Carbon Storage of Single Tree and Mixed Tree Dominant Species Stands in a Reserve Forest—Case Study of the Eastern Sub-Himalayan Region of India
Abstract
1. Introduction
2. Materials and Methods
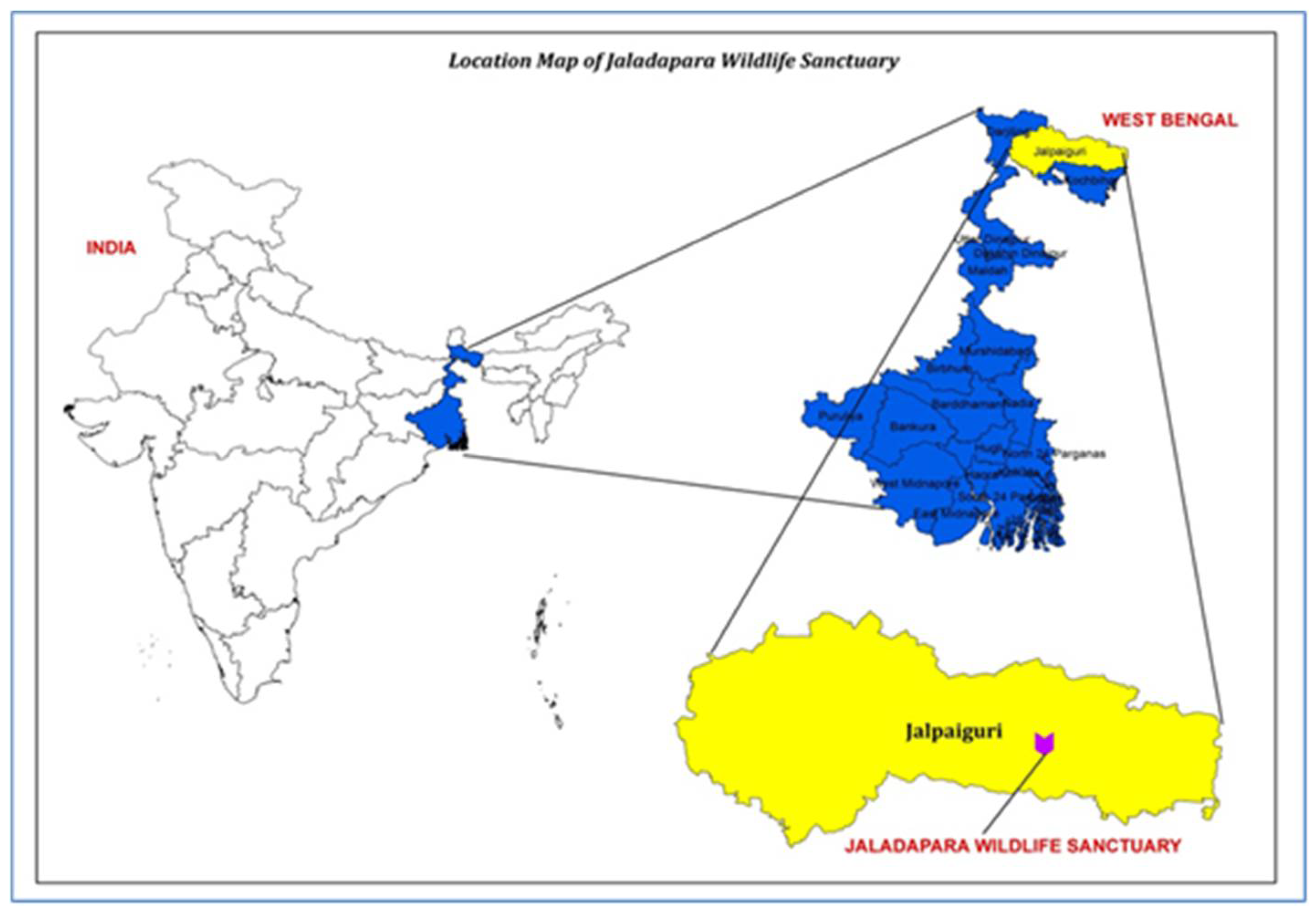
2.1. Site Description and Stand Structure
2.2. Computation of Indices
2.3. Soil Organic Carbon (SOC) Stock
2.4. Statistical Analysis
3. Results and Discussion
3.1. Litter Production and Decomposition
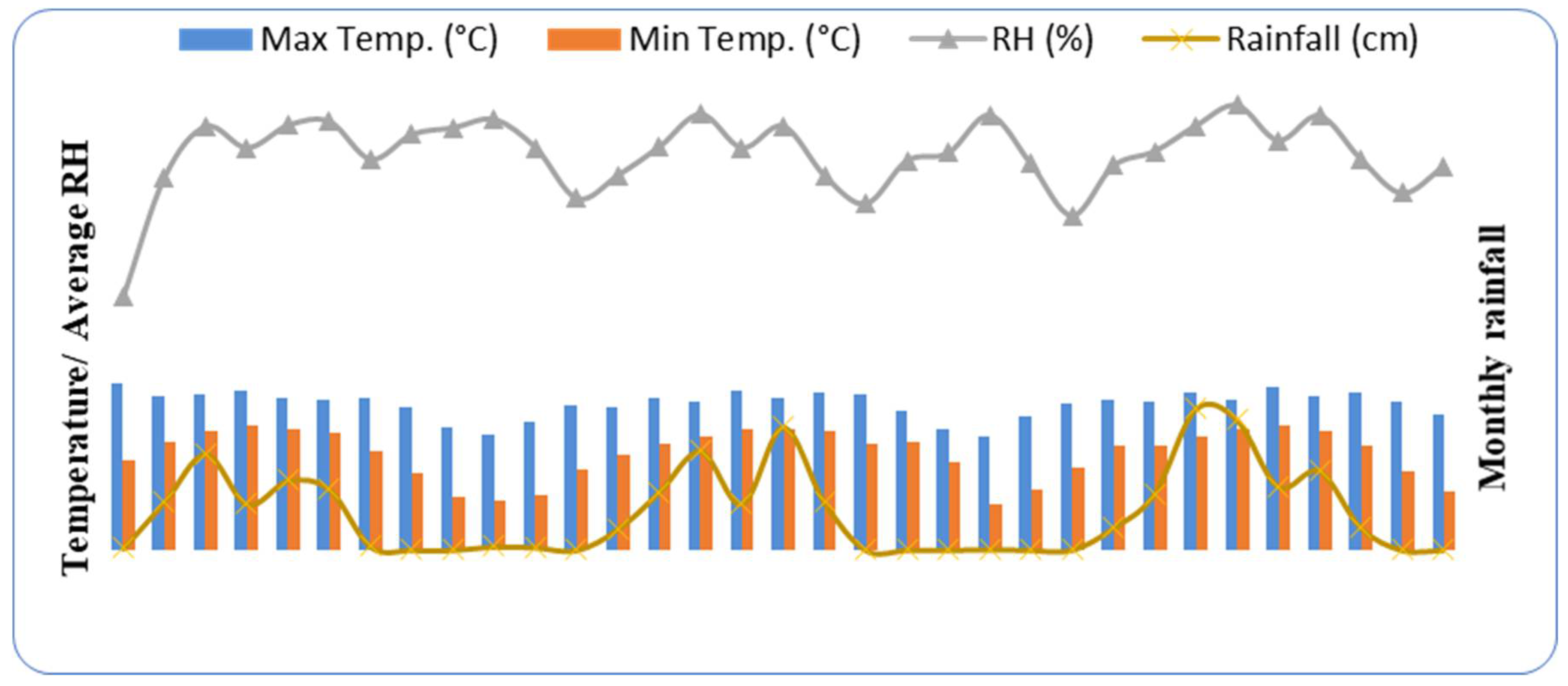
3.2. Soil pH, EC (m mhos Decisiemens m−1) and Moisture (%)
3.3. Litter Available ‘N’, ‘P’ and ‘K’ Release
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, G.; Chakravarty, S. Vegetation Analysis and Production Potential of Chilapatta Reserve Forest Ecosystem of West Bengal. Ph.D. Thesis, Uttar Banga Krishi Viswavidyalaya, Behar, India, 2012; pp. 12–18. [Google Scholar]
- Rawat, R.S. Studies on interrelationship of woody vegetation, density and soil characteristics along an altitudinal gradient in a montane forest of Garhwal Himalayas. Indian For. 2005, 131, 991–995. [Google Scholar]
- Schimel, D.S. Terrestrial ecosystems and the carbon cycle. Glob. Chang. Biol. 1994, 1, 77–91. [Google Scholar] [CrossRef]
- Jha, M. A preliminary study of ecosystems and biodiversity in Lonar Crater. Indian For. 2003, 129, 1192–1200. [Google Scholar]
- Pande, P.K. Litter nutrient dynamics of Shorea robusta Gaertn. plantation at Doon Valley (Uttaranchal), India. Indian For. 2001, 127, 980–994. [Google Scholar]
- Bhadwal, S.; Singh, R. Carbon sequestration estimates for forestry options under different land use scenarios in India. Curr. Sci. 2002, 83, 1380–1386. [Google Scholar]
- Chhabra, A.; Palria, S.; Dadhwal, V.K. Growing stock-based forest biomass estimates for India. Biomass Bioenergy 2002, 22, 187–194. [Google Scholar] [CrossRef]
- Shadangi, D.K.; Nath, V. Litter decomposition in eucalyptus and pines plantations and natural Sal forests related to micro-arthropods in different season in Amarkantak, Madhya Pradesh. Indian For. 2006, 132, 420–428. [Google Scholar]
- Chacon, N.; Dezzeo, N. Litter decomposition in primary forest and adjacent fire-disturbed forests in the Gran Sabana, southern Venezuela. Biol. Fertil. Soils 2007, 43, 815–821. [Google Scholar] [CrossRef]
- Hossain, M.; Hoque, A.F. Litter production and decomposition in mangroves—A review. Indian J. For. 2008, 31, 227–238. [Google Scholar]
- Shukla, G.; Biswas, R.; Das, A.P.; Chakravarty, S. Plant diversity at Chilapatta Reserve Forest of Terai Duars in sub-humid tropical foothills of Indian Eastern Himalayas. J. For. Res. 2014, 25, 591–596. [Google Scholar] [CrossRef]
- Paul, S.C. Land Use Effects on Soil Characteristics of Terai Region of West Bengal. Master’s Thesis, Uttar Banga Krishi Viswavidyalaya, West Bengal, India, 2004. [Google Scholar]
- Pande, P.K. Litter Production and Decomposition, Mineral Release and Biochemical Diversity of Four Forest Stands at FRI Demonstration Area. Ph.D. Thesis, Garhwal University, Srinagar (Garhwal), Indian, 1986. Unpublished. [Google Scholar]
