Assemblage Characteristics of Butterflies and Carabid Beetles as a Function of Soil Characteristics and Plant Diversity in Differently Managed Fields, Forests and Ecotones: A Case Study in Tuczno Forest District, Poland
Abstract
1. Introduction
- (1)
- The study site types differ in soil characteristics and plants, with ecotones characterized by a higher diversity of environmental characteristics than individual ecosystems, resulting in increased numbers of species, some of them solely found in these areas.
- (2)
- Carabid beetle and butterfly species assemblages differ between the study site types.
- (3)
- Carabid beetles and butterflies show differences in response to the studied factors of soil characteristics and plant diversity.
2. Materials and Methods
2.1. Study Sites
2.2. Field Methods
2.2.1. Soil Samples and Analyses
2.2.2. Inventory of Plants
2.2.3. Inventory of Carabids and Butterflies
2.3. Data Analysis
2.3.1. Study Site Characterization
2.3.2. Response of Carabids and Butterflies
3. Results
3.1. Study Site Characterization
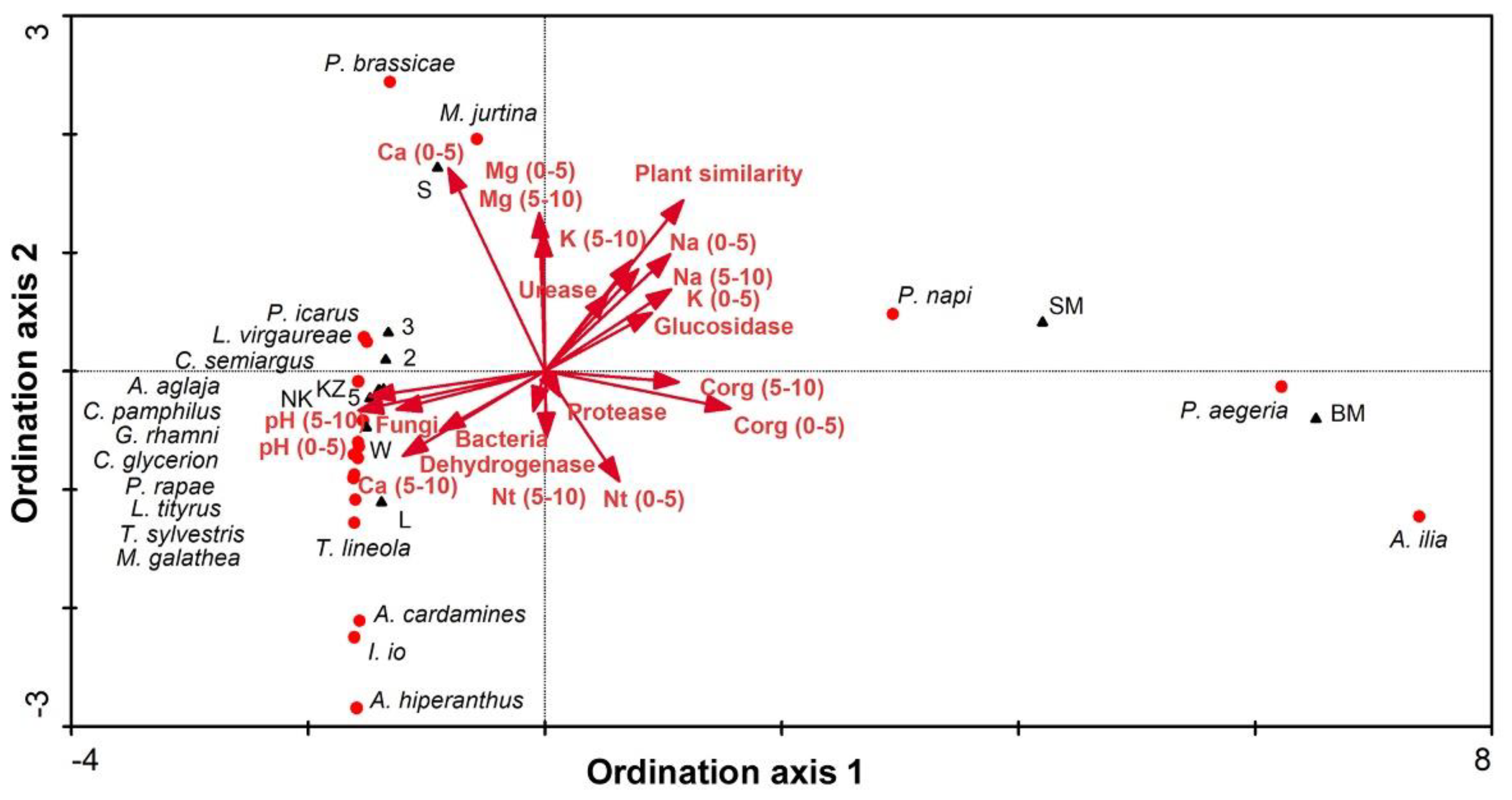
3.2. Carabids and Butterflies
4. Discussion
4.1. Limitation of the Study Design
4.2. Study Site Characterization
4.3. Carabids and Butterflies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristic | Study Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | L | W | NK | KZ | S | BM | SM | |
| Type | Fallow | Fallow | Fallow | Ecotone | Ecotone | Meadow | Meadow | Forest | Forest | Forest |
| Corg (0–5 cm) % | 1.890 ± 0.178 | 2.183 ± 0.427 | 2.249 ± 0.473 | 1.618 ± 0.844 | 2.384 ± 0.697 | 2.204 ± 0.238 | 2.431 ± 0.467 | 1.458 ± 0.261 | 3.772 ± 1.515 | 4.023 ± 1.790 |
| Corg (5–10 cm) % | 1.248 ± 0.124 | 1.231 ± 0.155 | 1.234 ± 0.311 | 1.046 ± 0.236 | 1.536 ± 0.553 | 1.613 ± 0.454 | 2.288 ± 0.546 | 1.048 ± 0.091 | 2.006 ± 0.273 | 2.877 ± 2.580 |
| Nt (0–5 cm) % | 0.126 ± 0.011 | 0.152 ± 0.025 | 0.168 ± 0.051 | 0.119 ± 0.056 | 0.182 ± 0.053 | 0.180 ± 0.029 | 0.195 ± 0.033 | 0.102 ± 0.020 | 0.181 ± 0.069 | 0.189 ± 0.079 |
| Nt (5–10 cm) % | 0.091 ± 0.010 | 0.093 ± 0.013 | 0.079 ± 0.021 | 0.080 ± 0.017 | 0.124 ± 0.047 | 0.140 ± 0.037 | 0.192 ± 0.054 | 0.066 ± 0.004 | 0.095 ± 0.012 | 0.136 ± 0.122 |
| Ca (0–5 cm) mg∙100 g−1 | 43.804 ± 4.595 | 53.519 ± 13.777 | 69.658 ± 21.384 | 32.544 ± 18.074 | 54.508 ± 28.990 | 7.382 ± 4.624 | 22.167 ± 9.881 | 134.876 ± 40.245 | 4.283 ± 2.298 | 4.752 ± 2.000 |
| Ca (5–10 cm) mg∙100 g−1 | 27.734 ± 6.597 | 25.868 ± 7.870 | 31.298 ± 11.955 | 21.803 ± 8.323 | 33.900 ± 16.500 | 4.078 ± 3.120 | 23.921 ± 14.851 | 9.447 ± 3.781 | 2.473 ± 0.471 | 3.199 ± 1.445 |
| Mg (0–5 cm) mg∙100 g−1 | 0.070 ± 0.018 | 0.084 ± 0.045 | 0.276 ± 0.237 | 0.088 ± 0.099 | 0.130 ± 0.084 | 12.751 ± 7.955 | 8.333 ± 4.537 | 12.851 ± 6.642 | 3.347 ± 1.542 | 4.110 ± 2.241 |
| Mg (5–10 cm) mg∙100 g−1 | 0.022 ± 0.007 | 0.014 ± 0.006 | 0.034 ± 0.022 | 0.023 ± 0.014 | 0.033 ± 0.012 | 4.532 ± 2.431 | 11.995 ± 7.132 | 9.389 ± 6.525 | 3.016 ± 1.491 | 2.657 ± 1.407 |
| K (0–5 cm) mg∙100 g−1 | 0.027 ± 0.007 | 0.039 ± 0.017 | 0.056 ± 0.021 | 0.020 ± 0.011 | 0.029 ± 0.010 | 1.866 ± 0.982 | 0.979 ± 0.889 | 1.329 ± 0.489 | 1.912 ± 0.619 | 1.328 ± 0.697 |
| K (5–10 cm) mg∙100 g−1 | 0.014 ± 0.003 | 0.010 ± 0.003 | 0.023 ± 0.015 | 0.010 ± 0.004 | 0.013 ± 0.002 | 0.811 ± 0.521 | 0.748 ± 0.542 | 0.769 ± 0.339 | 0.748 ± 0.235 | 0.519 ± 0.496 |
| Na (0–5 cm) mg∙100 g−1 | 0.013 ± 0.002 | 0.016 ± 0.005 | 0.020 ± 0.007 | 0.009 ± 0.004 | 0.012 ± 0.002 | 0.249 ± 0.001 | 0.271 ± 0.051 | 0.291 ± 0.064 | 0.333 ± 0.064 | 0.270 ± 0.051 |
| Na (5–10 cm) mg∙100 g−1 | 0.006 ± 0.002 | 0.006 ± 0.003 | 0.011 ± 0.005 | 0.006 ± 0.002 | 0.007 ± 0.001 | 0.270 ± 0.050 | 0.333 ± 0.064 | 0.249 ± 0.001 | 0.249 ± 0.001 | 0.270 ± 0.051 |
