Community Structure and Distribution Pattern of Woody Vegetation in Response to Soil properties in Semi-Arid Lowland District Kasur Punjab, Pakistan
Abstract
1. Introduction
2. Materials and Methods
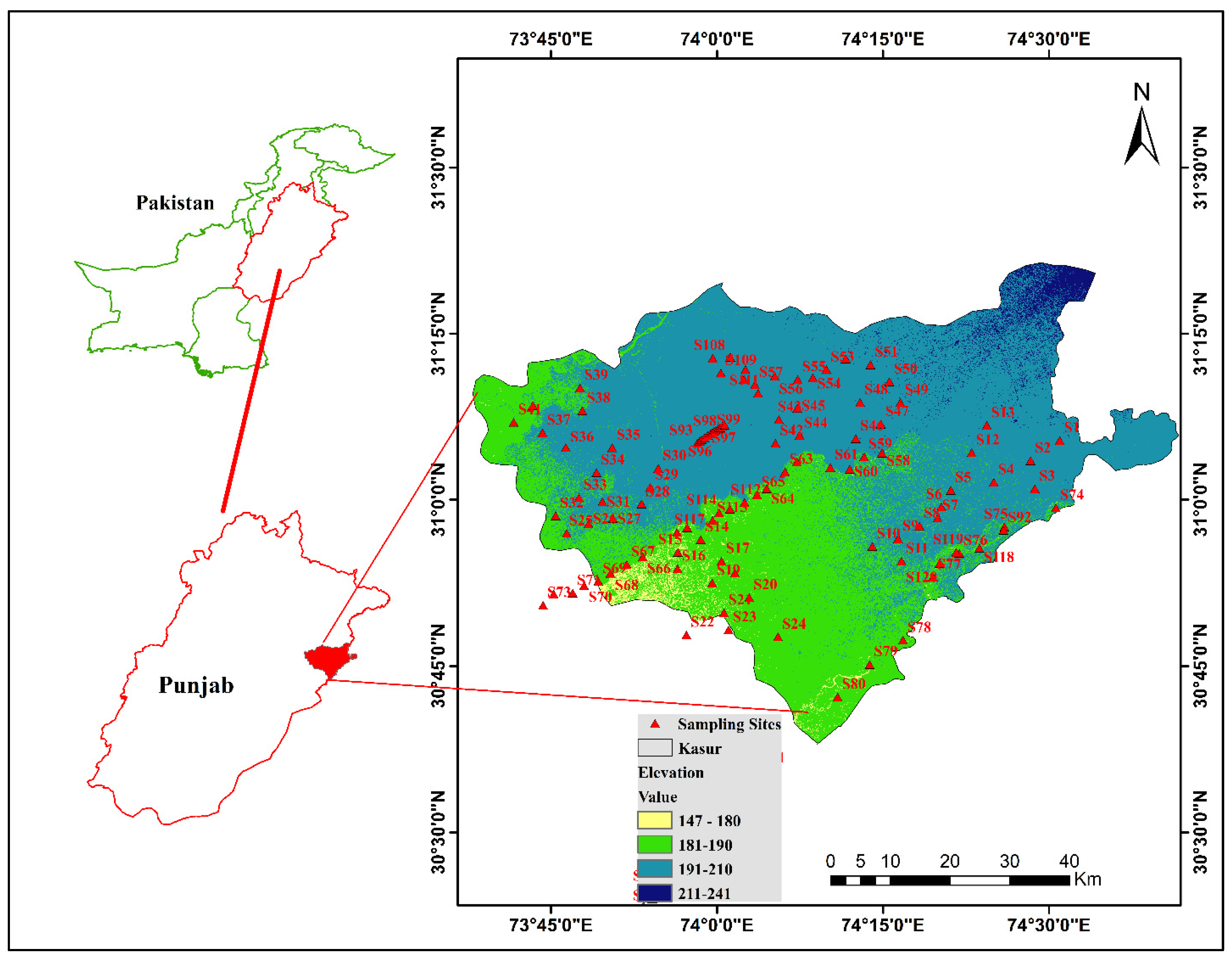
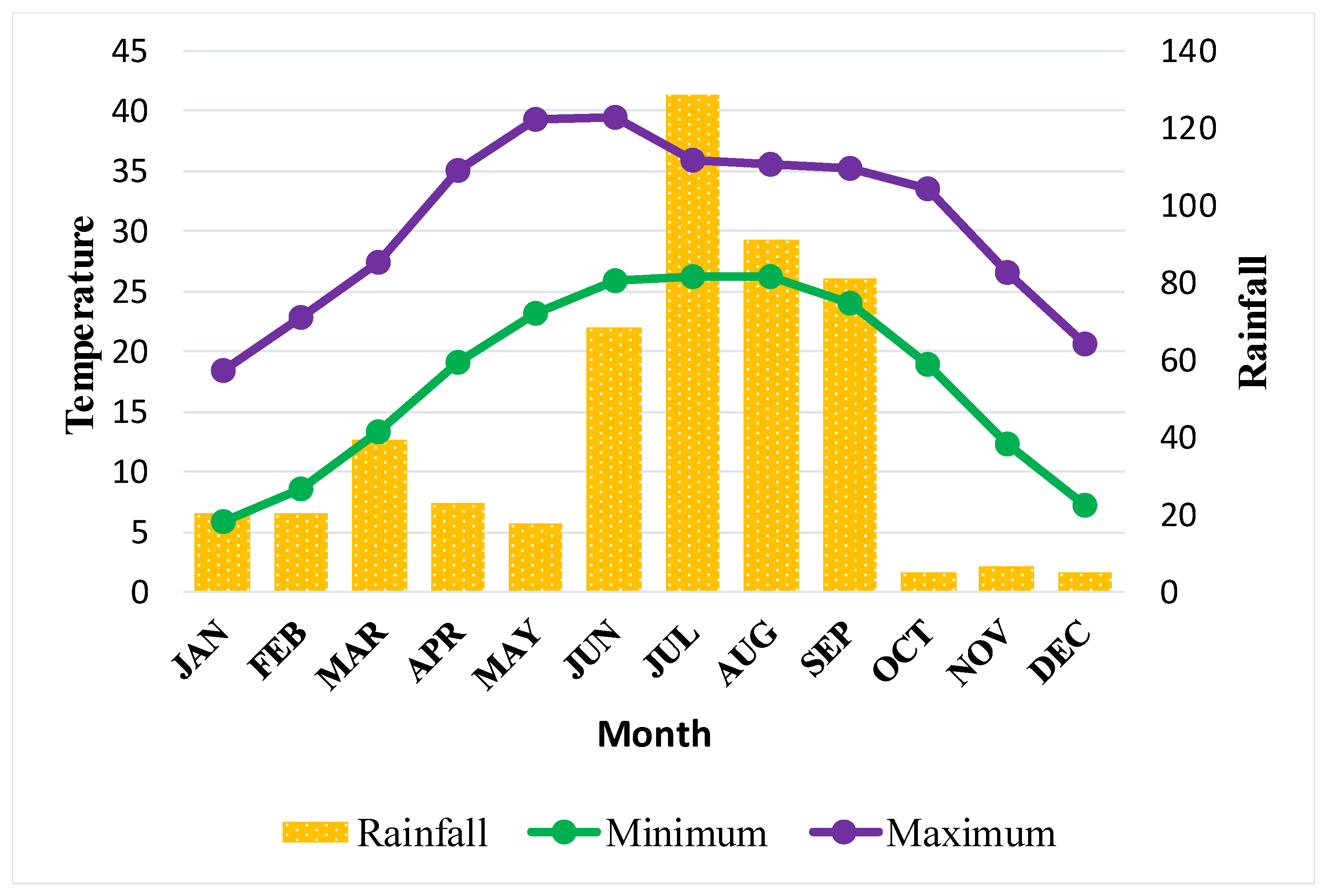
2.1. Study Area
2.2. Field Sampling
2.3. Soil Sampling
2.4. Indicator Species Analysis
2.5. Data Analysis
3. Results
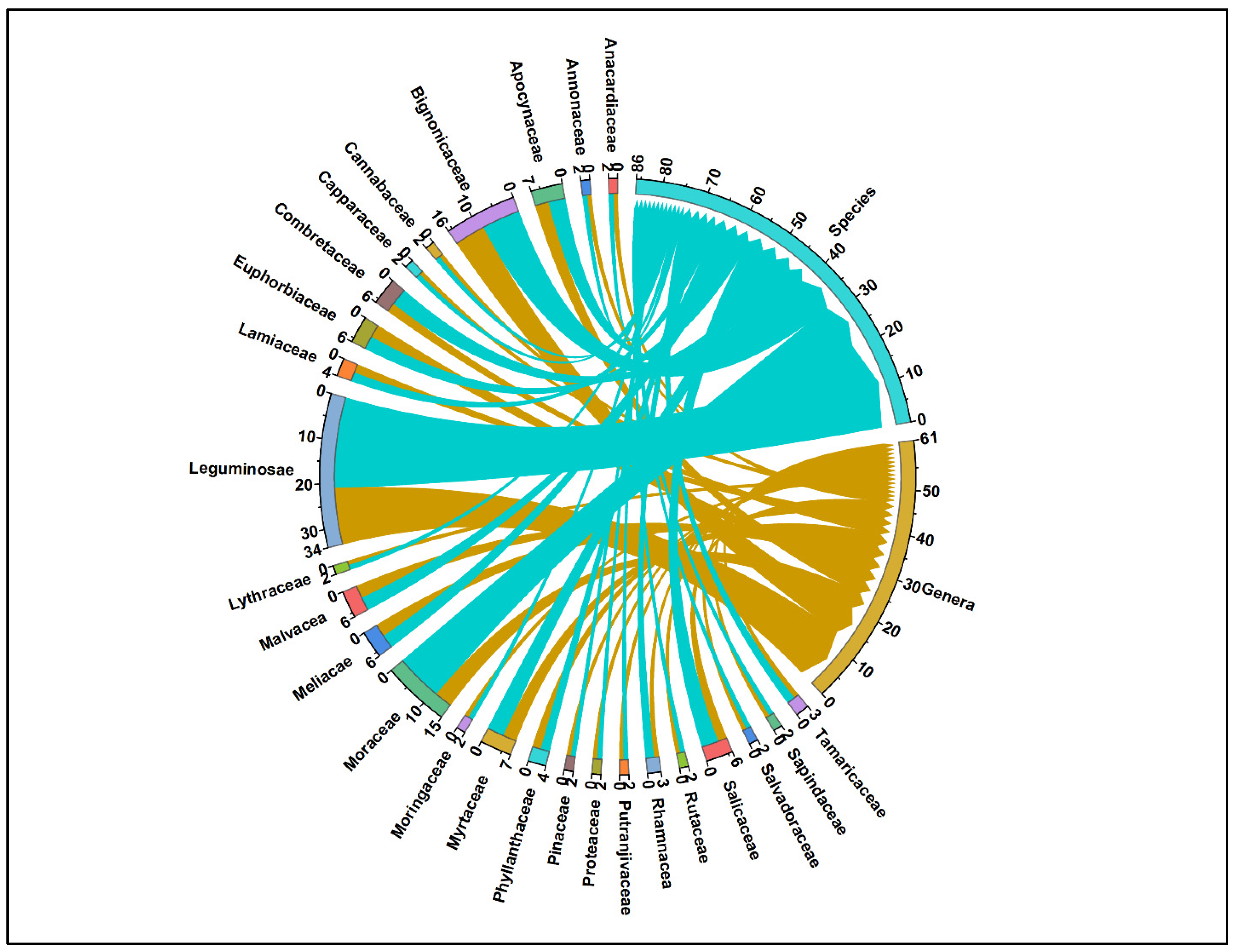
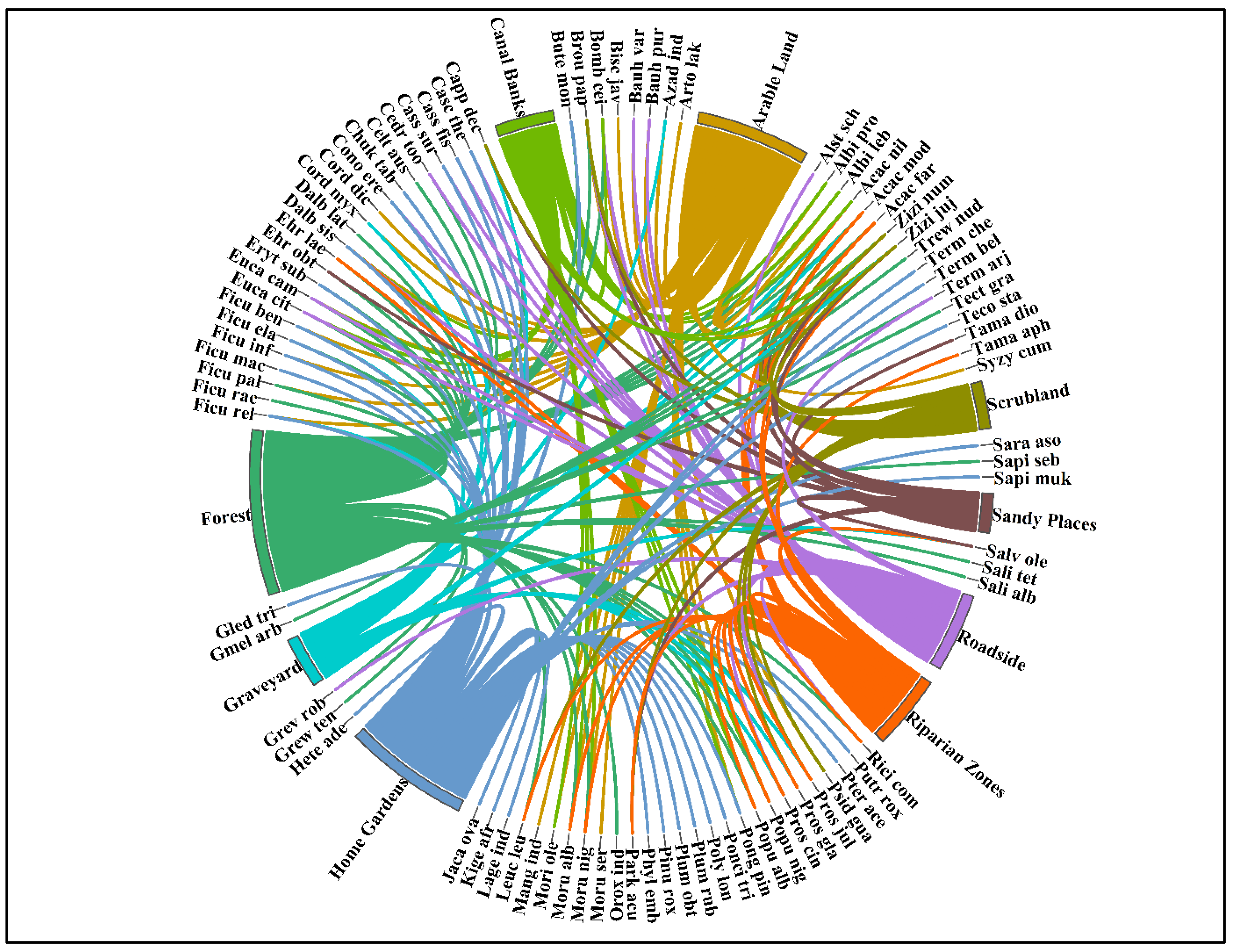
3.1. Woody Vegetation of Study Area
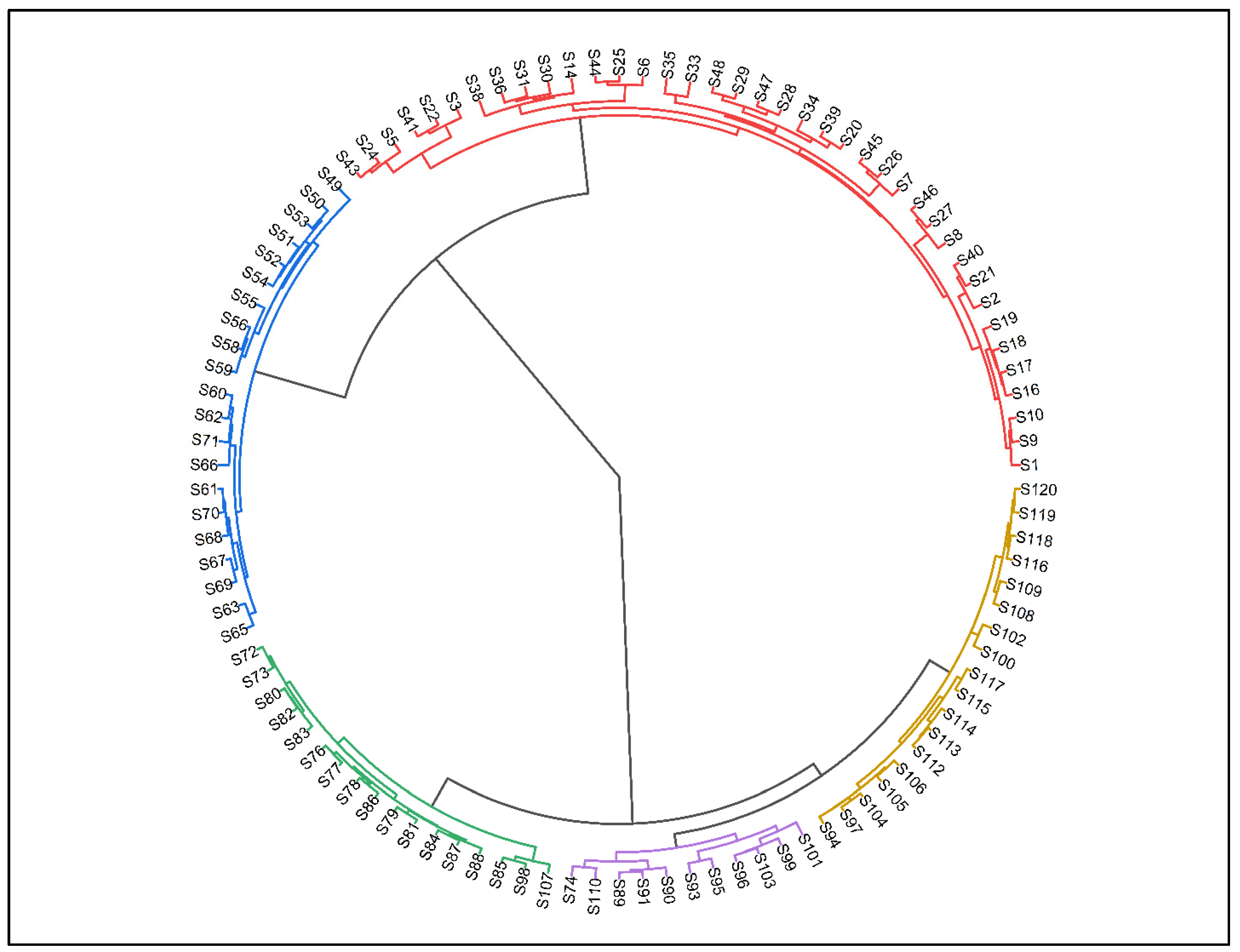
3.2. Classification of Woody Vegetation
3.3. Community 01 (EDM: Eucalyptus-Dalbergia-Morus Community)
3.4. Community 02 (PBM: Populus-Bombax-Morus Community)
3.5. Community 03 (ZTZ: Ziziphus-Tamarix- Ziziphus Community)
3.6. Community 04 (PAP: Prosopis-Acacia-Prosopis Community)
3.7. Community 05 (BCR: Broussonetia-Conocarpus-Ricinus Community)
3.8. Community 05 (BCR: Broussonetia-Conocarpus-Ricinus Community)
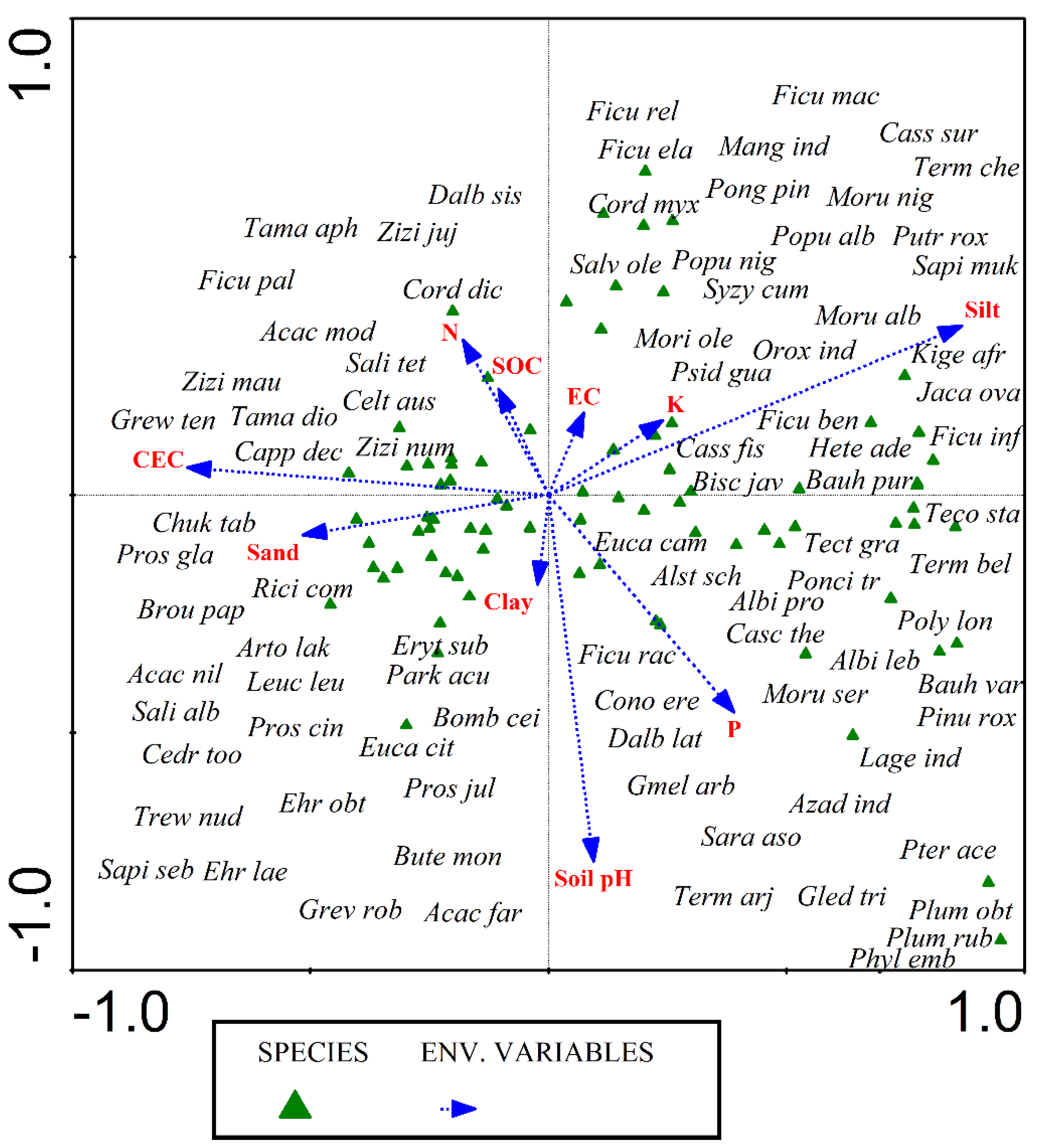
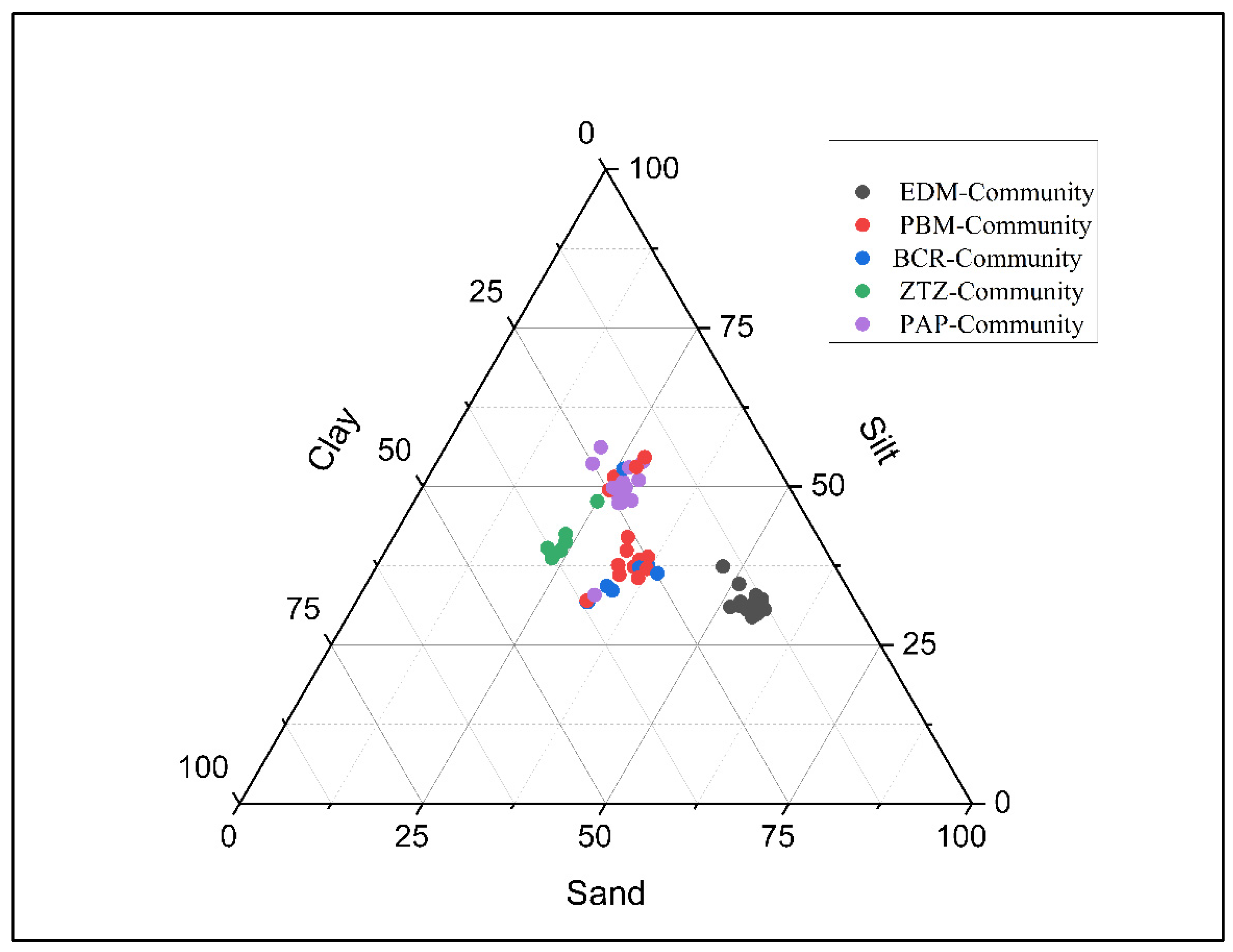
3.9. Role of Soil Properties on Distribution of Woody Vegetation
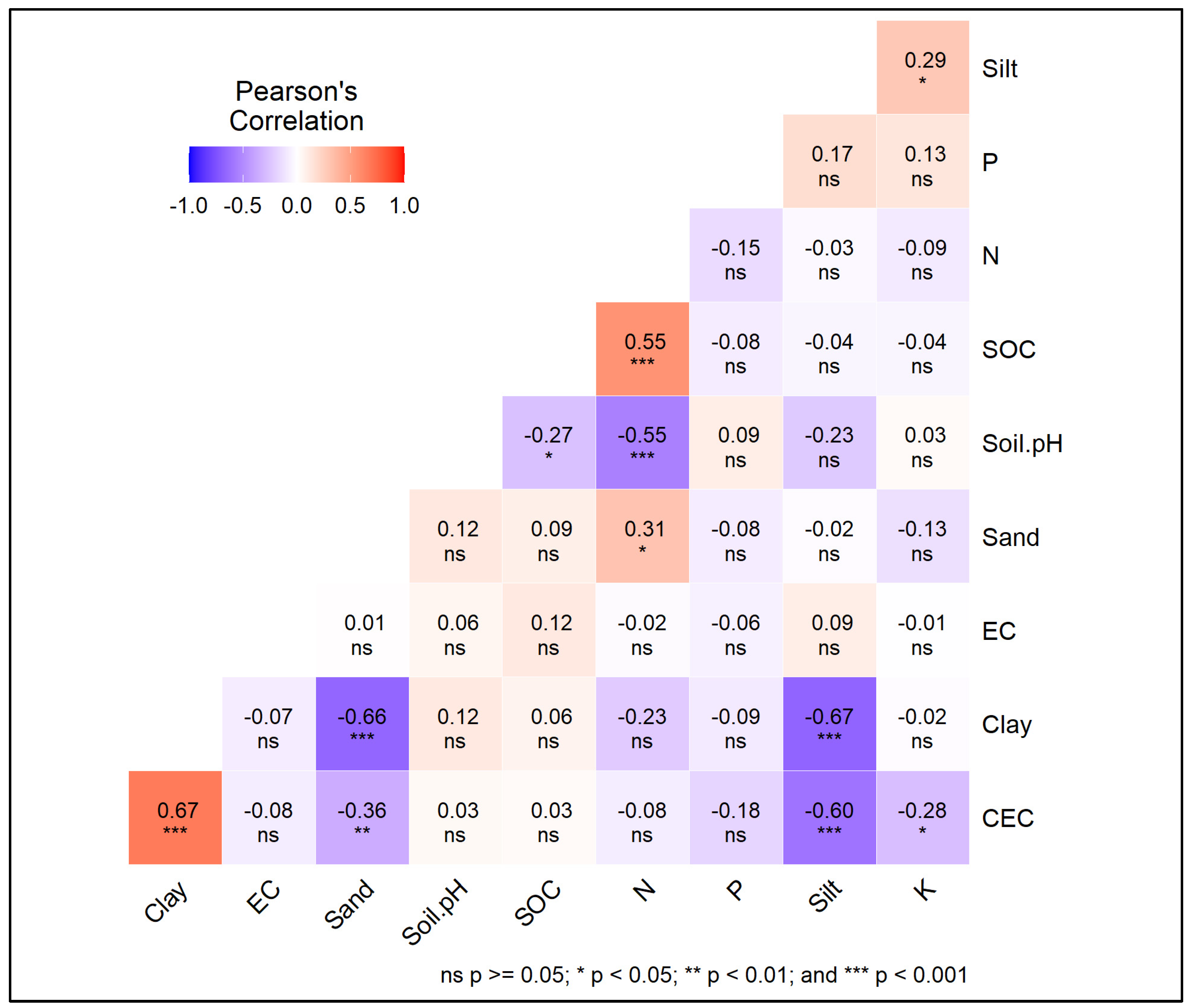
3.10. Correlation of Soil Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dar, J.A.; Sundarapandian, S. Patterns of plant diversity in seven temperate forest types of Western Himalaya, India. J. Asia-Pacific Biodivers. 2016, 9, 280–292. [Google Scholar] [CrossRef]
- Khan, A.M.; Qureshi, R.; Saqib, Z. Multivariate analyses of the vegetation of the western Himalayan forests of Muzaffarabad district, Azad Jammu and Kashmir, Pakistan. Ecol. Indic. 2019, 104, 723–736. [Google Scholar] [CrossRef]
- Nafeesa, Z.; Haq, S.M.; Bashir, F.; Gaus, G.; Mazher, M.; Anjum, M.; Rasool, A.; Rashid, N. Observations on the floristic, life-form, leaf-size spectra and habitat diversity of vegetation in the Bhimber hills of Kashmir Himalayas. Shengtai Xuebao/Acta Ecol. Sin. 2021, 41, 228–234. [Google Scholar] [CrossRef]
- Rahman, I.U.R.; Afzal, A.; Iqbal, Z.; Ijaz, F.; Ali, N.; Asif, M.; Alam, J.; Majid, A.; Hart, R.; Bussmann, R.W. First insights into the floristic diversity, biological spectra and phenology of manoor valley, Pakistan. Pak. J. Bot. 2018, 50, 1113–1124. [Google Scholar]
- Byrne, K.M.; Lauenroth, W.K.; Adler, P.B. Contrasting Effects of Precipitation Manipulations on Production in Two Sites within the Central Grassland Region, USA. Ecosystems 2013, 16, 1039–1051. [Google Scholar] [CrossRef]
- Coroi, M.; Skeffington, M.S.; Giller, P.; Smith, C.; Gormally, M.; O’Donovan, G. Vegetation diversity and stand structure in streamside forests in the south of Ireland. For. Ecol. Manag. 2004, 202, 39–57. [Google Scholar] [CrossRef]
