Ozone Response of Leaf Physiological and Stomatal Characteristics in Brassica juncea L. at Supraoptimal Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and O3 Fumigation Chamber
2.2. Physiological Characteristics
2.3. Chlorophyll Content
Chlorophyll b (mg g−1 FW) = 22.9 × A645−4.68 × A663
Total chlorophyll (mg g−1 FW) = 20.2 × A645 + 8.02 × A663.
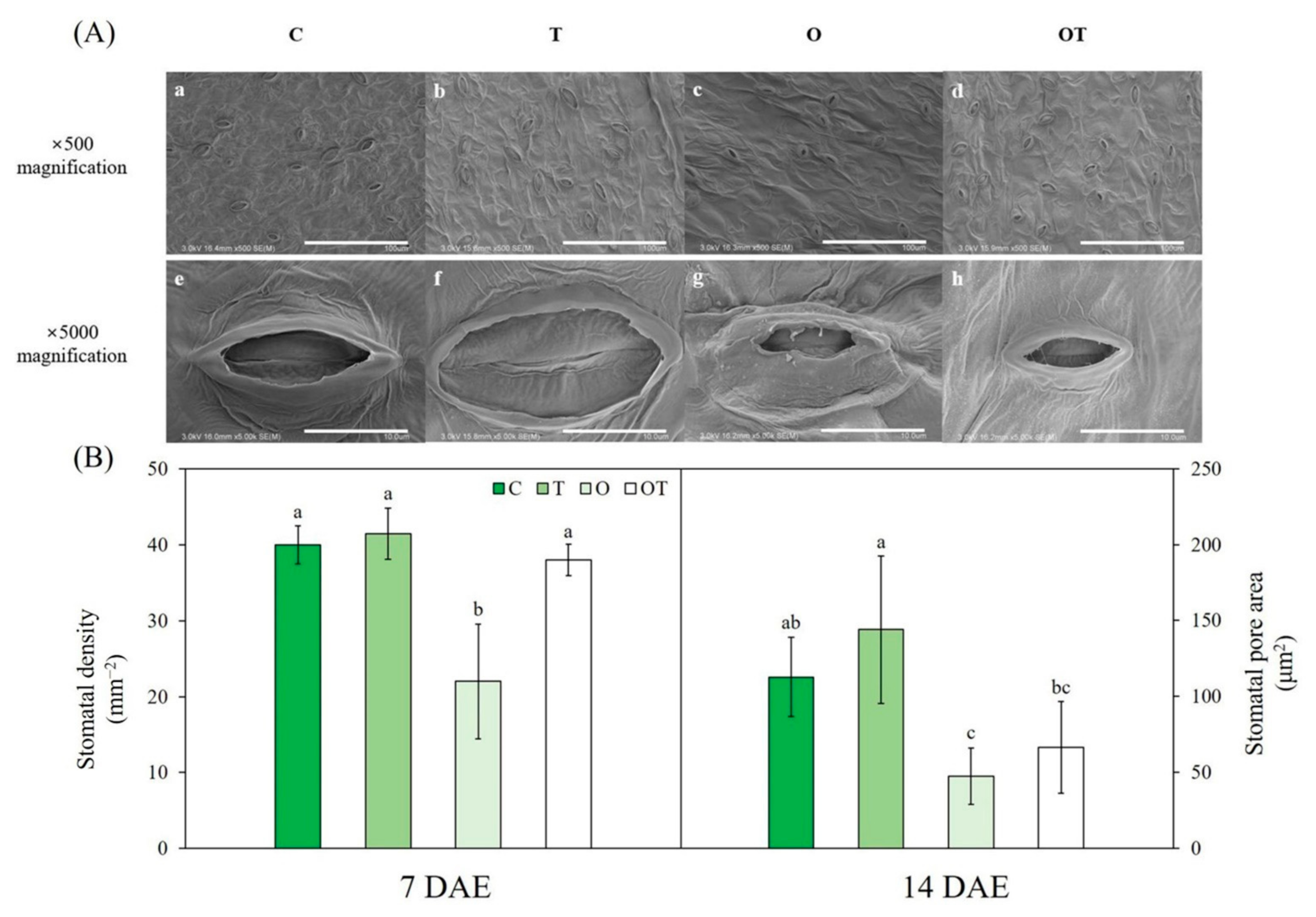
2.4. Stomatal Characteristics
2.5. Hydrogen Peroxide (H2O2) and Superoxide (O2−) Accumulation
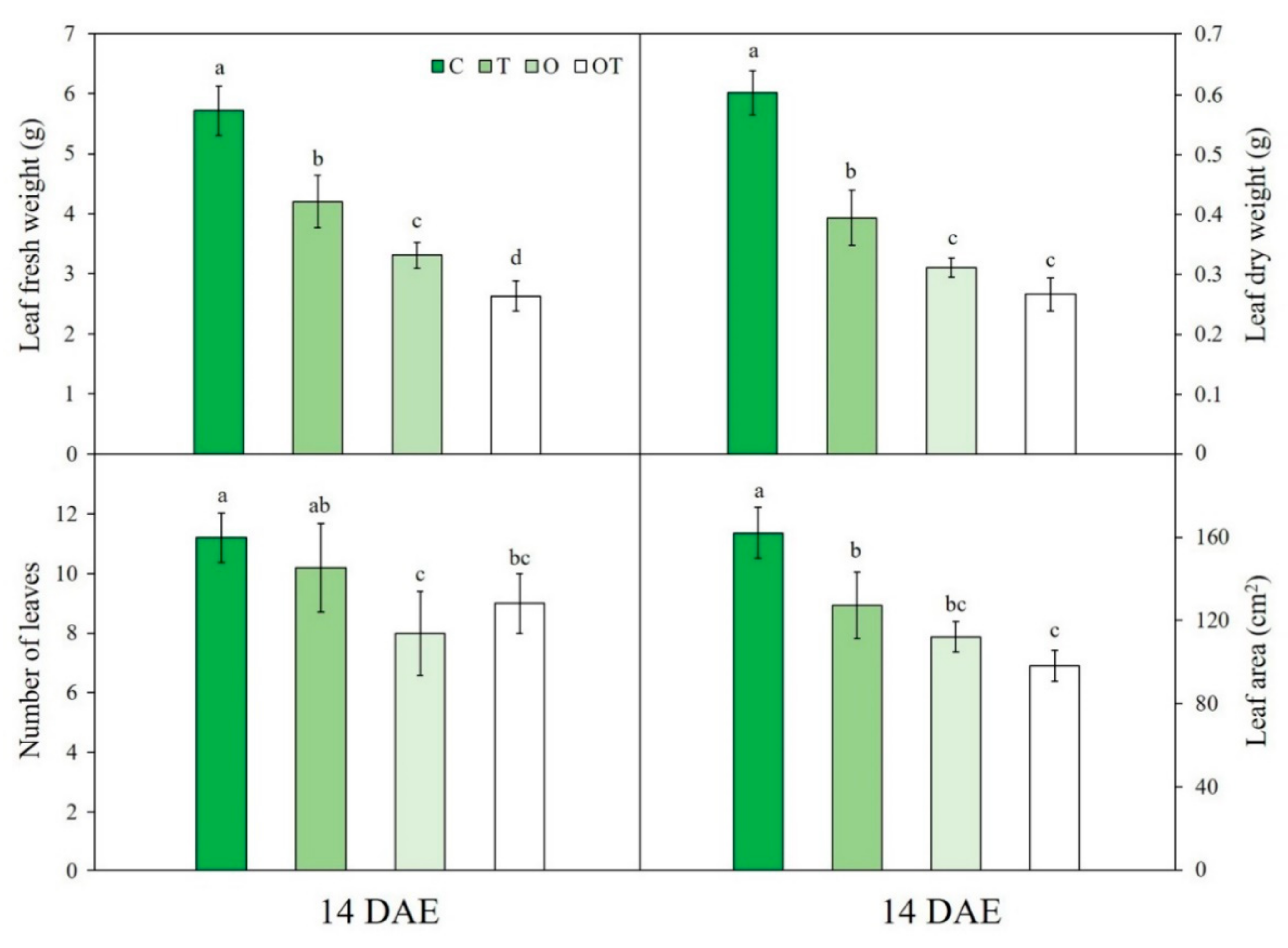
2.6. Growth Characteristics
2.7. Statistical Analysis
3. Results
3.1. A/Ci Curve Response
3.2. Chl Content
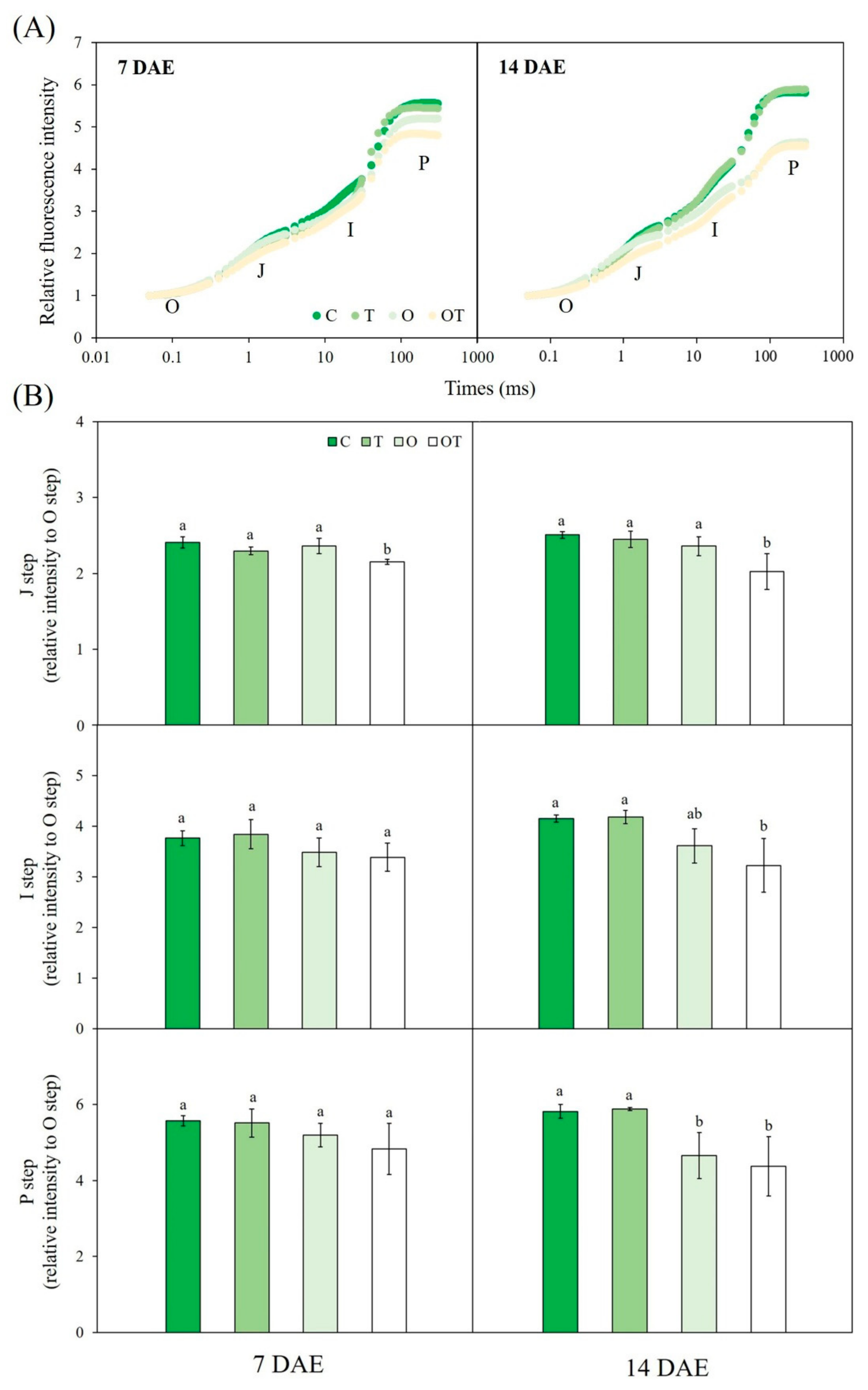
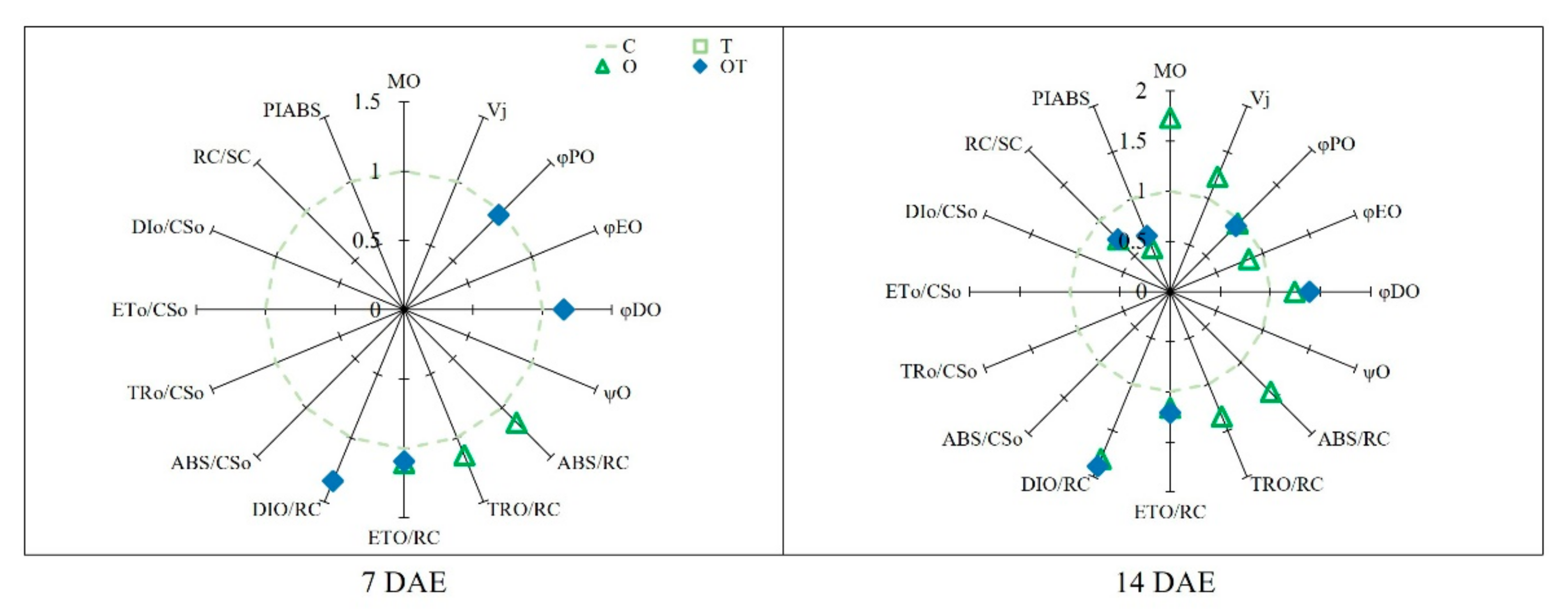
3.3. Chl a Fluorescence
3.4. Stomatal Characteristics
3.5. H2O2 and O2− Accumulation
3.6. Plant Growth Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tai, A.P.K.; Martin, M.V.; Heald, C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Chang. 2014, 4, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Battisti, D.S.; Naylor, R.L. Historical warnings of future food insecurity with unprecedented seasonal heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Ainsworth, E.A. Rice production in a changing climate: A meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob. Chang. Biol. 2008, 14, 1642–1650. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DaMatta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of climate changes on crop physiology and food quality. Food Res. Int. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Lawson, T.; Matthews, J. Guard Cell Metabolism and Stomatal Function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlesinger, W.H.; Jasechko, S. Transpiration in the global water cycle. Agric. For. Meteorol. 2014, 189–190, 115–117. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, C.; Gu, S.; Ma, Q.; Zhang, Y.; Li, X.; Shi, K. Stomatal movements are involved in elevated CO2 -mitigated high temperature stress in tomato. Physiol. Plant. 2019, 165, 569–583. [Google Scholar] [CrossRef]
- Drake, J.E.; Tjoelker, M.G.; Vårhammar, A.; Medlyn, B.E.; Reich, P.B.; Leigh, A.; Pfautsch, S.; Blackman, C.J.; López, R.; Aspinwall, M.J.; et al. Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Glob. Chang. Biol. 2018, 24, 2390–2402. [Google Scholar] [CrossRef] [PubMed]
- Duursma, R.A.; Blackman, C.J.; Lopéz, R.; Martin-StPaul, N.K.; Cochard, H.; Medlyn, B.E. On the minimum leaf conductance: Its role in models of plant water use, and ecological and environmental controls. New Phytol. 2019, 221, 693–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Fuhrer, J.; Val Martin, M.; Mills, G.; Heald, C.L.; Harmens, H.; Hayes, F.; Sharps, K.; Bender, J.; Ashmore, M.R. Current and future ozone risks to global terrestrial biodiversity and ecosystem processes. Ecol. Evol. 2016, 6, 8785–8799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, E.A.; Yendrek, C.R.; Sitch, S.; Collins, W.J.; Emberson, L.D. