Spatial Pattern and Ecological Process Difference Analyses of the Boundary Habitats of a Treeline Patch: A Case Study from the Li Mountain, North China
Abstract
1. Introduction
2. Materials and Methods
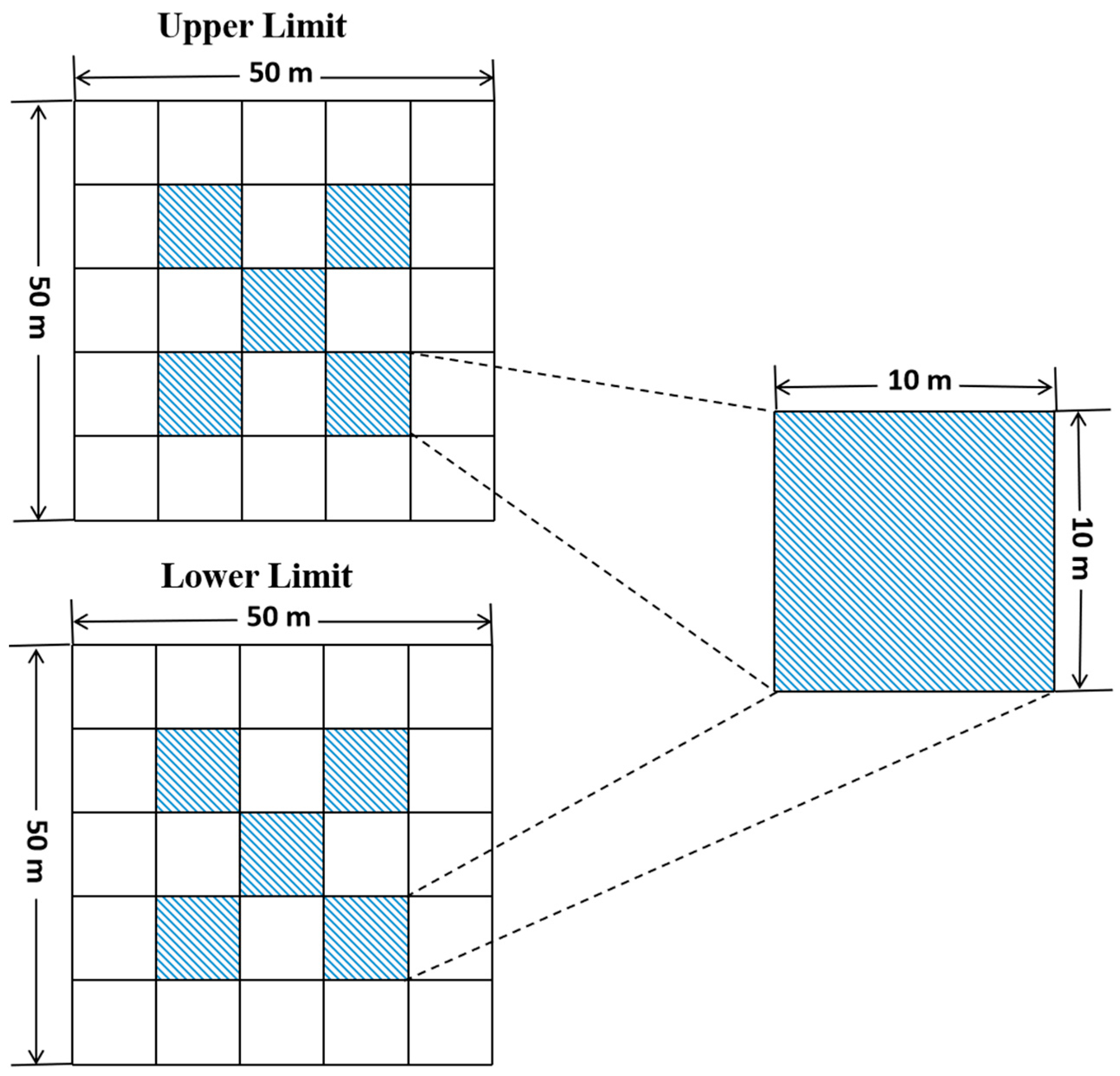
2.1. Study Area and Field Sampling
2.2. Data Analysis
2.2.1. Environmental Factors Analysis
2.2.2. Importance Value Calculation
2.2.3. Community Similarity Analysis
2.2.4. DBH Distribution Analysis
2.2.5. Point Pattern Analysis
2.2.6. Model Test
2.3. Software Analysis and Figure Processing
3. Results
3.1. Environmental Conditions within Sampling Plots
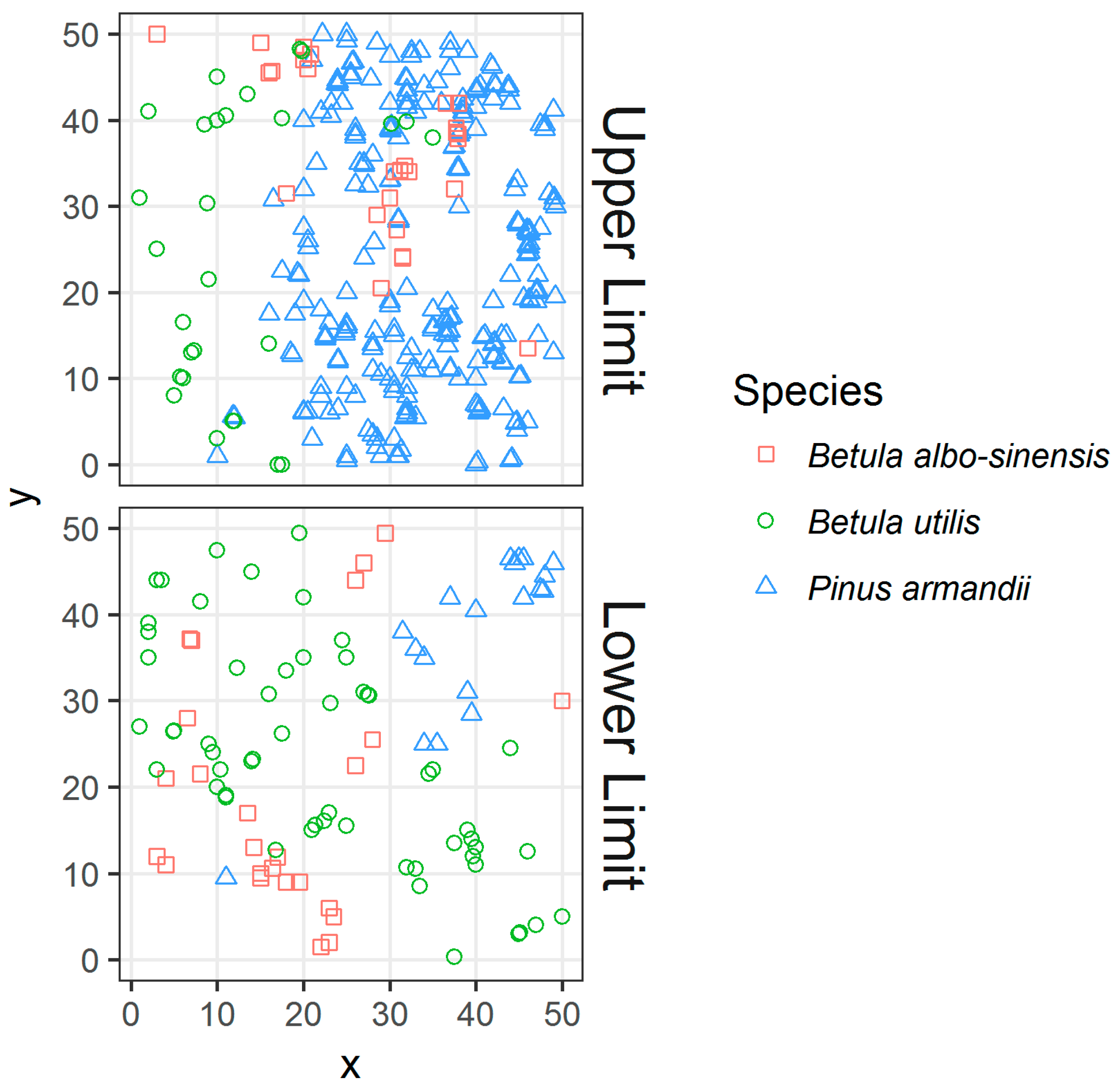
3.2. Community Structure and Dynamic
3.2.1. Composition and Similarity Differences in Tree Layer and Shrub Layer
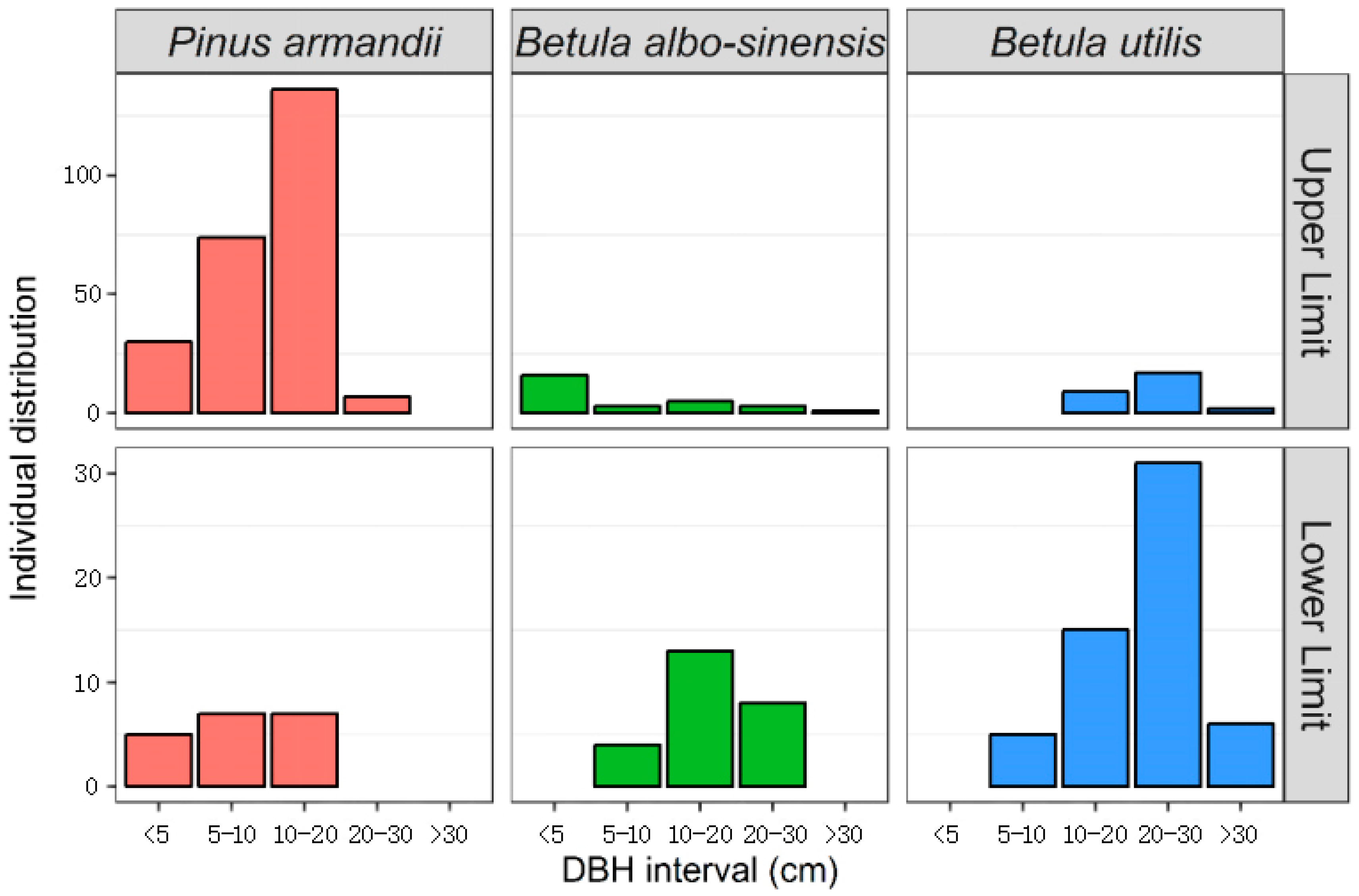
3.2.2. DBH Numerical Distribution
3.3. Null Model Analysis on Patterns, Processes of the Three Species
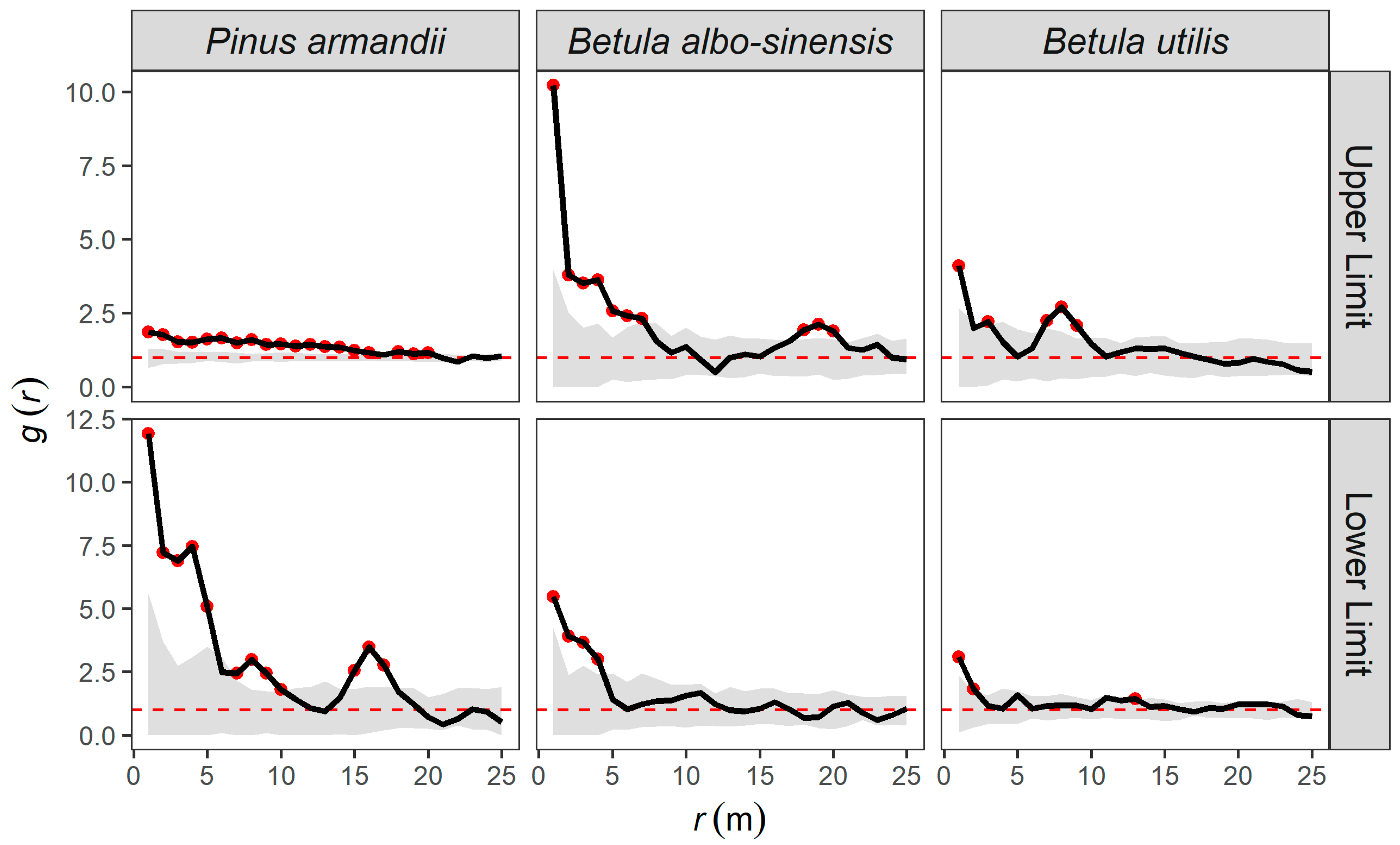
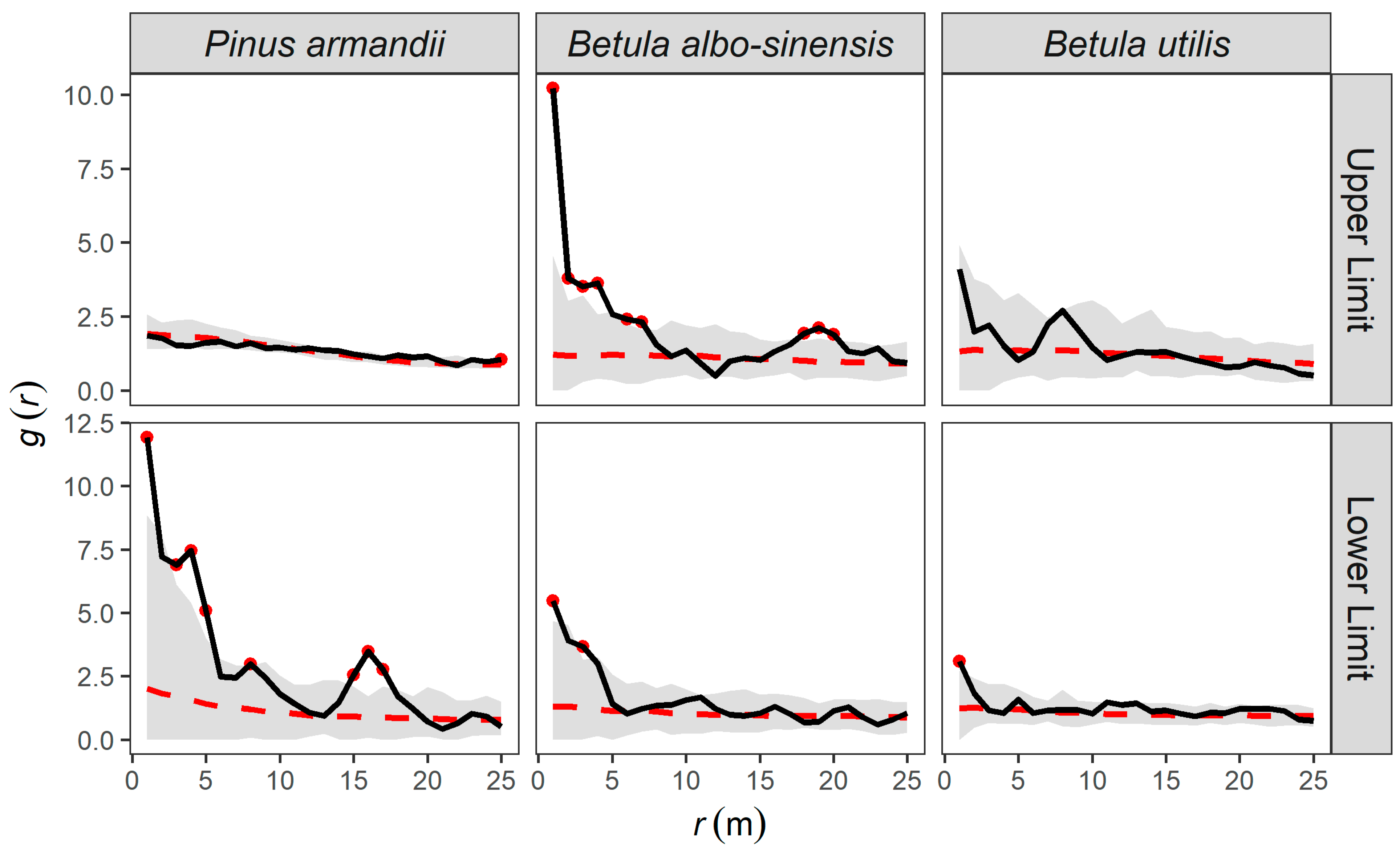
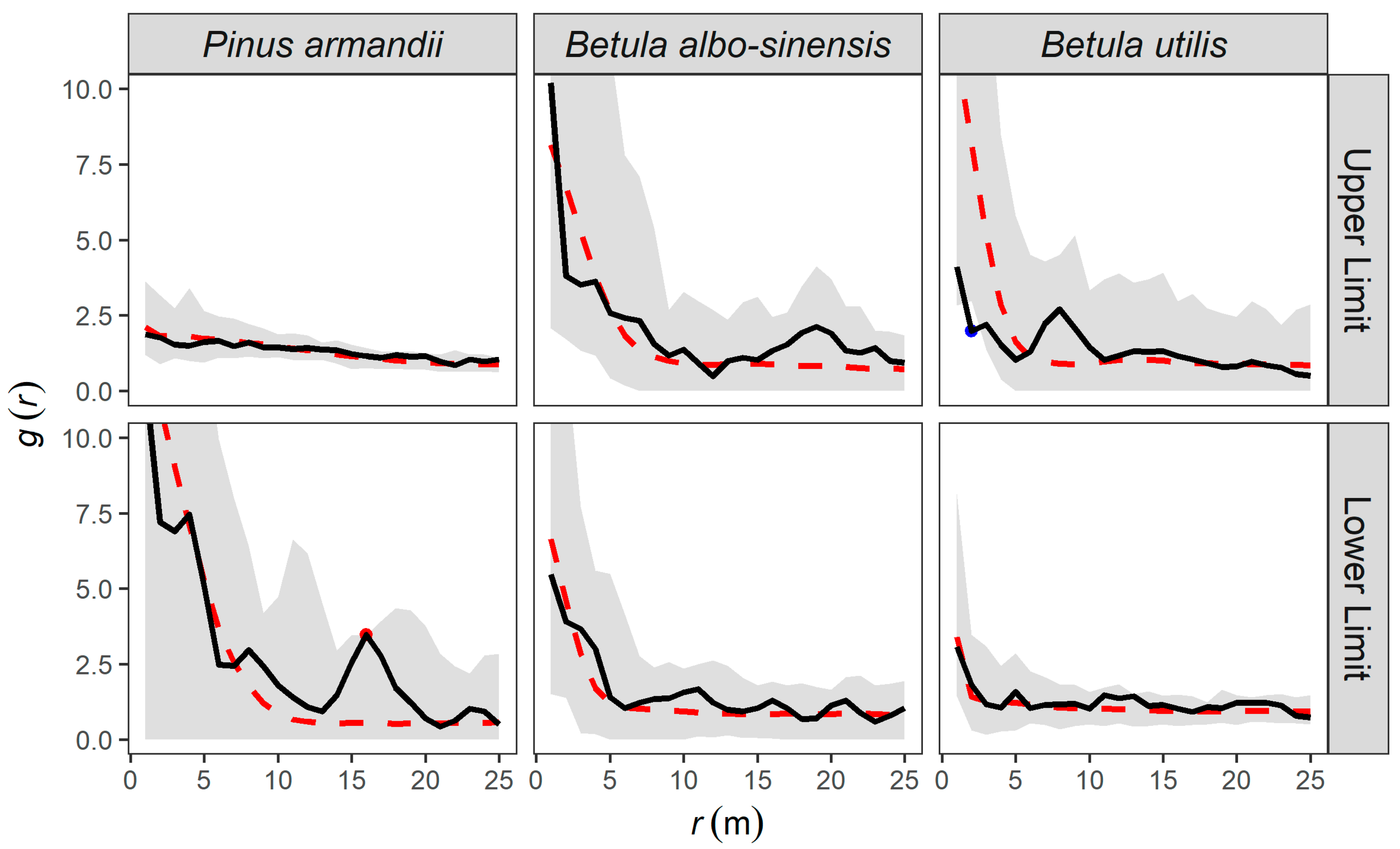
3.3.1. Completely Spatially Random Null Model Analysis
3.3.2. Heterogeneous Poisson (HP) Null Model Analysis
3.3.3. Homogeneous Thomas (HT) Null Model Analysis
3.3.4. Inhomogeneous Thomas (IT) Null Model Analysis
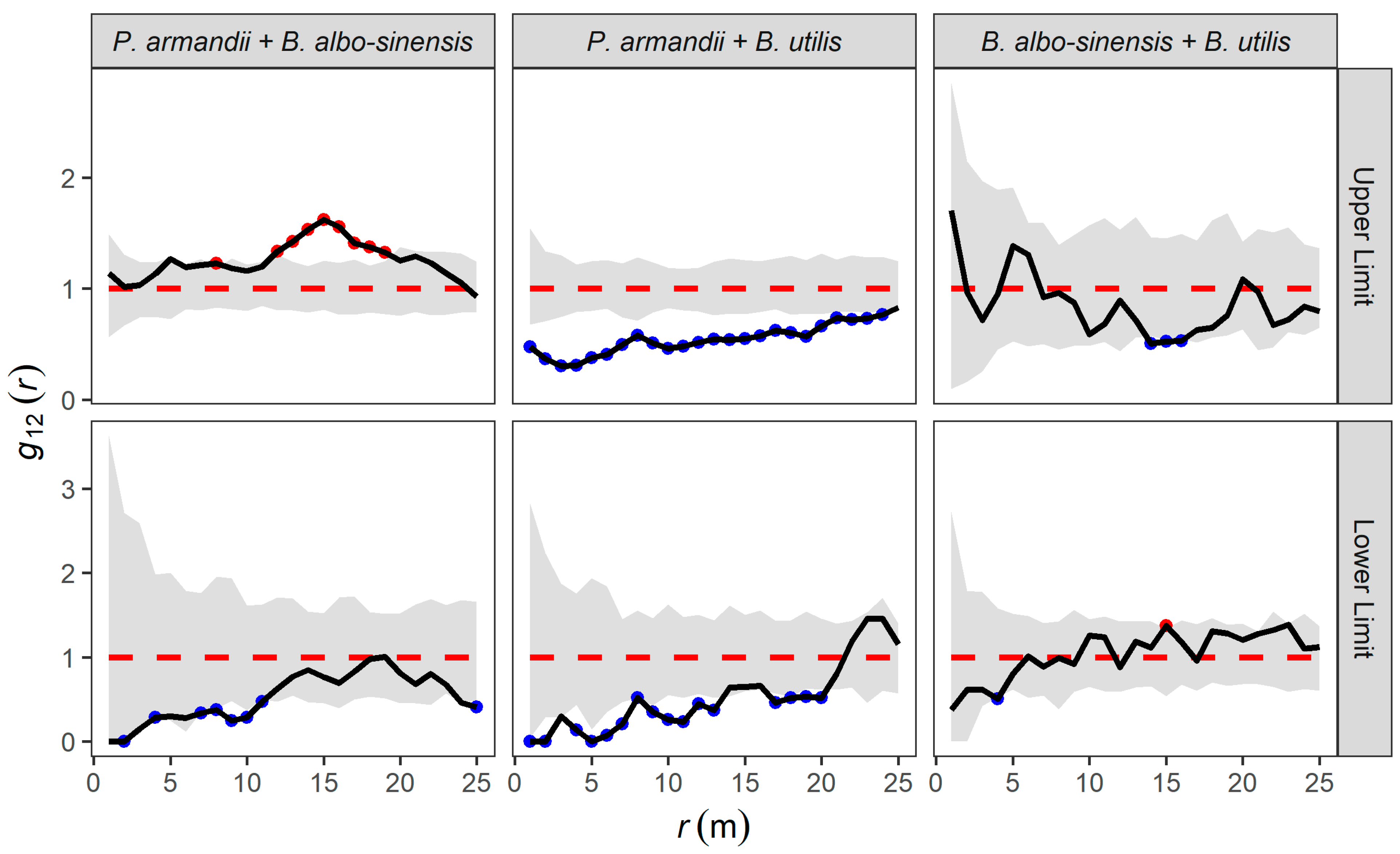
3.4. Homogeneous Poisson Null Model Analysis Inter-Specific Correlation
4. Discussion
4.1. The Differences of Community Structure and Species Spatial Pattern at the Upper and Lower Limits of Treeline Patch
4.2. Effect of Community Density Heterogeneity on Seedling Pattern
4.3. Seed Dispersal Effect and Size Effect on Species Patterns
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalhais, N.; Forkel, M.; Khomik, M.; Bellarby, J.; Jung, M.; Migliavacca, M.; Mu, M.; Saatchi, S.; Santoro, M.; Thurner, M.; et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 2014, 514, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Kullman, L. Tree line population monitoring of Pinus sylvestris in the Swedish Scandes, 1973–2005: Implications for tree line theory and climate change ecology. J. Ecol. 2007, 95, 41–52. [Google Scholar] [CrossRef]
- Tiwari, A.; Jha, P.K. An overview of treeline response to environmental changes in Nepal Himalaya. Trop. Ecol. 2018, 59, 273–285. [Google Scholar]