- Akhlaq, R.; Amjad, M.S.; Qaseem, M.F.; Fatima, S.; Chaudhari, S.K.; Khan, A.M.; Khan, S.; Malik, N.Z.; Gardazi, S.M.H.; Bibi, A.; et al. Species diversity and vegetation structure from different climatic zones of Tehsil Harighel, Bagh, Azad Kasmir, Pakistan analysed through multivariate techniques. Appl. Ecol. Environ. Res. 2018, 16, 5193–5211. [Google Scholar] [CrossRef]
- Khan, A.M.; Qureshi, R.; Saqib, Z.; Munir, M.; Shaheen, H.; Habib, T.; Dar, M.E.U.I.; Fatimah, H.; Afza, R.; Hussain, M.A. A first ever detailed ecological exploration of the western himalayan forests of sudhan gali and ganga summit, azad Jammu and Kashmir, Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 15477–15505. [Google Scholar] [CrossRef]
- Poulos, H.M.; Camp, A.E. Topographic influences on vegetation mosaics and tree diversity in the Chihuahuan Desert Borderlands. Ecology 2010, 91, 1140–1151. [Google Scholar] [CrossRef]
- Husain, T.; Hussain, A.; Ahmed, M. Studies of vegetative behavior and climatic effects on some pasture grasses growing wild in Pakistan. Pak. J. Bot. 2009, 41, 2379–2386. [Google Scholar]
- Schnitzler, A.; Amigo, J.; Hale, B.; Schnitzler, C. Patterns of climber distribution in temperate forests of the Americas. J. Plant Ecol. 2016, 9, 724–733. [Google Scholar] [CrossRef]
- Pueyo, Y.; Alados, C.L.; Maestro, M.; Komac, B. Gypsophile vegetation patterns under a range of soil properties induced by topographical position. Plant Ecol. 2007, 189, 301–311. [Google Scholar] [CrossRef]
- Dray, S.; Pélissier, R.; Couteron, P.; Fortin, M.J.; Legendre, P.; Peres-Neto, P.R.; Bellier, E.; Bivand, R.; Blanchet, F.G.; De Cáceres, M.; et al. Community ecology in the age of multivariate multiscale spatial analysis. Ecol. Monogr. 2012, 82, 257–275. [Google Scholar] [CrossRef]
- Majeed, M.; Bhatti, K.H.; Amjad, M.S. Impact of Climatic Variations on the Flowering Phenology of Plant Species in Jhelum District, Punjab, Pakistan. Appl. Ecol. Environ. Res. 2021, 19, 3343–3376. [Google Scholar] [CrossRef]
- Tassadduq, S.S.; Akhtar, S.; Waheed, M.; Bangash, N.; Nayab, D.; Majeed, M.; Abbasi, S.; Muhammad, M.; Alataway, A.; Dewidar, A.Z.; et al. Ecological Distribution Patterns of Wild Grasses and Abiotic Factors. Sustainability 2022, 14, 11117. [Google Scholar] [CrossRef]
- Waheed, M.; Arshad, F.; Iqbal, M.; Fatima, K.; Fatima, K. Ethnobotanical assessment of woody flora of district Kasur (Punjab), Pakistan. Ethnobot. Res. Appl. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Arshad, F.; Waheed, M.; Fatima, K.; Harun, N.; Iqbal, M.; Fatima, K.; Umbreen, S. Predicting the Suitable Current and Future Potential Distribution of the Native Endangered Tree Tecomella undulata (Sm.) Seem. in Pakistan. Sustainability 2022, 14, 7215. [Google Scholar] [CrossRef]