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu. Rev. Plant Biol. 2012, 63, 637–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Woo, S.Y.; Lee, J.K.; Kwak, M.J.; Khaine, I.; Jang, J.H.; Kim, H.N.; Kim, J.E.; Park, S.H.; Kim, H.D.; et al. Effects of elevated ozone on physiological, biochemical and morphological characteristics of eggplant. Hortic. Environ. Biotechnol. 2019, 60, 809–820. [Google Scholar] [CrossRef]
- Sarkar, A.; Rakwal, R.; Agrawal, S.B.; Shibato, J.; Ogawa, Y.; Yoshida, Y.; Kumar Agrawal, G.; Agrawal, M. Investigating the impact of elevated levels of ozone on tropical wheat using integrated phenotypical, physiological, biochemical, and proteomics approaches. J. Proteome Res. 2010, 9, 4565–4584. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; Grulke, N.E. Does living in elevated CO2 ameliorate tree response to ozone? A review on stomatal responses. Environ. Pollut. 2005, 137, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Schauberger, B.; Rolinski, S.; Schaphoff, S.; Müller, C. Global historical soybean and wheat yield loss estimates from ozone pollution considering water and temperature as modifying effects. Agric. For. Meteorol. 2019, 265, 1–15. [Google Scholar] [CrossRef]
- Hoshika, Y.; De Carlo, A.; Baraldi, R.; Neri, L.; Carrari, E.; Agathokleous, E.; Zhang, L.; Fares, S.; Paoletti, E. Ozone-induced impairment of night-time stomatal closure in O3-sensitive poplar clone is affected by nitrogen but not by phosphorus enrichment. Sci. Total Environ. 2019, 692, 713–722. [Google Scholar] [CrossRef] [PubMed]
- McAinsh, M.R.; Evans, N.H.; Montgomery, L.T.; North, K.A. Calcium signalling in stomatal responses to pollutants. New Phytol. 2002, 153, 441–447. [Google Scholar] [CrossRef]
- Hoshika, Y.; Carriero, G.; Feng, Z.; Zhang, Y.; Paoletti, E. Determinants of stomatal sluggishness in ozone-exposed deciduous tree species. Sci. Total Environ. 2014, 481, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Frenck, G.; van der Linden, L.; Mikkelsen, T.N.; Brix, H.; Jørgensen, R.B. Increased [CO2] does not compensate for negative effects on yield caused by higher temperature and [O3] in Brassica napus L. Eur. J. Agron. 2011, 35, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Steiner, A.L.; Davis, A.J.; Sillman, S.; Owen, R.C.; Michalak, A.M.; Fiore, A.M. Observed suppression of ozone formation at extremely high temperatures due to chemical and biophysical feedbacks. Proc. Natl. Acad. Sci. USA 2010, 107, 19685–19690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vukovich, F.M.; Sherwell, J. An examination of the relationship between certain meteorological parameters and surface ozone variations in the Baltimore-Washington corridor. Atmos. Environ. 2003, 37, 971–981. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to UV-B and CO2 under controlled environment conditions. Physiol. Plant. 2005, 125, 247–259. [Google Scholar] [CrossRef]
