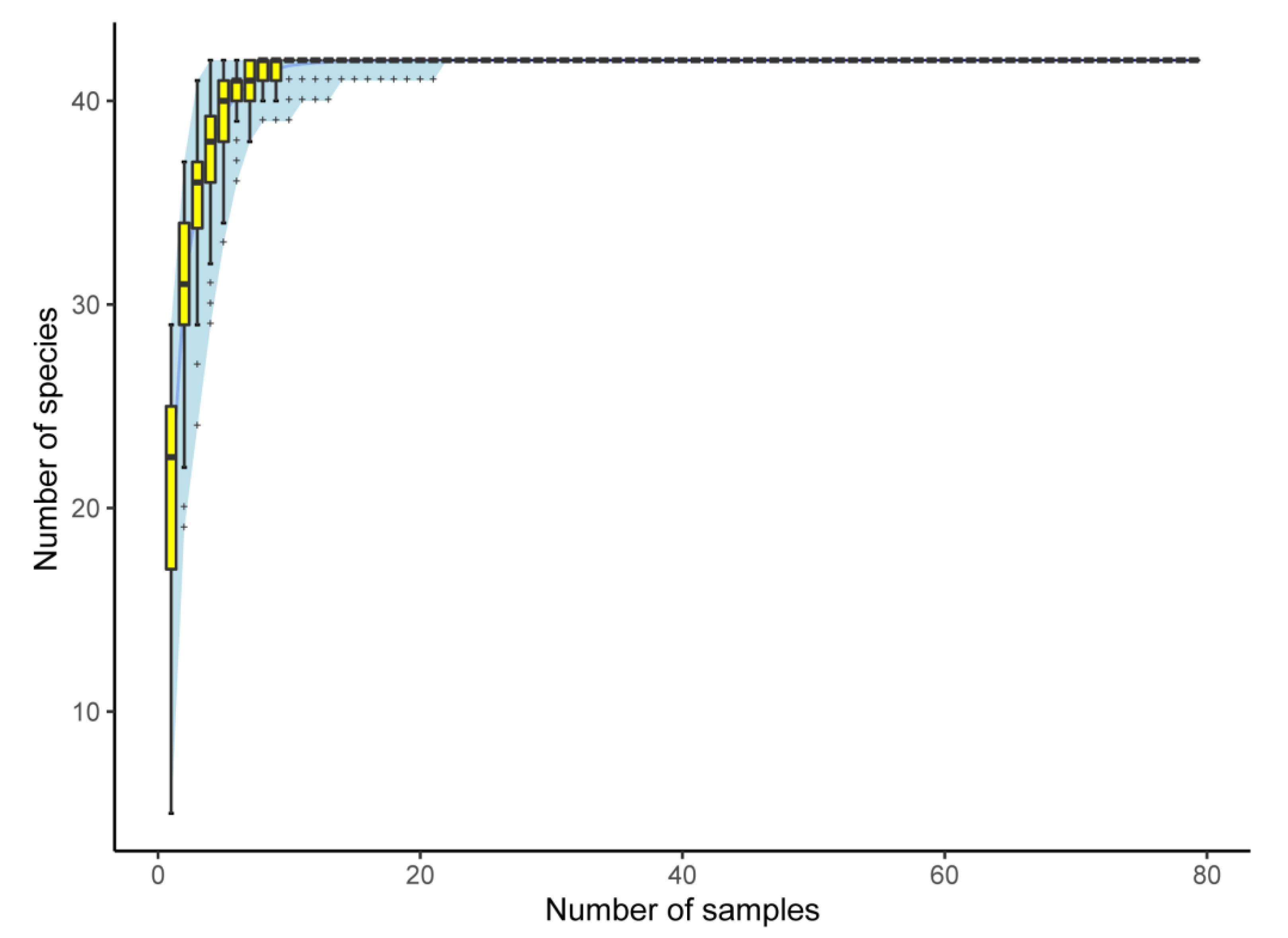
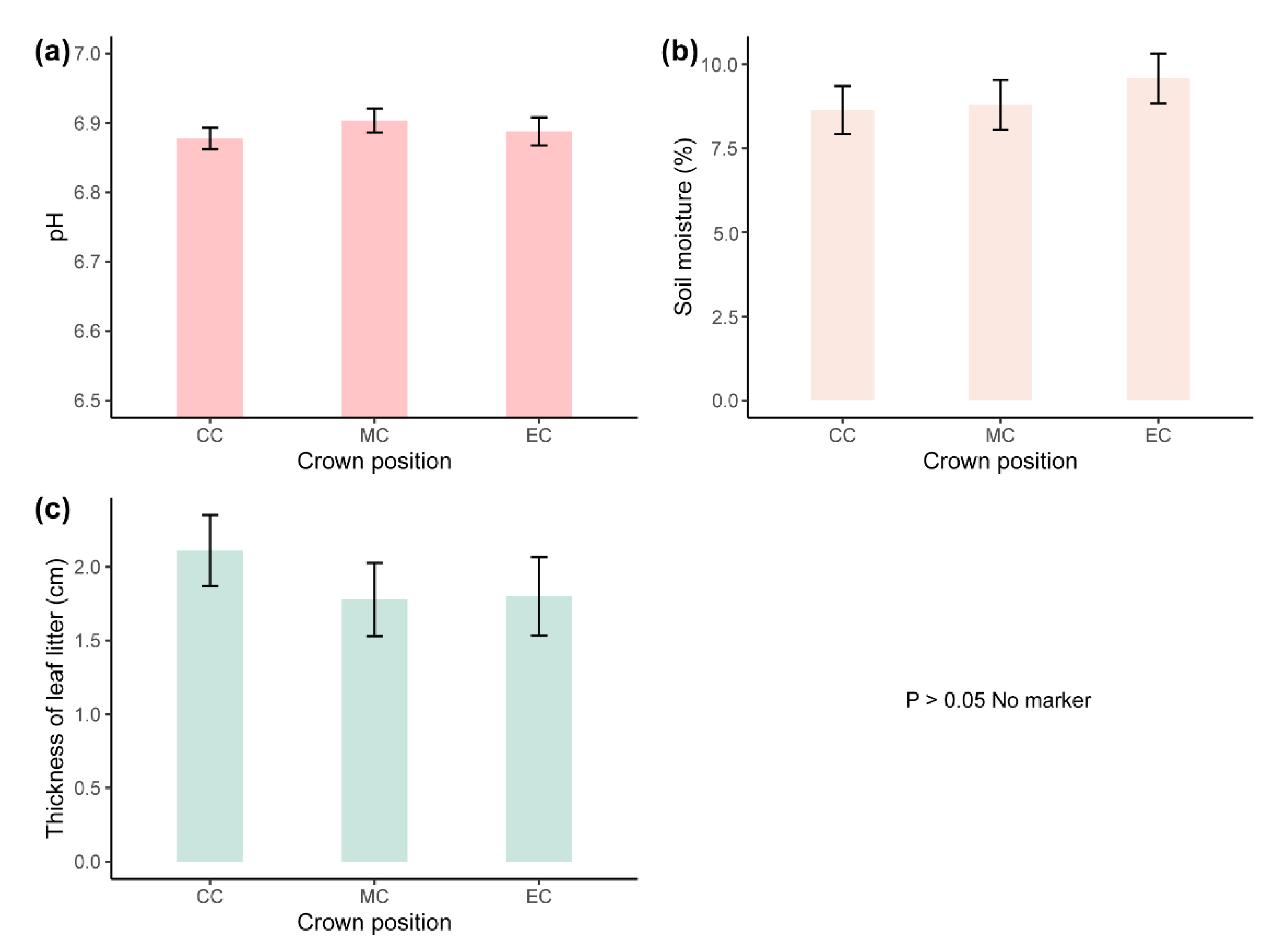
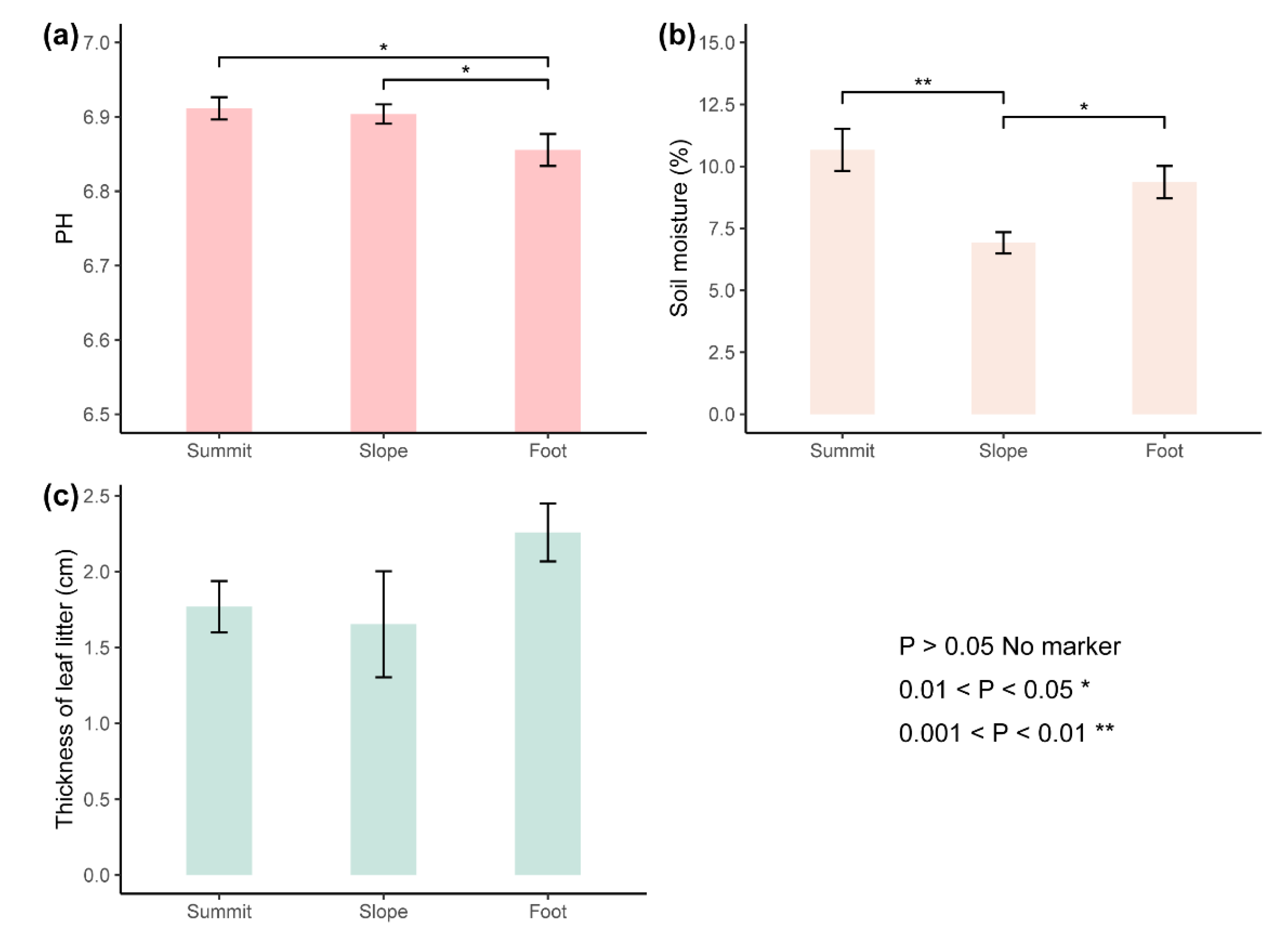

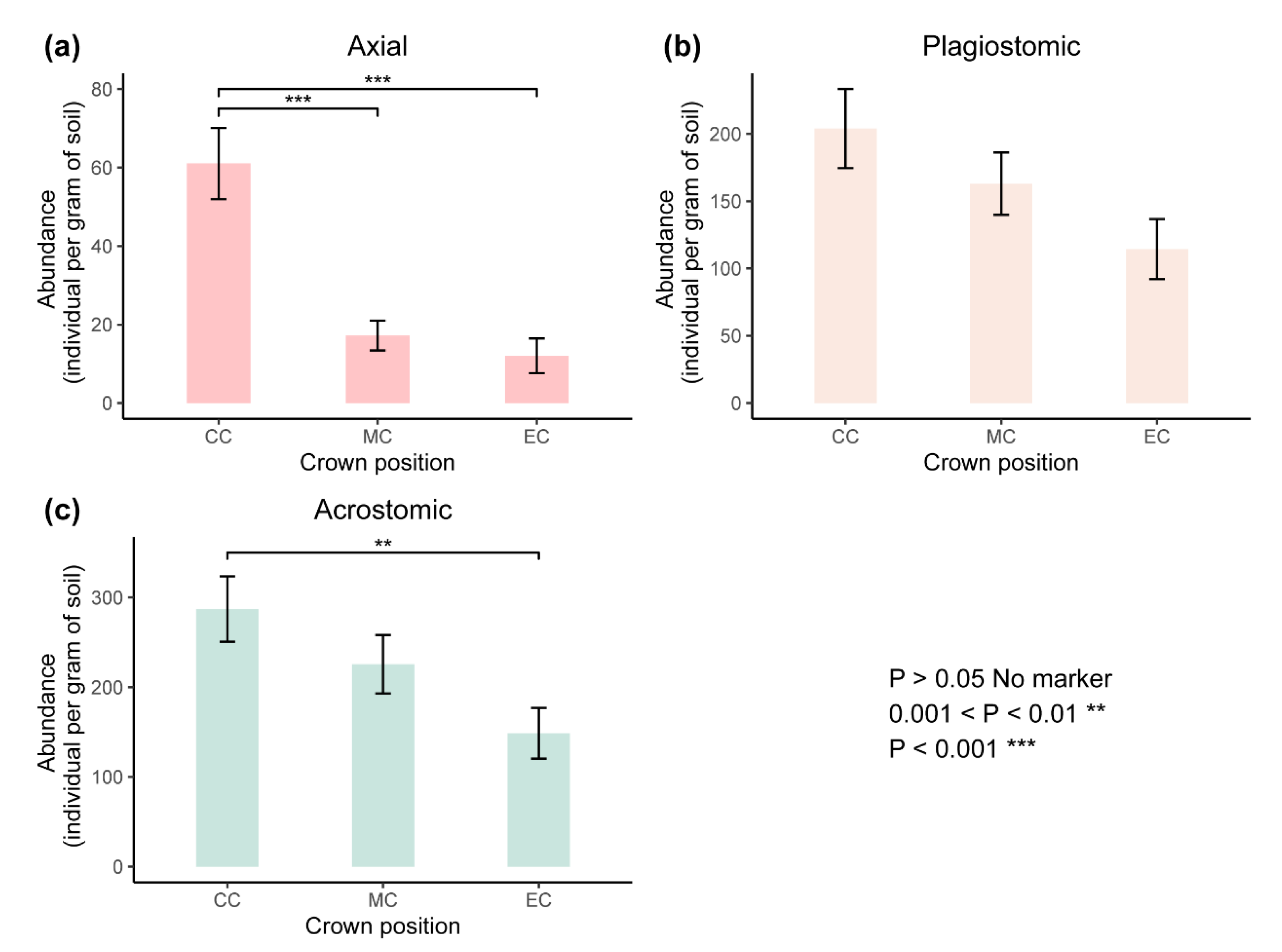

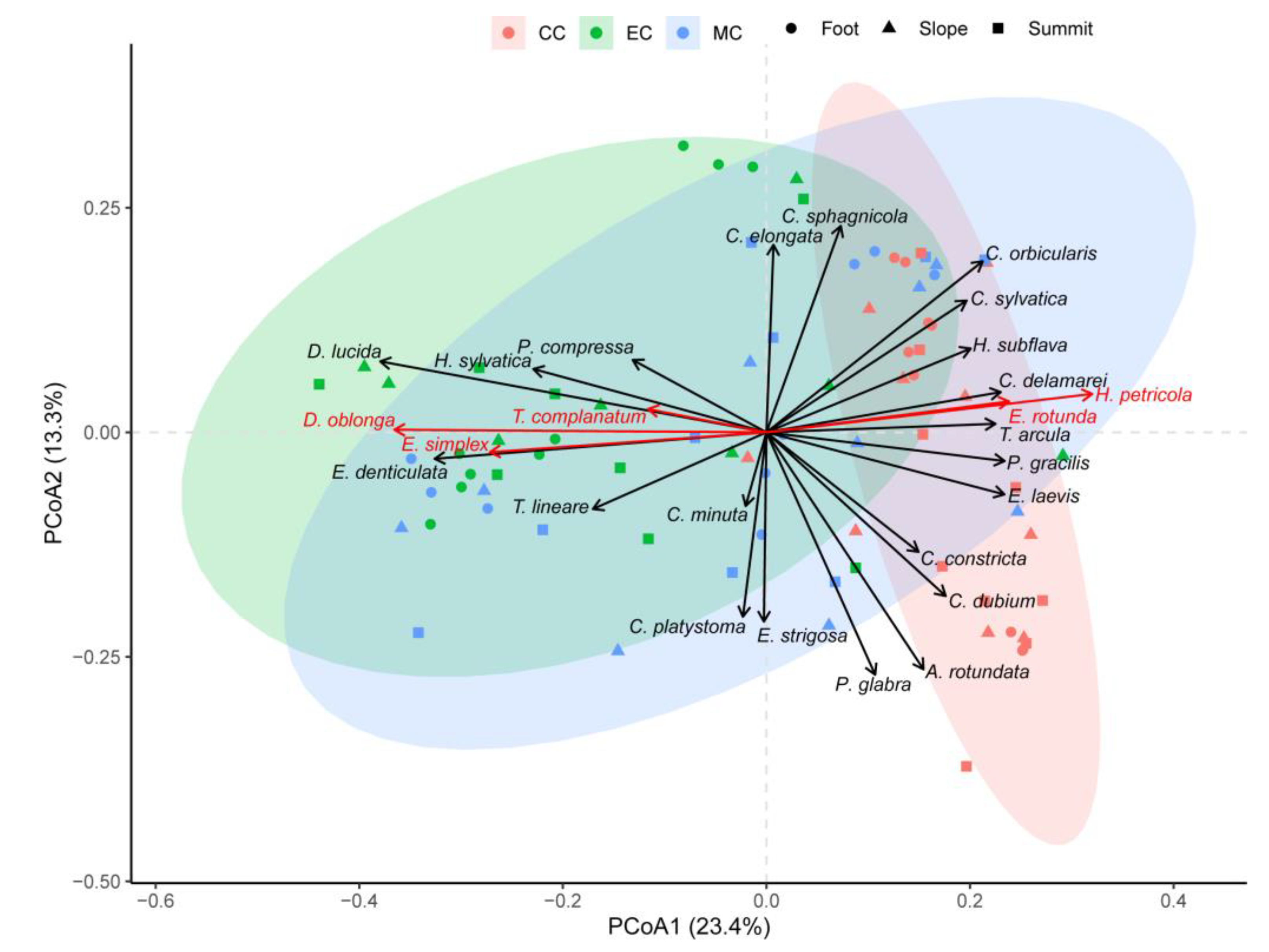
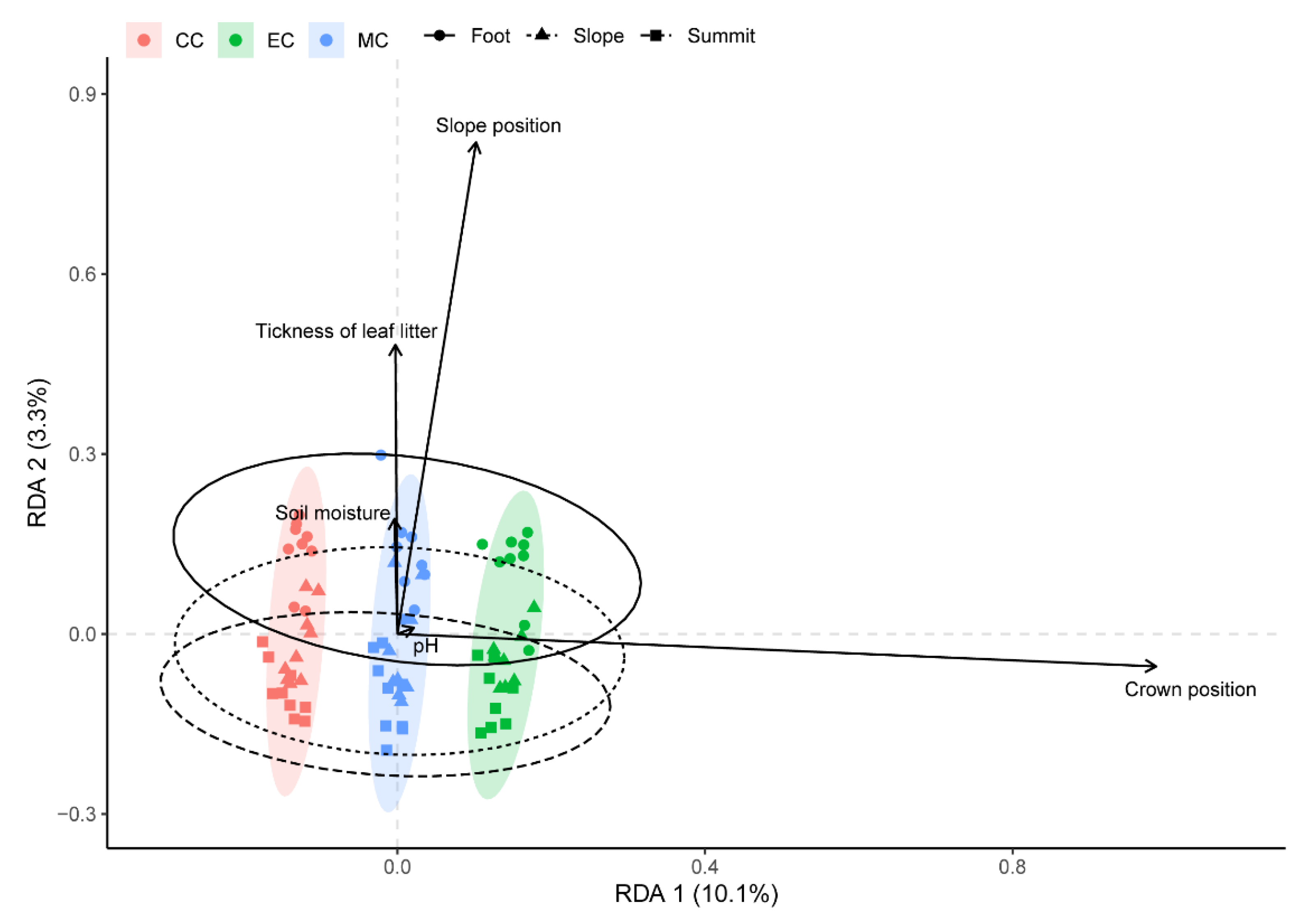
| Characteristics | Species | CC | MC | EC |
|---|---|---|---|---|
| N = 27 | N = 27 | N = 25 | ||
| Occurrence (>70%) | Centropyxis sylvatica | 85.19% | ||
| Centropyxis platystoma | 74.07% | |||
| Corythion dubium | 74.07% | |||
| Cyclopyxis eurystoma | 74.07% | |||
| Difflugia oblonga | 76.00% | |||