- Majeed, M.; Lu, L.; Haq, S.M.; Waheed, M.; Sahito, H.A.; Fatima, S.; Aziz, R.; Bussmann, R.W.; Tariq, A.; Ullah, I. Spatiotemporal Distribution Patterns of Climbers along an Abiotic Gradient in Jhelum District, Punjab, Pakistan. Forests 2022, 13, 1244. [Google Scholar] [CrossRef]
- Zeb, U.; Ali, S.; Li, Z.H.; Khan, H.; Shahzad, K.; Shuaib, M.; Ihsan, M. Floristic diversity and ecological characteristics of weeds at Atto Khel Mohmand Agency, KPK, Pakistan. Acta Ecol. Sin. 2017, 37, 363–367. [Google Scholar] [CrossRef]
- Majeed, M.; Tariq, A.; Haq, S.M.; Waheed, M.; Anwar, M.M.; Li, Q.; Aslam, M.; Abbasi, S.; Mousa, B.G. A Detailed Ecological Exploration of the Distribution Patterns of Wild Poaceae from the Jhelum District (Punjab), Pakistan. Sustainability 2022, 14, 3786. [Google Scholar] [CrossRef]
- Paudel, S.; Sah, J.P. Physiochemical characteristics of soil in tropical sal (Shorea robusta Gaertn.) forests in eastern Nepal. Himal. J. Sci. 1970, 1, 107–110. [Google Scholar] [CrossRef]
- Lü, X.T.; Hu, Y.Y.; Wolf, A.A.; Han, X.G. Species richness mediates within-species nutrient resorption: Implications for the biodiversity–productivity relationship. J. Ecol. 2019, 107, 2346–2352. [Google Scholar] [CrossRef]
- Saeed, S.; Irfan Ashraf, M.; Ahmad, A.; Rahman, Z. The bela forest ecosystem of district jhelum, a potential carbon sink. Pak. J. Bot. 2016, 48, 121–129. [Google Scholar]
- Soltanpour, P.N. Determination of Nutrient Availability and Elemental Toxicity by AB-DTPA Soil Test and ICPS. In Advances in Soil Science; Springer: New York, NY, USA, 1991; Volume 16, pp. 165–190. [Google Scholar] [CrossRef]
- Hepper, E.N.; Buschiazzo, D.E.; Hevia, G.G.; Urioste, A.; Antón, L. Clay mineralogy, cation exchange capacity and specific surface area of loess soils with different volcanic ash contents. Geoderma 2006, 135, 216–223. [Google Scholar] [CrossRef]
- Rasheed, S.; Khan, S.M.; Ahmad, Z.; Mustafa, G.; Haq, Z.U.; Shah, H.; Ansari, L.; Jatt, T. Ecological assessment and indicator species analyses of the Cholistan desert using multivariate statistical tools. Pak. J. Bot. 2022, 54, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Mahmood, A.; Habib, T.; Mushahid, M.; Ali, H.; Khan, N.; Ali, K. Vegetation analysis and environmental indicators of an arid tropical forest ecosystem of Pakistan. Ecol. Indic. 2022, 142, 109291. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, S.M.; Ullah, Z.; Ahmad, Z.; Alam, N.; Shah, S.N.; Khan, R.; Zada, M. Phytogeographic classification using multivariate approach; a case study from the Jambil valley swat, Pakistan. Pak. J. Bot. 2020, 52, 279–290. [Google Scholar] [CrossRef]
- Ilyas, M.; Qureshi, R.; Akhtar, N.; Ziaul-Haq, Z.; Khan, A.M. Floristic diversity and vegetation structure of the remnant subtropical broad leaved forests from Kabal Valley, Swat, Pakistan. Pak. J. Bot. 2018, 50, 217–230. [Google Scholar]