- Jackson, M.L. Soil Chemistry Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1967. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic and available form of phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Moran, E.F.; Brondizion, E.S.; Tucker, J.M.; Da Silva-Forsberg, M.C.; McCracken, S.; Falesi, I. Effects of soil fertility and land use on forest succession in Amazônia. For. Ecol. Manag. 2000, 139, 93–108. [Google Scholar] [CrossRef]
- Lu, D.; Moran, E.; Mausel, P. Linking Amazonian secondary succession forest growth to soil properties. Land Degrad. Dev. 2002, 13, 331–343. [Google Scholar] [CrossRef]
- De M. Sá, J.C.; Cerri, C.C.; Dick, W.A.; Lal, R.; Filho, S.P.V.; Piccolo, M.C.; Feigl, B.E. Organic matter dynamics and carbon sequestration rates for a tillage chronosequence in Brazilian Oxisol. Soil Sci. Soc. Am. J. 2001, 65, 1486–1499. [Google Scholar] [CrossRef]
- Takeda, H. A 5 year study of pine needle litter decomposition in relation to mass loss and faunal abundances. Pedobiologia 1988, 32, 221–226. [Google Scholar]
- Pande, P.K.; Sharma, S.C. Litter decomposition in some plantation (India). Ann. For. 1993, 1, 90–101. [Google Scholar]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; University of California Press: Los Angeles, CA, USA, 1979; p. 361. [Google Scholar]
- Xiao-wen, D.; Ying, L.; Shi-jie, H. Carbon and nitrogen dynamics in early stages of forest litter decomposition as affected by nitrogen addition. J. For. Res. 2009, 20, 111–116. [Google Scholar]
- Uriarte, M.; Turner, B.L.; Thompson, J.; Zimmerman, J.K. Linking spatial patter ns of leaf litter fall and soil nutrients in a tropical forest: A neighborhood approach. Ecol. Appl. 2015, 25, 2022–2034. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.A.; Kumar, M.; Kumar, A.; Negi, A.K.; Todaria, N.P.; Malik, Z.A.; Pala, N.A.; Kumar, A.; Shukla, G. Altitudinal gradient of species diversity and community of woody vegetation in the Western Himalayas. Glob. Ecol. Conserv. 2020, 24, e01302. [Google Scholar] [CrossRef]
- Ashton, C.E.; Hogarth, P.J.; Ormond, R. Breakdown of mangrove leaf litter in a managed mangrove forest in Peninsular, Malaysia. Hydrobiology 1999, 413, 77–88. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Wong, S.Y.; Lan, Y.C.; Wang, N.L. Litter production and decomposition in a subtropical mangrove swamp receiving wastewater. J. Exp. Mar. Biol. Ecol. 1999, 226, 1–18. [Google Scholar] [CrossRef]
- Hagen-Thron, A.; Callesen, I.; Armolaitis, K.; Nihlgard, B. The impact of six European tree species on the chemistry of mineral topsoil in forest plantations on former agricultural land. For. Ecol. Manag. 2006, 195, 373–384. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Purahong, W.; Kapturska, D.; Pecyna, M.J.; Schulz, E.; Schloter, M.; Buscot, F.; Hofrichter, M.; Dirk Kruger, D. Influence of different forest system management practices on leaf litter decomposition rates, nutrient dynamics and the activity of ligninolytic enzymes: A case study from central European forests. PLoS ONE 2014, e93700. [Google Scholar] [CrossRef]
- Aponte, C.; Garcia, L.V.; Maranon, T. Tree species effect on litter decomposition and nutrient release in Mediterranean Oak Forests changes over time. Ecosystem 2012, 15, 1204–1218. [Google Scholar] [CrossRef]
- Berg, B.; Davey, M.; De Marco, A.; Emmett, B.; Faituri, M.; Hobbie, S.; Johansson, M.B.; Liu, C.; McClaugherty, C.; Norell, L.; et al. Factors influencing limit values for pine needle litter decomposition: A synthesis for boreal and temperate pine forest systems. Biogeochemistry 2010, 100, 57–73. [Google Scholar] [CrossRef]
