Assessing Climate Change Exposure for the Adaptation of Conservation Management: The Importance of Scale in Mountain Landscapes
Abstract
:1. Introduction
2. Materials and Methods
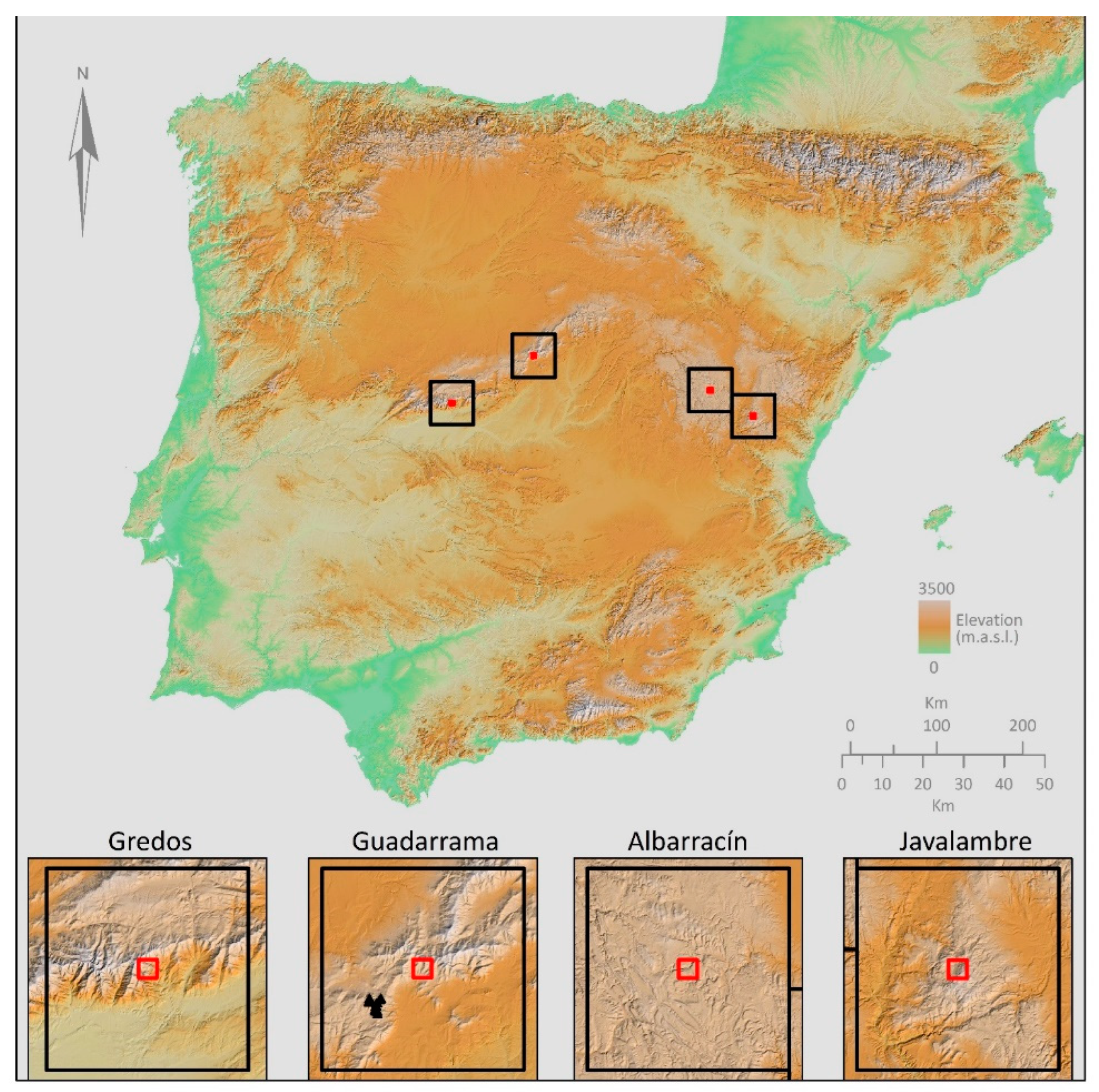
2.1. Study Area
2.2. Mesoclimate and Microclimate Data
2.3. Field Validation of Mesoclimate and Microclimate
2.4. Exposure to Analogous and Non-Analogous Conditions
2.5. Statistical Analysis
3. Results
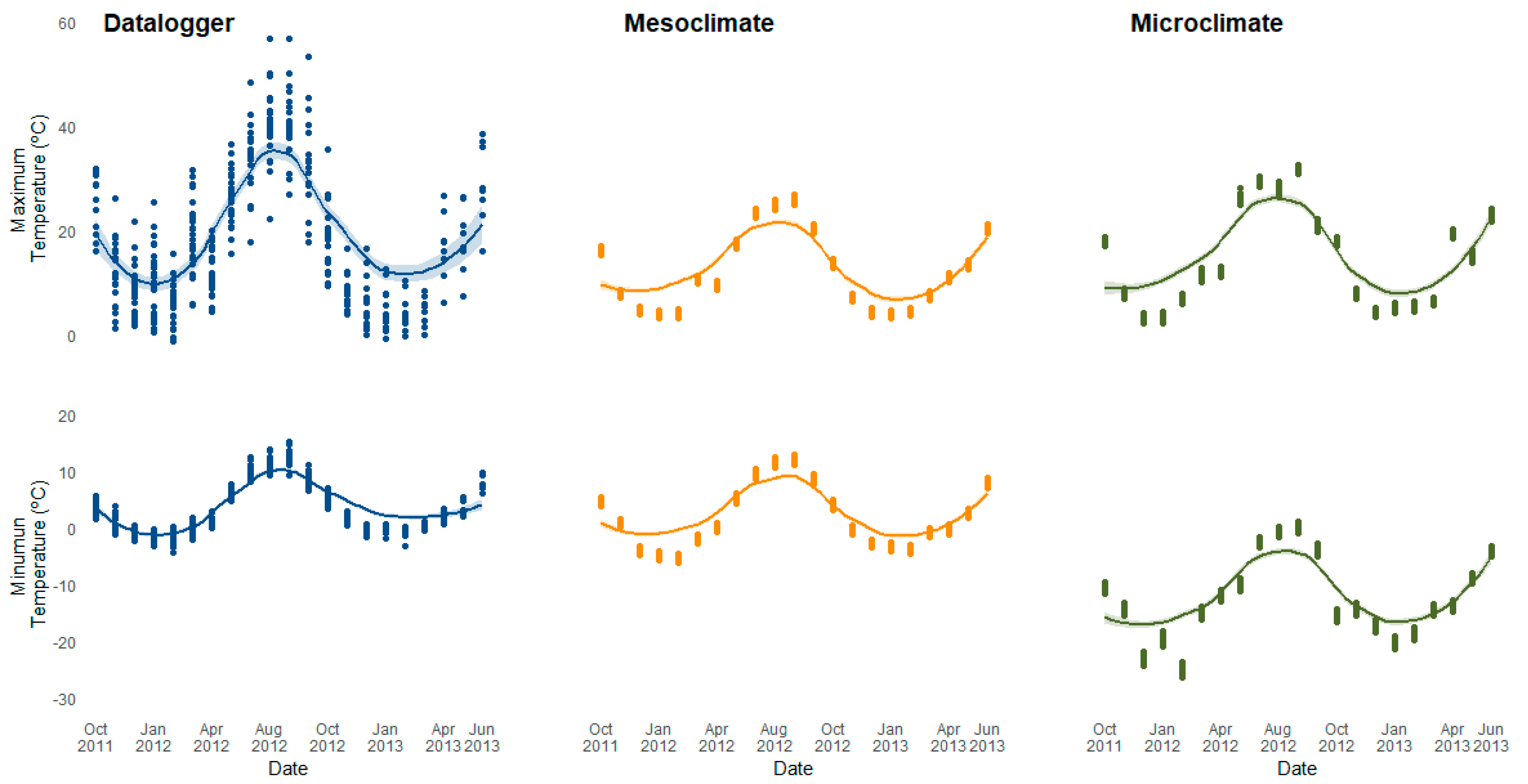
3.1. Field Validation of Mesoclimate and Microclimate Data
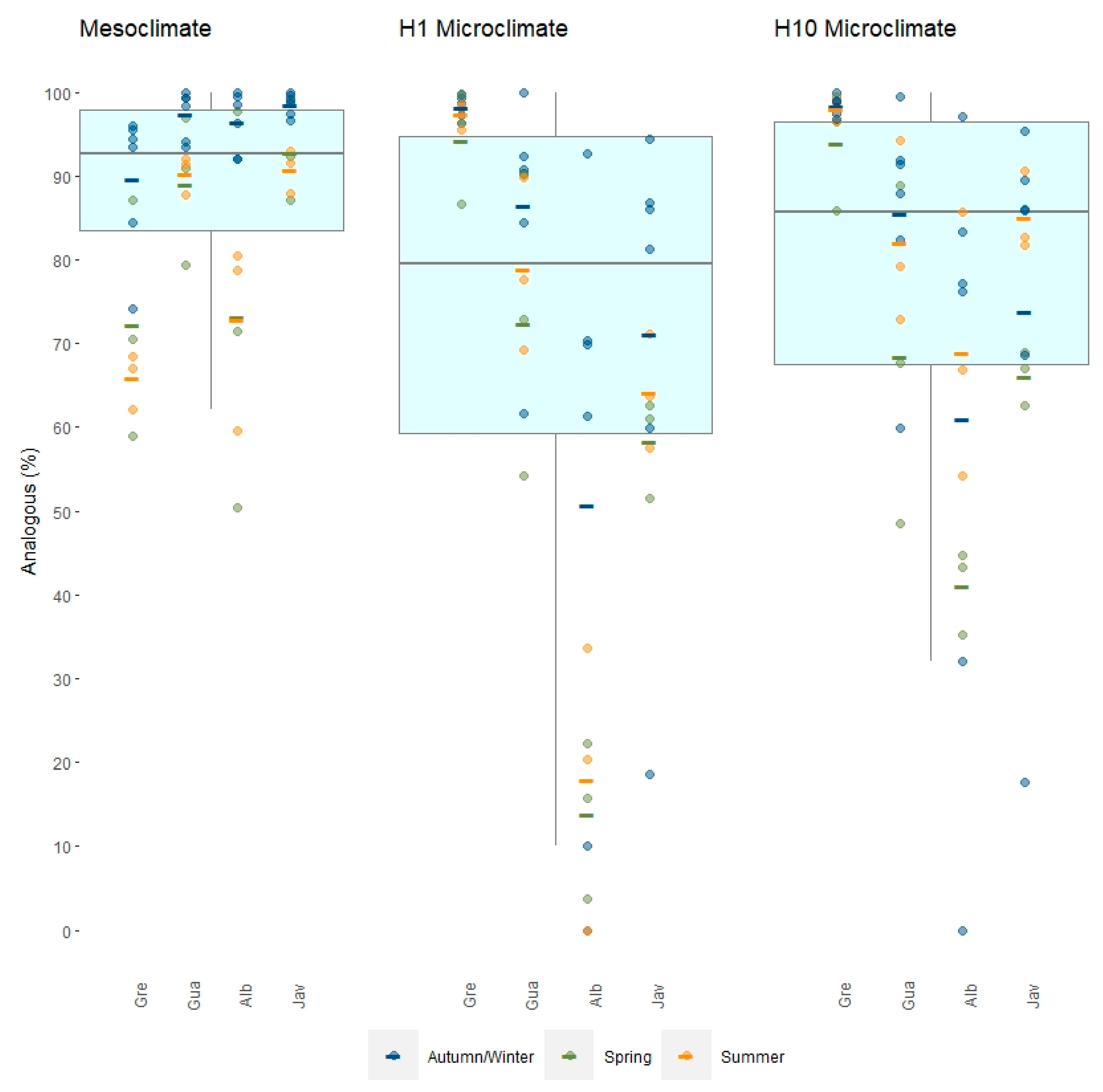
3.2. Exposure to Non-Analogous Temperatures
4. Discussion
4.1. Field Validation of Microclimate Model
4.2. Exposure to Non-Analogous Temperatures over Time
4.3. Implications for Adapting Conservation to Climate Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Morecroft, M.; Trisurat, Y.; Adrian, R.; Anshari, G.Z.; Arneth, A.; Gao, Q.; Gonzalez, P.; Harris, R.; Price, J.; et al. Terrestrial and Freshwater Ecosystems and Their Services; Cambridge University Press: Cambridge, UK, 2022; ISBN 9781009325844. [Google Scholar] [CrossRef]
- Ohlemüller, R.; Gritti, E.S.; Sykes, M.T.; Thomas, C.D. Towards European Climate Risk Surfaces: The Extent and Distribution of Analogous and Non-Analogous Climates 1931-2100. Glob. Ecol. Biogeogr. 2006, 15, 395–405. [Google Scholar] [CrossRef]