- Yadav, D.S.; Mishra, A.K.; Rai, R.; Chaudhary, N.; Mukherjee, A.; Agrawal, S.B.; Agrawal, M. Responses of an old and a modern Indian wheat cultivar to future O3 level: Physiological, yield and grain quality parameters. Environ. Pollut. 2020, 259, 113939. [Google Scholar] [CrossRef]
- Farrell, K.T. Spices, Condiments, and Seasonings, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; pp. 138–140. [Google Scholar]
- Black, V.J.; Stewart, C.A.; Roberts, J.A.; Black, C.R. Timing of exposure to ozone affects reproductive sensitivity and compensatory ability in Brassica campestris. Environ. Exp. Bot. 2012, 75, 225–234. [Google Scholar] [CrossRef]
- Bosac, C.; Robert, J.A.; Black, V.J.; Black, C.R. Impact of O3 and SO 2 on reproductive development in oilseed rape (Brassica napus L.). New Phytol. 1994, 126, 71–79. [Google Scholar] [CrossRef]
- Singh, S.; Agrawal, S.B. Use of ethylene diurea (EDU) in assessing the impact of ozone on growth and productivity of five cultivars of Indian wheat (Triticum aestivum L.). Environ. Monit. Assess. 2009, 159, 125–141. [Google Scholar] [CrossRef]
- Singh, P.; Agrawal, M.; Agrawal, S.B. Evaluation of physiological, growth and yield responses of a tropical oil crop (Brassica campestris L. var. Kranti) under ambient ozone pollution at varying NPK levels. Environ. Pollut. 2009, 157, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhatia, A.; Tomer, R.; Kumar, V.; Singh, B.; Singh, S.D. Synergistic action of tropospheric ozone and carbon dioxide on yield and nutritional quality of Indian mustard (Brassica juncea (L.) Czern.). Environ. Monit. Assess. 2013, 185, 6517–6529. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Woo, S.Y.; Kwak, M.J.; Park, S.H.; Kim, H.D.; Lim, Y.J.; Park, J.H.; Lee, K.A. Effects of elevated temperature and ozone in Brassica juncea L.: Growth, physiology, and ROS accumulation. Forests 2020, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Environmental Research. Annual Report of Air Quality in Korea 2016; National Institute of Environmental Research: Incheon, Korea, 2017.
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Angadi, S.V.; Cutforth, H.W.; Miller, P.R.; McConkey, B.G.; Entz, M.H.; Brandt, S.A.; Volkmar, K.M. Response of three brassica species to high temperature stress during reproductive growth. Can. J. Plant Sci. 2000, 80, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant. 2006, 128, 710–721. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant. Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Jiang, H.X.; Chen, L.S.; Zheng, J.G.; Han, S.; Tang, N.; Smith, B.R. Aluminum-induced effects on Photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008, 28, 1863–1871. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venketeswaran, S. Studies on the isolation of green pigmented callus tissue of tobacco and its continued maintenance in suspension cultures. Physiol. Plant. 1965, 18, 776–789. [Google Scholar] [CrossRef]