- Iqbal, M.; Khan, S.M.; Ahmad, Z.; Hussain, M.; Shah, S.N.; Kamran, S.; Manan, F.; Ul Haq, Z.; Ullah, S. Vegetation classification of the margalla foothills, islamabad under the influence of edaphic factors and anthropogenic activities using modern ecological tools. Pak. J. Bot. 2021, 53, 1831–1843. [Google Scholar] [CrossRef]
- Tolera, F.F.; Moa, M.S.; Tilahun, B.H.; Tena, R.; Nebiyu, K.K. Ethnobotanical study of ethnoveterinary plants in Kelem Wollega Zone, Oromia Region, Ethiopia. J. Med. Plants Res. 2017, 11, 307–317. [Google Scholar] [CrossRef][Green Version]
- Sang, W. Plant diversity patterns and their relationships with soil and climatic factors along an altitudinal gradient in the middle Tianshan Mountain area, Xinjiang, China. Ecol. Res. 2009, 24, 303–314. [Google Scholar] [CrossRef]
- Majeed, M.; Bhatti, K.H.; Pieroni, A.; Sõukand, R.; Bussmann, R.W.; Khan, A.M.; Chaudhari, S.K.; Aziz, M.A.; Amjad, M.S. Gathered wild food plants among diverse religious groups in Jhelum District, Punjab, Pakistan. Foods 2021, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Burnside, N.G.; Joyce, C.B.; Puurmann, E.; Scott, D.M. Use of vegetation classification and plant indicators to assess grazing abandonment in Estonian coastal wetlands. J. Veg. Sci. 2007, 18, 645–654. [Google Scholar] [CrossRef]
- Charles Goebel, P.; Palik, B.J.; Pregitzer, K.S. Structure and composition of riparian forests in an old-growth northern hardwood-hemlock watershed. For. Ecol. Manag. 2012, 280, 52–61. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J.; Hoehn, R.E.; Lapoint, E. Carbon storage and sequestration by trees in urban and community areas of the United States. Environ. Pollut. 2013, 178, 229–236. [Google Scholar] [CrossRef]
- Majeed, M.; Tariq, A.; Anwar, M.M.; Khan, A.M.; Arshad, F.; Mumtaz, F.; Farhan, M.; Zhang, L.; Zafar, A.; Aziz, M.; et al. Monitoring of land use–Land cover change and potential causal factors of climate change in Jhelum district, Punjab, Pakistan, through GIS and multi-temporal satellite data. Land 2021, 10, 1026. [Google Scholar] [CrossRef]
- Hassan, M.; Haq, S.M.; Majeed, M.; Umair, M.; Sahito, H.A.; Shirani, M.; Waheed, M.; Aziz, R.; Ahmad, R.; Bussmann, R.W.; et al. Traditional Food and Medicine: Ethno-Traditional Usage of Fish Fauna across the Valley of Kashmir: A Western Himalayan Region. Diversity 2022, 14, 455. [Google Scholar] [CrossRef]
- Wazir, S.M.; Dasti, A.A.; Saima, S.; Shah, J.; Hussain, F. Multivariate analysis of vegetation of Chapursan valley: An alpine meadow in Pakistan. Pak. J. Bot. 2008, 40, 615–626. [Google Scholar]
