How Does Land Consolidation Affect Soil Fungal Community Structure? Take Heavy Metal Contaminated Areas in Eastern China for Example
Abstract
:1. Introduction
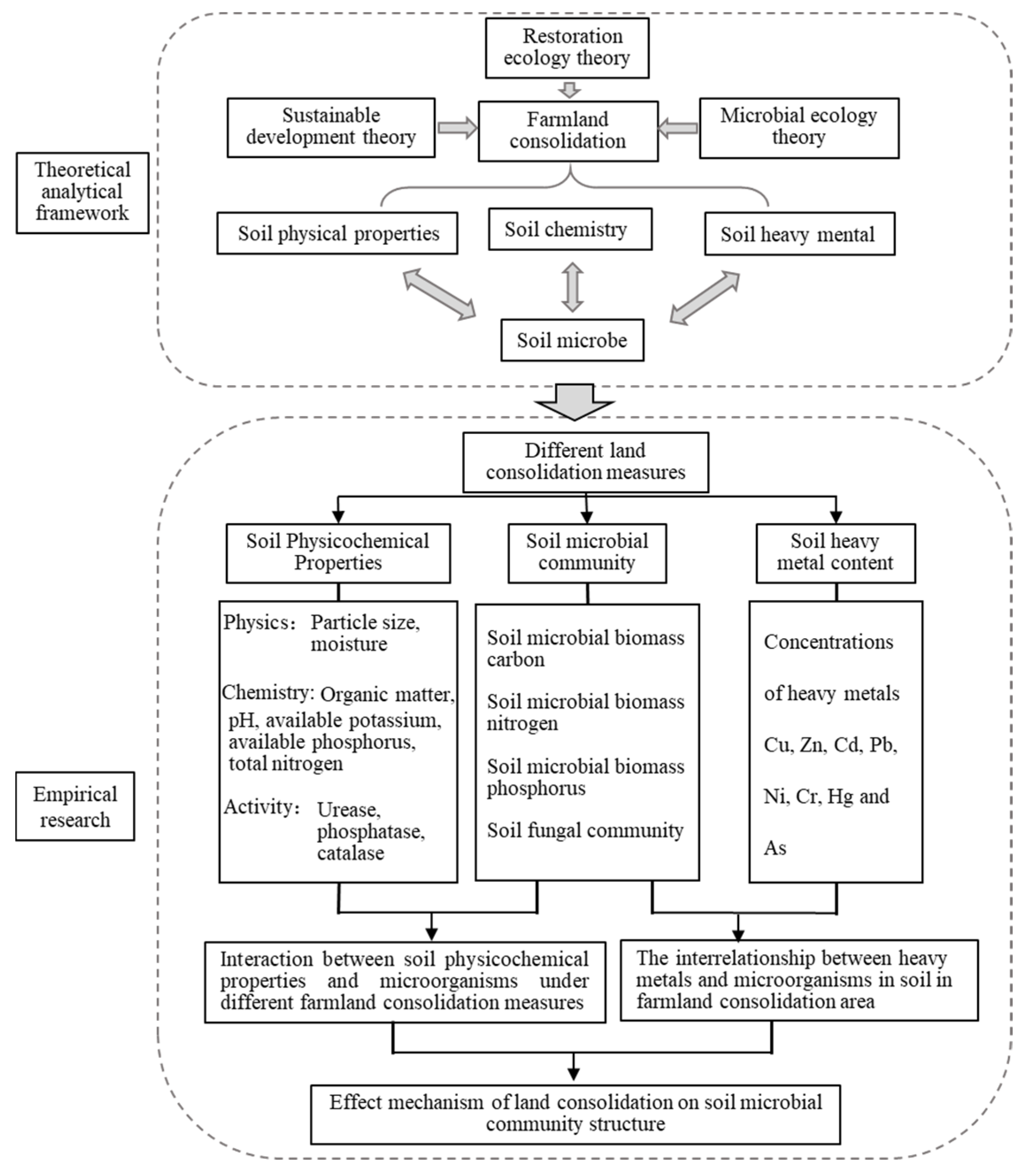
2. Framework and Data Collection
2.1. Research Framework
2.2. Study Area
2.3. Soil Collection and Analysis
2.3.1. Soil Collection
2.3.2. Soil Basic Physical and Chemical Properties Test
2.3.3. Heavy Metal Soil Content Test
2.3.4. Soil Microbial Properties Determination
2.4. Statistic Analysis
3. Results and Discussion
3.1. Effect of Land Consolidation on Soil Fungal Community
3.1.1. Changes in Soil Fungal Diversity
Analysis of α Diversity of Fungal Community
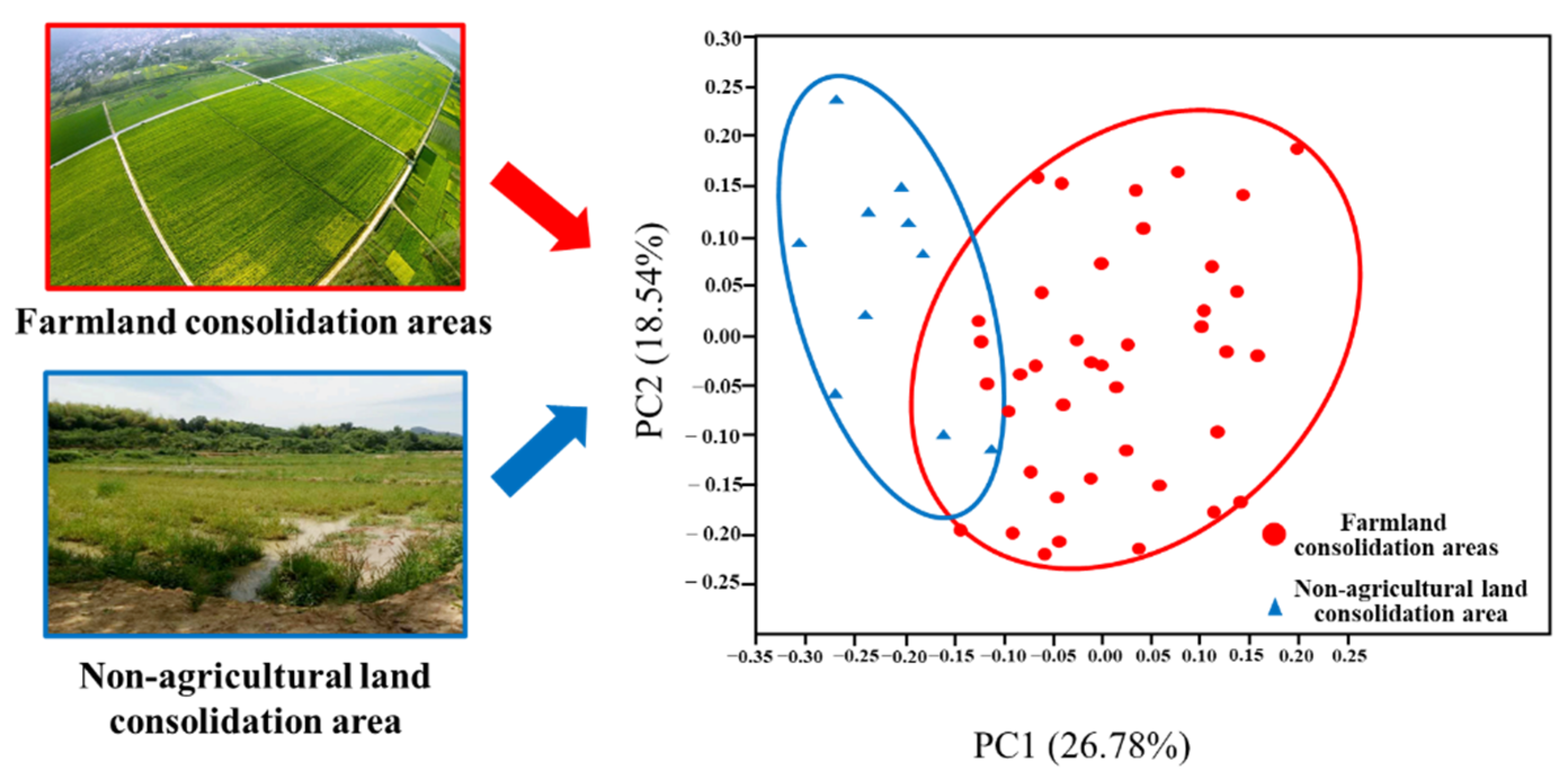
Analysis of β Diversity of Fungal Community
3.1.2. Changes in Soil Fungal Community Structure
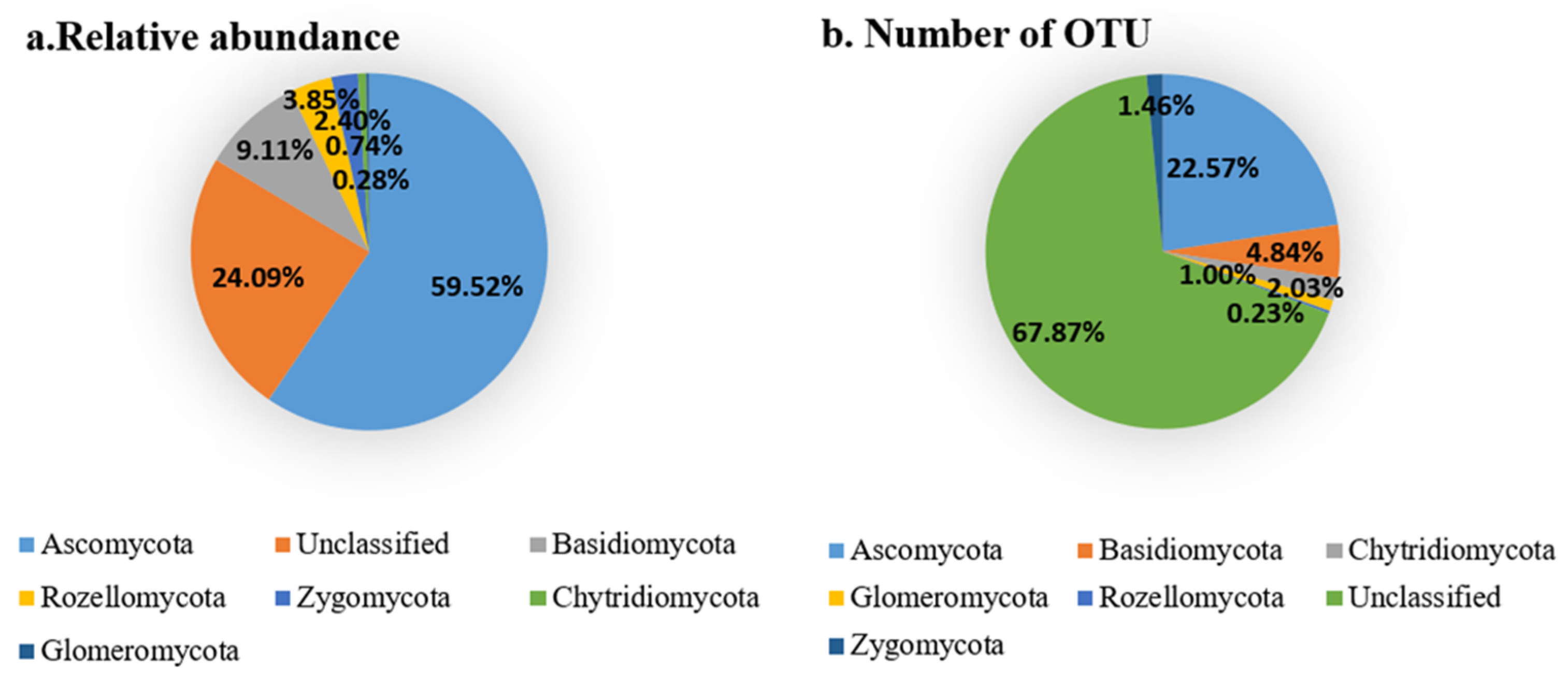
Analysis on the Changes of Fungal Community at the Phylum Level
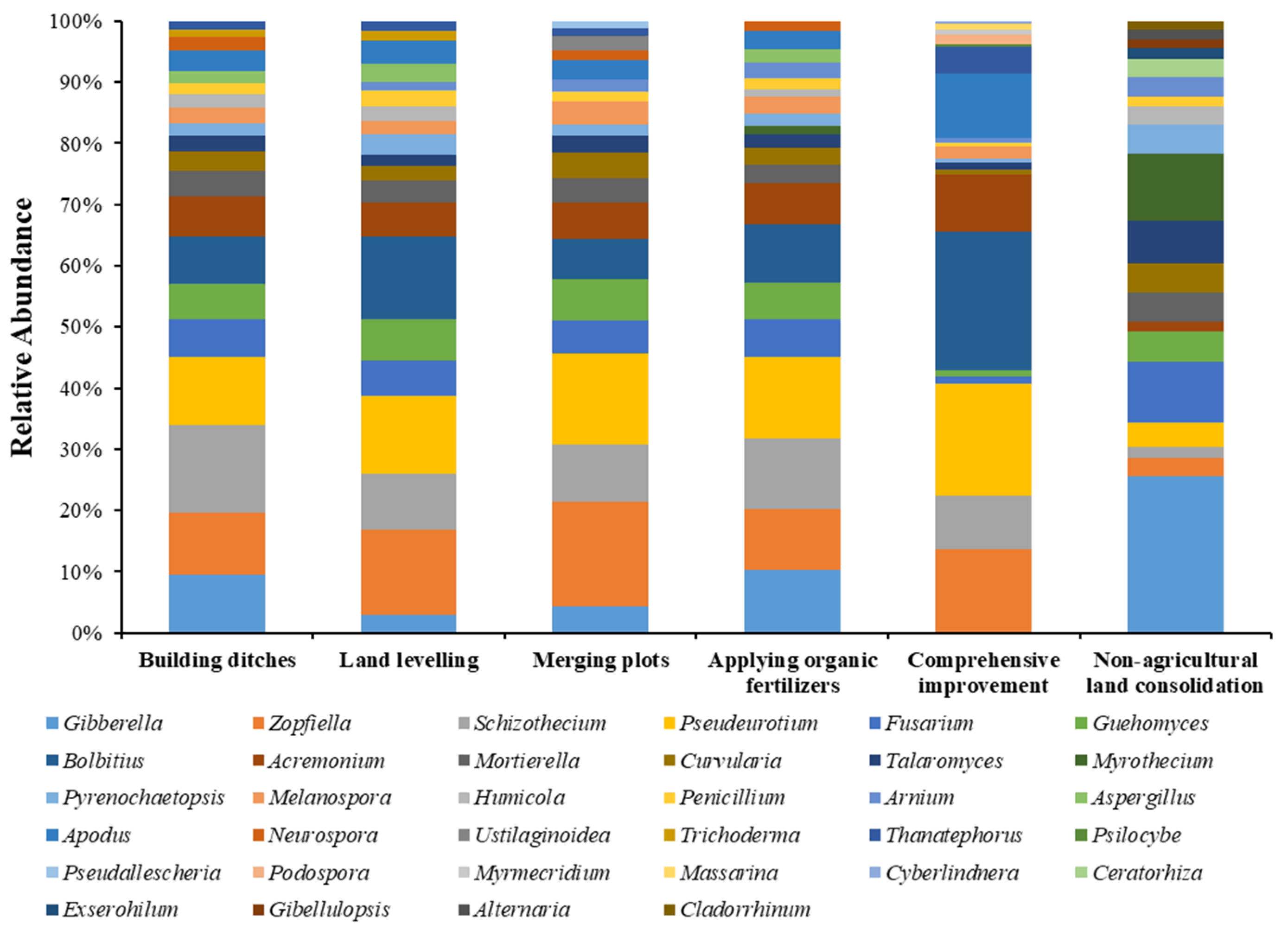
Analysis on the Changes of Fungal Community at the Genus Level
Analysis of Changes in Fungal Community Function
3.2. Farmland Consolidation Regulates the Basic Physical and Chemical Properties of Soil and Its Mechanism of Action on Fungi
3.2.1. Farmland Consolidation Promotes Changes in Basic Physical and Chemical Properties of Soil
Soil Physical Properties
Soil Chemistry
Soil Enzyme Activity
3.2.2. The Mechanism of Basic Physical and Chemical Properties of Soil on Fungal Community
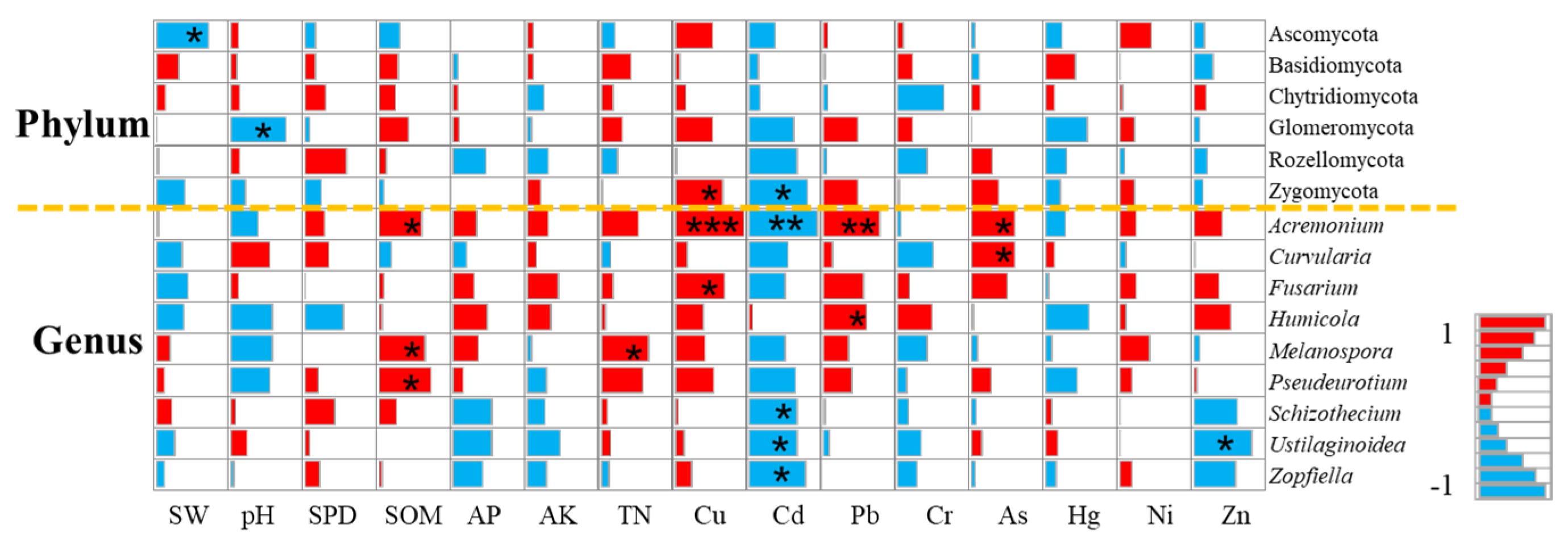
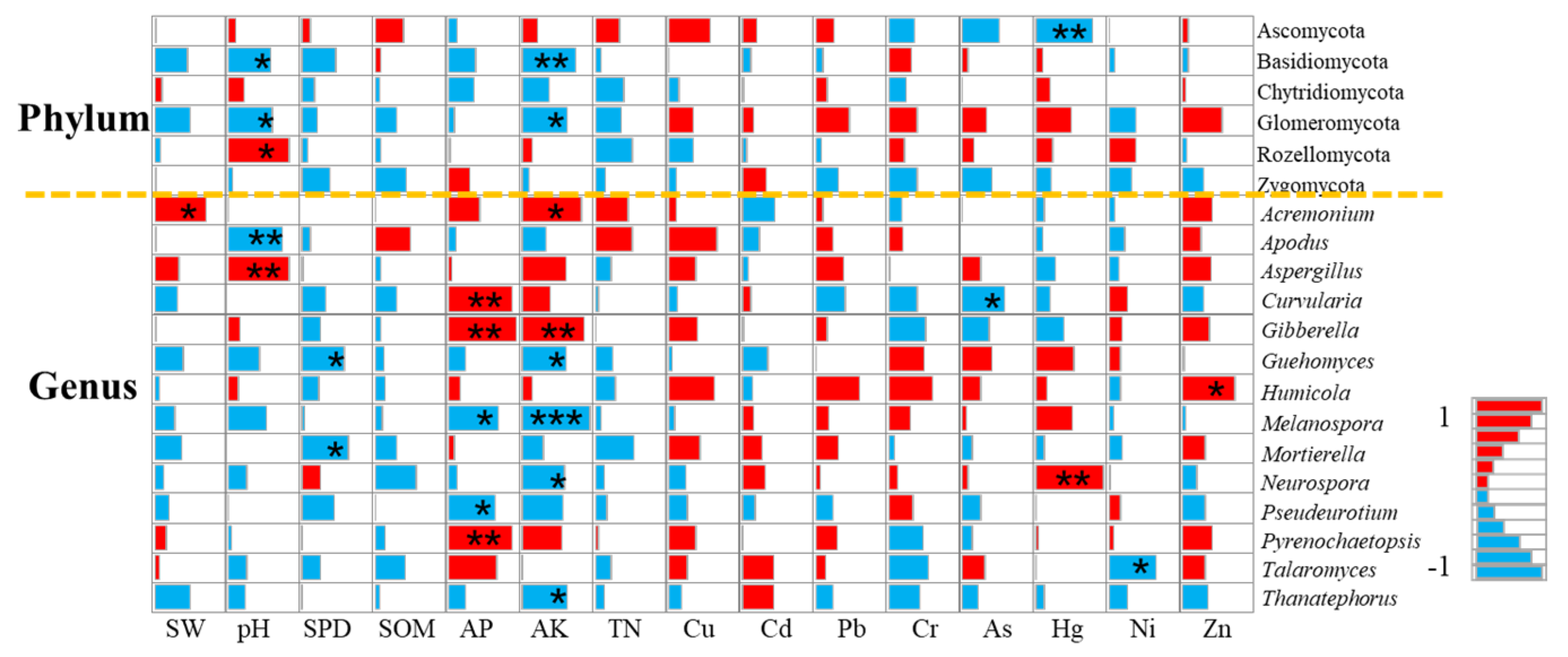
Soil Physical Properties and Fungal Community
Soil Chemical Properties and Fungal Communities
Soil Enzyme Activity and Fungal Community
3.3. Farmland Consolidation Regulates Heavy Metal Soil Content and Its Mechanism of Action on Fungi
3.3.1. Effects of Farmland Consolidation on Heavy Metal Soil Content
Heavy Metal Content of Farmland Soil
Heavy Metal Pollution Level of Farmland Soil
3.3.2. The Mechanism of Heavy Metal Soil Pollution on Fungal Communities
Fungal Communities at Low Pollution Levels
Fungal Communities at Light Pollution Levels
Fungal Communities at Moderate Pollution Levels
Fungal Communities under Heavy Pollution Levels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Compliance with Ethical Standards
References
- Djanibekov, U.; Finger, R. Agricultural risks and farm land consolidation process in transition countries: The case of cotton production in Uzbekistan. Agric. Syst. 2018, 164, 223–235. [Google Scholar] [CrossRef]
- Demetriou, D. The assessment of land valuation in land consolidation schemes: The need for a new land valuation framework. Land Use Policy 2016, 54, 487–498. [Google Scholar] [CrossRef]