- Hu, X.; Tanaka, A.; Tanaka, R. Simple extraction methods that prevent the artifactual conversion of chlorophyll to chlorophyllide during pigment isolation from leaf samples. Plant Methods 2013, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Tao, Y.; Mao, W.H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.Q. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, V.; García-Breijo, F.J.; Cervero, J.; Reig-Armiñana, J.; Sanz, M.J. Physiological, anatomical and biomass partitioning responses to ozone in the Mediterranean endemic plant Lamottea dianae. Ecotoxicol. Environ. Saf. 2011, 74, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Calatayud, V.; García-Breijo, F.; Reig-Armiñana, J.; Feng, Z. Effects of elevated ozone on physiological, anatomical and ultrastructural characteristics of four common urban tree species in China. Ecol. Indic. 2016, 67, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Ser-Oddamba, B.; Batkhuu, N.-O.; Seok Kim, H. Comparison of water use efficiency and biomass production in 10-year-old Populus sibirica and Ulmus pumila plantations in Lun soum, Mongolia. For. Sci. Technol. 2019, 15, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Pell, E.J.; Eckardt, N.A.; Glick, R.E. Biochemical and molecular basis for impairment of photosynthetic potential. Photosynth. Res. 1994, 39, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, V.; Marco, F.; Cerveró, J.; Sánchez-Peña, G.; Sanz, M.J. Contrasting ozone sensitivity in related evergreen and deciduous shrubs. Environ. Pollut. 2010, 158, 3580–3587. [Google Scholar] [CrossRef] [PubMed]
- Glick, R.E.; Schlagnhaufer, C.D.; Arteca, R.N.; Pell, E.J. Ozone-induced ethylene emission accelerates the loss of ribulose-1,5-bisphosphate carboxylase/oxygenase and nuclear-encoded mRNAs in senescing potato leaves. Plant Physiol. 1995, 109, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thwe, A.A.; Vercambre, G.; Gautier, H.; Gay, F.; Phattaralerphong, J.; Kasemsap, P. Response of photosynthesis and chlorophyll fluorescence to acute ozone stress in tomato (Solanum lycopersicum Mill.). Photosynthetica 2014, 52, 105–116. [Google Scholar] [CrossRef]