| pH (0–5 cm) | 4.655 ± 0.132 | 4.582 ± 0.226 | 4.673 ± 0.405 | 4.313 ± 0.072 | 4.453 ± 0.548 | 4.000 ± 0.110 | 4.480 ± 0.351 | 4.058 ± 0.122 | 3.317 ± 0.059 | 3.157 ± 0.076 |
| pH (5–10 cm) | 4.650 ± 0.156 | 4.613 ± 0.518 | 4.292 ± 0.237 | 4.235 ± 0.144 | 4.232 ± 0.307 | 4.030 ± 0.065 | 4.445 ± 0.353 | 4.060 ± 0.204 | 3.557 ± 0.067 | 3.557 ± 0.067 |
| Dehydrogenase (0–20 cm) µg TFP 24 h 10 g−1 | 0.153 ± 0.052 | 0.174 ± 0.052 | 0.197 ± 0.065 | 0.119 ± 0.029 | 0.123 ± 0.030 | 0.322 ± 0.133 | 0.315 ± 0.108 | 0.122 ± 0.049 | 0.182 ± 0.086 | 0.187 ± 0.068 |
| Protease (0–20 cm) mg tyrosine kg−1 h−1 | 0.263 ± 0.041 | 0.272 ± 0.075 | 0.348 ± 0.153 | 0.206 ± 0.070 | 0.246 ± 0.122 | 0.304 ± 0.118 | 0.334 ± 0.029 | 0.244 ± 0.108 | 0.320 ± 0.337 | 0.235 ± 0.085 |
| Glukosidase(0–20 cm) mM pN P∙kg−1 h−1 | 0.446 ± 0.161 | 0.491 ± 0.086 | 0.617 ± 0.248 | 0.404 ± 0.146 | 0.316 ± 0.095 | 1.242 ± 0.507 | 1.412 ± 0.516 | 0.799 ± 0.272 | 1.072 ± 0.358 | 1.585 ± 1.032 |
| Urease (0–20 cm) mg NH3 g−1 24 h−1 | 0.555 ± 0.238 | 0.668 ± 0.138 | 0.704 ± 0.183 | 0.572 ± 0.176 | 0.548 ± 0.221 | 3.730 ± 0.791 | 3.563 ± 1.246 | 2.595 ± 0.805 | 2.620 ± 0.631 | 2.380 ± 0.937 |
| Bacteria (0–20 cm) CFU/g−1 | 82.500 ± 29.912 | 97.000 ± 37.342 | 103.000 ± 57.838 | 47.500 ± 37.023 | 81.667 ± 20.801 | 7.317 ± 3.975 | 21.300 ± 8.080 | 12.567 ± 4.668 | 9.683 ± 1.869 | 12.100 ± 11.606 |
| Fungi (0–20 cm) CFU/g−1 | 79.667 ± 25.216 | 62.333 ± 28.069 | 128.333 ± 37.425 | 82.333 ± 21.658 | 65.500 ± 43.514 | 138.833 ± 45.305 | 86.000 ± 30.509 | 81.833 ± 44.853 | 37.833 ± 29.728 | 11.000 ± 5.288 |
| Plant similarity | 0.503 ± 0.100 | 0.599 ± 0.088 | 0.511 ± 0.094 | 0.550 ± 0.101 | 0.366 ± 0.157 | 0.400 ± 0.188 | 0.474 ± 0.099 | 0.745 ± 0.236 | 0.701 ± 0.207 | 0.759 ± 0.083 |
| Parameter | Study Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | L | W | NK | KZ | S | BM | SM | |
| Type | Fallow | Fallow | Fallow | Ecotone | Ecotone | Meadow | Meadow | Forest | Forest | Forest |
| Carabidae (species) | 20 | 18 | 18 | 23 | 20 | 22 | 23 | 13 | 16 | 9 |
| Carabidae (individuals) | 445 | 627 | 380 | 197 | 68 | 130 | 211 | 69 | 188 | 72 |
| Lepidoptera (species) | 21 | 17 | 15 | 20 | 13 | 17 | 16 | 3 | 2 | 3 |
| Lepidoptera (individuals) | 398 | 233 | 114 | 256 | 68 | 164 | 126 | 5 | 4 | 5 |
| Species | Habitat Preference |
|---|---|
| Carabid Beetles (Carabidae) | |
| Agonum fuliginosum | Forest habitats |
| Amara aenea | Open habitats |
| Amara communis | Eurytopic species |
| Amara consularis | Open habitats |
| Amara convexior | Open habitats |
| Amara equestris | Open habitats |
| Amara familiaris | Eurytopic species |
| Amara lunicollis | Eurytopic species |
| Amara ovata | Open habitats |
| Amara plebeja | Open habitats |
| Amara similata | Open habitats |
| Anisodactylus nemorivagus | Open habitats |
| Badister bullatus | Open habitats |
| Badister lacertosus | Forest habitats |
| Bembidion lampros | Open habitats |
| Calathus erratus | Eurytopic species |
| Calathus fuscipes | Open habitats |
| Calathus melanocephalus | Eurytopic species |
| Calathus micropterus | Forest habitats |
| Carabus granulatus | Eurytopic species/moist |
| Carabus hortensis | Forest habitats |
| Carabus nemoralis | Forest habitats |
| Carabus violaceus | Forest habitats |
| Clivina fossor | Open habitats |
| Cychrus caraboides | Forest habitats |
| Elaphrus riparius | Open habitats/moist |
| Harpalus griseus | Open habitats |
| Harpalus latus | Eurytopic species |
| Harpalus luteicornis | Eurytopic species |
| Harpalus pumilus | Open habitats |
| Harpalus rubripes | Open habitats |
| Harpalus rufipalpis | Eurytopic species |
| Harpalus rufipes | Open habitats |
| Harpalus tardus | Eurytopic species |
| Harpalus xanthopus | Forest habitats |
| Lebia chlorocephala | Eurytopic species |
| Leistus terminatus | Forest habitats |