- Ohlemüller, R.; Anderson, B.J.; Araújo, M.B.; Butchart, S.H.M.; Kudrna, O.; Ridgely, R.S.; Thomas, C.D. The Coincidence of Climatic and Species Rarity: High Risk to Small-Range Species from Climate Change. Biol. Lett. 2008, 4, 568–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampe, A.; Petit, R.J. Conserving Biodiversity under Climate Change: The Rear Edge Matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The Velocity of Climate Change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Morelli, T.L.; Daly, C.; Dobrowski, S.Z.; Dulen, D.M.; Ebersole, J.L.; Jackson, S.T.; Lundquist, J.D.; Millar, C.I.; Maher, S.P.; Monahan, W.B.; et al. Managing Climate Change Refugia for Climate Adaptation. PLoS ONE 2016, 11, e0159909. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for Distribution Models in Biodiversity Assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [Green Version]
- Lenoir, J.; Bertrand, R.; Comte, L.; Bourgeaud, L.; Hattab, T.; Murienne, J.; Grenouillet, G. Species Better Track Climate Warming in the Oceans than on Land. Nat. Ecol. Evol. 2020, 4, 1044–1059. [Google Scholar] [CrossRef]
- Taheri, S.; Naimi, B.; Rahbek, C.; Araújo, M.B. Improvements in Reports of Species Redistribution under Climate Change Are Required. Sci. Adv. 2021, 7, eabe1110. [Google Scholar] [CrossRef]