- Stańczuk-Gałwiaczek, M.; Sobolewska-Mikulska, K.; Ritzema, H.; van Loon-Steensma, J.M. Integration of water management and land consolidation in rural areas to adapt to climate change: Experiences from Poland and the Netherlands. Land Use Policy 2018, 77, 498–511. [Google Scholar] [CrossRef]
- Johansen, P.H.; Ejrnæs, R.; Kronvang, B.; Olsen, J.V.; Præstholm, S.; Schou, J.S. Pursuing collective impact: A novel indicator-based approach to assessment of shared measurements when planning for multifunctional land consolidation. Land Use Policy 2018, 73, 102–114. [Google Scholar] [CrossRef]
- Muchová, Z.; Konc, Ľ.; Petrovič, F. Land plots valuation in land consolidation in slovakia: A need for a new approach. Int. J. Strateg. Prop. Manag. 2018, 22, 372–380. [Google Scholar] [CrossRef]
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Carof, M.; Chen, W.; Le Floch, G. Effect of tillage and static abiotic soil properties on microbial diversity. Appl. Soil Ecol. 2018, 132, 135–145. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The microbial engines that drive Earth′s biogeochemical cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef] [Green Version]
- Frey, S.D.; Lee, J.; Melillo, J.M.; Six, J. The temperature response of soil microbial efficiency and its feedback to climate. Nat. Clim. Change 2013, 3, 395–398. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.-A.; Wookey, P.A.; Ågren, G.I.; Sebastià, M.T.; et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef]
- Ferreira, D.A.; da Silva, T.F.; Pylro, V.S.; Salles, J.F.; Andreote, F.D.; Dini-Andreote, F. Soil Microbial Diversity Affects the Plant-Root Colonization by Arbuscular Mycorrhizal Fungi. Microb. Ecol. 2020, 82, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.J.; Wan, J. Effects of soil microbes on plant competition: A perspective from modern coexistence theory. Ecol. Monogr. 2020, 90, e01391. [Google Scholar] [CrossRef]
- Bourles, A.; Guentas, L.; Charvis, C.; Gensous, S.; Majorel, C.; Crossay, T.; Cavaloc, Y.; Burtet-Sarramegna, V.; Jourand, P.; Amir, H. Co-inoculation with a bacterium and arbuscular mycorrhizal fungi improves root colonization, plant mineral nutrition, and plant growth of a Cyperaceae plant in an ultramafic soil. Mycorrhiza 2020, 30, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, L.; Fei, S.; Ma, Z.; Hao, R.; Zhao, Z. Effect of Soil Layer and Plant–Soil Interaction on Soil Microbial Diversity and Function after Canopy Gap Disturbance. Forests 2018, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Lüneberg, K.; Schneider, D.; Siebe, C.; Daniel, R. Drylands soil bacterial community is affected by land use change and different irrigation practices in the Mezquital Valley, Mexico. Sci. Rep. 2018, 8, 1413. [Google Scholar] [CrossRef] [PubMed]