- Adel, M.N.; Pourbabaei, H.; Dey, D.C. Ecological species group-Environmental factors relationships in unharvested beech forests in the north of Iran. Ecol. Eng. 2014, 69, 1–7. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Erum, S.; Khan, S.M.; Nawaz, M. An appraisal of ecological distribution of herbaceous flora at Changa Manga park Lahore, Pakistan. Pak. J. Bot. 2014, 46, 19–25. [Google Scholar]
- Salama, F.; Abd El-Ghani, M.; Gadallah, M.; El-Naggar, S.; Amro, A. Characteristics of desert vegetation along four transects in the arid environment of southern Egypt. Turk. J. Bot. 2014, 40, 59–73. [Google Scholar] [CrossRef]
- Zheng, Z.; Zeng, Y.; Schneider, F.D.; Zhao, Y.; Zhao, D.; Schmid, B.; Schaepman, M.E.; Morsdorf, F. Mapping functional diversity using individual tree-based morphological and physiological traits in a subtropical forest. Remote Sens. Environ. 2021, 252, 112170. [Google Scholar] [CrossRef]
- Khan, A.M.; Li, Q.; Saqib, Z.; Khan, N.; Habib, T.; Khalid, N.; Majeed, M.; Tariq, A. MaxEnt Modelling and Impact of Climate Change on Habitat Suitability Variations of Economically Important Chilgoza Pine (Pinus gerardiana Wall.) in South Asia. Forests 2022, 13, 715. [Google Scholar] [CrossRef]
- Abella, S.R.; Covington, W.W. Forest ecosystems of an Arizona Pinus ponderosa landscape: Multifactor classification and implications for ecological restoration. J. Biogeogr. 2006, 33, 1368–1383. [Google Scholar] [CrossRef]
- Mahmood, A.; Mahmood, A.; Naveed, I.; Memon, M.M.; Bux, H.; Younas Majeed, M.; Mujtaba, G.; Saqlain Mumtaz, M. Indigenous medicinal knowledge of common plants used by local people of Hattian Bala District, Azad Jammu and Kashmir (AJK), Pakistan. J. Med. Plant Res. 2011, 5, 5517–5521. [Google Scholar]
- Divakara, B.N.; Nikitha, C.U.; Nölke, N.; Tewari, V.P.; Kleinn, C. Tree Diversity and Tree Community Composition in Northern Part of Megacity Bengaluru, India. Sustainability 2022, 14, 1295. [Google Scholar] [CrossRef]
- Shaheen, H.; Khan, S.M.; Harper, D.M.; Ullah, Z.; Allem Qureshi, R. Species Diversity, Community Structure, and Distribution Patterns in Western Himalayan Alpine Pastures of Kashmir, Pakistan. Mt. Res. Dev. 2011, 31, 153. [Google Scholar] [CrossRef]
- Furusawa, T.; Sirikolo, M.Q.; Sasaoka, M.; Ohtsuka, R. Interaction between forest biodiversity and people’s use of forest resources in Roviana, Solomon Islands: Implications for biocultural conservation under socioeconomic changes. J. Ethnobiol. Ethnomed. 2014, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, H.; Arshad, M.; Bibi, Y.; Ahmad, R.; Osunkoya, O.O.; Adkins, S.W. Multivariate impact analysis of parthenium hysterophorus invasion on above-ground plant diversity in pothwar region of Pakistan. Appl. Ecol. Environ. Res. 2018, 16, 5799–5813. [Google Scholar] [CrossRef]

| Plant Name | Species Code | Community 1 | Community 2 | Community 3 | Community 4 | Community 5 |