| Notiophilus palustris | Forest habitats |
| Oodes helopioides | Open habitats/moist |
| Oxypselaphus obscurus | Forest habitats |
| Poecilus cupreus | Open habitats |
| Poecilus lepidus | Open habitats |
| Poecilus versicolor | Open habitats |
| Pterostichus diligens | Forest habitats/moist |
| Pterostichus melanarius | Eurytopic species |
| Pterostichus niger | Forest habitats |
| Pterostichus nigrita | Eurytopic species/moist |
| Pterostichus oblongopunctatus | Forest habitats |
| Pterostichus rhaeticus | Eurytopic species/moist |
| Pterostichus strenuus | Forest habitats |
| Pterostichus vernalis | Eurytopic species |
| Syntomus foveatus | Open habitats |
| Syntomus truncatellus | Open habitats |
| Synuchus vivalis | Eurytopic species |
| Zabrus tenebrioides | Open habitats |
| Butterflies (Lepidoptera) | |
| Anthocharis cardamines | Open areas, forest edges |
| Apatura ilia | Open areas, forest edges, forests/moist |
| Aphantopus hyperanthus | Open areas |
| Araschnia levana | Open areas, forest edges, forests |
| Argynnis aglaja | Open areas, forest edges |
| Argynnis paphia | Open areas, forest edges |
| Coenonympha glycerion | Open areas, forest edges |
| Coenonympha pamphilus | Open areas, forest edges |
| Colias hyale | Open areas |
| Cyaniris semiargus | Open areas, forest edges |
| Gonepteryx rhamni | Open areas, forest edges, forests |
| Inachis io | Open areas, forest edges |
| Issoria lathonia | Open areas |
| Lycaena dispar | Open areas |
| Lycaena tityrus | Open areas, forest edges |
| Lycaena virgaureae | Open areas, forest edges |
| Maniola jurtina | Open areas, forest edges |
| Melanargia galathea | Open areas, forest edges |
| Nymphalis antiopa | Open areas, forest edges, forests |
| Papilio machaon | Open areas |
| Pararge aegeria | Forests/moist |
| Pieris brassicae | Open areas, forest edges |
| Pieris daplidice | Open areas |
| Pieris napi | Open areas, forest edges |
| Pieris rapae | Open areas, forest edges |
| Polyommatus icarus | Open areas, forest edges |
| Thymelicus sylvestris | Open areas, forest edges |
| Thymelicus lineola | Open areas, forest edges |
| Vanessa atalanta | Open areas, forest edges |
References
- Kotze, D.J.; O’Hara, R.B. Species decline—But why? Explanations of carabid beetle (Coleoptera, Carabidae) declines in Europe. Oecologia 2003, 135, 138–148. [Google Scholar] [CrossRef]
- Brooks, D.R.; Bater, J.E.; Clark, S.J.; Monteith, D.T.; Andrews, C.; Corbett, S.J.; Beaumont, D.A.; Chapman, J.W. Large carabid beetle declines in a United Kingdom monitoring network increases evidence for a widespread loss in insect biodiversity. J. Appl. Ecol. 2012, 49, 1009–1019. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Muller, A.; Sumser, H.; Horren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Homburg, K.; Drees, C.; Boutaud, E.; Nolte, D.; Schuett, W.; Zumstein, P.; von Ruschkowski, E.; Assmann, T. Where have all the beetles gone? Long-term study reveals carabid species decline in a nature reserve in Northern Germany. Insect Conserv. Divers. 2019, 12, 268–277. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Ryszkowski, L.; Karg, J.; Kujawa, K.; Goldyn, H.; Arczynska-Chudy, E. Influence of landscape mosaic structure on diversity of wild plant and animal communities in agricultural landscapes of Poland. In Landscape Ecology in Agrosystems Management; Ryszkowski, L., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2002; pp. 185–217. [Google Scholar] [CrossRef]
- Weibull, A.-C.; Östman, Ö.; Granqvist, Å. Species richness in agroecosystems: The effect of landscape, habitat and farm management. Biodivers. Conserv. 2003, 12, 1335–1355. [Google Scholar] [CrossRef]