- Baldoncini, M.; Albéri, M.; Bottardi, C.; Chiarelli, E.; Raptis, K.G.C.; Strati, V.; Mantovani, F. Biomass water content effect on soil moisture assessment via proximal gamma-ray spectroscopy. Geoderma 2019, 335, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Song, X.D.; Yang, F.; Ju, B.; Li, D.C.; Zhao, Y.G.; Yang, J.L.; Zhang, G.L. The influence of the conversion of grassland to cropland on changes in soil organic carbon and total nitrogen stocks in the Songnen Plain of Northeast China. Catena 2018, 171, 588–601. [Google Scholar] [CrossRef]
- Shi, Z.J.; Lu, Y.; Xu, Z.G.; Fu, S.L. Enzyme activities of urban soils under different land use in the Shenzhen city, China. Plant Soil Environ. 2008, 54, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhou, S.; Bao, H.; Chen, D.; Wang, C.; Li, B.; Tong, G.; Yuan, Y.; Xu, B. Improving risk management by using the spatial interaction relationship of heavy metals and PAHs in urban soil. J. Hazard. Mater. 2019, 364, 108–116. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Caban, J.R.; Kuppusamy, S.; Kim, J.H.; Yoon, Y.E.; Kim, S.Y.; Lee, Y.B. Green manure amendment enhances microbial activity and diversity in antibiotic-contaminated soil. Appl. Soil Ecol. 2018, 129, 72–76. [Google Scholar] [CrossRef]
- Jiang, B.; Adebayo, A.; Jia, J.; Xing, Y.; Deng, S.; Guo, L.; Liang, Y.; Zhang, D. Impacts of heavy metals and soil properties at a Nigerian e-waste site on soil microbial community. J. Hazard. Mater. 2019, 362, 187–195. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Fierer, N.; Turner, B.L.; Whitaker, J.; Ostle, N.J.; McNamara, N.P.; Bardgett, R.D.; Leff, J.W.; Salinas, N.; Silman, M.R.; et al. Microbes follow Humboldt: Temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology 2018, 99, 2455–2466. [Google Scholar] [CrossRef] [Green Version]
- Barnes, A.D.; Allen, K.; Kreft, H.; Corre, M.D.; Jochum, M.; Veldkamp, E.; Clough, Y.; Daniel, R.; Darras, K.; Denmead, L.H.; et al. Direct and cascading impacts of tropical land-use change on multi-trophic biodiversity. Nat. Ecol. Evol. 2017, 1, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bai, J.; Wang, W.; Zhang, G.; Cui, B.; Liu, X.; Li, X. Comprehensive assessment of soil quality for different wetlands in a Chinese delta. Land Degrad. Dev. 2018, 29, 3783–3794. [Google Scholar] [CrossRef]
- Kong, M.; Zhong, H.; Wu, Y.; Liu, G.; Xu, Y.; Wang, G. Developing and validating intrinsic groundwater vulnerability maps in regions with limited data: A case study from Datong City in China using DRASTIC and Nemerow pollution indices. Environ. Earth Sci. 2019, 78, 262. [Google Scholar] [CrossRef]
- Keshavarzi, A.; Kumar, V. Ecological risk assessment and source apportionment of heavy metal contamination in agricultural soils of Northeastern Iran. Int. J. Environ. Health Res. 2018, 29, 544–560. [Google Scholar] [CrossRef]