|---|---|---|---|---|---|---|
| Acacia farnesiana (L.) Wild | Acac far | 10.23 | 9.32 | 13.08 | 69.64 | 35.66 |
| Acacia modesta Wall | Acac mod | 9 | 0 | 58.24 | 15.99 | 13.56 |
| Acacia nilotica (L.) Delile. | Acac nil | 31.23 | 62.05 | 51.39 | 78.56 | 35.25 |
| Albizia lebbek (L.) Benth. | Albi leb | 14.67 | 0 | 3.45 | 7.79 | 2.79 |
| Albizia procera (Roxb.) Benth. | Albi pro | 15.11 | 0 | 0 | 5.3 | 3.57 |
| Alstonia scholaris (L.) R. Br. | Alst sch | 32.79 | 0 | 5.67 | 0 | 16.66 |
| Artocarpus lakucha Buch. Ham. | Arto lak | 0 | 0 | 0 | 1.45 | 0 |
| Azadirachta indica (L.) A. Juss | Azad ind | 3.79 | 0 | 0 | 0 | 0 |
| Bauhinia purpurea L. | Bauh pur | 5.07 | 0 | 2.43 | 0 | 0 |
| Bauhinia variegata L. | Bauh var | 2.51 | 0 | 0 | 0 | 0 |
| Bischofia javanica Blume | Bisc jav | 3.1 | 0 | 0 | 2.12 | 0 |
| Bombax ceiba L. | Bomb cei | 23 | 85.13 | 7.54 | 38.8 | 3.45 |
| Broussonetia papyrifera (L.) L’Herit. ex Vent. | Brou pap | 1.23 | 5.78 | 36.07 | 57.1 | 65.01 |
| Butea monosperma (Lain.) Taubert. | Bute mon | 0 | 0 | 0 | 2.31 | 0 |
| Capparis decidua (Forssk.) Edgew. | Capp dec | 0 | 0 | 0 | 4.32 | 0 |
| Cascabela thevetia (L.) Lippold | Casc the | 31.02 | 0 | 0 | 0 | 10.33 |
| Cassia fistula L. | Cass fis | 9.68 | 0 | 2.11 | 0 | 4.32 |
| Cassia surattensis Burn. f. | Cass sur | 8.65 | 0 | 0 | 0 | 0 |
| Cedrela toona Roxb.ex Wild. | Cedr too | 0 | 6.4 | 0 | 3.43 | 0 |
| Celtis australis L. | Celt aus | 9.43 | 6.5 | 0 | 27.25 | 0 |
| Chukrasia tabularis Adr. Juss. | Chuk tab | 0 | 0 | 0 | 3.234 | 0 |
| Conocarpus erectus L. | Cono ere | 58.66 | 0 | 18.89 | 0 | 63.86 |
| Cordia dichotoma G.Forst. | Cord dic | 2.46 | 0 | 0 | 2.15 | 0 |
| Cordia myxa L. | Cord myx | 8.03 | 0 | 0 | 1.65 | 0 |
| Dalbergia latifolia Roxb. | Dalb lat | 2.98 | 2.33 | 0 | 2.34 | 0 |
| Dalbergia sisso Roxb. | Dalb sis | 82.66 | 5.88 | 50.88 | 60.45 | 2.34 |
| Ehretia laevis Roxb. | Ehr lae | 14.07 | 32.79 | 0 | 40.69 | 0 |
| Ehretia obtusifolia Hochst. ex A.DC. | Ehr obt | 0 | 0 | 1.23 | 12.11 | 0 |
| Erythrina suberosa Roxb. | Eryt sub | 0 | 0 | 5.64 | 0 | 18.91 |
| Eucalyptus camaldulensis Dehnh. | Euca cam | 88.22 | 6.64 | 7.48 | 60.31 | 24.05 |
| Eucalyptus citriodora Hook. | Euca cit | 0 | 0 | 0 | 2.34 | 15.66 |
| Ficus benghalensis L. | Ficu ben | 7.79 | 15 | 0 | 0 | 0 |
| Ficus elastica Roxb. ex Hornem. | Ficu ela | 3.66 | 0 | 0 | 0 | 0 |
| Ficus infectoria Roxb. | Ficu inf | 19.68 | 0 | 0 | 0 | 0 |
| Ficus macrophylla Huegel ex Miq. | Ficu mac | 3.21 | 0 | 0 | 0 | 0 |
| Ficus palmata Forssk. | Ficu pal | 1.68 | 8.65 | 30.71 | 39.79 | 0 |
| Ficus racemosa L. | Ficu rac | 0 | 8.01 | 0 | 0 | 0 |
| Ficus religiosa L. | Ficu rel | 19.77 | 0 | 2.34 | 0 | 0 |
| Gmelina arborea Roxb. ex Sm. | Gmel arb | 5.53 | 0 | 0 | 5.43 | 0 |