- Chhetri, P.K.; Bista, R.; Shrestha, K.B. How does the stand structure of treeline-forming species shape the treeline ecotone in different regions of the Nepal Himalayas? J. Mt. Sci. 2020, 17, 2354–2368. [Google Scholar] [CrossRef]
- Shi, C.; Masson-Delmotte, V.; Daux, V.; Li, Z.S.; Carré, M.; Moore, J.C. Unprecedented recent warming rate and temperature variability over the east Tibetan Plateau inferred from Alpine treeline dendrochronology. Clim. Dyn. 2015, 45, 1367–1380. [Google Scholar] [CrossRef]
- Bader, M.Y.; van Geloof, I.; Rietkerk, M. High solar radiation hinders tree regeneration above the alpine treeline in northern Ecuador. Plant Ecol. 2007, 191, 33–45. [Google Scholar] [CrossRef]
- Kaspar, J.; Treml, V. Thermal characteristics of alpine treelines in Central Europe north of the Alps. Clim. Res. 2016, 68, 1–12. [Google Scholar] [CrossRef]
- Gruber, A.; Baumgartner, D.; Zimmermann, J.; Oberhuber, W. Temporal dynamic of wood formation in Pinus cembra along the alpine treeline ecotone and the effect of climate variables. Trees-Struct. Funct. 2009, 23, 623–635. [Google Scholar] [CrossRef]
- Renard, S.M.; McIntire, E.J.B.; Fajardo, A.; Woods, K. Winter conditions—Not summer temperature—Influence establishment of seedlings at white spruce alpine treeline in Eastern Quebec. J. Veg. Sci. 2016, 27, 29–39. [Google Scholar] [CrossRef]
- Greenwood, S.; Chen, J.-C.; Chen, C.-T.; Jump, A.S. Strong topographic sheltering effects lead to spatially complex treeline advance and increased forest density in a subtropical mountain region. Glob. Chang. Biol. 2014, 20, 3756–3766. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, H.-Y.; Hou, G.-L.; Zhao, J.-J.; Liu, D.-Y.; Guo, X.-Y. Topographic controls on alpine treeline patterns on Changbai Mountain, China. J. Mt. Sci. 2014, 11, 429–441. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Germino, M.J.; Smith, W.K. Photosynthesis during an episodic drought in Abies lasiocarpa and Picea engelmannii across an alpine treeline. Arct. Antarct. Alp. Res. 2006, 38, 34–41. [Google Scholar] [CrossRef]
- Stueve, K.M.; Cerney, D.L.; Rochefort, R.M.; Kurth, L.L. Post-fire tree establishment patterns at the alpine treeline ecotone: Mount Rainier National Park, Washington, USA. J. Veg. Sci. 2009, 20, 107–120. [Google Scholar] [CrossRef]