- Liu, C.Z.; Huang, Y.Z.; Lei, M.; Hao, X.W.; Li, X.; Tie, B.Q.; Xie, J.Z. Soil Contamination and Assessment of Heavy Metals of Xiangjiang River Basin. Environ. Sci. 2012, 33, 260–265. [Google Scholar]
- Cheng, Y.Y.; Jin, Z.J.; Wang, X.T.; Jia, Y.H.; Yuan, W.; Zhou, J.B. Effect of Land-use on Soil Fungal Community Structure and Associated Functional Group in Huixian Karst Wetland. Environ. Sci. 2020, 41, 4294–4304. [Google Scholar]
- Zhang, W.H.; Sun, R.B.; Xu, L.; Liang, J.N.; Wu, T.Y.; Zhou, J. Effects of micro-/nano-hydroxyapatite and phytoremediation on fungal community structure in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 174, 100–109. [Google Scholar] [CrossRef]
- Orr, R.; Nelson, P.N. Impacts of soil abiotic attributes on Fusarium wilt, focusing on bananas. Appl. Soil Ecol. 2018, 132, 20–33. [Google Scholar] [CrossRef]
- Schneider, F.; Don, A.; Hennings, I.; Schmittmann, O.; Seidel, S.J. The effect of deep tillage on crop yield—What do we really know? Soil Tillage Res. 2017, 174, 193–204. [Google Scholar] [CrossRef]
- Vanderlinden, K.; Vereecken, H.; Hardelauf, H.; Herbst, M.; Martínez, G.; Cosh, M.H.; Pachepsky, Y.A. Temporal Stability of Soil Water Contents: A Review of Data and Analyses. Vadose Zone J. 2012, 11, vzj2011.0178. [Google Scholar] [CrossRef]
- Li, M.M.; Nie, S.A.; Chen, X.J.; Luo, L.; Zhu, H.H.; Shi, H.; Ge, T.D.; Tong, C.L.; Wu, J.S. Distribution Characteristics of Rice Photosynthesized Carbon in Soil Aggregates of Different Size and Density. Environ. Sci. 2013, 34, 1568–1575. [Google Scholar]
- Spohn, M. Phosphorus and carbon in soil particle size fractions: A synthesis. Biogeochemistry 2020, 147, 225–242. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.Y.; Liu, L. Spatial variation of soil moisture content of a stepped slope under sprinkler irrigation and its affecting factors. Appl. Ecol. Environ. Res. 2020, 18, 3161–3175. [Google Scholar] [CrossRef]
- Vogel, S.; Conedera, M. Effects of land use-induced vegetation and topography changes on soil chemistry in the Southern Alps (Ticino, Switzerland). Plant Soil Environ. 2020, 66, 73–80. [Google Scholar] [CrossRef]
- Ozlu, E.; Kumar, S. Response of Soil Organic Carbon, pH, Electrical Conductivity, and Water Stable Aggregates to Long-Term Annual Manure and Inorganic Fertilizer. Soil Sci. Soc. Am. J. 2018, 82, 1243–1251. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Wang, X.; Shao, H.; Yang, J.; Wang, X. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar]