- Potter, K.A.; Arthur Woods, H.; Pincebourde, S. Microclimatic Challenges in Global Change Biology. Glob. Chang. Biol. 2013, 19, 2932–2939. [Google Scholar] [CrossRef]
- Gonzalez-Hidalgo, J.C.; Peña-Angulo, D.; Brunetti, M.; Cortesi, N. MOTEDAS: A New Monthly Temperature Database for Mainland Spain and the Trend in Temperature (1951–2010). Int. J. Climatol. 2015, 35, 4444–4463. [Google Scholar] [CrossRef]
- Maclean, I.M.D.; Suggitt, A.J.; Wilson, R.J.; Duffy, J.P.; Bennie, J.J. Fine-Scale Climate Change: Modelling Spatial Variation in Biologically Meaningful Rates of Warming. Glob. Chang. Biol. 2017, 23, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Notivoli, R.; Beguería, S.; De Luis, M. STEAD: A High-Resolution Daily Gridded Temperature Dataset for Spain. Earth Syst. Sci. Data Discuss. 2019, 11, 1171–1188. [Google Scholar] [CrossRef] [Green Version]
- Oldfather, M.F.; Kling, M.M.; Sheth, S.N.; Emery, N.C.; Ackerly, D.D. Range Edges in Heterogeneous Landscapes: Integrating Geographic Scale and Climate Complexity into Range Dynamics. Glob. Chang. Biol. 2020, 26, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Brischke, C.; Bayerbach, R.; Otto Rapp, A. Decay-Influencing Factors: A Basis for Service Life Prediction of Wood and Wood-Based Products. Wood Mater. Sci. Eng. 2006, 1, 91–107. [Google Scholar] [CrossRef]