- Adhikari, B.S.; Kumar, R.; Singh, S.P. Early snowmelt impact on herb species composition, diversity and phenology in a western Himalayan treeline ecotone. Trop. Ecol. 2018, 59, 365–382. [Google Scholar]
- Pereg, D.; Payette, S. Development of black spruce growth forms at treeline. Plant Ecol. 1998, 138, 137–147. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Jacoby, G.; Buckley, B.; Sakulich, J.; Frank, D.; Wilson, R.; Curtis, A.; Anchukaitis, K. Tree growth and inferred temperature variability at the North American Arctic treeline. Glob. Planet. Chang. 2009, 65, 71–82. [Google Scholar] [CrossRef]
- Dawes, M.A.; Haettenschwiler, S.; Bebi, P.; Hagedorn, F.; Handa, I.T.; Köerner, C.; Rixen, C. Species-specific tree growth responses to 9 years of CO2 enrichment at the alpine treeline. J. Ecol. 2011, 99, 383–394. [Google Scholar] [CrossRef]
- Barbeito, I.; Bruecker, R.L.; Rixen, C.; Bebi, P. Snow Fungi-Induced Mortality of Pinus cembra at the Alpine Treeline: Evidence from Plantations. Arct. Antarct. Alp. Res. 2013, 45, 455–470. [Google Scholar] [CrossRef]
- Hiemstra, C.A.; Liston, G.E.; Reiners, W.A. Snow redistribution by wind and interactions with vegetation at upper treeline in the Medicine Bow Mountains, Wyoming, USA. Arct. Antarct. Alp. Res. 2002, 34, 262–273. [Google Scholar] [CrossRef]
- Hattenschwiler, S.; Smith, W.K. Seedling occurrence in alpine treeline conifers: A case study from the central Rocky Mountains, USA. Acta Oecolog.-Int. J. Ecol. 1999, 20, 219–224. [Google Scholar] [CrossRef]
- Hughes, N.M.; Johnson, D.M.; Akhalkatsi, M.; Abdaladze, O. Characterizing Betula litwinowii Seeding Microsites at the Alpine-Treeline Ecotone, Central Greater Caucasus Mountains, Georgia. Arct. Antarct. Alp. Res. 2009, 41, 112–118. [Google Scholar] [CrossRef][Green Version]
- Germino, M.J.; Smith, W.K.; Resor, A.C. Conifer seedling distribution and survival in an alpine-treeline ecotone. Plant Ecol. 2002, 162, 157–168. [Google Scholar] [CrossRef]
- Holtmeier, F.K.; Broll, G. Sensitivity and response of northern hemisphere altitudinal and polar treelines to environmental change at landscape and local scales. Glob. Ecol. Biogeogr. 2005, 14, 395–410. [Google Scholar] [CrossRef]