- Kravkaz Kuscu, I.S.; Cetin, M.; Yigit, N.; Savaci, G.; Sevik, H. Relationship between Enzyme Activity (Urease-Catalase) and Nutrient Elementin Soil Use. Pol. J. Environ. Stud. 2018, 27, 2107–2112. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of beta-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Hao, B.; Jing, X.; He, J.S.; Ma, W.; Zhu, B. Minor responses of soil microbial biomass, community structure and enzyme activities to nitrogen and phosphorus addition in three grassland ecosystems. Plant Soil 2019, 444, 21–37. [Google Scholar] [CrossRef]
- Latkovic, D.; Maksimovic, J.; Dinic, Z.; Pivic, R.; Stanojkovic, A.; Stanojkovic-Sebic, A. Case Study upon Foliar Application of Biofertilizers Affecting Microbial Biomass and Enzyme Activity in Soil and Yield Related Properties of Maize and Wheat Grains. Biology 2020, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Cenini, V.L.; Fornara, D.A.; McMullan, G.; Ternan, N.; Carolan, R.; Crawley, M.J.; Clément, J.-C.; Lavorel, S. Linkages between extracellular enzyme activities and the carbon and nitrogen content of grassland soils. Soil Biol. Biochem. 2016, 96, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, J.; Pu, S.; Blagodatskaya, E.; Kuzyakov, Y.; Razavi, B.S. Impact of manure on soil biochemical properties: A global synthesis. Sci. Total Environ. 2020, 745, 141003. [Google Scholar] [CrossRef]
- de Oliveira, T.B.; de Lucas, R.C.; Scarcella, A.S.D.A.; Contato, A.G.; Pasin, T.M.; Martinez, C.A.; Polizeli, M.D.L.T.D.M. Fungal communities differentially respond to warming and drought in tropical grassland soil. Mol. Ecol. 2020, 29, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, Z.; Mou, H.; Zhang, P.; Jia, Z. Effects of different long-term farmland mulching practices on the loessial soil fungal community in a semiarid region of China. Appl. Soil Ecol. 2019, 137, 111–119. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Christensen, B.T.; Tebbe, C.C.; Hartmann, M. Taxon-specific fungal preference for distinct soil particle size fractions. Eur. J. Soil Biol. 2019, 94, 103103. [Google Scholar] [CrossRef]
- Zarei, M.; Hempel, S.; Wubet, T.; Schäfer, T.; Savaghebi, G.; Jouzani, G.S.; Nekouei, M.K.; Buscot, F. Molecular diversity of arbuscular mycorrhizal fungi in relation to soil chemical properties and heavy metal contamination. Environ. Pollut. 2010, 158, 2757–2765. [Google Scholar] [CrossRef]
- An, N.; Gao, J.; Han, Y.; Guo, Q.; Xu, Y.; Zhang, J.; Guan, S. Effects of Manure Application on Soil Physicochemical Properties and Fungal Community Structure in Ginseng-planted Soil. J. Jilin Agric. Univ. 2019, 41, 695–706. [Google Scholar]
- Onufrak, A.; Rua, M.A.; Hossler, K. The Missing Metric: An Evaluation of Fungal Importance in Wetland Assessments. Wetlands 2020, 40, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Guo, B.; Liu, C.; Lin, Y.; Fu, Q.; Li, N.; Li, H. Soil fertility, enzyme activity, and microbial community structure diversity among different soil textures under different land use types in coastal saline soil. J. Soils Sediments 2021, 21, 2240–2252. [Google Scholar] [CrossRef]