- Spahić, M. Microclimate and (or) Topoclimate-Representation of Climate Factors in Defining the Climate Elements. Acta Geogr. Bosniae Herzeg. 2018, 9, 17–26. [Google Scholar]
- Suggitt, A.J.; Gillingham, P.K.; Hill, J.K.; Huntley, B.; Kunin, W.E.; Roy, D.B.; Thomas, C.D. Habitat Microclimates Drive Fine-Scale Variation in Extreme Temperatures. Oikos 2011, 120, 1–8. [Google Scholar] [CrossRef]
- Bramer, I.; Anderson, B.J.; Bennie, J.; Bladon, A.J.; De Frenne, P.; Hemming, D.; Hill, R.A.; Kearney, M.R.; Körner, C.; Korstjens, A.H.; et al. Advances in Monitoring and Modelling Climate at Ecologically Relevant Scales. In Advances in Ecological Research; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 58, pp. 101–161. [Google Scholar] [CrossRef]
- Bütikofer, L.; Anderson, K.; Bebber, D.P.; Bennie, J.J.; Early, R.I.; Maclean, I.M.D. The Problem of Scale in Predicting Biological Responses to Climate. Glob. Chang. Biol. 2020, 26, 6657–6666. [Google Scholar] [CrossRef]
- Araújo, M.B.; Guilhaumon, F.; Neto, D.R.; Pozo, I.; Calmaestra, R.G. Impactos, Vulnerabilidad y Adaptación Al Cambio Climático de La Biodiversidad Española. Fauna de Vertebrados; Dirección general de medio Natural y Política Forestal, Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2011.