- Elliottt, G.P.; Kipfmueller, K.E. Multi-scale Influences of Slope Aspect and Spatial Pattern on Ecotonal Dynamics at Upper Treeline in the Southern Rocky Mountains, USA. Arct. Antarct. Alp. Res. 2010, 42, 45–56. [Google Scholar] [CrossRef]
- Liptzin, D.; Sanford, R.L., Jr.; Seastedt, T.R. Spatial patterns of total and available N and P at alpine treeline. Plant Soil 2013, 365, 127–140. [Google Scholar] [CrossRef]
- Thebault, A.; Clement, J.-C.; Ibanez, S.; Roy, J.; Geremia, R.A.; Perez, C.A.; Buttler, A.; Estienne, Y.; Lavorel, S. Nitrogen limitation and microbial diversity at the treeline. Oikos 2014, 123, 729–740. [Google Scholar] [CrossRef]
- Jameson, R.G.; Trant, A.J.; Hermanutz, L. Insects can limit seed productivity at the treeline. Can. J. For. Res. 2015, 45, 286–296. [Google Scholar] [CrossRef]
- Anschlag, K.; Broll, G.; Holtmeier, F.-K. Mountain Birch Seedlings in the Treeline Ecotone, Subarctic Finland: Variation in Above- and Below-Ground Growth Depending on Microtopography. Arct. Antarct. Alp. Res. 2008, 40, 609–616. [Google Scholar] [CrossRef]
- Brooker, R.W.; Scott, D.; Palmer, S.C.F.; Swaine, E. Transient facilitative effects of heather on Scots pine along a grazing disturbance gradient in Scottish moorland. J. Ecol. 2010, 94, 637–645. [Google Scholar] [CrossRef]
- Brooker, R.W.; Callaghan, T.V. The balance between positive and negative plant interactions and its relationship to environmental gradients: A model. Oikos 1998, 81, 196–207. [Google Scholar] [CrossRef]
- le Roux, P.C.; McGeoch, M.A. Spatial variation in plant interactions across a severity gradient in the sub-Antarctic. Oecologia 2008, 155, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Miao, N.; Liu, S.R.; Shi, Z.M.; Xue, Z.B.; Yu, H.; He, F. 青藏高原东缘林线杜鹃-岷江冷杉原始林的空间格局 [Spatial pattern analysis of a Rhododendron-Abies virginal forest near timberline on the eastern edge of Qinghai-Tibetan Plateau, China]. Acta Ecol. Sin. 2011, 31, 1–9. [Google Scholar]
- Zhang, Q.Y. 青藏高原东缘高山林线乔木种群生态特征研究 [Ecological Characteristics of Timberline Tree Populations in Eastern Qinghai—Tibetan Plateau]; Graduate School, Chinese Academy of Sciences: Chengdu, China, 2006. [Google Scholar]
- Wang, H.T. 山西历山自然生态风景区的开发利用 [Development and Utilization of Li Mountain Natural Ecological Scenic Area in Shanxi Province]. Shanxi Agric. Econ. 2017, 5, 69–71. [Google Scholar] [CrossRef]