- Makino, A.; Osmond, B. Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol. 1991, 96, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, B.; Xu, Y.; Dai, L.; Yuan, X.; Feng, Z. Elevated ozone reduced leaf nitrogen allocation to photosynthesis in poplar. Sci. Total Environ. 2019, 657, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Feller, U.; Crafts-Brandner, S.J.; Salvucci, M.E. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol. 1998, 116, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.N.; McAusland, L.; Smith, K.E.; Price, A.H.; Wilson, Z.A.; Murchie, E.H. Rapid temperature responses of photosystem II efficiency forecast genotypic variation in rice vegetative heat tolerance. Plant J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sharwood, R.E.; Crous, K.Y.; Whitney, S.M.; Ellsworth, D.S.; Ghannoum, O. Linking photosynthesis and leaf N allocation under future elevated CO 2 and climate warming in Eucalyptus globulus. J. Exp. Bot. 2017, 68, erw484. [Google Scholar] [CrossRef] [Green Version]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Huntingford, C.; Atkin, O.K.; Martinez-De La Torre, A.; Mercado, L.M.; Heskel, M.A.; Harper, A.B.; Bloomfield, K.J.; O’Sullivan, O.S.; Reich, P.B.; Wythers, K.R.; et al. Implications of improved representations of plant respiration in a changing climate. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Booker, F.L.; Burkey, K.O.; Pursley, W.A.; Heagle, A.S. Elevated carbon dioxide and ozone effects on peanut: I. Gas-exchange, biomass, and leaf chemistry. Crop Sci. 2007, 47, 1475–1487. [Google Scholar] [CrossRef]
- Sun, J.; Feng, Z.; Ort, D.R. Impacts of rising tropospheric ozone on photosynthesis and metabolite levels on field grown soybean. Plant Sci. 2014, 226, 147–161. [Google Scholar] [CrossRef]
- Eichelmann, H.; Oja, V.; Rasulov, B.; Padu, E.; Bichele, I.; Pettai, H.; Möls, T.; Kasparova, I.; Vapaavuori, E.; Laisk, A. Photosynthetic parameters of birch (Betula pendula Roth) leaves growing in normal and in CO2- and O3-enriched atmospheres. Plant Cell Environ. 2004, 27, 479–495. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, Z.; Tarvainen, L.; Shang, B.; Dai, L.; Uddling, J. Mesophyll conductance limitation of photosynthesis in poplar under elevated ozone. Sci. Total Environ. 2019, 657, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ribas-Carbó, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef]
- Paoletti, E.; Contran, N.; Bernasconi, P.; Günthardt-Goerg, M.S.; Vollenweider, P. Structural and physiological responses to ozone in Manna ash (Fraxinus ornus L.) leaves of seedlings and mature trees under controlled and ambient conditions. Sci. Total Environ. 2009, 407, 1631–1643. [Google Scholar] [CrossRef]
- Xiong, D.; Douthe, C.; Flexas, J. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant. Cell Environ. 2018, 41, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Agrawal, M.; Agrawal, S.B. Effects of ambient O3 on wheat during reproductive development: Gas exchange, photosynthetic pigments, chlorophyll fluorescence, and carbohydrates. Photosynthetica 2011, 49, 285–294. [Google Scholar] [CrossRef]