- García-Ruiz, R.; Ochoa, V.; Hinojosa, M.B.; Carreira, J.A. Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biol. Biochem. 2008, 40, 2137–2145. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, K.-F.; Yang, S.-S.; Zhang, M. Enzyme Activities in Perfluorooctanoic Acid (PFOA)-Polluted Soils. Pedosphere 2013, 23, 120–127. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Y.; Li, X.; Chen, W. Effects of chlorimuron-ethyl on soil microorganisms and enzyme activities under moderate salt stress. Fresenius Environ. Bull. 2018, 27, 2358–2365. [Google Scholar]
- Kahkonenn, M.A.; Lankinen, P.; Hatakka, A. Hydrolytic and ligninolytic enzyme activities in the Pb contaminated soil inoculated with litter-decomposing fungi. Chemosphere 2008, 72, 708–714. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Z.; Li, F. Using ensemble models to identify and apportion heavy metal pollution sources in agricultural soils on a local scale. Environ. Pollut. 2015, 206, 227–235. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Solgun, E.; Horasan, B.Y.; Ozturk, A. Heavy metal accumulation and potential ecological risk assessment in sediments from the southwestern Konya district (Turkey). Arab. J. Geosci. 2021, 14, 730. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, M. Source identification and exchangeability of heavy metals accumulated in vegetable soils in the coastal plain of eastern Zhejiang province, China. Ecotoxicol. Environ. Saf. 2017, 142, 410–416. [Google Scholar]
- Zhao, X.; Huang, J.; Lu, J.; Sun, Y. Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef] [PubMed]



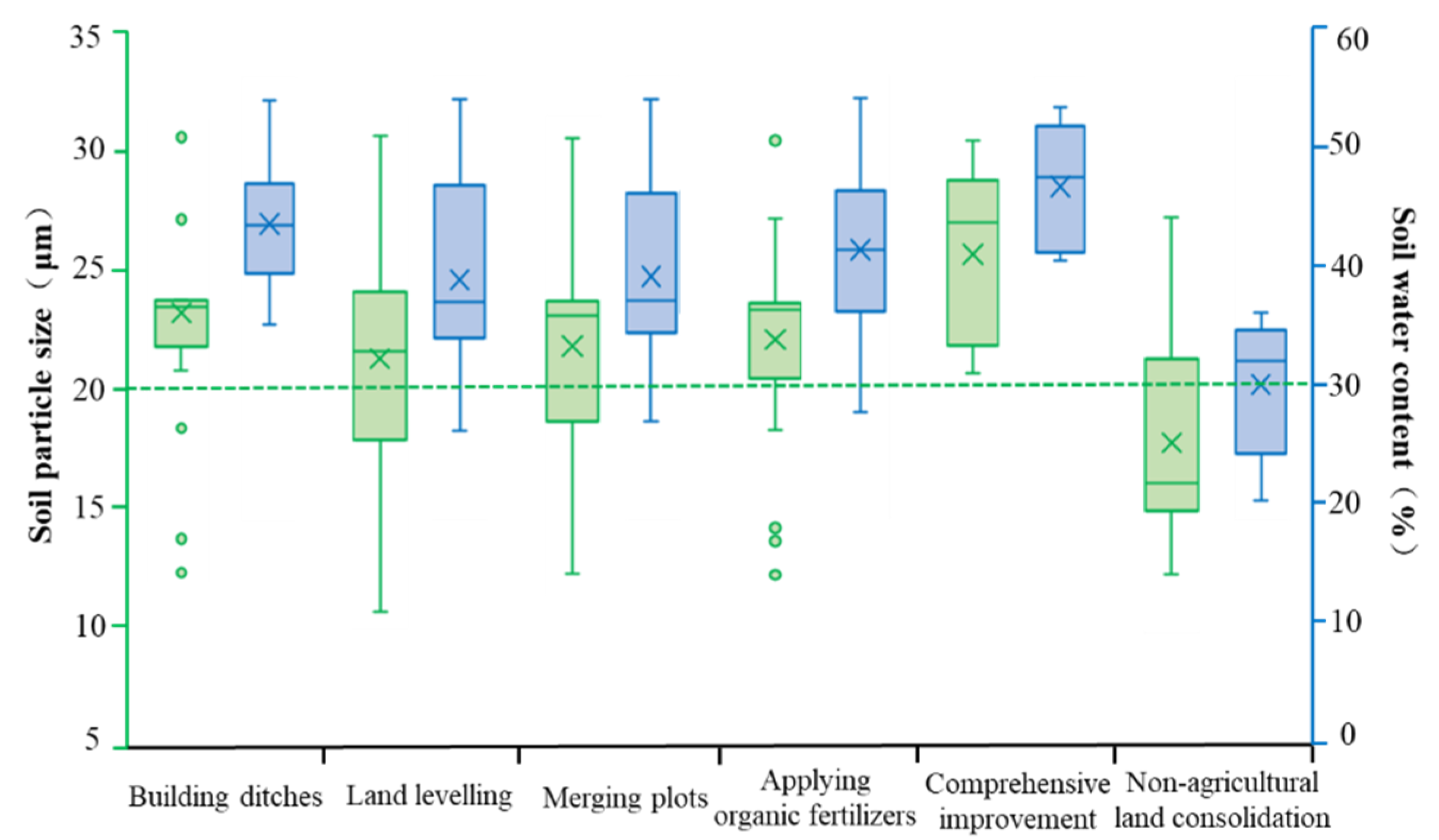
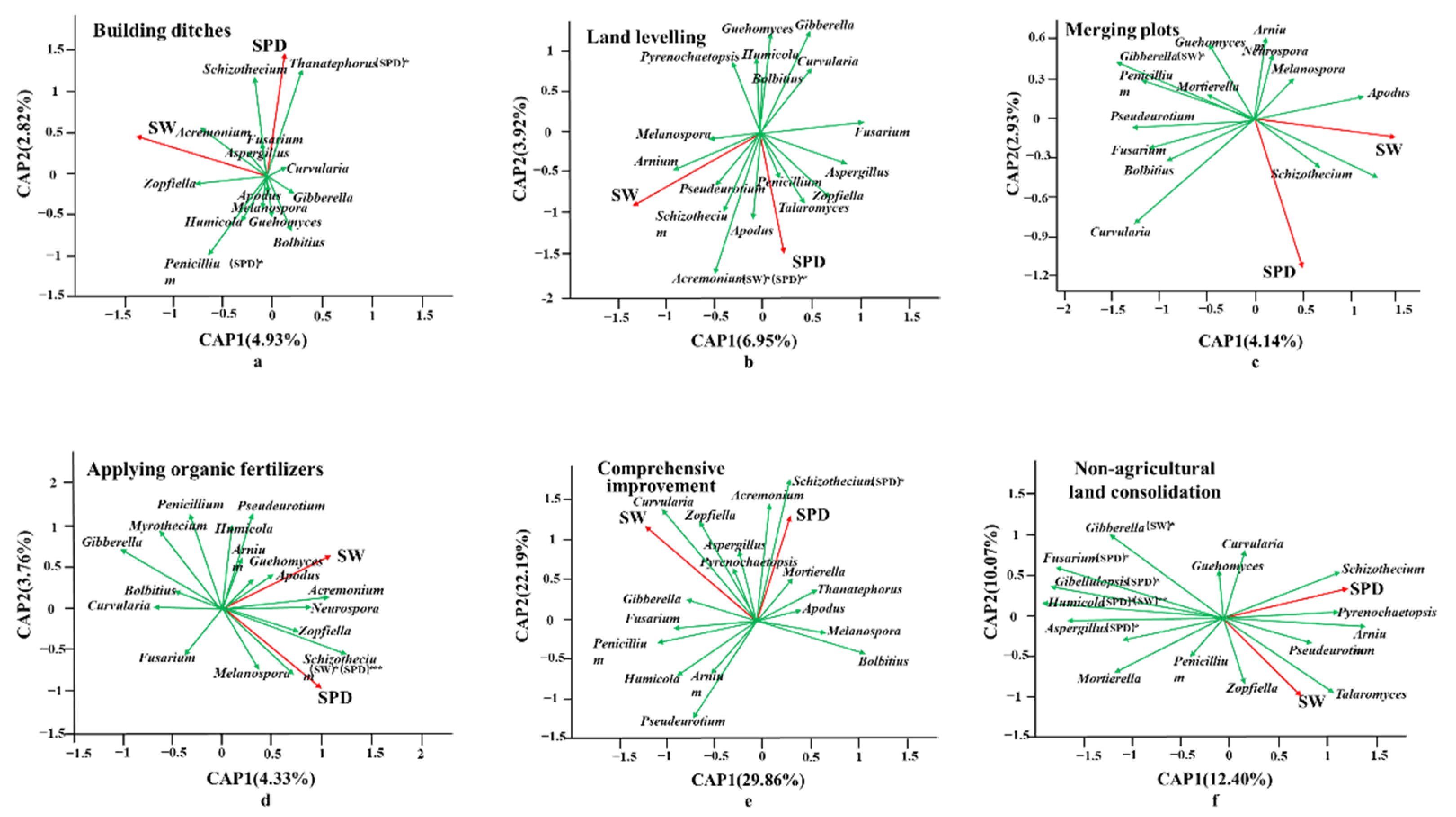

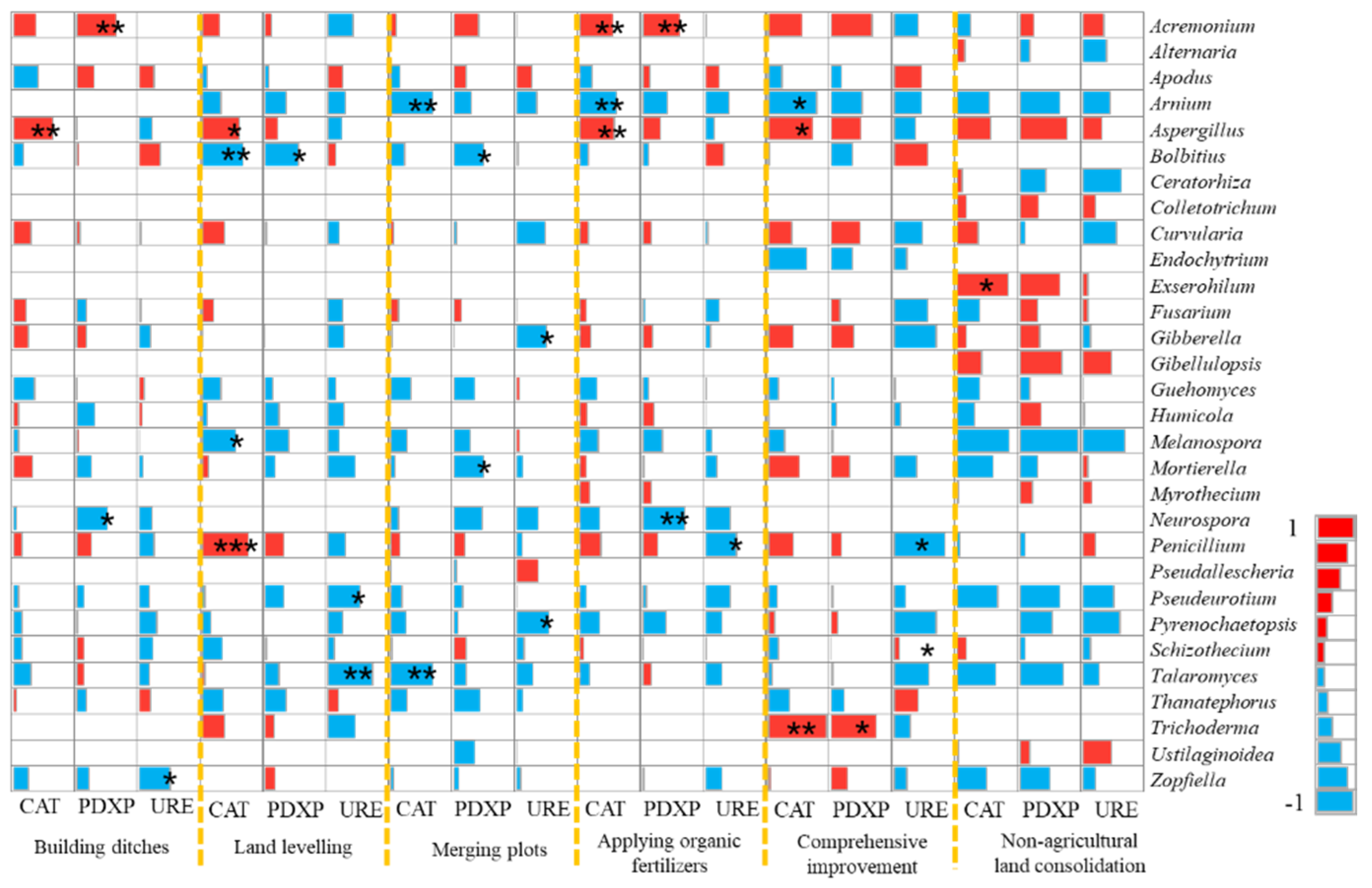
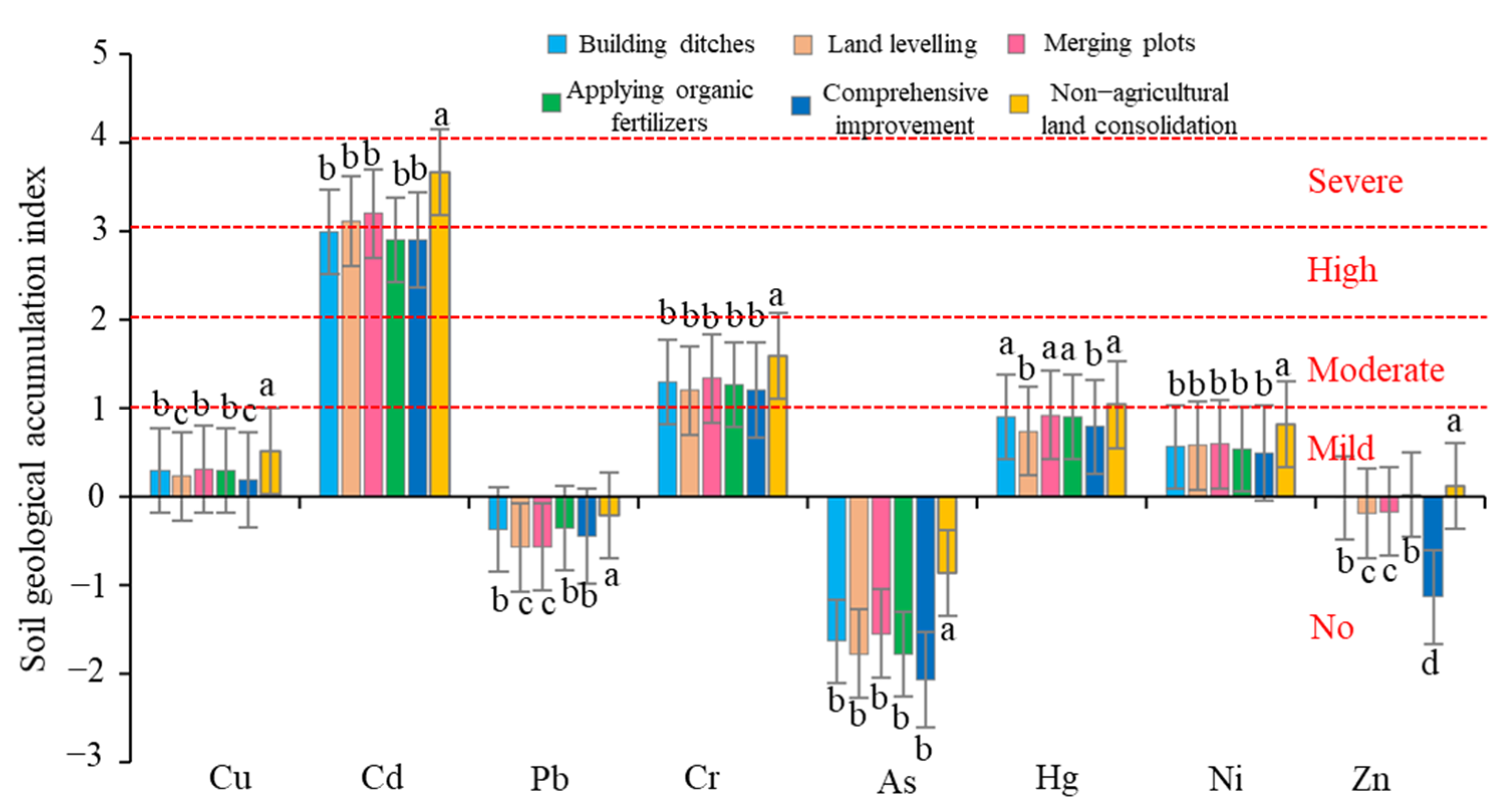



| Land Consolidation Measures | Sample | Main Methods | Sum |
|---|---|---|---|
| Building ditches | A04 A05 A06 A07 A08 A10 A11 A14 A15 A19 A21 A23 A24 A25 A27 A28 A30 A33 A35 A36 A37 A38 A39 A40 | Digging and constructing waterways in the field to facilitate irrigation or drainage. | 24 |
| Merging plots | A01 A02 A04 A05 A06 A08 A13 A14 A18 A20 A24 A25 A26 A28 A29 A30 A31 A32 A34 A37 A39 | Including plowing, deep loosening, harrowing, drowning, suppression, land levelling, ridging, and upsetting. | 21 |
| Land levelling | A02 A03 A04 A05 A06 A07 A08 A09 A12 A13 A14 A15 A16 A17 A20 A22 A23 A24 A25 A27 A28 A30 A33 A35 A37 A38 A39 | Through ownership adjustment and mechanical means, the scattered plots will be merged into large plots of cultivated land for unified farming management. | 27 |