- Felicísimo, Á.M.; Muñoz, J.; Villalba, C.J.; Mateo, R.G. Impactos, Vulnerabilidad y Adaptación Al Cambio Climático de La Biodiversidad Española. 1. Flora y Vegetación.; Oficina Española de Cambio Climático, Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2011.
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Jähnig, S.; Sander, M.M.; Caprio, E.; Rosselli, D.; Rolando, A.; Chamberlain, D. Microclimate Affects the Distribution of Grassland Birds, but Not Forest Birds, in an Alpine Environment. J. Ornithol. 2020, 161, 677–689. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Nijs, I.; Lenoir, J. Incorporating Microclimate into Species Distribution Models. Ecography 2019, 42, 1267–1279. [Google Scholar] [CrossRef]
- Mingarro, M.; Cancela, J.P.; BurÓn-Ugarte, A.; García-Barros, E.; Munguira, M.L.; Romo, H.; Wilson, R.J. Butterfly Communities Track Climatic Variation over Space but Not Time in the Iberian Peninsula. Insect Conserv. Divers. 2021, 14, 647–660. [Google Scholar] [CrossRef]
- Shahgedanova, M.; Adler, C.; Gebrekirstos, A.; Grau, H.R.; Huggel, C.; Marchant, R.; Pepin, N.; Vanacker, V.; Viviroli, D.; Vuille, M. Mountain Observatories: Status and Prospects for Enhancing and Connecting a Global Community. Mt. Res. Dev. 2021, 41, A1–A15. [Google Scholar] [CrossRef]
- Maclean, I.M.D.; Mosedale, J.R.; Bennie, J.J. Microclima: An r Package for Modelling Meso- and Microclimate. Methods Ecol. Evol. 2019, 10, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Kearney, M.R.; Gillingham, P.K.; Bramer, I.; Duffy, J.P.; Maclean, I.M.D. A Method for Computing Hourly, Historical, Terrain-Corrected Microclimate Anywhere on Earth. Methods Ecol. Evol. 2020, 11, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.J.; Dickson, C.R.; Bergstrom, D.M.; Whinam, J.; Maclean, I.M.D.; McGeoch, M.A. Evaluating Models for Predicting Microclimates across Sparsely Vegetated and Topographically Diverse Ecosystems. Divers. Distrib. 2021, 27, 2093–2103. [Google Scholar] [CrossRef]
- Wilson, R.J.; Bennie, J.; Lawson, C.R.; Pearson, D.; Ortúzar-Ugarte, G.; Gutiérrez, D. Population Turnover, Habitat Use and Microclimate at the Contracting Range Margin of a Butterfly. J. Insect Conserv. 2015, 19, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Billman, P.D.; Beever, E.A.; McWethy, D.B.; Thurman, L.L.; Wilson, K.C. Factors Influencing Distributional Shifts and Abundance at the Range Core of a Climate-Sensitive Mammal. Glob. Chang. Biol. 2021, 27, 4498–4515. [Google Scholar] [CrossRef]
- Illán, J.G.; Gutiérrez, D.; Wilson, R.J. The Contributions of Topoclimate and Land Cover to Species Distributions and Abundance: Fine-Resolution Tests for a Mountain Butterfly Fauna. Glob. Ecol. Biogeogr. 2010, 19, 159–173. [Google Scholar] [CrossRef]