- Ru, W.; Zhang, J.T.; Zhang, F.; Zhang, G.; Liu, R. 历山森林群落物种多样性与群落结构研究 [Species diversity and community structure of forest communities in Lishan Mountain]. Chin. J. Appl. Ecol. 2006, 17, 561–566. [Google Scholar] [CrossRef]
- Ru, W.M.; Zhang, F. 山西中条山东部种子植物区系分析 [Analysis on the flora of eastern Zhongtiao Mountains, Shanxi]. J. Shanxi Univ. 2000, 23, 82–87. [Google Scholar] [CrossRef]
- Wang, X.G.; Wiegand, T.; Hao, Z.Q.; Li, B.H.; Ye, J.; Lin, F. Species associations in an old-growth temperate forest in north-eastern China. J. Ecol. 2010, 98, 674–686. [Google Scholar] [CrossRef]
- Nguyen, H.; Lamb, D.; Herbohn, J.; Firn, J. Designing Mixed Species Tree Plantations for the Tropics: Balancing Ecological Attributes of Species with Landholder Preferences in the Philippines. PLoS ONE 2014, 9, E95267. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. A Handbook of Spatial Point Pattern Analysis in Ecology; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Liang, Y.; Yang, X.X.; Zhang, X.Y.; Zhang, J.T.; Zhang, D.Y.; Liao, W.J. Effects of plant interactions on the populations of the endangered Fagus pashanica. Plant Ecol. Divers. 2022, 15, 25–37. [Google Scholar] [CrossRef]
- Dixon, P.M. Ripley’s K function. Encycl. Environ. 2002, 3, 1796–1803. [Google Scholar]
- Stoyan, D.; Stoyan, H. Fractals, Random Shapes, and Point Fields: Methods of Geometrical Statistics; John Wiley and Sons: Chichester, UK, 1994; Volume 302. [Google Scholar]
- Jia, M.Y.; Zhang, J.T.; Song, Z.H.; Liang, Y. Size-gradient differences in the spatial patterns and processes of Pinus armandii in forest lines of Li Mountains, North Central China. Acta Ecol. Sin. 2022, 42, 162–170. [Google Scholar] [CrossRef]
- Waagepetersen, R.P. An estimating function approach to inference for inhomogeneous Neyman-Scott processes. Biometrics 2007, 63, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, T.; Gunatilleke, S.; Gunatilleke, N.; Okuda, T. Analyzing the spatial structure of a Sri Lankan tree species with multiple scales of clustering. Ecology 2007, 88, 3088–3102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Wiegand, T.; Wolf, A.; Howe, R.; Davies, S.J.; Hao, Z. Spatial patterns of tree species richness in two temperate forests. J. Ecol. 2011, 99, 1382–1393. [Google Scholar] [CrossRef]