- Welfare, K.; Flowers, T.J.; Taylor, G.; Yeo, A.R. Additive and antagonistic effects of ozone and salinity on the growth, ion contents and gas exchange of five varieties of rice (Oryza sativa L.). Environ. Pollut. 1996, 92, 257–266. [Google Scholar] [CrossRef]
- Agrawal, M.; Krizek, D.T.; Agrawal, S.B.; Kramer, G.F.; Lee, E.H.; Mirecki, R.M.; Rowland, R.A. Influence of Inverse Day/Night Temperature on Ozone Sensitivity and Selected Morphological and Physiological Responses of Cucumber. J. Am. Soc. Hortic. Sci. 1993, 118, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, A.; Jones, M.B.; Burke, J.I.; Schnieders, B. Elevated CO2 provides protection from O3 induced photosynthetic damage and chlorophyll loss in flag leaves of spring wheat (Triticum aestivum L., cv. ‘Minaret’). Agric. Ecosyst. Environ. 2000, 80, 159–168. [Google Scholar] [CrossRef]
- Hassan, I.A. Physiological and biochemical response of potato (Solanum tuberosum L. cv. Kara) to O3 and antioxidant chemicals: Possible roles of antioxidant enzymes. Ann. Appl. Biol. 2006, 148, 197–206. [Google Scholar] [CrossRef]
- Pandey, A.K.; Ghosh, A.; Agrawal, M.; Agrawal, S.B. Effect of elevated ozone and varying levels of soil nitrogen in two wheat (Triticum aestivum L.) cultivars: Growth, gas-exchange, antioxidant status, grain yield and quality. Ecotoxicol. Environ. Saf. 2018, 158, 59–68. [Google Scholar] [CrossRef]
- Singh, E.; Rai, R.; Pandey, B.; Agrawal, M. Development of resistance in two wheat cultivars against constant fumigation of ozone. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1121–1134. [Google Scholar] [CrossRef]
- Atherton, J.; Olascoaga, B.; Alonso, L.; Porcar-Castell, A. Spatial variation of leaf optical properties in a boreal forest is influenced by species and light environment. Front. Plant Sci. 2017, 8, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czuba, M.; Ormrod, D.P. Effects of cadmium and zinc on ozone-induced phytotoxicity in cress and lettuce. Can. J. Bot. 1974, 52, 645–649. [Google Scholar] [CrossRef]
- Pellegrini, E.; Nali, C.; Lorenzini, G. Ecophysiology of Tilia Americana under ozone fumigation. Atmos. Pollut. Res. 2013, 4, 142–146. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzuoli, R.; Finco, A.; Chiesa, M.; Gerosa, G. A dose-response relationship for marketable yield reduction of two lettuce (Lactuca sativa L.) cultivars exposed to tropospheric ozone in Southern Europe. Environ. Sci. Pollut. Res. 2017, 24, 26249–26258. [Google Scholar] [CrossRef] [PubMed]
- Maliba, B.G.; Inbaraj, P.M.; Berner, J.M. The use of OJIP fluorescence transients to monitor the effect of elevated ozone on biomass of Canola Plants. Water. Air. Soil Pollut. 2019, 230, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, A.; Iglesias, D.J.; Talon, M.; Barreno, E. Effects of long-term ozone exposure on citrus: Chlorophyll a fluorescence and gas exchange. Photosynthetica 2006, 44, 548–554. [Google Scholar] [CrossRef]