- Gutiérrez, D. Butterfly Richness Patterns and Gradients. In Butterflies in Europe; Settele, J., Shreeve, T., Konvicka, M., van Dyck, H., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 250–280. [Google Scholar]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate Warming and the Decline of Amphibians and Reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Wilson, R.J.; Gutiérrez, D.; Gutiérrez, J.; Martínez, D.; Agudo, R.; Monserrat, V.J. Changes to the Elevational Limits and Extent of Species Ranges Associated with Climate Change. Ecol. Lett. 2005, 8, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Elsen, P.R.; Tingley, M.W. Global Mountain Topography and the Fate of Montane Species under Climate Change. Nat. Clim. Chang. 2015, 5, 772–776. [Google Scholar] [CrossRef]
- Kearney, M.R.; Porter, W.P. NicheMapR—An R Package for Biophysical Modelling: The Microclimate Model. Ecography 2017, 40, 664–674. [Google Scholar] [CrossRef]
- Ashton, S.; GutiÉrrez, D.; Wilson, R.J. Effects of Temperature and Elevation on Habitat Use by a Rare Mountain Butterfly: Implications for Species Responses to Climate Change. Ecol. Entomol. 2009, 34, 437–446. [Google Scholar] [CrossRef]
- Stewart, J.E.; Illán, J.G.; Richards, S.A.; Gutiérrez, D.; Wilson, R.J. Linking Inter-Annual Variation in Environment, Phenology, and Abundance for a Montane Butterfly Community. Ecology 2020, 101, e02906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colom, P.; Ninyerola, M.; Pons, X.; Traveset, A.; Stefanescu, C. Phenological Sensitivity and Seasonal Variability Explain Climate-Driven Trends in Mediterranean Butterflies. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220251. [Google Scholar] [CrossRef]
- Gómez–Déniz, E.; Gallardo, D.I.; Gómez, H.W. Quasi-Binomial Zero-Inflated Regression Model Suitable for Variables with Bounded Support. J. Appl. Stat. 2020, 47, 2208–2229. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2003. [Google Scholar]
- Maclean, I.M.D.; Duffy, J.P.; Haesen, S.; Govaert, S.; De Frenne, P.; Vanneste, T.; Lenoir, J.; Lembrechts, J.J.; Rhodes, M.W.; Van Meerbeek, K. On the Measurement of Microclimate. Methods Ecol. Evol. 2021, 12, 1397–1410. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Wilson, R.J. Intra- and Interspecific Variation in the Responses of Insect Phenology to Climate. J. Anim. Ecol. 2021, 90, 248–259. [Google Scholar] [CrossRef]
- Antão, L.H.; Weigel, B.; Strona, G.; Hällfors, M.; Kaarlejärvi, E.; Dallas, T.; Opedal, Ø.H.; Heliölä, J.; Henttonen, H.; Huitu, O.; et al. Climate Change Reshuffles Northern Species within Their Niches. Nat. Clim. Chang. 2022, 12, 587–592. [Google Scholar] [CrossRef]
- Bennie, J.; Wilson, R.J.; Maclean, I.M.D.; Suggitt, A. Seeing the Woods for the Trees—When Is Microclimate Important in Species Distribution Models? Glob. Chang. Biol. 2014, 20, 2699–2700. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, O.; Mossman, H.L.; Suggitt, A.J.; Curtis, R.J.; Maclean, I.M.D. Using in Situ Management to Conserve Biodiversity under Climate Change. J. Appl. Ecol. 2016, 53, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Barrows, C.W.; Ramirez, A.R.; Sweet, L.C.; Morelli, T.L.; Millar, C.I.; Frakes, N.; Rodgers, J.; Mahalovich, M.F. Validating Climate-Change Refugia: Empirical Bottom-up Approaches to Support Management Actions. Front. Ecol. Environ. 2020, 18, 298–306. [Google Scholar] [CrossRef]