- Jacob, M.; Viedenz, K.; Polle, A.; Thomas, F.M. Leaf litter decomposition in temperate deciduous forest stands with a decreasing fraction of beech (Fagus sylvatica). Oecologia 2010, 164, 1083–1094. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photo degradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Aponte, C.; García, L.V.; Pérez-Ramos, I.M.; Gutiérrez, E.; Marañó, T. Oak trees and soil interactions in Mediterranean forests: A positive feedback model. J. Veg. Sci. 2011, 22, 856–867. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, M.P.; Yang, T. Estimation of carbon stock for greenhouse gas emissions from hydropower reservoirs. Stoch. Environ. Res. Risk Assess. 2018, 32, 3183–3193. [Google Scholar] [CrossRef]
- Champion, H.G.; Seth, S.K. A Revised Survey of the Forest Types of India; Manager of Publications: New Delhi, India, 1968. [Google Scholar]
- Kumar, A.; Sharma, M.P. Estimation of carbon stock of Balganga reserve forest, Uttarakhand, India. For. Sci. Technol. 2015, 11, 177–181. [Google Scholar]
- Pastor, J. Successional changes in nitrogen availability as a potential factor contributing to spruce declines in boreal North America. Can. J. For. Res. 1987, 17, 1394–1400. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Li, X.R.; Liu, L.C.; Jia, R.L.; Zhang, J.G.; Wang, T. Distribution, biomass and dynamics of root in revegetated stand of Caragana korshinskii in the Tengger Desert, northwestern China. J. Plant Res. 2009, 122, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Gupta, S.R. Plant decomposition and soil respiration in terrestrial ecosystems. Bot. Rev. 1977, 43, 449–528. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Song, Y.C.; Wu, H.Q.; You, W.H. Dynamics of litter amount and its decomposition in different successional stage of evergreen broad-leaved forest in Tiantong, Zhejiang, Province. Acta Phytoecol. Sin. 1999, 23, 250–255. [Google Scholar]
- Wang, J.; Huang, J.H. Comparison of major nutrient release patterns in leaf litter decomposition in warm temperate zone of China. Acta Phytoecol. Sin. 2001, 25, 375–380. [Google Scholar]
- Scott, N.A.; Binkley, D. Foliage litter quality and annual net N mineralization: Comparison across North America forest site. Oecologia 1997, 111, 151–159. [Google Scholar] [CrossRef]
- Pande, P.K.; Meshram, P.B.; Banerjee, S.K. Litter production and nutrient return in tropical dry deciduous teak forests of Satpura Plateau in central India. Trop. Ecol. 2002, 43, 337–344. [Google Scholar]
- Gairola, S.; Sharma, C.M.; Ghildiyal, S.K.; Suyal, S. Chemical properties of soils in relation to forest composition in moist temperate valley slopes of Garhwal Himalaya, India. Environmentalist 2012, 32, 512–523. [Google Scholar] [CrossRef]
- Jha, M.N.; Gupta, M.K.; Dimri, B.M. Soil organic matter status under different social forestry plantations. Indian For. 1999, 125, 883–890. [Google Scholar]
- Paudel, S.; Sah, J.P. Physiochemical characteristic of soil in Sal (Shorea robusta) forests in eastern Nepal. Himal. J. Sci. 2003, 1, 107–110. [Google Scholar]