- Walter, J.; Nagy, L.; Hein, R.; Rascher, U.; Beierkuhnlein, C.; Willner, E.; Jentsch, A. Do plants remember drought? Hints towards a drought-memory in grasses. Environ. Exp. Bot. 2011, 71, 34–40. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Vainonen, J.P.; Kangasjärvi, J. Plant signalling in acute ozone exposure. Plant Cell Environ. 2015, 38, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Xu, Y.; Peng, J.; Agathokleous, E.; Feng, Z. High nitrogen addition decreases the ozone flux by reducing the maximum stomatal conductance in poplar saplings. Environ. Pollut. 2021, 272, 115979. [Google Scholar] [CrossRef]
- Vollenweider, P.; Ottiger, M.; Günthardt-Goerg, M.S. Validation of leaf ozone symptoms in natural vegetation using microscopical methods. Environ. Pollut. 2003, 124, 101–118. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ashmore, M.R.; Bell, J.N.B. Effects of O3 on the stomatal behaviour of Egyptian varieties of radish (Raphanus sativus L. cv. Baladey) and turnip (Brassica rapa L. cv. Sultani). New Phytol. 1994, 128, 243–249. [Google Scholar] [CrossRef]
- Neufeld, H.S.; Sullins, A.; Sive, B.C.; Lefohn, A.S. Spatial and temporal patterns of ozone at Great Smoky Mountains National Park and implications for plant responses. Atmos. Environ. X 2019, 2, 100023. [Google Scholar] [CrossRef]
- Baek, S.G.; Park, J.H.; Na, C.S.; Lee, B.; Cheng, H.C.; Woo, S.Y. The morphological characteristics of Pterocarpus indicus induced by elevated ozone under well-watered and drought conditions. For. Sci. Technol. 2018, 14, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.; Scheidegger, C.; Günthardt-Goerg, M.S.; Matyssek, R. The effects of ozone and nutrient supply on stomatal response in birch (Betula pendula) leaves as determined by digital image-analysis and X-ray microanalysis. New Phytol. 1996, 132, 135–143. [Google Scholar] [CrossRef]
- Wu, G.; Liu, H.; Hua, L.; Luo, Q.; Lin, Y.; He, P.; Feng, S.; Liu, J.; Ye, Q. Differential responses of stomata and photosynthesis to elevated temperature in two co-occurring subtropical forest tree species. Front. Plant Sci. 2018, 9, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apple, M.E.; Olszyk, D.M.; Ormrod, D.P.; Lewis, J.; Southworth, D.; Tingey, D.T. Morphology and stomatal function of Douglas fir needles exposed to climate change: Elevated CO2 and temperature. Int. J. Plant Sci. 2000, 161, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S.; Pandey, G.P. Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean. Photosynth. Res 2017, 3, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.G.; Park, J.H.; Kwak, M.J.; Lee, J.K.; Na, C.S.; Lee, B.; Woo, S.Y. Physiological and biochemical responses of elevated ozone on Pterocarpus indicus under well-watered and drought conditions. For. Sci. Technol. 2018, 14, 153–159. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, S.B.; Agrawal, M. Responses of tropical tree species to urban air pollutants: ROS/RNS formation and scavenging. Sci. Total Environ. 2020, 710, 136363. [Google Scholar] [CrossRef] [PubMed]