| Grevillea robusta A. Cunn. ex R. Br. | Grev rob | 0 | 6.54 | 2.34 | 0 | 4.3 |
| Grewia tenax (Forssk.) Fiori | Grew ten | 0 | 0 | 11.17 | 9.07 | 0 |
| Heterophragma adenophylla (Wall. ex G. Don) Dop | Hete ade | 7.77 | 0 | 0 | 0 | 0 |
| Jacaranda ovalifolia R. Br | Jaca ova | 4.32 | 0 | 0 | 0 | 0 |
| Kigelia africana (Lam.) Benth. | Kige afr | 4.32 | 0 | 0 | 0 | 0 |
| Lagerstroemia indica L. | Lage ind | 31.05 | 0 | 0 | 0 | 3.41 |
| Leucaena leucocephala (Lam.) de Wit | Leuc leu | 0 | 8.54 | 0 | 52.13 | 0 |
| Mangifera indica L. | Mang ind | 4.69 | 0 | 7.78 | 0 | 0 |
| Moringa oleifera Lam. | Mori ole | 5.11 | 10.32 | 0 | 0 | 0 |
| Morus alba L. | Moru alb | 27.77 | 77.43 | 3.35 | 47.99 | 0 |
| Morus nigra L. | Moru nig | 71.57 | 2.3 | 3.23 | 54.96 | 0 |
| Morus serrata Roxb. | Moru ser | 6.43 | 0 | 0 | 2.11 | 0 |
| Oroxylum indicum (L.) Kurz | Orox ind | 3.21 | 0 | 3.1 | 0 | 0 |
| Parkinsonia aculeata L. | Park acu | 0 | 0 | 31.91 | 0 | 31.69 |
| Phyllanthus emblica L. | Phyl emb | 3.21 | 0 | 0 | 0 | 0 |
| Pinus roxburghii Sargent | Pinu rox | 7.55 | 0 | 0 | 0 | 0 |
| Plumeria obtusa L. | Plum obt | 3.74 | 0 | 0 | 0 | 0 |
| Plumeria rubra L. | Plum rub | 2.12 | 0 | 0 | 0 | 0 |
| Polyalthia longifolia Benth, & Hook. f. | Poly lon | 40.39 | 0 | 0 | 0 | 0 |
| Poncirus trifoliate (L.) Raf | Ponci tri | 1.32 | 0 | 0 | 0 | 0 |
| Pongamia pinnata (L.) Merrill. | Pong pin | 13.87 | 27.64 | 12.21 | 11.55 | 23.32 |
| Populus alba L. | Popu alb | 31.98 | 50.88 | 0 | 18.18 | 0 |
| Populus nigra L. | Popu nig | 29.11 | 95.28 | 22.56 | 15.19 | 0 |
| Prosopis cineraria (L.) Druce | Pros cin | 0 | 0 | 5.61 | 72.21 | 45.57 |
| Prosopis glandulosa Torr. | Pros gla | 0 | 3.23 | 17.68 | 2.54 | 2.23 |
| Prosopis juliflora (Sw.) DC. | Pros jul | 0 | 30.79 | 40.89 | 85.32 | 45.78 |
| Psidium guajava L. | Psid gua | 47.65 | 24.77 | 0 | 0 | 0 |
| Pterospermum acerifolium (L.) Willd | Pter ace | 3.96 | 0 | 0 | 0 | 0 |
| Putranjiva roxburghii (Wall.) Hurusawa | Putr rox | 2.34 | 0 | 0 | 0 | 0 |
| Ricinus communis L. | Rici com | 6.9 | 7.65 | 9.43 | 21.02 | 54.66 |
| Salix alba L. | Sali alb | 0 | 3.45 | 0 | 15.2 | 0 |
| Salix tetrasperma Roxb. | Sali tet | 0 | 3.25 | 0 | 7.54 | 0 |
| Salvadora oleoides Decne. | Salv ole | 27.92 | 0 | 0 | 0 | 7.77 |
| Sapindus mukorossi Gaertn | Sapi muk | 4.22 | 0 | 0 | 0 | 0 |
| Sapium sebiferum (L.) Roxb. | Sapi seb | 0 | 32.9 | 0 | 30.24 | 0 |
| Saraca asoca (Roxb.) De Wilde. | Sara aso | 3.23 | 0 | 0 | 0 | 0 |
| Syzygium cumini (L.) Skeels | Syzy cum | 14.76 | 0 | 3.56 | 0 | 0 |
| Tamarix aphylla (Linn.) Karst. | Tama aph | 0 | 0 | 64.68 | 35.8 | 0 |
| Tamarix dioica Roxb. ex Roth | Tama dio | 0 | 0 | 32.34 | 0 | 0 |
| Tecoma stans L. | Teco sta | 66.87 | 0 | 0 | 0 | 0 |