- De Hann, S. Humus, its formation, its relation with the mineral part of the soil and its significance for soil productivity. In Organic Matter Studies; International Atomic Energy Agency: Vienna, Austria, 1977; pp. 21–30. [Google Scholar]
- Chakraborthy, R.N.; Chakraborthy, D. Changes in soil properties under Acacia auriculiformis plantation in Tripura. Indian For. 1989, 115, 272–273. [Google Scholar]
- Sharma, B.D.; Gupta, I.C. Effect of tree cover on soil fertility in western Rajasthan. Indian For. 1989, 115, 57–68. [Google Scholar]
- Stevenson, F.J. Humus Chemistry, 2nd ed.; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Hattenschwiler, S.; Gasser, P. Soil animals alter plant litter diversity effects on decomposition. Proc. Natl. Acad. Sci. USA 2005, 102, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.B.L.; Kaul, O.N.; Mathur, R.N. Seasonal variation of nutrient in foliage and their return through leaf-litter in some plantation ecosystem. In Proceedings of the Symposium on Man-Made Forests in India, Dehradun, India, 8–10 June 1972. [Google Scholar]
- Kumar, A.; Sharma, M.P. Carbon stock estimation in the catchment of Kotli Bhel 1A Hydroelectric Reservoir, Uttarakhand, India. Ecotoxicol. Environ. Saf. 2016, 134, 365–369. [Google Scholar] [CrossRef]
- Upadhyay, S.D.; Singh, V.P. Microbial turnover of organic matter in litter decomposition in semi-arid grassland. Ecology 1981, 21, 100–109. [Google Scholar]
- Kumar, A.; Sharma, M.P. Estimation of Soil Organic Carbon in the Forest Catchment of two Hydroelectric Reservoirs in Uttarakhand, India. Hum. Ecol. Risk Assess. 2016, 22, 991–1001. [Google Scholar] [CrossRef]
- Tandon, H.L.S. Methods of Analysis of Soils, Plants, Waters, Fertilizers and Organic Manures; Fertilizer Development and Consultation Organization: New Delhi, India, 2005; p. 204. [Google Scholar]
- Jha, M.N.; Dimri, B.M. Soil nutrient and forest productivity in four stands. J. Indian Soc. Soil Sci. 1991, 39, 735–738. [Google Scholar]
- Srinivasan, U.M.; Caulfield, I. Agroforestry land management system in developing countries: An overview. Indian For. 1989, 115, 57–68. [Google Scholar]
- Panwar, P.; Pal, S.; Reza, S.K.; Sharma, B. Soil fertility index, soil evaluation factor, and microbial indices under different land use in acidic soil of humid subtropical India. Commun. Soil Sci. Plant Anal. 2011, 42, 2724–2737. [Google Scholar] [CrossRef]
- Koul, D.N. Carbon Sequestration Estimates of Various Land Uses in Terai Zone of West Bengal. Master’s Thesis, Uttar Banga Krishi Viswavidyalaya, Pundibari, India, 2004. [Google Scholar]
- Jerabkova, L.; Prescott, C.E.; Kishchuk, B.E. Nitrogen availability in soil and forest floor of contrasting types of boreal mixed wood forests. Can. J. For. Res. 2006, 36, 112–122. [Google Scholar] [CrossRef]
- Johnston, A.E. Soil organic matter, effects on soil and crops. Soil Use Manag. 1986, 2, 97–105. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, M.P.; Taxak, A.K. Effect of vegetation communities and altitudes on the SOC stock in Kotli Bhel-1A Catchment, India. Clean Soil Air Water 2017, 45, 1600650. [Google Scholar] [CrossRef]
- Binkly, D.; Vitousek, P.M. Soil nutrient availability. In Plant Physiological, Field Methods and Instrumentation; Pearcy, R.W., Ehleringer, J.R., Mooney, H., Rundel, P.W., Eds.; Champan and Hall: London, UK, 1989; pp. 75–96. [Google Scholar]