- del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.J.; Gharibeshghi, A.; Mewis, I.; Förster, N.; Beck, W.; Ulrichs, C. Plant responses to ozone: Effects of different ozone exposure durations on plant growth and biochemical quality of Brassica campestris L. ssp. chinensis. Sci. Hortic. (Amst.) 2020, 262, 108921. [Google Scholar] [CrossRef]
- Menéndez, A.I.; Gundel, P.E.; Lores, L.M.; Martínez-Ghersa, M.A. Assessing the impacts of intra-and interspecific competition between Triticum aestivum and Trifolium repens on the species’ responses to ozone. Botany 2017, 95, 923–932. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Hu, E.; Wang, X.; Jiang, L.; Liu, X. Ground-level O3 pollution and its impacts on food crops in China: A review. Environ. Pollut. 2015, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
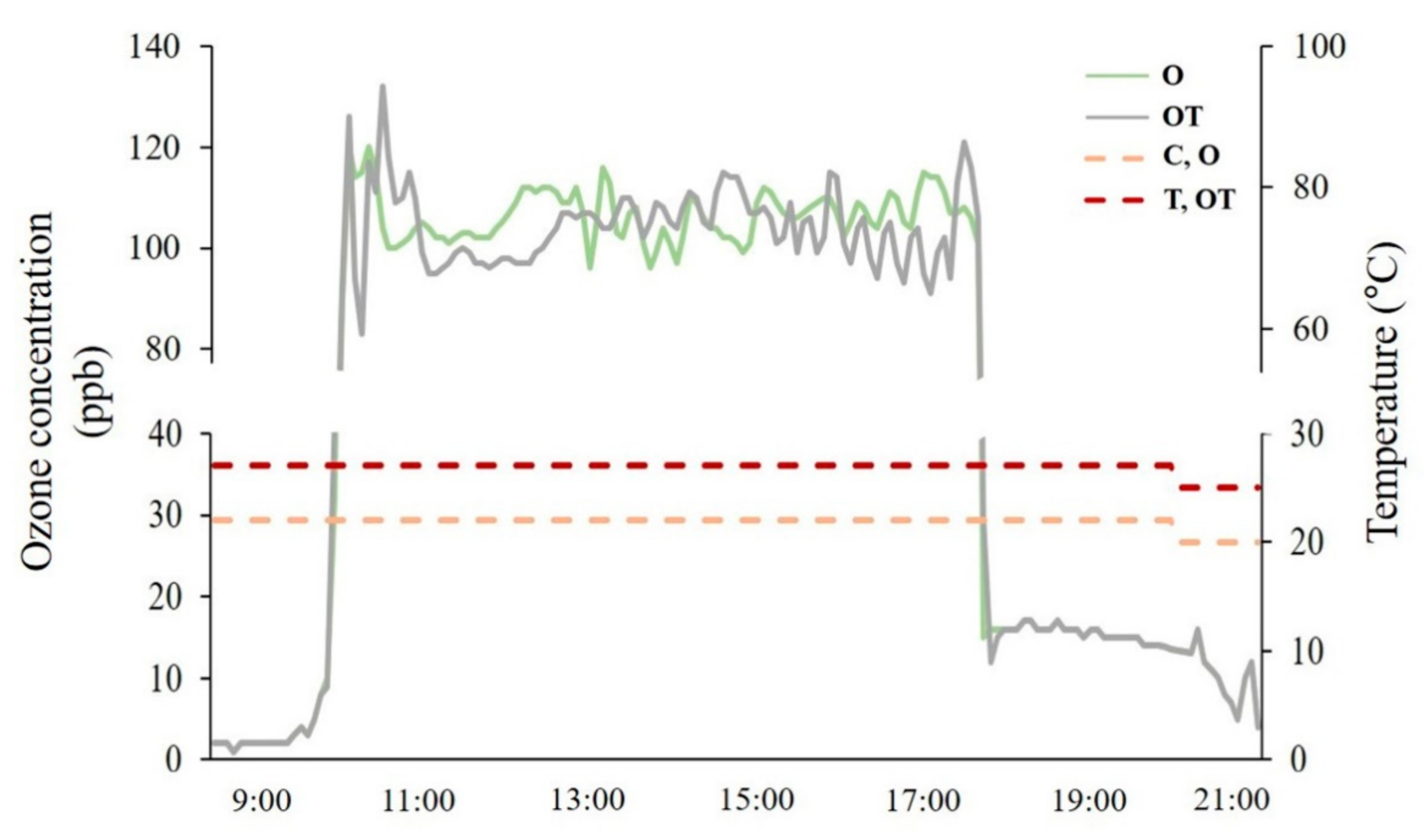
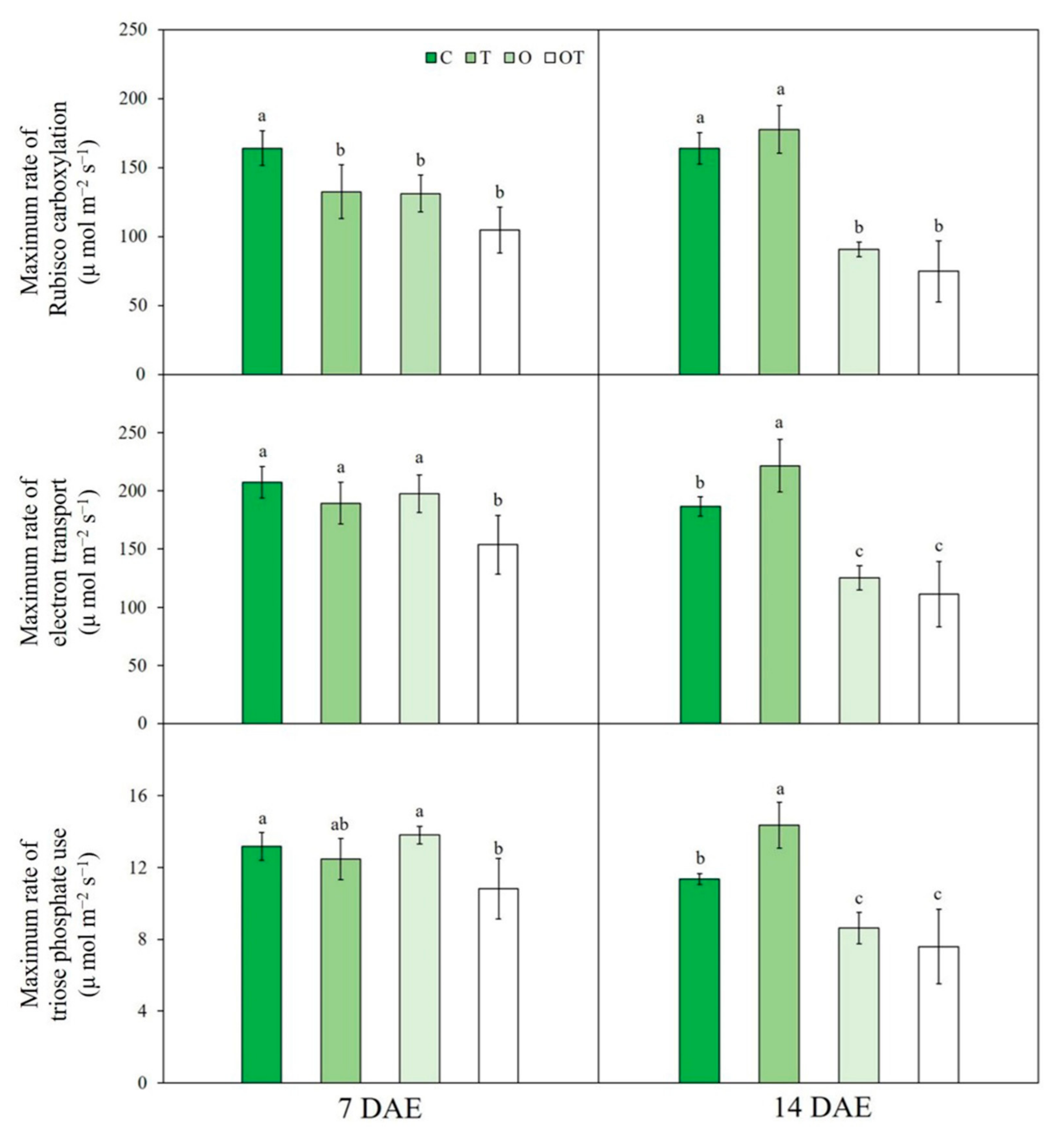
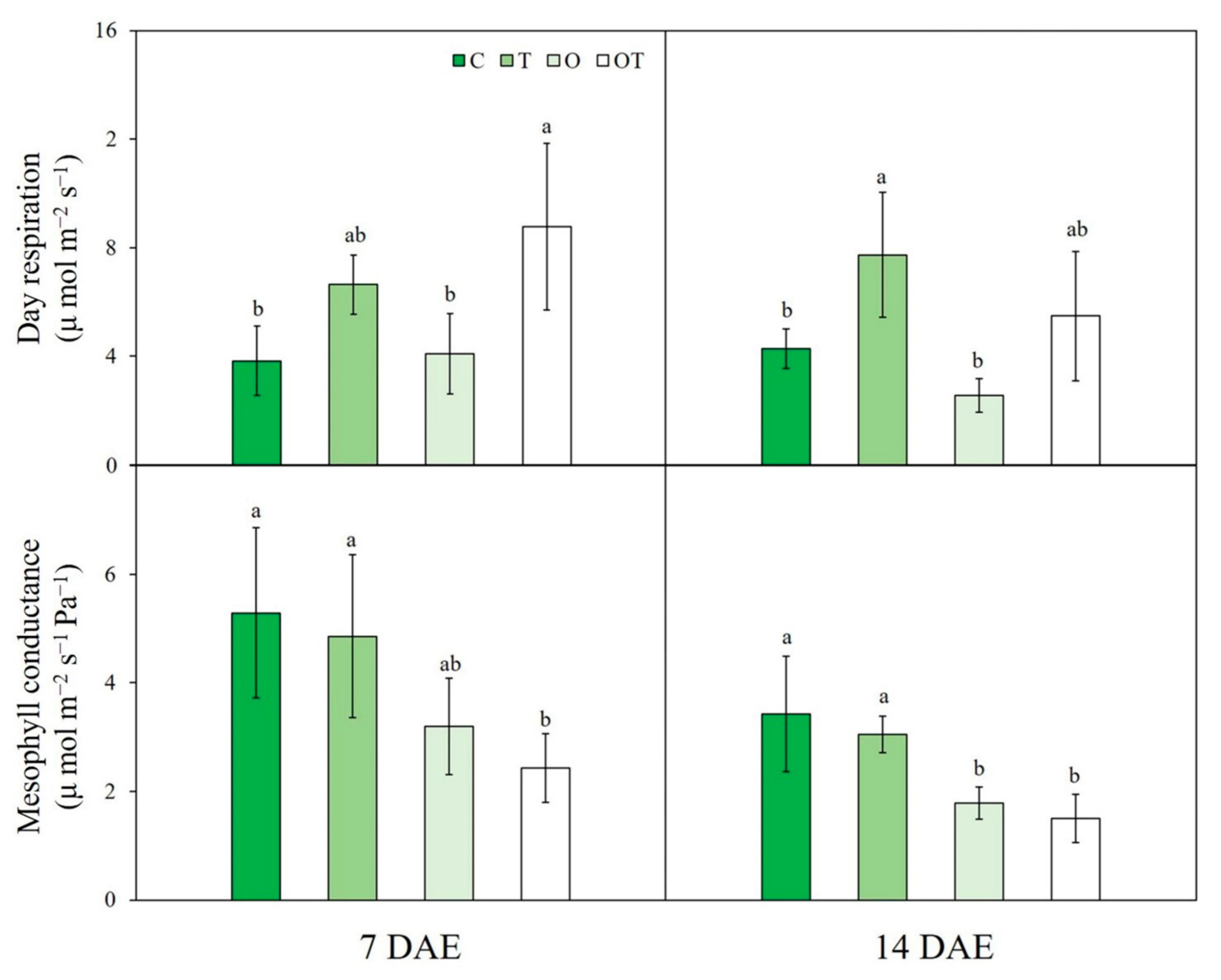
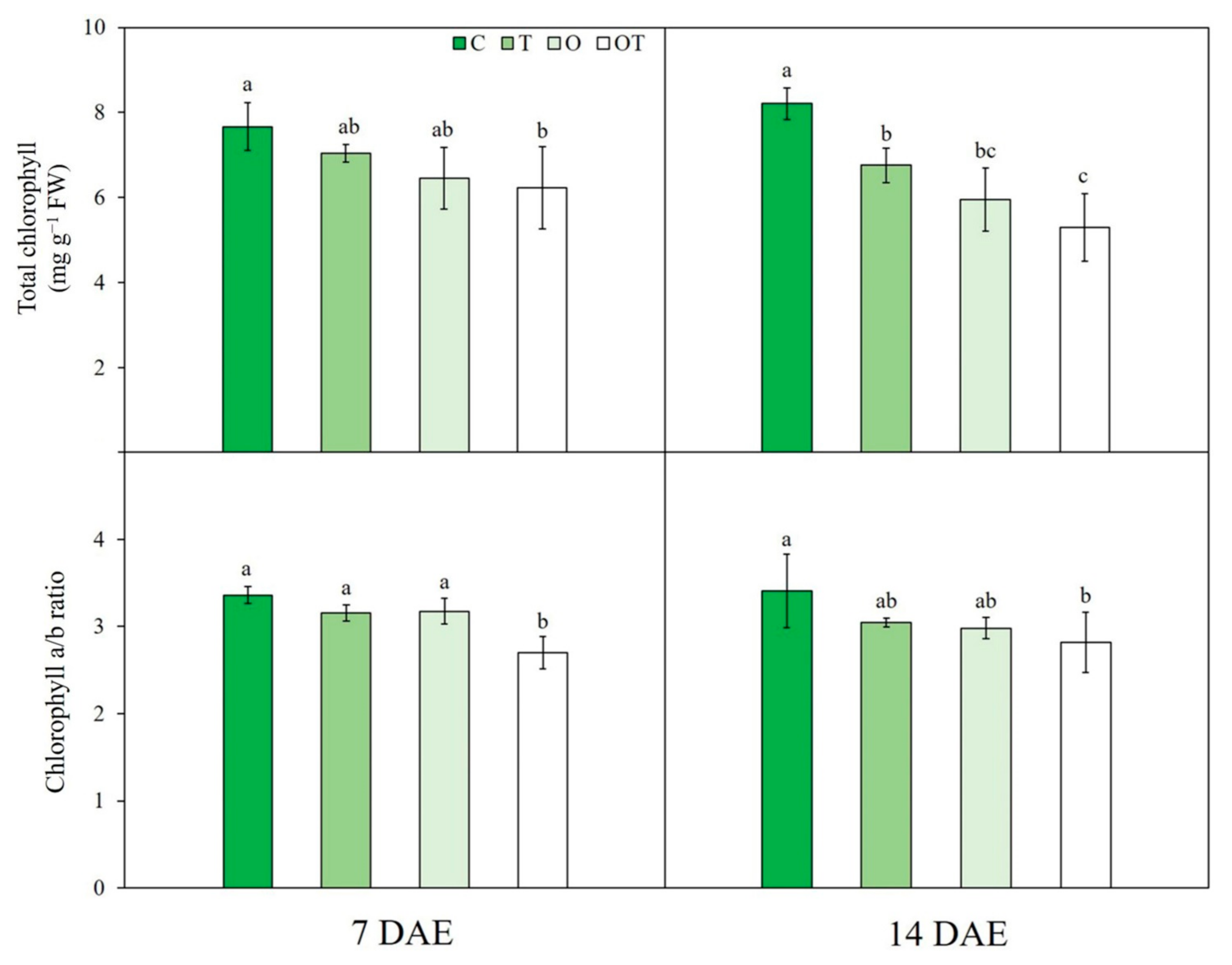

| Treatment | Notation | Ozone Concentration (ppb) | Temperature (°C, Day/Night) |
|---|---|---|---|
| Charcoal-filtered O3 + optimal temperature | C | 11 ± 3.4 | 22/20 |
| Charcoal-filtered O3 + supraoptimal temperature | T | 13 ± 3.9 | 27/25 |
| Elevated O3 + optimal temperature | O | 105.6 ± 9.2 | 22/20 |
| Elevated O3 + supraoptimal temperature | OT | 103 ± 9.9 | 27/25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.K.; Kwak, M.J.; Park, S.H.; Kim, H.D.; Lim, Y.J.; Jeong, S.G.; Choi, Y.S.; Woo, S.Y. Ozone Response of Leaf Physiological and Stomatal Characteristics in Brassica juncea L. at Supraoptimal Temperatures. Land 2021, 10, 357. https://doi.org/10.3390/land10040357
Lee JK, Kwak MJ, Park SH, Kim HD, Lim YJ, Jeong SG, Choi YS, Woo SY. Ozone Response of Leaf Physiological and Stomatal Characteristics in Brassica juncea L. at Supraoptimal Temperatures. Land. 2021; 10(4):357. https://doi.org/10.3390/land10040357
Chicago/Turabian StyleLee, Jong Kyu, Myeong Ja Kwak, Sang Hee Park, Han Dong Kim, Yea Ji Lim, Su Gyeong Jeong, Yun Soo Choi, and Su Young Woo. 2021. "Ozone Response of Leaf Physiological and Stomatal Characteristics in Brassica juncea L. at Supraoptimal Temperatures" Land 10, no. 4: 357. https://doi.org/10.3390/land10040357
APA StyleLee, J. K., Kwak, M. J., Park, S. H., Kim, H. D., Lim, Y. J., Jeong, S. G., Choi, Y. S., & Woo, S. Y. (2021). Ozone Response of Leaf Physiological and Stomatal Characteristics in Brassica juncea L. at Supraoptimal Temperatures. Land, 10(4), 357. https://doi.org/10.3390/land10040357