- Purtauf, T.; Dauber, J.; Wolters, V. Carabid communities in the spatio-temporal mosaic of a rural landscape. Landsc. Urban Plan. 2004, 67, 185–193. [Google Scholar] [CrossRef]
- Hendrickx, F.; Maelfait, J.-P.; van Wingerden, W.; Schweiger, O.; Speelmans, M.; Aviron, I.; Augenstein, I.; Billeter, R.; Bailey, D.; Bukacek, R.; et al. How landscape structure, land-use intensity and habitat diversity affect components of total arthropod diversity in agricultural landscapes. J. Appl. Ecol. 2007, 44, 340–351. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the multiple meanings of ß diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef]
- Amoros, C. The concept of habitat diversity between and within ecosystems applied to river side-arm restoration. Environ. Manag. 2001, 28, 805–817. [Google Scholar] [CrossRef]
- Kark, S. Effects of ecotones on biodiversity. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Elsevier: Cham, Switzerland, 2017; pp. 142–148. [Google Scholar]
- Langhans, S.D.; Tockner, K. Edge effects are important in supporting beetle biodiversity in a gravel-bed river floodplain. PLoS ONE 2014, 9, e114415. [Google Scholar] [CrossRef]
- Rastelli, F.; Staffolani, L.; Hruska, K. Ecological study of the vegetal component in the terrestrial ecotones of central Italy. J. Mediterr. Ecol. 2003, 4, 39–43. [Google Scholar]
- Bruce, T.J.A. Interplay between insects and plants: Dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. J. Exp. Bot. 2015, 66, 455–465. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Heil, M. Multitrophic interactions below and above ground: En route to the next level. J. Ecol. 2011, 99, 77–88. [Google Scholar] [CrossRef]
- Ehrnsberger, R. (Ed.) Bodenmesofauna und Naturschutz. Bedeutung und Auswirkungen von anthropogenen Maβnahmen. Informationen zu Naturschutz und Landschaftspflege in Nordwestdeutschland; Verlag Günter Runge: Cloppenburg, Germany, 1993; p. 452. [Google Scholar]
- Kotze, D.J.; Brandmayr, P.; Casale, A.; Dauffey-Richard, E.; Dekoninck, W.; Koivula, M.; Lövei, G.; Mossakowski, D.; Noordijk, J.; Paarmann, W.; et al. Forty years of carabid beetle research in Europe—From taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. ZooKeys 2011, 100, 55–148. [Google Scholar] [CrossRef]
- Morris, M.G. The effects of structure and its dynamics on the ecology and conservation of arthropods in British grasslands. Biol. Conserv. 2000, 95, 129–142. [Google Scholar] [CrossRef]
- Schwerk, A.; Dymitryszyn, I. Mowing intensity influences degree of changes in carabid beetle assemblages. Appl. Ecol. Environ. Res. 2017, 15, 427–440. [Google Scholar] [CrossRef]
- Küster, H. Cultural landscapes. In Cultural Landscapes and Land Use. The Nature Conservation—Society Interface; Dieterich, M., van der Straaten, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 1–11. [Google Scholar] [CrossRef]
- Eriksson, O. Species pools in cultural landscapes—Niche construction, ecological opportunity and niche shifts. Ecography 2013, 36, 403–413. [Google Scholar] [CrossRef]
- Valkó, O.; Tóth, K.; Kelemen, A.; Miglécz, T.; Radócz, S.; Sonkoly, J.; Tóthmérész, B.; Török, P.; Deák, B. Cultural heritage and biodiversity conservation—Plant introduction and practical restoration on ancient burial mounds. Nat. Conserv. 2018, 24, 65–80. [Google Scholar] [CrossRef]
- Koivula, M.J. Useful model organisms, indicators, or both? Ground beetles (Coleoptera, Carabidae) reflecting environmental conditions. ZooKeys 2011, 100, 287–317. [Google Scholar] [CrossRef] [PubMed]
- Rainio, J.; Niemelä, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Avgın, S.S.; Luff, M.L. Ground beetles (Coleoptera: Carabidae) as bioindicators of human impact. Munis Entomol. Zool. 2010, 5, 209–215. [Google Scholar]