| Euglypha rotunda | 85.19% | |||
| Euglypha ciliata | 81.48% | |||
| Euglypha compressa | 81.48% | |||
| Euglypha strigosa | 70.37% | |||
| Heleopera petricola | 100.00% | |||
| Heleopera sylvatica | 81.48% | 72.00% | ||
| Hyalosphenia subflava | 77.78% | |||
| Pseudodifflugia gracilis | 96.30% | |||
| Pseudodifflugia compressa | 81.48% | |||
| Plagiopyxis callida | 88.89% | 80.00% | ||
| Trinema complanatum | 88.89% | 77.78% | 80.00% | |
| Trinema enchelys | 85.19% | 88.89% | 80.00% | |
| Trigonopyxis arcula | 92.59% | |||
| Relative abundance (>5%) | Difflugia oblonga | 8.04% | ||
| Heleopera sylvatica | 6.66% | 8.48% | ||
| Hyalosphenia subflava | 5.31% | 5.15% | ||
| Pseudodifflugia gracilis | 6.50% | |||
| Pseudodifflugia compressa | 6.34% | 5.53% | ||
| Plagiopyxis callida | 6.31% | |||
| Trinema enchelys | 5.30% | 6.27% | 6.68% | |
| Trinema complanatum | 6.59% | |||
| Trinema lineare | 5.05% | |||
| Trigonopyxis arcula | 5.48% |
| Characteristics | Species | Summit | Slope | Foot |
|---|---|---|---|---|
| N = 26 | N = 26 | N = 27 | ||