- Loosmore, N.B.; Ford, E.D. Statistical inference using the G or K point pattern spatial statistics. Ecology 2006, 87, 1925–1931. [Google Scholar] [CrossRef]
- Zhang, J.-T.; Xiang, C.L.; Li, M. Effects of Tourism and Topography on Vegetation Diversity in the Subalpine Meadows of the Dongling Mountains of Beijing, China. Environ. Manag. 2012, 49, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Sun, Q.; Chen, Z.; Pang, L.; Long, F. 道路边坡坡度对植被恢复中物种多样性的影响研究 [Effects of Roadside Slope Gradient on Plant Diversty during Revegetation]. Bulletion Bot. Res. 2013, 33, 447–483. [Google Scholar]
- Dirr, M.A. Manual of Woody Landscape Plants: Their Identification, Ornamental Characteristics, Culture, Propagation and Uses, 3rd ed.; Stipes Publishing Co.: Champaign, IL, USA, 1983. [Google Scholar]
- Wang, X.Y.; Yu, D.P.; Zhou, L.; Zhou, W.M.; Wu, Z.J.; Guo, Y.; Bao, Y.; Meng, Y.Y.; Dai, L.M. 长白山北坡林线岳桦种群空间分布格局 [Spatial patterns of a treeline Betula ermanii Cham. population on the north slope of Changbai Mountain]. Acta Ecol. Sin. 2015, 35, 116–124. [Google Scholar] [CrossRef][Green Version]
- Guo, Y.-X.; Li, G.; Hu, Y.-N.; Kang, D.; Wang, D.X.; Yang, G.H. Regeneration of Betula albosinensis in Strip Clearcut and Uncut Forests of the Qinling Mountains in China. PLoS ONE 2013, 8, e59375. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.-Y.; Zhao, X.-H. 内蒙古黑里河天然油松林主要树种的空间分布格局 [Spatial Distribution Pattern of Main Tree Species in Pinus tabulae formis Forest of Heilihe in Inner-Mongolia]. Acta Bot. Boreal-Occident. Sin. 2009, 29, 167–173. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. Global controls of forest line elevation in the northern and southern hemispheres. Glob. Ecol. Biogeogr. 2000, 9, 253–268. [Google Scholar] [CrossRef]
- Bharti, R.R.; Adhikari, B.S.; Rawat, G.S. Assessing vegetation changes in timberline ecotone of Nanda Devi National Park, Uttarakhand. Int. J. Appl. Earth Obs. Geoinf. 2012, 18, 472–479. [Google Scholar] [CrossRef]
- Frei, E.R.; Bianchi, E.; Bernareggi, G.; Bebi, P.; Dawes, M.A.; Brown, C.D.; Trant, A.J.; Mamet, S.D.; Rixen, C. Biotic and abiotic drivers of tree seedling recruitment across an alpine treeline ecotone. Sci. Rep. 2018, 8, 10894. [Google Scholar] [CrossRef]