- Skłodowski, J. Manual soil preparation and piles of branches can support ground beetles (Coleoptera, Carabidae) better than four different mechanical soil treatments in a clear-cut area of a closed-canopy pine forest in northern Poland. Scand. J. For. Res. 2017, 32, 123–133. [Google Scholar] [CrossRef]
- Schwerk, A.; Wińska-Krysiak, M.; Przybysz, A.; Zaraś-Januszkiewicz, E.; Sikorski, P. Carabid beetle (Coleoptera: Carabidae) response to soil properties of urban wasteland in Warsaw, Poland. Sustainability 2020, 12, 10673. [Google Scholar] [CrossRef]
- Thomas, C.D.; Hanski, I. Metapopulation dynamics in changing environments: Butterfly responses to habitat climate change. In Ecology, Genetics and Evolution of Metapopulations; Hanski, I., Gaggiotti, O.E., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2004; pp. 489–514. [Google Scholar] [CrossRef]
- Wilson, R.J.; Maclean, I.M.D. Recent evidence for the climate change threat to Lepidoptera and other insects. J. Insect Conserv. 2011, 15, 259–268. [Google Scholar] [CrossRef]
- Maurer, J.A.; Shepard, J.H.; Crabo, L.G.; Hammond, P.C.; Zack, R.S.; Peterson, M.A. Phenological responses of 215 moth species to interannual climate variation in the Pacific Northwest from 1895 through 2013. PLoS ONE 2018, 13, e0202850. [Google Scholar] [CrossRef]
- Szyszko-Podgórska, K. Characteristics of the butterflies on various forms of land uses. Environ. Prot. Nat. Resour. Ochr. Sr. Zasobów Nat. 2019, 30, 15–22. [Google Scholar] [CrossRef]
- Hill, G.M.; Kawahara, A.Y.; Daniels, J.C.; Bateman, C.C.; Scheffers, B.R. Climate change effects on animal ecology: Butterflies and moths as a case study. Biol. Rev. 2021, 96, 2113–2126. [Google Scholar] [CrossRef]
- Brown, K.S.; Freitas, A.V.L. Atlantic forest butterflies: Indicators for landscape conservation 1. Biotropica 2000, 32, 934–956. [Google Scholar] [CrossRef]
- Sielezniew, M.; Stankiewicz, A.M. Ekologiczne, prawne i praktyczne aspekty ochrony motyli w Polsce na przykładzie modraszków Maculinea spp. (Lepidoptera: Lycaenidae). Wiad. Entomol. 2006, 25, 179–188. [Google Scholar]
- Møller, A.P.; Mousseau, T.A. Reduced abundance of insects and spiders linked to radiation at Chernobyl 20 years after the accident. Biol. Lett. 2009, 5, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Nohara, C.; Kinjo, S.; Taira, W.; Gima, S.; Tanahara, A.; Otaki, J.M. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012, 2, 570. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Nishiumi, I.; Suzuki, H.; Ueda, K.; Mouseeau, T.A. Differences in effects of radiation on abundance of animals in Fukushima and Chernobyl. Ecol. Indicat. 2012, 24, 75–78. [Google Scholar] [CrossRef]
- Dymitryszyn, I.; Szyszko, J.; Rylke, J. (Eds.) Field Methods of Evaluation and Assessment of Natural Resources; Warsaw University of Life Sciences Press—Wydawnictwo SGGW: Warszawa, Poland, 2013; 264p. [Google Scholar]
- Biały, K.; Brożek, S.; Chojnicki, J.; Czępińska-Kamińska, D.; Januszek, K.; Kowalkowski, A.; Krzyżanowski, A.; Okołowicz, M.; Sienkiewicz, A.; Skiba, S.; et al. Klasyfikacja Gleb Leśnych Polski; Centrum Informacyjne Lasów Państwowych: Warsaw, Poland, 2000; p. 127. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 192. [Google Scholar]
- Ostrowska, A.; Gawlinski, S.; Szczubiałka, Z. Metody Analizy i Oceny Właściwości Gleb i Roślin; Instytut Ochrony Środowiska: Warsaw, Poland, 1991; p. 334. [Google Scholar]
- Bednarek, R.; Charzyński, I.P.; Kabała, C. (Eds.) Klasyfikacja Zasobów Glebowych Świata 2006; Wydawnictwo UMK: Toruń, Poland, 2009; p. 145. [Google Scholar]
- Karczewska, A.; Szopka, K.; Bogacz, A.; Kabała, C.; Daszyńska, D. Rozważania nad metodyką monitoringu gleb strefy leśnej Karkonoskiego Parku Narodowego (KPN)—w świetle zróżnicowania właściwości tych gleb. Opera Corcon. 2007, 44, 95–105. [Google Scholar]