| Occurrence (>70%) | Centropyxis platystoma | 70.37% | ||
| Centropyxis sphagnicola | 77.78% | |||
| Euglypha compressa | 74.07% | |||
| Galeripora arenaria | 74.07% | |||
| Galeripora artocrea | 81.48% | |||
| Heleopera sylvatica | 77.78% | |||
| Hyalosphenia subflava | 77.78% | |||
| Pseudodifflugia gracilis | 77.78% | |||
| Pseudodifflugia compressa | 88.89% | |||
| Plagiopyxis callida | 76.92% | 88.89% | ||
| Trinema complanatum | 80.77% | 100% | ||
| Trinema enchelys | 88.46% | 100% | ||
| Trigonopyxis arcula | 74.07% | |||
| Relative abundance (>5%) | Heleopera sylvatica | 5.92% | ||
| Pseudodifflugia compressa | 6.27% | |||
| Plagiopyxis callida | 5.15% | |||
| Trinema enchelys | 5.96% | 6.07% | ||
| Trinema complanatum | 5.57% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Ivanovskii, A.; Ndayishimiye, J.C.; Tsyganov, A.N.; Babeshko, K.; Saldaev, D.; Mazei, Y. Distribution of Soil Microbes in Urban Parks: An Effect of Under-Tree Crown and Hillside Position on Testate Amoeba Assemblages in Subtropics (Shenzhen, China). Land 2022, 11, 2250. https://doi.org/10.3390/land11122250