- Chauhan, M.; Kumar, M.; Kumar, A. Impact of carbon stocks of Anogeissus latifolia on climate change and socio-economic development: A case study of Garhwal Himalaya, India. Water Air Soil Pollut. 2020, 231, 1–15. [Google Scholar] [CrossRef]
- Rana, K.; Kumar, M.; Kumar, A. Assessment of annual shoot biomass and carbon storage potential of Grewia optiva: An approach to combat Climate Change in Garhwal Himalaya. Water Air Soil Pollut. 2020, 231, 1–13. [Google Scholar] [CrossRef]
- Dimri, B.M.; Jha, M.N.; Gupta, M.K. Status of soil nitrogen at different altitudes in Garhwal Himalaya. Van Vigyan 1997, 359, 77–84. [Google Scholar]
- Kumar, M.; Kumar, A. Carbon stock potential in Pinus roxburghii forests of Indian Himalayan regions. Environ. Dev. Sustain. 2021. [Google Scholar] [CrossRef]
- Sariyildiz, T.; Anderson, J.M. Interactions between litter quality, decomposition and soil fertility: A laboratory study. Soil Biol. Biochem. 2003, 35, 391–399. [Google Scholar] [CrossRef]
- Loreau, M. Biodiversity and ecosystem functioning: Recent theoretical advances. Oikos 2000, 91, 3–17. [Google Scholar] [CrossRef]
- Spehn, E.M.; Hector, A.; Joshi, J.; Scherer-Lorenzen, M.; Schmid, B.; Bazeley-White, E.; Beierkuhnlein, C.; Caldeira, M.C.; Diemer, M.; Dimitrakopoulos, P.G. Ecosystem effects of biodiversity manipulations in European grasslands. Ecol. Monogr. 2005, 75, 37–63. [Google Scholar] [CrossRef]
- Erskine, P.D.; Lamb, D.; Bristow, M. Tree species diversity and ecosystem function: Can tropical multi-species plantations generate greater productivity? For. Ecol. Manag. 2006, 233, 205–210. [Google Scholar] [CrossRef]
- Vila, M.; Vayreda, J.; Comas, L.; Ibanez, J.J.; Mata, T.; Obon, B. Species richness and wood production: A positive association in Mediterranean forests. Ecol. Lett. 2007, 10, 241–250. [Google Scholar] [CrossRef]
- Fornara, D.A.; Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- Healy, C.; Gotelli, N.J.; Potvin, C. Partitioning the effects of biodiversity and environmental heterogeneity for productivity and mortality in a tropical tree plantation. J. Ecol. 2008, 96, 903–913. [Google Scholar] [CrossRef]
- Piotto, D. A meta-analysis comparing tree growth in monocultures and mixed plantations. For. Ecol. Manag. 2008, 255, 781–786. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Potvin, C. Can we predict carbon stocks in tropical ecosystems from tree diversity? Comparing species and functional diversity in a plantation and a natural forest. New Phytol. 2010, 189, 978–987. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, B.; Xian, J.R.; Korpelainen, H.; Li, C. Links between plant diversity, carbon stocks and environmental factors along a successional gradient in a subalpine coniferous forest in Southwest China. For. Ecol. Manag. 2011, 262, 361–369. [Google Scholar] [CrossRef]
- Vandermeer, J. The Ecology of Intercropping; Cambridge University Press: Cambridge, UK, 1989; p. 249. [Google Scholar]
- Catovsky, S.; Bradford, M.A.; Hector, A. Biodiversity and ecosystem productivity: Implications for carbon storage. Oikos 2002, 97, 443–448. [Google Scholar] [CrossRef]
- Kirby, K.R.; Potvin, C. Variation in carbon storage among tree species implications for the management of a small scale carbon sink project. For. Ecol. Manag. 2007, 246, 208–221. [Google Scholar] [CrossRef]