- Polish Soil Science Society (PTG). Klasyfikacja uziarnienia gleb i utworów mineralnych—PTG 2008. Soil Sci. Annu. 2009, 60, 5–16. [Google Scholar]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrate. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M.; Paul, W.; Ronikier, M.; Bernacki, L.; Cieślak, E.; Głowacki, Z.; Leda, M.; et al. Flowering Plants and Pteridophytes of Poland. A Checklist. Biodiversity of Poland; Władysław Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002; Volume 1, p. 442. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1964; p. 631. [Google Scholar] [CrossRef]
- Freude, H.; Harde, K.-W.; Lohse, G.A.; Klausnitzer, B. Die Käfer Mitteleuropas. Bd. 2, Adephaga 1, Carabidae (Laufkäfer). 2. (Erweiterte) Aufl.; Spektrum: Heidelberg/Berlin, Germany, 2004; p. 521. [Google Scholar]
- Pollard, E. A method for assessing changes in the abundance of butterflies. Biol. Conserv. 1977, 12, 115–134. [Google Scholar] [CrossRef]
- Buszko, J.; Masłowski, J. Atlas Motyli Polski Part 1. Motyle Dzienne; Wydawnictwo IMAGE: Warsaw, Poland, 1993; p. 272. [Google Scholar]
- Sielezniew, M.; Dziekańska, I. Fauna Polski. Motyle Dzienne; Wydawnicza Multico Oficyna Wydawnicza: Warsaw, Poland, 2010; p. 335. [Google Scholar]
- Hammer, Ø.; Harperd, A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Ter Braak, C.J.F. CANOCO—A FORTRAN Program. for Canonical Community Ordination by [Partial][Detrended][Canonical] Correspondence Analysis, Principal Components Analysis and Redundancy Analysis; Version 2.1; DLO Agricultural Mathematics Group: Wageningen, The Netherland, 1987; p. 95. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination; Version 4.5; Microcomputer Power: Ithaca, NY, USA, 2002; p. 499. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 268. [Google Scholar] [CrossRef]
- Burel, F. Landscape structure effects on carabid beetles spatial patterns in western France. Lands. Ecol. 1989, 2, 215–226. [Google Scholar] [CrossRef]
- Holland, J.; Fahrig, L. Effect of woody borders on insect density and diversity in crop fields: A landscape-scale analysis. Agric. Ecosyst. Environ. 2000, 78, 115–122. [Google Scholar] [CrossRef]
- Boetzl, F.A.; Ries, E.; Schneider, G.; Krauss, J. It’s a matter of design—how pitfall trap design affects trap samples and possible predictions. PeerJ 2018, 6, e5078. [Google Scholar] [CrossRef]
- Kenngott, K.G.J.; Riess, K.; Muñoz, K.; Schaumann, G.E.; Buhk, C.; Diehl, D. Flood pulse irrigation of meadows shapes soil chemical and microbial parameters more than mineral fertilization. Soil Syst. 2021, 5, 24. [Google Scholar] [CrossRef]
- Chazdon, R.L. Tropical forest recovery: Legacies of human impact and natural disturbances. Prospect. Plant. Ecol. Evol. Syst. 2003, 6, 51–71. [Google Scholar] [CrossRef]
- Yuanjie, X.; ·Yaning, C.; ·Weihong, L.; ·Aihong, F.; ·Xiaodong, M.; ·Dongwei, G.; ·Yapeng, C. Distribution pattern of plant species diversityin the mountainous Region of Ili River Valley, Xinjiang. Environ. Monit. Assess. 2011, 177, 681–694. [Google Scholar] [CrossRef]
- Kouba, Y.; Martínez-García, F.; de Frutos, Á.; Alados, C.L. Effects of Previous Land-Use on Plant Species Composition and Diversity in Mediterranean Forests. PLoS ONE 2015, 10, e0139031. [Google Scholar] [CrossRef]
- Hettenbergerová, E.; Hájek, M.; Zelený, D.; Jiroušková, J.; Mikulášková, E. Changes in species richness and species composition of vascular plants and bryophytes along a moisture gradient. Preslia 2013, 85, 369–388. [Google Scholar]
- Fischer, F.M.; Wright, A.J.; Eisenhauer, N.; Ebeling, A.; Roscher, C.; Wagg, C.; Weigelt, A.; Weisser, W.W.; Pillar, V.D. Plant species richness and functional traits affect community stability after a flood event. Phil. Trans. R. Soc. B 2016, 371, 20150276. [Google Scholar] [CrossRef] [PubMed]