| Applying organic fertilizers | A02 A03 A05 A06 A08 A10 A11 A14 A15 A16 A17 A18 A19 A21 A22 A23 A24 A25 A28 A30 A32 A36 A37 A38 A39 A40 B01 | In the study area, organic fertilizers were mainly applied in addition to chemical fertilizers. | 27 |
| Comprehensive improvement | A05 A06 A08 A14 A24 A25 A28 A30 A37 A39 | Simultaneously implement the four measures of building ditches, merging land, levelling land and applying organic fertilizer. | 10 |
| Non-agricultural land consolidation | B01 B02 B03 B04 B05 B06 B07 B08 B09 B10 | It is a traditional farming method and mainly uses chemical fertilizers. | 10 |
| Land Consolidation Measures | The Community Richness Index | The Community Evenness Index | The Community Diversity Index | The Community Coverage | |||
|---|---|---|---|---|---|---|---|
| Sobs | Chao | Shannoneven | Simpsoneven | Shannon | Invsimpson | Coverage | |
| Comprehensive improvement | 4312.00 ± 452.33 a | 5866.69 ± 523.61 a | 0.89 ± 0.01 a | 0.17 ± 0.02 a | 7.44 ± 0.12 a | 730.73 ± 118.61 a | 0.95 ± 0.01 a |
| Applying organic fertilizers | 4318.92 ± 447.21 a | 5768.07 ± 530.29 a | 0.88 ± 0.01 a | 0.15 ± 0.04 a | 7.36 ± 0.16 a | 658.19 ± 189.31 a | 0.96 ± 0.01 a |
| Building ditches | 4370.70 ± 443.19 a | 5808.02 ± 493.61 a | 0.88 ± 0.01 a | 0.16 ± 0.03 a | 7.39 ± 0.14 a | 693.77 ± 161.98 a | 0.95 ± 0.01 a |
| Merging plots | 4349.68 ± 440.11 a | 5823.19 ± 528.11 a | 0.88 ± 0.01 a | 0.15 ± 0.04 a | 7.38 ± 0.15 a | 675.83 ± 176.20 a | 0.96 ± 0.01 a |
| Land levelling | 4405.47 ± 396.52 a | 5963.16 ± 483.81 a | 0.88 ± 0.01 a | 0.16 ± 0.04 a | 7.39 ± 0.17 a | 696.92 ± 202.67 a | 0.96 ± 0.01 a |
| Non-agricultural land consolidation | 3338.20 ± 675.30 b | 4348.85 ± 922.49 b | 0.87 ± 0.01 a | 0.13 ± 0.04 a | 7.01 ± 0.25 b | 439.17 ± 166.72 b | 0.96 ± 0.01 a |
| Group | pH | Organic Matter(g/kg) | Available Phosphorus (mg/kg) | Available Potassium (μg/mL) | Total Nitrogen (g/kg) |
|---|---|---|---|---|---|
| Building ditches (n = 24) | 7.09 ± 0.63 b | 46.63 ± 14.22 b | 90.78 ± 83.78 a | 29.65 ± 13.31 a | 2.41 ± 0.73 b |
| Land levelling (n = 27) | 7.23 ± 0.63 a | 41.97 ± 15.33 b | 72.85 ± 54.01 b | 28.99 ± 12.00 a | 2.20 ± 0.77 b |
| Merging plots (n = 21) | 7.11 ± 0.57 b | 41.52 ± 15.30 b | 70.24 ± 57.77 b | 28.60 ± 15.25 a | 2.23 ± 0.77 b |
| Applying organic fertilizers (n = 27) | 7.11 ± 0.64 b | 52.49 ± 11.59 a | 93.67 ± 84.50 a | 31.38 ± 14.57 a | 2.51 ± 0.64 b |
| Comprehensive improvement (n = 10) | 6.93 ± 0.51 b | 57.98 ± 11.66 a | 79.80 ± 58.37 b | 37.78 ± 7.61 a | 3.08 ± 0.45 a |
| Non-agricultural land consolidation (n = 10) | 7.38 ± 0.71 a | 30.21 ± 9.80 c | 53.43 ± 37.13 c | 24.00 ± 14.93 b | 1.69 ± 0.45 c |
| Group | Catalase (mg/g) | Phosphatase (mg/g) | Urease (mg/g) |
|---|---|---|---|
| Building ditches | 207.66 ± 38.30 a | 16.017 ± 8.44 a | 0.25 ± 0.16 a |