| Tectona grandis L. f. | Tect gra | 2.54 | 0 | 0 | 0 | 0 |
| Terminalia belerica Roxb. | Term bel | 2.34 | 0 | 0 | 0 | 0 |
| Terminalia chebula Retz. | Term che | 2.11 | 0 | 0 | 0 | 0 |
| Terminalia. arjuna (Roxb. Ex DC) Wt. & Arn. | Term arj | 39.64 | 6.36 | 34.2 | 16.29 | 0 |
| Trewia nudiflora L. | Trew nud | 1.23 | 0 | 0 | 5.62 | 0 |
| Ziziphus jujuba Mill. | Zizi juj | 20.33 | 21.45 | 92.63 | 19.4 | 2.12 |
| Ziziphus nummularia (Burm.f.) Wight & Arn. | Zizi num | 15.83 | 21.43 | 61.83 | 45.3 | 3.23 |
| Axes | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Eigen values | 0.437 | 0.24 | 0.18 | 0.167 |
| Species-environment associations | 0.907 | 0.768 | 0.821 | 0.755 |
| Accumulative percentage variance of wild grass species data | 6.3 | 9.8 | 12.4 | 14.8 |
| Accumulative percentage variance of species-environment relation | 26.1 | 40.4 | 51.2 | 61.2 |
| Total inertia | 6.912 | |||
| Sum of all eigen values | 6.912 | |||
| Sum of all canonical eigenvalues | 1.675 | |||
| Monte-Carlo test | ||||
| Test of significance of first canonical axis: eigenvalue | 0.437 | |||
| F-ratio | 3.240 | |||
| p-value | 0.0020 | |||
| Test of significance of total canonical axes; Trace | 1.675 | |||
| F-ratio | 1.396 | |||
| p-value | 0.0020 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waheed, M.; Arshad, F.; Majeed, M.; Fatima, S.; Mukhtar, N.; Aziz, R.; Mangrio, W.M.; Almohamad, H.; Al Dughairi, A.A.; Al-Mutiry, M.; et al. Community Structure and Distribution Pattern of Woody Vegetation in Response to Soil properties in Semi-Arid Lowland District Kasur Punjab, Pakistan. Land 2022, 11, 2145. https://doi.org/10.3390/land11122145
Waheed M, Arshad F, Majeed M, Fatima S, Mukhtar N, Aziz R, Mangrio WM, Almohamad H, Al Dughairi AA, Al-Mutiry M, et al. Community Structure and Distribution Pattern of Woody Vegetation in Response to Soil properties in Semi-Arid Lowland District Kasur Punjab, Pakistan. Land. 2022; 11(12):2145. https://doi.org/10.3390/land11122145
Chicago/Turabian StyleWaheed, Muhammad, Fahim Arshad, Muhammad Majeed, Sammer Fatima, Naila Mukhtar, Robina Aziz, Wali Muhammad Mangrio, Hussein Almohamad, Ahmed Abdullah Al Dughairi, Motrih Al-Mutiry, and et al. 2022. "Community Structure and Distribution Pattern of Woody Vegetation in Response to Soil properties in Semi-Arid Lowland District Kasur Punjab, Pakistan" Land 11, no. 12: 2145. https://doi.org/10.3390/land11122145
APA StyleWaheed, M., Arshad, F., Majeed, M., Fatima, S., Mukhtar, N., Aziz, R., Mangrio, W. M., Almohamad, H., Al Dughairi, A. A., Al-Mutiry, M., & Abdo, H. G. (2022). Community Structure and Distribution Pattern of Woody Vegetation in Response to Soil properties in Semi-Arid Lowland District Kasur Punjab, Pakistan. Land, 11(12), 2145. https://doi.org/10.3390/land11122145