- Schwerk, A.; Jojczyk, A.; Dymitryszyn, I. Impact of different habitat parameters on carabid beetle assemblages in selected areas of a forest-field landscape in Poland—10 years of Data. Acta Zool. Acad. Sci. Hung. 2020, 66, 169–184. [Google Scholar] [CrossRef]
- Zumstein, P.; Bruelheide, H.; Fichtner, A.; Schuldt, A.; Staab, M.; Härdtle, W.; Zhou, H.; Assmann, T. What shapes ground beetle assemblages in a tree species-rich subtropical forest? ZooKeys 2021, 1044, 907–927. [Google Scholar] [CrossRef] [PubMed]
- Nietupski, M.; Sowiński, P.; Sądej, W.; Kosewska, A. Content of organic C and pH of bog and post-bog soils versus the presence of ground beetles Carabidae in Stary Dwor near Olsztyn. J. Elementol. 2010, 15, 581–591. [Google Scholar] [CrossRef]
- Kondras, M.; Czępińska-Kamińska, D.; Osińska, E.; Osiński, M. Zapas węgla organicznego oraz właściwości fizykochemiczne gleb w kompleksie leśnym „Dąbrowy Krotoszyńskie. Rocz. Glebozn. 2010, 61, 113–122. [Google Scholar]
- Łabęda, D.; Kondras, M. Influence of forest management on soil organic carbon stocks. Soil Sci. Annu. 2020, 71, 165–173. [Google Scholar] [CrossRef]
- Hyvönen, T.; Huusela, E.; Kuussaari, M.; Niemi, M.; Uusitalo, R.; Nuutinen, V. Aboveground and belowground biodiversity responses to seed mixtures and mowing in a long-term set-aside experiment. Agric. Ecosyst. Environ. 2021, 322, 107656. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Görn, S.; Dobner, B.; Suchanek, A.; Fischer, K. Assessing human impact on fen biodiversity: Effects of different management regimes on butterfly, grasshopper, and carabid beetle assemblages. Biodivers. Conserv. 2014, 23, 309–326. [Google Scholar] [CrossRef]
- Šálek, M.; Kučera, T.; Zimmermann, K.; Bartůšková, I.; Plátek, M.; Grill, S.; Konvička, M. Edges within farmland: Management implications of taxon specific species richness correlates. Basic Appl. Ecol. 2015, 16, 714–725. [Google Scholar] [CrossRef]
- Koivula, M.; Niemelä, J. Boreal carabid beetles (Coleoptera, Carabidae) in managed spruce forests—A summary of Finnish case studies. Silva. Fenn. 2002, 36, 423–436. [Google Scholar] [CrossRef][Green Version]
- Kosewska, A. Conventional and non-inversion tillage systems as a factor causing changes in ground beetle (Col. Carabidae) assemblages in oilseed rape (Brassica napus) fields. Period. Biol. 2016, 118, 231–239. [Google Scholar] [CrossRef]
- Marini, L.; Fontana, P.; Battisti, A.; Gaston, K.J. Agricultural management, vegetation traits and landscape drive orthopteran and butterfly diversity in a grassland–forest mosaic: A multi-scale approach. Insect Conserv. Divers. 2009, 2, 213–220. [Google Scholar] [CrossRef]
- Swengel, A.B. Effects of management on butterfly abundance in tallgrass prairie and pine barrens. Biol. Conserv. 1998, 93, 77–89. [Google Scholar] [CrossRef]
- Aviron, S.; Jeanneret, P.; Schüpbach, B.; Herzog, F. Effects of agri-environmental measures, site and landscape conditions on butterfly diversity of Swiss grassland. Agric. Ecosyst. Environ. 2007, 122, 295–304. [Google Scholar] [CrossRef]
- Scheper, J.; Holzschuh, A.; Kuussaari, M.; Potts, S.G.; Rundlöf, M.; Smith, H.G.; Kleijn, D. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss—A meta-analysis. Ecol. Lett. 2013, 16, 912–920. [Google Scholar] [CrossRef]
- Szyszko, J.; Schwerk, A.; Malczyk, J. Animals as an indicator of carbon sequestration and valuable landscapes. ZooKeys 2011, 100, 565–573. [Google Scholar] [CrossRef]
- Burakowski, B.; Mroczkowski, M.; Stefańska, J. Katalog Fauny Polski (Catalogus Faunae Poloniae). Część XXIII, Tom 2. Chrząszcze (Coleoptera). Biegaczowate (Carabidae), Część 1; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1973; 232p. [Google Scholar]