- Singh, P.; Dubey, P.; Jha, K.K. Biomass production and carbon storage at harvest age in superior Dendrocalamus strictus plantation in dry deciduous forest region in India. In Proceedings of the Abstract Volume of VIIth World Bamboo Congress, Proceedings of the VIIth World Bamboo Congress, New Delhi, India, 27 February–4 March 2004. [Google Scholar]
- Chhabra, A.; Dhadwal, V.K. Forest soil organic carbon pool: An estimate and review of Indian studies. Indian For. 2005, 131, 201–214. [Google Scholar]
- Jha, M.N.; Gupta, M.K.; Saxena, A.; Kumar, R. Soil organic carbon store in different forest in India. Indian For. 2003, 129, 715–724. [Google Scholar]
- Scharlemann, J.P.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global soil carbon: Under-standing and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Gederma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Bhat, J.A.; Kumar, M.; Negi, A.K.; Pala, N.A.; Todaria, N.P. Soil organic carbon stock and sink potential in high mountain temperate Himalayan forests of India. Int. J. Curr. Res. 2012, 4, 206–209. [Google Scholar]
- Woomer, P.L.; Martin, A.; Albrecht, A.; Reseck, D.V.S.; Scharpenseel, H.W. The importance and management of soil organic matter in the tropics. In The Biological Management of Tropical Soil Fertility; Woomer, P.L., Swift, M.J., Eds.; Wiley: Chichester, UK, 1994. [Google Scholar]

| Stand | ILM | Decomposition (of Total Litter %) | Litter Decomposed (Mg ha−1/3 Months) | TD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mar | Jun | Sept | Dec | Mar | Jun | Sept | Dec | |||
| LPDS | 13.2a | 39.5 | 67.6 | 93.9 | 99.3 | 5.2ab | 3.7a | 3.5a | 0.7b | 13.1a |
| MCDS | 13.9a | 43.1 | 72.2 | 88.3 | 96.5 | 6.0a | 4.0a | 2.3b | 1.1a | 13.4a |
| SRDS | 10.6b | 40.3 | 69.9 | 90.3 | 98.5 | 4.3c | 3.1c | 2.2b | 0.8b | 10.4b |
| TGDS | 8.90c | 37.0 | 71.1 | 93.1 | 98.8 | 3.3d | 3.0c | 2.0b | 0.5c | 8.8c |
| MS | 13.4a | 41.6 | 68.0 | 91.4 | 98.2 | 5.6a | 3.5ab | 3.2a | 0.9b | 13.2a |
| SEm | 0.13 | 0.11 | 0.12 | 0.02 | 0.06 | 0.09 | 0.07 | 0.05 | 0.05 | 0.08 |
| SEd | 0.18 | 0.15 | 0.16 | 0.02 | 0.09 | 0.12 | 0.10 | 0.07 | 0.07 | 0.11 |
| P = 0.05 | 0.40 | 0.33 | 0.36 | 0.05 | 0.19 | 0.27 | 0.23 | 0.16 | 0.16 | 0.24 |
| Stands | Soil Parameter/Soil Depth | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | EC (m mhos Decisiemens m−1) | Soil Moisture (%) | |||||||
| 0–20 cm | 20–40 cm | 40–60 cm | 0–20 cm | 20–40 cm | 40–60 cm | 0–20 cm | 20–40 cm | 40–60 cm | |
| LPDS | 4.61 | 4.83 | 5.13 | 0.37 | 0.42 | 0.44 | 26.03 | 33.53 | 34.35 |
| MCDS | 4.74 | 4.93 | 5.18 | 0.24 | 0.39 | 0.38 | 22.91 | 26.87 | 31.26 |
| SRDS | 4.76 | 5.20 | 5.52 | 0.35 | 0.40 | 0.33 | 24.76 | 32.63 | 34.57 |
| TGDS | 4.91 | 4.98 | 5.09 | 0.45 | 0.31 | 0.37 | 23.15 | 27.76 | 30.55 |
| MS | 4.98 | 5.12 | 5.32 | 0.41 | 0.65 | 0.43 | 28.61 | 30.23 | 34.92 |
| SEm | 0.14 | 0.10 | 0.08 | 0.01 | 0.02 | 0.01 | 0.02 | 0.54 | 0.01 |
| SEd | 0.20 | 0.14 | 0.11 | 0.02 | 0.03 | 0.02 | 0.03 | 0.76 | 0.02 |
| P = 0.05 | 0.44 | 0.30 | 0.23 | 0.04 | 0.06 | 0.04 | 0.07 | 1.66 | 0.05 |
| Stands | Jan | Mar | Jun | Sept | RS * | ||||
|---|---|---|---|---|---|---|---|---|---|
| IC | IQ | DC | DQ 1 | DC | DQ 2 | DC | DQ 3 | ||