| Land levelling | 203.62 ± 38.48 a | 17.35 ± 10.65 a | 0.28 ± 0.22 a |
| Merging plots | 201.61 ± 32.37 a | 15.28 ± 9.97 b | 0.23 ± 0.15 a |
| Applying organic fertilizers | 198.61 ± 41.72 a | 17.54 ± 9.68 a | 0.25 ± 0.16 a |
| Comprehensive improvement | 206.72 ± 28.98 a | 20.88 ± 10.58 a | 0.33 ± 0.16 a |
| Non-agricultural land consolidation | 183.58 ± 50.72 a | 12.32 ± 11.87 c | 0.22 ± 0.30 a |
| Cu | Cd | Pb | Cr | As | Hg | Ni | Zn | |
|---|---|---|---|---|---|---|---|---|
| Building ditches | 45.95 ± 12.74 | 2.28 ± 0.23 | 43.39 ± 13.23 | 214.65 ± 11.67 | 4.66 ± 3.83 | 0.57 ± 0.28 | 53.15 ± 5.60 | 125.75 ± 26.70 |
| Land levelling | 39.44 ± 7.92 | 2.23 ± 0.27 | 37.54 ± 12.81 | 214.61 ± 11.34 | 4.21 ± 3.51 | 0.45 ± 0.15 | 54.06 ± 7.63 | 110.47 ± 18.39 |
| Merging plots | 41.72 ± 8.83 | 2.27 ± 0.33 | 37.62 ± 12.06 | 212.64 ± 11.22 | 4.99 ± 3.91 | 0.53 ± 0.25 | 54.55 ± 7.44 | 112.51 ± 21.58 |
| Applying organic fertilizers | 46.55 ± 12.25 | 2.27 ± 0.31 | 42.98 ± 10.38 | 216.45 ± 11.12 | 3.89 ± 2.90 | 0.53 ± 0.21 | 55.00 ± 6.86 | 128.36 ± 24.72 |
| Comprehensive improvement | 39.90 ± 9.17 | 2.28 ± 0.13 | 40.29 ± 14.98 | 208.70 ± 6.59 | 2.93 ± 3.94 | 0.47 ± 0.14 | 51.10 ± 6.93 | 117.82 ± 20.45 |
| Non-agricultural land consolidation | 48.97 ± 6.71 | 3.30 ± 0.55 | 46.83 ± 8.81 | 252.96 ± 11.05 | 7.88 ± 5.29 | 0.65 ± 0.37 | 64 ± 10.85 | 136.82 ± 21.17 |
| Maximum | 84 | 3.98 | 77.5 | 268.8 | 14.4 | 1.38 | 80.4 | 198 |
| Minimum | 25 | 1.57 | 20 | 195 | 0.81 | 0.1 | 39 | 70.6 |
| Average value | 45.17 | 2.5 | 41.94 | 223.79 | 5.29 | 0.55 | 56.77 | 123.27 |
| Variation coefficient (%) | 22.38 | 22.86 | 26.4 | 8.72 | 77.91 | 49.99 | 15.96 | 20.7 |
| National standard | 100 | 0.3 | 300 | 300 | 25 | 0.5 | 50 | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Yang, H.; Ye, Y.; Wen, J.; Chen, D. How Does Land Consolidation Affect Soil Fungal Community Structure? Take Heavy Metal Contaminated Areas in Eastern China for Example. Land 2022, 11, 142. https://doi.org/10.3390/land11010142
Lin Y, Yang H, Ye Y, Wen J, Chen D. How Does Land Consolidation Affect Soil Fungal Community Structure? Take Heavy Metal Contaminated Areas in Eastern China for Example. Land. 2022; 11(1):142. https://doi.org/10.3390/land11010142
Chicago/Turabian StyleLin, Yaoben, Haoran Yang, Yanmei Ye, Jiahao Wen, and Danling Chen. 2022. "How Does Land Consolidation Affect Soil Fungal Community Structure? Take Heavy Metal Contaminated Areas in Eastern China for Example" Land 11, no. 1: 142. https://doi.org/10.3390/land11010142
APA StyleLin, Y., Yang, H., Ye, Y., Wen, J., & Chen, D. (2022). How Does Land Consolidation Affect Soil Fungal Community Structure? Take Heavy Metal Contaminated Areas in Eastern China for Example. Land, 11(1), 142. https://doi.org/10.3390/land11010142