Zhong Y, Ivanovskii A, Ndayishimiye JC, Tsyganov AN, Babeshko K, Saldaev D, Mazei Y. Distribution of Soil Microbes in Urban Parks: An Effect of Under-Tree Crown and Hillside Position on Testate Amoeba Assemblages in Subtropics (Shenzhen, China). Land. 2022; 11(12):2250. https://doi.org/10.3390/land11122250
Chicago/Turabian StyleZhong, Yuantan, Aleksandr Ivanovskii, Jean Claude Ndayishimiye, Andrey N. Tsyganov, Kirill Babeshko, Damir Saldaev, and Yuri Mazei. 2022. "Distribution of Soil Microbes in Urban Parks: An Effect of Under-Tree Crown and Hillside Position on Testate Amoeba Assemblages in Subtropics (Shenzhen, China)" Land 11, no. 12: 2250. https://doi.org/10.3390/land11122250
APA StyleZhong, Y., Ivanovskii, A., Ndayishimiye, J. C., Tsyganov, A. N., Babeshko, K., Saldaev, D., & Mazei, Y. (2022). Distribution of Soil Microbes in Urban Parks: An Effect of Under-Tree Crown and Hillside Position on Testate Amoeba Assemblages in Subtropics (Shenzhen, China). Land, 11(12), 2250. https://doi.org/10.3390/land11122250