| Nitrogen | |||||||||
| LPDS | 1.55 | 0.20 | 1.2 | 0.16 | 0.93 | 0.12 | 0.09 | 0.01 | 0.19 |
| MCDS | 1.2 | 0.17 | 0.92 | 0.13 | 0.63 | 0.09 | 0.12 | 0.02 | 0.15 |
| SRDS | 2.02 | 0.21 | 1.67 | 0.17 | 0.85 | 0.09 | 0.14 | 0.02 | 0.19 |
| TGDS | 1.96 | 0.17 | 1.46 | 0.13 | 0.81 | 0.07 | 0.14 | 0.01 | 0.16 |
| MS | 1.98 | 0.27 | 1.43 | 0.19 | 0.83 | 0.11 | 0.21 | 0.03 | 0.24 |
| SEm | 0.07 | 0.01 | 0.05 | 0.004 | 0.04 | 0.01 | 0.01 | 0.01 | 0.02 |
| SEd | 0.10 | 0.02 | 0.07 | 0.01 | 0.06 | 0.01 | 0.01 | 0.01 | 0.02 |
| P = 0.05 | 0.23 | 0.04 | 0.16 | 0.01 | 0.13 | 0.02 | 0.02 | 0.02 | 0.05 |
| Phosphorus | |||||||||
| LPDS | 1.15 | 0.15 | 0.94 | 0.12 | 0.58 | 0.08 | 0.10 | 0.01 | 0.14 |
| MCDS | 0.90 | 0.13 | 0.75 | 0.10 | 0.42 | 0.06 | 0.07 | 0.01 | 0.12 |
| SRDS | 1.17 | 0.12 | 0.95 | 0.10 | 0.38 | 0.04 | 0.11 | 0.01 | 0.11 |
| TGDS | 1.02 | 0.09 | 0.95 | 0.08 | 0.38 | 0.03 | 0.11 | 0.01 | 0.08 |
| MS | 1.23 | 0.16 | 1.11 | 0.15 | 0.92 | 0.12 | 0.19 | 0.03 | 0.13 |
| SEm | 0.07 | 0.01 | 0.03 | 0.03 | 0.02 | 0.01 | 0.02 | 0.10 | 0.01 |
| SEd | 0.09 | 0.01 | 0.05 | 0.04 | 0.03 | 0.03 | 0.03 | 0.12 | 0.01 |
| P = 0.05 | 0.21 | 0.03 | 0.10 | 0.01 | 0.06 | 0.04 | 0.07 | 0.25 | 0.03 |
| Potassium | |||||||||
| LPDS | 1.36 | 0.18 | 1.06 | 0.14 | 0.81 | 0.11 | 0.16 | 0.02 | 0.16 |
| MCDS | 1.05 | 0.15 | 0.95 | 0.13 | 0.62 | 0.09 | 0.18 | 0.03 | 0.12 |
| SRDS | 1.80 | 0.19 | 1.29 | 0.14 | 0.73 | 0.08 | 0.21 | 0.02 | 0.17 |
| TGDS | 1.65 | 0.15 | 1.29 | 0.11 | 0.73 | 0.06 | 0.21 | 0.02 | 0.13 |
| MS | 1.75 | 0.23 | 1.39 | 0.19 | 0.97 | 0.13 | 0.20 | 0.03 | 0.20 |
| SEm | 0.14 | 0.05 | 0.01 | 0.04 | 0.20 | 0.03 | 0.04 | 0.01 | 0.04 |
| SEd | 0.20 | 0.07 | 0.02 | 0.05 | 0.33 | 0.05 | 0.06 | 0.01 | 0.06 |
| P = 0.05 | 0.40 | 0.15 | 0.04 | 0.11 | 0.06 | 0.11 | 0.12 | 0.03 | 0.13 |
| Stands | Soil Nutrients/Depth | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Available N (Mg ha−1) | Available P (Mg ha−1) | Available K (Mg ha−1) | |||||||
| 0–20 cm | 20–40 cm | 40–60 cm | 0–20 cm | 20–40 cm | 40–60 cm | 0–20 cm | 20–40 cm | 40–60 cm | |
| LPDS | 0.27 | 0.23 | 0.19 | 0.13 | 0.11 | 0.10 | 0.15 | 0.15 | 0.125 |
| MCDS | 0.26 | 0.22 | 0.16 | 0.12 | 0.10 | 0.08 | 0.16 | 0.13 | 0.115 |
| SRDS | 0.26 | 0.22 | 0.15 | 0.12 | 0.10 | 0.08 | 0.15 | 0.13 | 0.126 |
| TGDS | 0.23 | 0.21 | 0.19 | 0.11 | 0.09 | 0.06 | 0.14 | 0.13 | 0.108 |
| MS | 0.29 | 0.26 | 0.22 | 0.13 | 0.13 | 0.11 | 0.16 | 0.13 | 0.096 |
| SEm | 0.07 | 0.06 | 0.05 | 0.03 | 0.03 | 0.03 | 0.04 | 0.04 | 0.03 |
| SEd | 0.10 | 0.09 | 0.07 | 0.05 | 0.05 | 0.04 | 0.06 | 0.05 | 0.05 |
| P = 0.05 | 0.23 | 0.19 | 0.16 | 0.11 | 0.09 | 0.08 | 0.13 | 0.13 | 0.10 |
| Stands | Soil Depth | ||
|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | |
| Exchangeable Al (c mol Kg−1) | |||
| LPDS | 1.16 | 1.38 | 1.09 |
| MCDS | 1.11 | 1.19 | 1.09 |
| SRDS | 1.03 | 0.96 | 0.91 |
| TGDS | 0.95 | 0.88 | 0.77 |
| MS | 1.26 | 1.68 | 1.49 |
| SEm | 0.06 | 0.05 | 0.02 |
| SEd | 0.08 | 0.07 | 0.04 |
| P = 0.05 | 0.18 | 0.15 | 0.08 |
| Exchangeable Ca (c mol Kg−1) | |||
| LPDS | 0.63 | 0.61 | 0.68 |
| MCDS | 0.61 | 0.61 | 0.57 |
| SRDS | 0.57 | 0.55 | 0.62 |
| TGDS | 0.43 | 0.40 | 0.32 |
| MS | 0.71 | 0.69 | 0.72 |
| SEm | 0.04 | 0.02 | 0.02 |
| SEd | 0.05 | 0.03 | 0.02 |
| P = 0.05 | 0.12 | 0.06 | 0.05 |