| Model | Residual Deviance | Residual DF | Deviance | DF | Factor | Coefficient (±S.E.) |
|---|---|---|---|---|---|---|
| H1—Pine forest | ||||||
| Null | 979,888 | 95 | ||||
| Region | 501,952 | 92 | 477,935 | 3 | Intercept | 4.07 *** (±0.53) |
| Albarracín | −4.32 *** (±0.55) | |||||
| Guadarrama | −2.05 *** (±0.56) | |||||
| Javalambre | −2.87 *** (±0.54) | |||||
| Scale | 457,320 | 91 | 44,633 | 1 | Mesoclimate | −2.16 * (±0.84) |
| Season | 383,453 | 89 | 73,867 | 2 | Spring | −1.12 *** (±0.27) |
| Summer | −0.86 ** (±0.27) | |||||
| Region:Scale | 326,851 | 86 | 56,602 | 3 | Albarracín | 4.70 *** (±1.15) |
| Guadarrama | 3.41 * (±1.41) | |||||
| Javalambre | 4.55 ** (±1.53) | |||||
| H10—Short grassland | ||||||
| Null | 626,672 | 95 | ||||
| Region | 416,599 | 92 | 210,073 | 3 | Intercept | 3.74 *** (±0.54) |
| Albarracín | −3.26 *** (±0.55) | |||||
| Guadarrama | −2.14 *** (±0.57) | |||||
| Javalambre | −2.48 *** (±0.56) | |||||
| Scale | 401,853 | 91 | 14,746 | 1 | Mesoclimate | −2.21 * (±0.85) |
| Season | 358,896 | 89 | 42,957 | 2 | Spring | −0.77 ** (±0.25) |
| Summer | 0.15 n.s. (±0.28) | |||||
| Region:Scale | 317,410 | 86 | 41,486 | 3 | Albarracín | 3.64 ** (±1.15) |
| Guadarrama | 3.49 * (±1.41) | |||||
| Javalambre | 4.14 ** (±1.54) |
| Model | Residual Deviance | Residual DF | Deviance | DF | Factor | Coefficient (±S.E.) |
|---|---|---|---|---|---|---|
| Null | 2,158,894 | 143 | ||||
| Region | 1,135,085 | 140 | 1,023,809 | 3 | Intercept | 3.56 *** (±0.44) |
| Guadarrama | −2.12 *** (±0.45) | |||||
| Albarracín | −3.67 *** (±0.44) | |||||
| Javalambre | −2.65 *** (±0.44) | |||||
| Habitat | 1,091,680 | 138 | 43,405 | 2 | Short grassland | 0.52 * (±0.22) |
| Open scrubland | 0.41 † (±0.21) | |||||
| Season | 966,869 | 136 | 124,812 | 2 | Spring | −0.90 *** (±0.21) |
| Summer | −0.21 n.s. (±0.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Vadillo, M.; Mingarro, M.; Ursul, G.; Wilson, R.J. Assessing Climate Change Exposure for the Adaptation of Conservation Management: The Importance of Scale in Mountain Landscapes. Land 2022, 11, 2052. https://doi.org/10.3390/land11112052
Gómez-Vadillo M, Mingarro M, Ursul G, Wilson RJ. Assessing Climate Change Exposure for the Adaptation of Conservation Management: The Importance of Scale in Mountain Landscapes. Land. 2022; 11(11):2052. https://doi.org/10.3390/land11112052
Chicago/Turabian StyleGómez-Vadillo, Mónica, Mario Mingarro, Guim Ursul, and Robert J. Wilson. 2022. "Assessing Climate Change Exposure for the Adaptation of Conservation Management: The Importance of Scale in Mountain Landscapes" Land 11, no. 11: 2052. https://doi.org/10.3390/land11112052
APA StyleGómez-Vadillo, M., Mingarro, M., Ursul, G., & Wilson, R. J. (2022). Assessing Climate Change Exposure for the Adaptation of Conservation Management: The Importance of Scale in Mountain Landscapes. Land, 11(11), 2052. https://doi.org/10.3390/land11112052