- Lett, S.; Dorrepaal, E. Global drivers of tree seedling establishment at alpine treelines in a changing climate. Funct. Ecol. 2018, 32, 1666–1680. [Google Scholar] [CrossRef]
- Kambo, D.; Danby, R.K. Constraints on treeline advance in a warming climate: A test of the reproduction limitation hypothesis. J. Plant Ecol. 2018, 11, 411–422. [Google Scholar] [CrossRef]
- Bai, X.J.; Queenborough, S.A.; Wang, X.G.; Zhang, J.; Li, B.H.; Yuan, Z.Q.; Xing, D.L.; Lin, F.; Ye, J.; Hao, Z.Q. Effects of local biotic neighbors and habitat heterogeneity on tree and shrub seedling survival in an old-growth temperate forest. Oecologia 2012, 170, 755–765. [Google Scholar] [CrossRef] [PubMed]
- HilleRisLambers, J.; Clark, J.S.; Beckage, B. Density-dependent mortality and the latitudinal gradient in species diversity. Nature 2002, 417, 732–735. [Google Scholar] [CrossRef]
- Malanson, G.P.; Resler, L.M. A size-gradient hypothesis for alpine treeline ecotones. J. Mt. Sci. 2016, 13, 1154–1161. [Google Scholar] [CrossRef]
- Šrůtek, M.; Doležal, J.; Hara, T. Spatial structure and associations in a Pinus canariensis population at the treeline, Pico del Teide, Tenerife, Canary Islands. Arct. Antarct. Alp. Res. 2002, 34, 201–210. [Google Scholar] [CrossRef]
- Wang, W.; Franklin, S.B.; Ren, Y.; Ouellette, J.R. Growth of bamboo Fargesia qinlingensis and regeneration of trees in a mixed hardwood-conifer forest in the Qinling Mountains, China. For. Ecol. Manag. 2006, 234, 107–115. [Google Scholar] [CrossRef]


| Environmental Factors | Upper Limit | Lower Limit | Significance |
|---|---|---|---|
| Altitude (m) | 2300 | 2250 | None |
| Slope aspect | North by east 45° | True north | None |
| Number of dead trees Slope * (°) | 14 28.6 A ± 2.27 | 7 6.6 B ± 1.03 | None p < 0.05 |
| Soil layer thickness (cm) | 27.8 ± 9.09 | 19.0 ± 4.54 | p > 0.05 |
| Litter layer thickness (cm) | 4.0 ± 3.39 | 1.4 ± 0.77 | p > 0.05 |
| pH of surface soil layer | 5.9 ± 0.10 | 5.9 ± 0.15 | p > 0.05 |
| Temperature of surface soil layer (°C) | 12.3 ± 0.97 | 13.6 ± 0.89 | p > 0.05 |
| Tree coverage (%) | 51.0 ± 11.40 | 43.8.0 ± 10.50 | p > 0.05 |
| Life Form | Species | Upper Limit | Lower Limit |
|---|---|---|---|
| Tree layer | Pinus armandii | 0.361 | 0.012 |
| p = 0.049 * | Betula albo-sinensis | 0.331 | 0.095 |
| Betula utilis | 0.237 | 0.804 | |
| Padus racemosa | 0.034 | 0.034 | |