| Exchangeable Mg (c mol Kg−1) | |||
| LPDS | 0.19 | 0.20 | 0.20 |
| MCDS | 0.17 | 0.17 | 0.17 |
| SRDS | 0.18 | 0.18 | 0.19 |
| TGDS | 0.14 | 0.12 | 0.11 |
| MS | 0.22 | 0.23 | 0.23 |
| SEm | 0.01 | 0.02 | 0.02 |
| SEd | 0.02 | 0.02 | 0.02 |
| P = 0.05 | 0.04 | 0.05 | 0.05 |
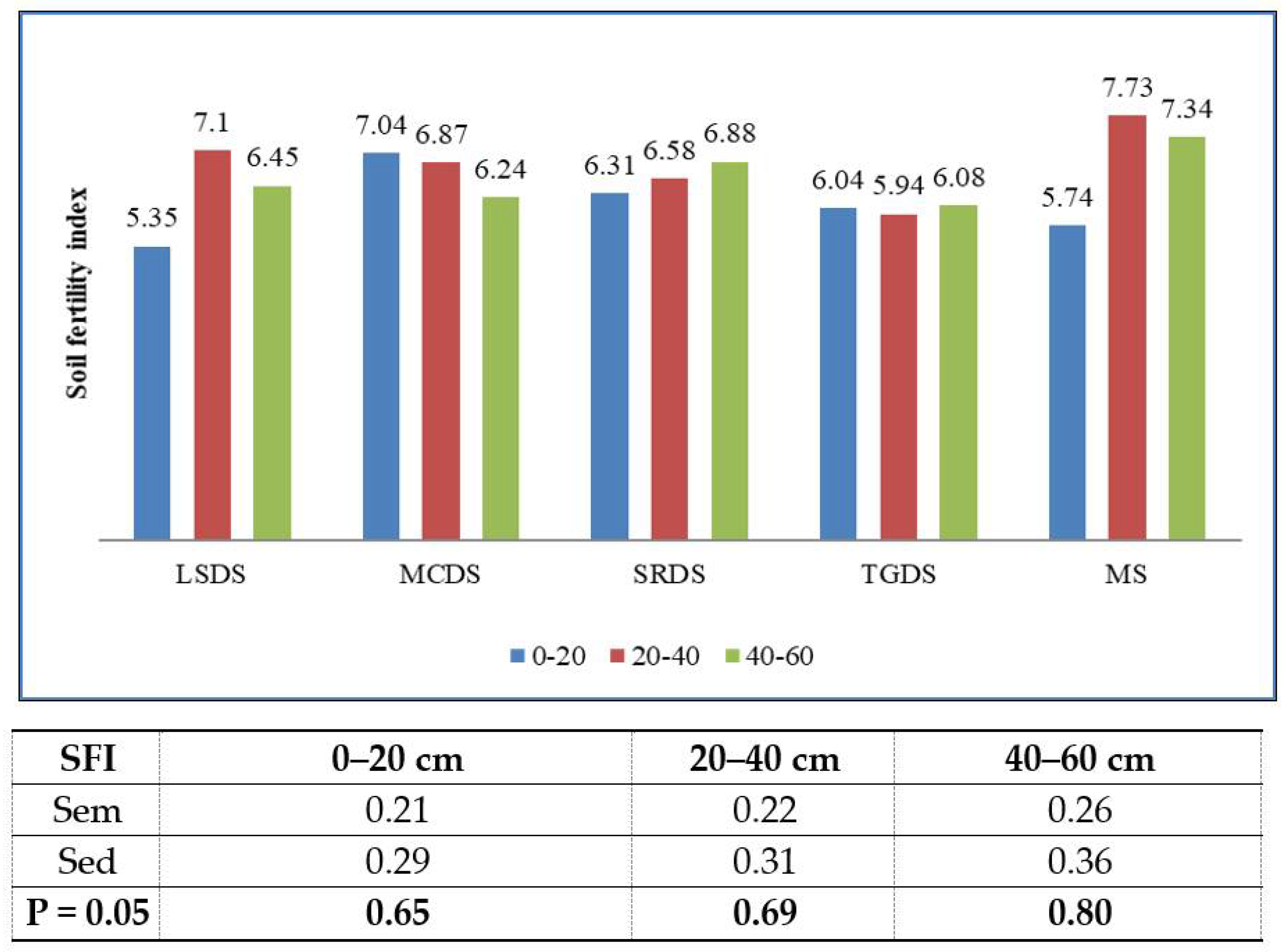
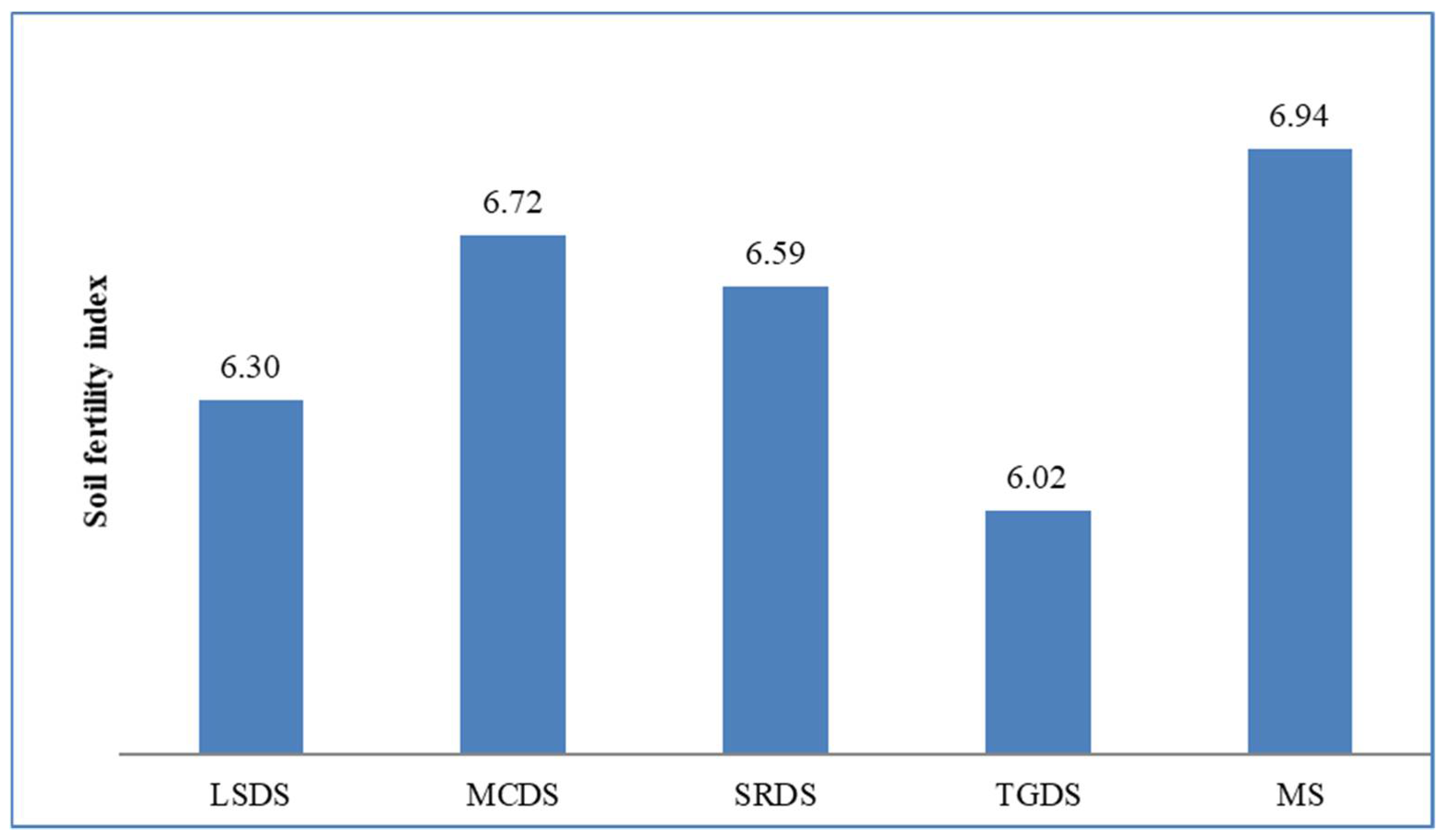
| OC | N | P | K | Mg | Ca | Al | pH | EC | SM | SEF | SFI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (0–60 cm Soil Depth) | ||||||||||||
| OC | 1 | |||||||||||
| N | 0.8 ** | 1 | ||||||||||
| P | 0.3 | NS | 1 | |||||||||
| K | 0.8 ** | 0.7 ** | 0.6 * | 1 | ||||||||
| Mg | −0.1 | −0.1 | −0.4 | −0.1 | 1 | |||||||
| Ca | 0.1 | −0.1 | −0.2 | 0.1 | 0.9 ** | 1 | ||||||
| Al | 0.2 | 0.3 | 0.2 | 0.2 | −0.5 * | −0.7 ** | 1 | |||||
| pH | 0.4 | 0.1 | 0.3 | 0.6 * | 0.1 | 0.1 | 0.1 | 1 | ||||
| EC | −0.1 | 0.2 | −0.5 | −0.2 | 0.1 | −0.2 | 0.2 | −0.1 | 1 | |||
| SM | 0.5 | 0.2 | 0.1 | 0.3 | −0.2 | −0.2 | 0.2 | 0.4 | 0.1 | 1 | ||
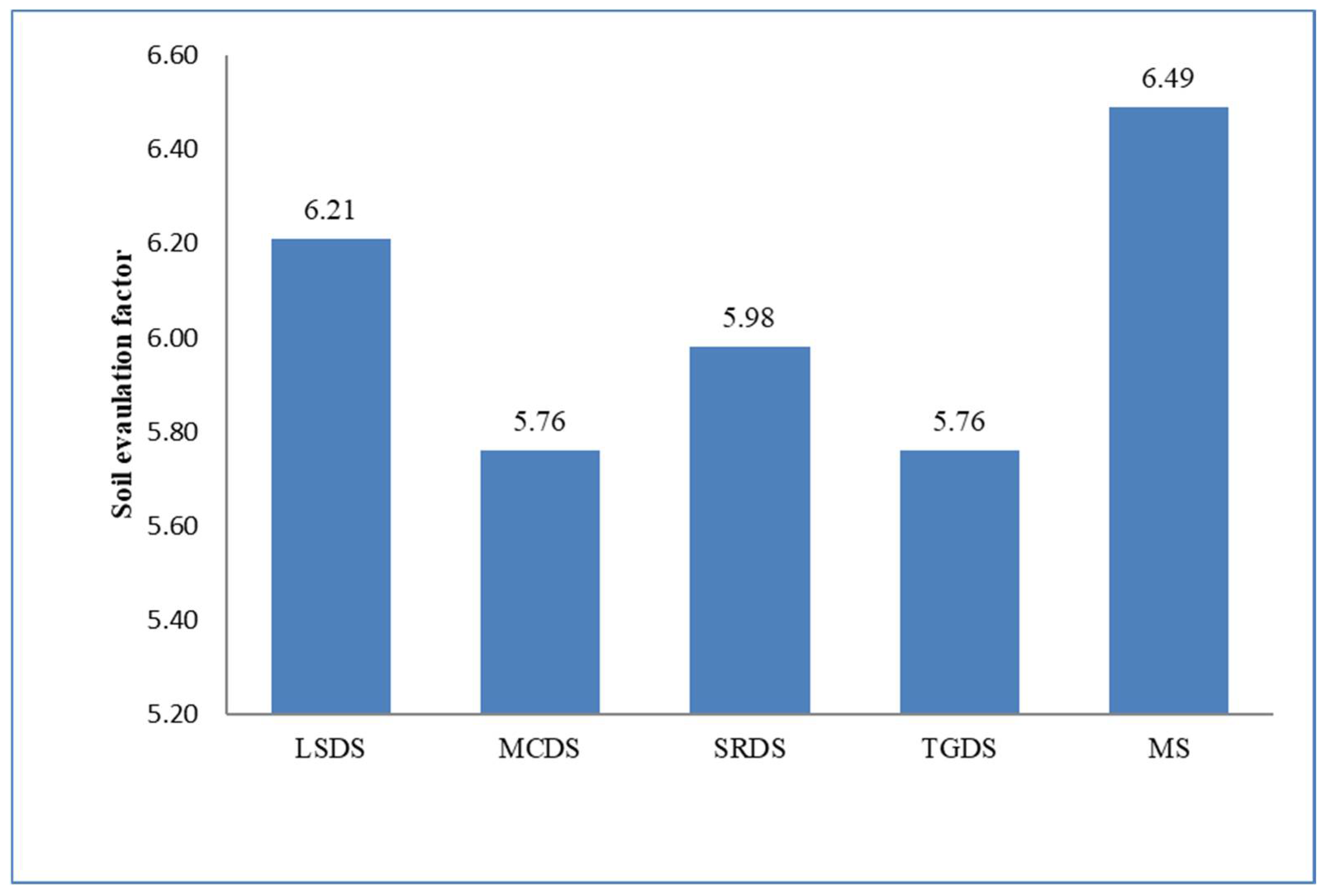
| SEF | −0.2 | 0.1 | −0.3 | −0.1 | 0.3 | 0.1 | −0.1 | −0.1 | 0.7 ** | 0.1 | 1 | |
| SFI | −0.4 | −0.2 | −0.3 | −0.3 | 0.3 | 0.1 | −0.2 | −0.2 | 0.4 | −0.1 | 0.7 ** | 1 |
| Stands | Soil Parameter/Soil Depth | ||||||
|---|---|---|---|---|---|---|---|
| SOC (%) | SOC (Mg ha−1) | ||||||
| 0–20 cm | 20–40 cm | 40–60 cm | 0–20 cm | 20–40 cm | 40–60cm | Total | |
| LPDS | 1.52 | 1.35 | 1.21 | 34.05 | 30.24 | 27.10 | 91.4 |
| MCDS | 1.36 | 1.25 | 1.14 | 30.46 | 28.00 | 25.54 | 84.0 |
| SRDS | 1.44 | 1.34 | 1.19 | 32.27 | 30.02 | 26.66 | 88.9 |
| TGDS | 1.26 | 1.10 | 1.03 | 28.22 | 24.64 | 23.07 | 75.9 |
| MS | 1.81 | 1.63 | 1.38 | 40.54 | 36.23 | 30.91 | 107.7 |
| SEm | 0.007 | 0.037 | 0.046 | 0.70 | 0.99 | 0.94 | 3.78 |
| SEd | 0.010 | 0.052 | 0.065 | 0.99 | 1.40 | 1.33 | 5.35 |
| P = 0.05 | 0.023 | 0.114 | 0.142 | 2.16 | 3.05 | 2.90 | 11.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, P.; Vineeta; Shukla, G.; Manohar K, A.; Bhat, J.A.; Kumar, A.; Kumar, M.; Cabral-Pinto, M.; Chakravarty, S. Carbon Storage of Single Tree and Mixed Tree Dominant Species Stands in a Reserve Forest—Case Study of the Eastern Sub-Himalayan Region of India. Land 2021, 10, 435. https://doi.org/10.3390/land10040435
Rai P, Vineeta, Shukla G, Manohar K A, Bhat JA, Kumar A, Kumar M, Cabral-Pinto M, Chakravarty S. Carbon Storage of Single Tree and Mixed Tree Dominant Species Stands in a Reserve Forest—Case Study of the Eastern Sub-Himalayan Region of India. Land. 2021; 10(4):435. https://doi.org/10.3390/land10040435
Chicago/Turabian StyleRai, Prakash, Vineeta, Gopal Shukla, Abha Manohar K, Jahangeer A Bhat, Amit Kumar, Munesh Kumar, Marina Cabral-Pinto, and Sumit Chakravarty. 2021. "Carbon Storage of Single Tree and Mixed Tree Dominant Species Stands in a Reserve Forest—Case Study of the Eastern Sub-Himalayan Region of India" Land 10, no. 4: 435. https://doi.org/10.3390/land10040435
APA StyleRai, P., Vineeta, Shukla, G., Manohar K, A., Bhat, J. A., Kumar, A., Kumar, M., Cabral-Pinto, M., & Chakravarty, S. (2021). Carbon Storage of Single Tree and Mixed Tree Dominant Species Stands in a Reserve Forest—Case Study of the Eastern Sub-Himalayan Region of India. Land, 10(4), 435. https://doi.org/10.3390/land10040435