- Burakowski, B.; Mroczkowski, M.; Stefańska, J. Katalog Fauny Polski (Catalogus Faunae Poloniae). Część XXIII, Tom 3. Chrząszcze (Coleoptera). Biegaczowate (Carabidae), Część 2; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1974; 430p. [Google Scholar]
- Hurka, K. Carabidae of Czech. and Slowak Republics; Kabournek: Zlin, Czech Republic, 1996; 565p. [Google Scholar]
- Szyszko-Podgórska, K. Motyle dzienne. In Podstawy Kompensacji Przyrodniczej; Szyszko, J., Tobolski, K., Eds.; Wydawnictwoi WSKiM: Toruń, Poland, 2010; pp. 238–244. [Google Scholar]
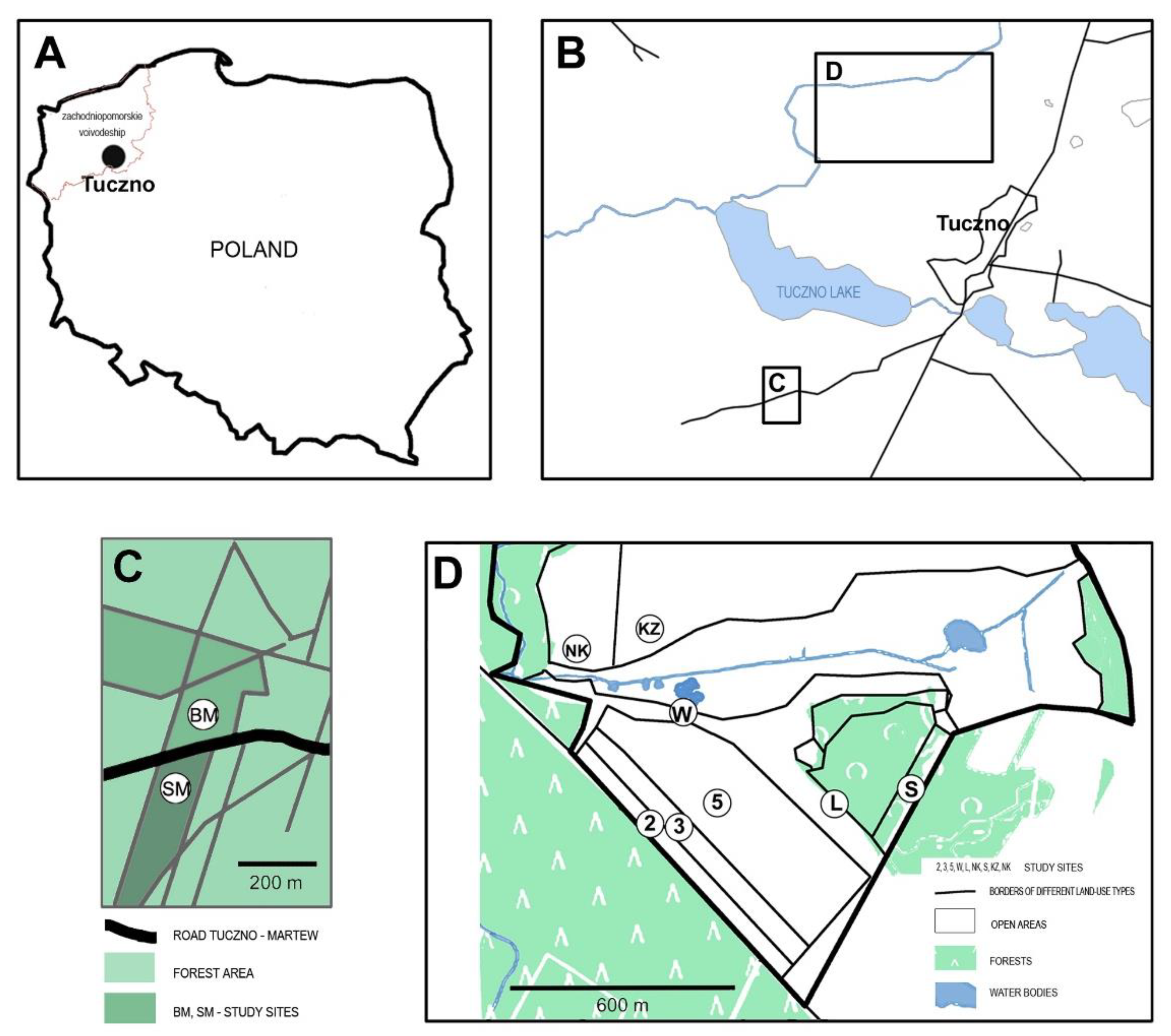
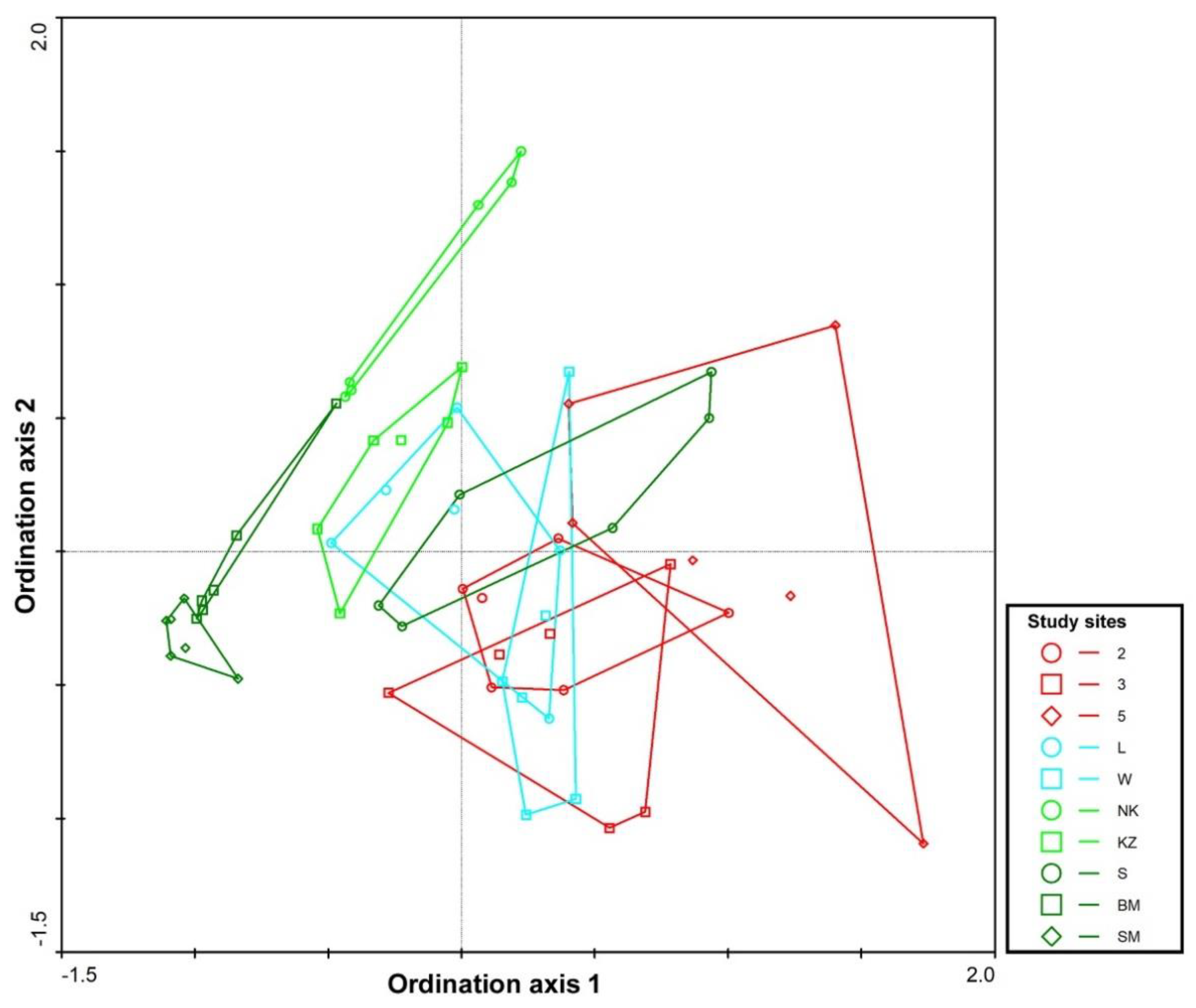
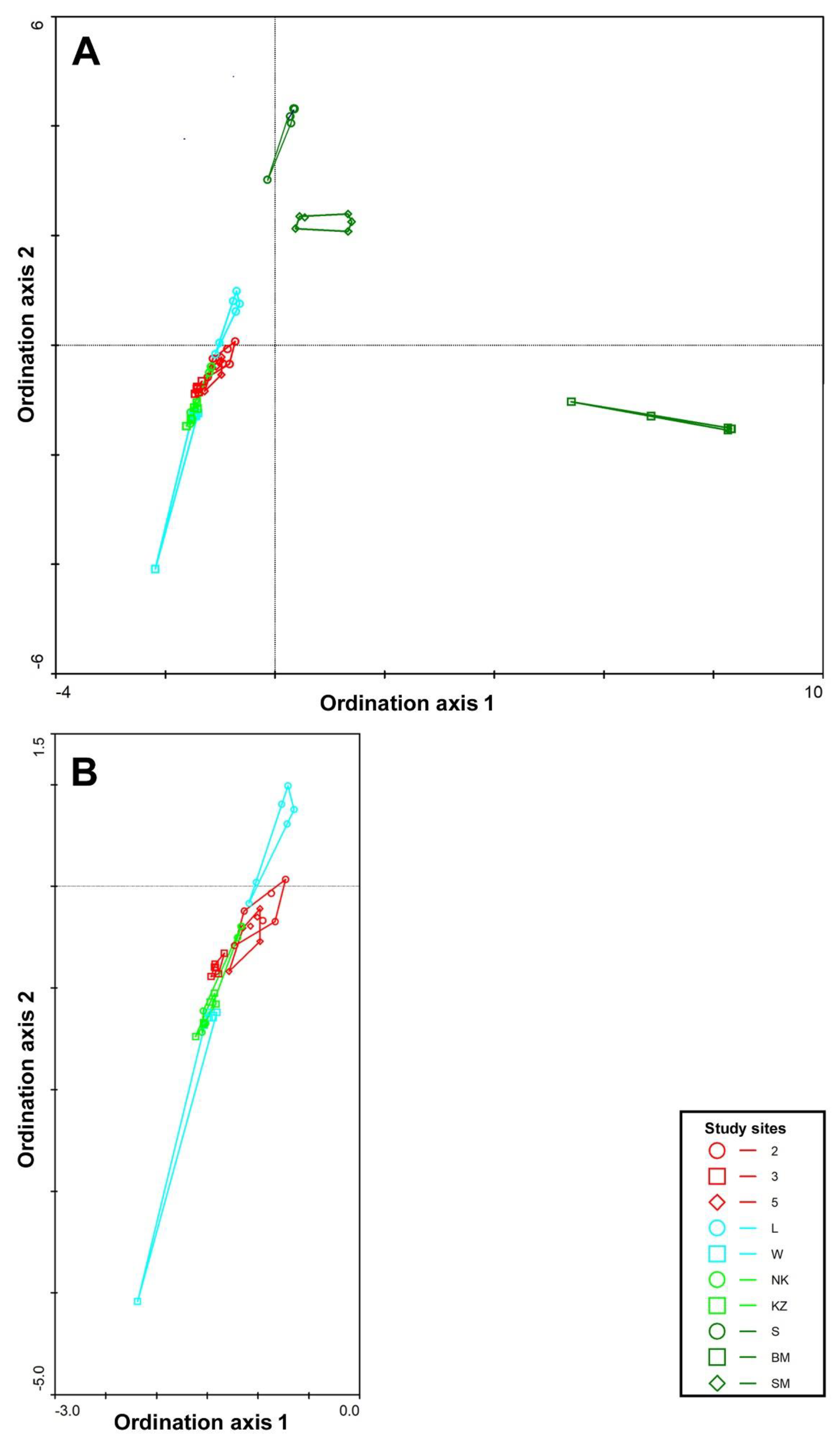
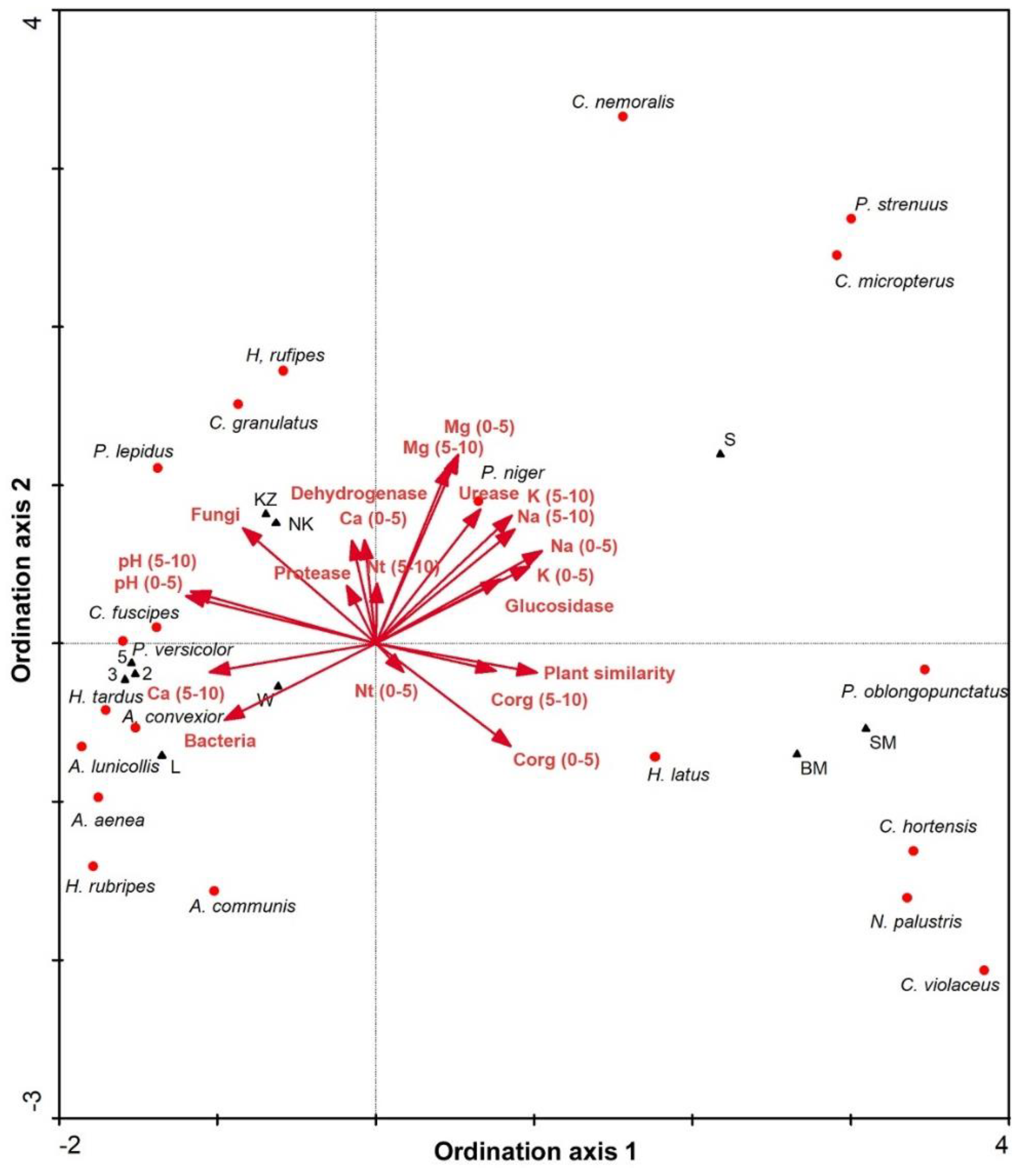
| Study Site | Type | Description | Dominant Plant Species |
|---|---|---|---|
| 2 | Fallow | Mown post-agricultural ground without biomass removal. | Anthoxanthum odoratum, Pleurozium schreberi, Holcus lanatus, Deschampsia flexuosa |
| 3 | Fallow | Mown post-agricultural ground with biomass removal. | Anthoxanthum odoratum, Hieracium pilosella, Festuca rubra, Armeria elongata |
| 5 | Fallow | Non-mown post-agricultural ground. | Anthoxanthum odoratum, Pleurozium schreberi, Deschampsia flexuosa, Phleum pretense |
| L | Ecotone | Ecotone between forest and fallow ground. | Sarothamnus scoparius, Anthoxanthum odoratum, Pinus silvestris, Agrostis capillaris |
| W | Ecotone | Ecotone between swamp and fallow ground. | Agrostis capillaris, Arrhenatherum elatius, Festuca rubra, Phalaris arundinacea |
| NK | Meadow | Non-mown meadow. | Festuca rubra, Pleurozium schreberi, Arrhenatherum elatius, Agrostis capillaris |
| KZ | Meadow | Mown meadow with biomass removal. | Agrostis capillaris, Arrhenatherum elatius, Anthoxanthum odoratum, Dactylis glomerata |
| S | Forest | Approximately 19 year old pine forest resulting from natural succession. | Pinus silvestris, Padus serotina |
| BM | Forest | Approximately 95 year old beech forest. | Fagus sylvatica, Polytrichastrum formosum, Carex pilulifera, Deschampsia flexuosa |
| SM | Forest | Approximately 46 year old pine forest. | Pinus silvestris, Pleurozium schreberi, Vaccinium myrtillus, Deschampsia flexuosa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szyszko-Podgórska, K.; Dymitryszyn, I.; Jankiewicz, U.; Kondras, M.; Żyfka-Zagrodzińska, E.; Schwerk, A. Assemblage Characteristics of Butterflies and Carabid Beetles as a Function of Soil Characteristics and Plant Diversity in Differently Managed Fields, Forests and Ecotones: A Case Study in Tuczno Forest District, Poland. Land 2022, 11, 25. https://doi.org/10.3390/land11010025
Szyszko-Podgórska K, Dymitryszyn I, Jankiewicz U, Kondras M, Żyfka-Zagrodzińska E, Schwerk A. Assemblage Characteristics of Butterflies and Carabid Beetles as a Function of Soil Characteristics and Plant Diversity in Differently Managed Fields, Forests and Ecotones: A Case Study in Tuczno Forest District, Poland. Land. 2022; 11(1):25. https://doi.org/10.3390/land11010025
Chicago/Turabian StyleSzyszko-Podgórska, Katarzyna, Izabela Dymitryszyn, Urszula Jankiewicz, Marek Kondras, Ewa Żyfka-Zagrodzińska, and Axel Schwerk. 2022. "Assemblage Characteristics of Butterflies and Carabid Beetles as a Function of Soil Characteristics and Plant Diversity in Differently Managed Fields, Forests and Ecotones: A Case Study in Tuczno Forest District, Poland" Land 11, no. 1: 25. https://doi.org/10.3390/land11010025
APA StyleSzyszko-Podgórska, K., Dymitryszyn, I., Jankiewicz, U., Kondras, M., Żyfka-Zagrodzińska, E., & Schwerk, A. (2022). Assemblage Characteristics of Butterflies and Carabid Beetles as a Function of Soil Characteristics and Plant Diversity in Differently Managed Fields, Forests and Ecotones: A Case Study in Tuczno Forest District, Poland. Land, 11(1), 25. https://doi.org/10.3390/land11010025