| Cerasus clarofolia | 0.017 | 0.056 | |
| Salix wallichiana | 0.020 | 0.000 | |
| Shrub layer | Betula albo-sinensis | 0.477 | 0.137 |
| p = 0.31 | Cotoneaster acutifolius | 0.073 | 0.038 |
| Lonicera szechuanica | 0.043 | 0.064 | |
| Lonicera chrysantha | 0.037 | 0.079 | |
| Pertya sinensis | 0.036 | 0.148 | |
| Salix wallichiana | 0.032 | 0.050 | |
| Spiraea trilobata | 0.100 | 0.000 | |
| Rubus flosculosus | 0.063 | 0.000 | |
| Ribes vilmorinii | 0.051 | 0.000 | |
| Sorbus koehneana | 0.000 | 0.077 | |
| Viburnum mongolicum | 0.000 | 0.062 | |
| Eleutherococcus henryi | 0.000 | 0.048 | |
| Abelia biflora | 0.000 | 0.032 | |
| Cerasus clarofolia | 0.000 | 0.200 |
| Test Item | CSR Null Model | IP Null Model | HT Null Model | IT Null Model |
|---|---|---|---|---|
| Upper Limit—Pinus armandii | 0.005 | 0.115 | 0.005 ** | 0.875 |
| Upper Limit—Betula albo-sinensis | 0.005 | 0.005 ** | 0.400 | 0.435 |
| Upper Limit—Betula utilis | 0.005 | 0.015 * | 0.350 | 0.090 |
| Lower Limit—Pinus armandii | 0.005 | 0.005 * | 0.675 | 0.510 |
| Lower Limit—Betula albo-sinensis | 0.005 | 0.010 * | 0.920 | 0.805 |
| Lower Limit—Betula utilis | 0.005 | 0.020 * | 0.335 | 0.710 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Zhang, J.; Song, Z.; Sadia, S. Spatial Pattern and Ecological Process Difference Analyses of the Boundary Habitats of a Treeline Patch: A Case Study from the Li Mountain, North China. Land 2022, 11, 2064. https://doi.org/10.3390/land11112064
Jia M, Zhang J, Song Z, Sadia S. Spatial Pattern and Ecological Process Difference Analyses of the Boundary Habitats of a Treeline Patch: A Case Study from the Li Mountain, North China. Land. 2022; 11(11):2064. https://doi.org/10.3390/land11112064
Chicago/Turabian StyleJia, Meiyu, Jintun Zhang, Zhenhui Song, and Sehrish Sadia. 2022. "Spatial Pattern and Ecological Process Difference Analyses of the Boundary Habitats of a Treeline Patch: A Case Study from the Li Mountain, North China" Land 11, no. 11: 2064. https://doi.org/10.3390/land11112064
APA StyleJia, M., Zhang, J., Song, Z., & Sadia, S. (2022). Spatial Pattern and Ecological Process Difference Analyses of the Boundary Habitats of a Treeline Patch: A Case Study from the Li Mountain, North China. Land, 11(11), 2064. https://doi.org/10.3390/land11112064






