Greenhouse Gas Emissions from Forest Soils Reduced by Straw Biochar and Nitrapyrin Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Biochar
2.2. Experimental Design and Incubation Procedure
2.3. Gas Sampling and Analysis
2.4. Soil Analysis
2.5. Data Analysis
3. Results
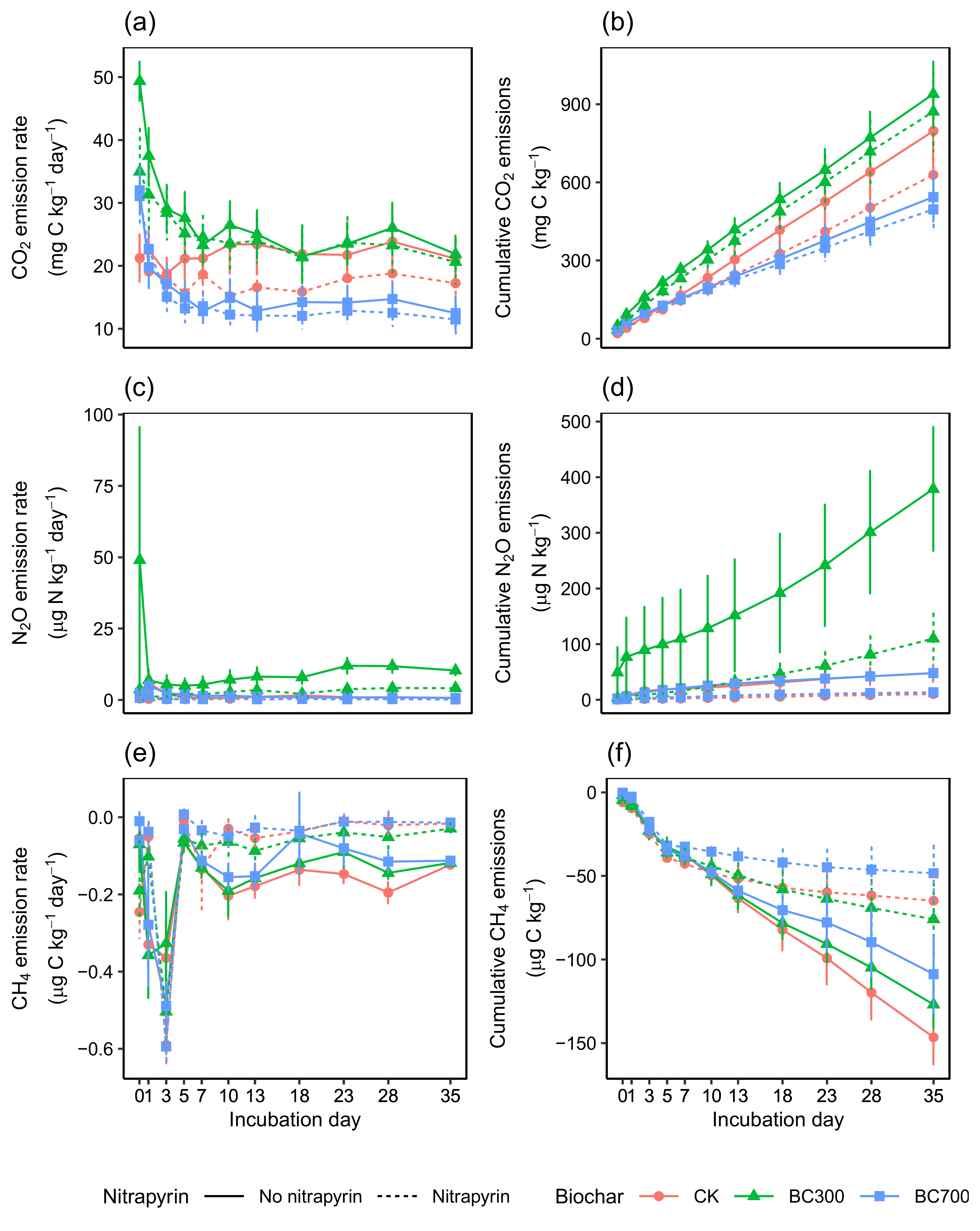
3.1. Biochar and Nitrapyrin Effects on CO2 Emissions
3.2. Biochar and Nitrapyrin Effects on N2O Emissions
3.3. Biochar and Nitrapyrin Effects on CH4 Emissions
3.4. Biochar and Nitrapyrin Effects on Global Warming Potential (GWP)
3.5. Biochar and Nitrapyrin Effects on Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amadi, C.C.; van Rees, K.C.J.; Farrell, R.E. Greenhouse gas mitigation potential of shelterbelts: Estimating farm-scale emission reductions using the Holos model. Can. J. Soil Sci. 2016. [Google Scholar] [CrossRef]
- Toensmeier, E.; Herren, D.H. The Carbon Farming Solution: A Global Toolkit of Perennial Crops and Regenerative Agriculture Practices for Climate Change Mitigation and Food Security, Illustrated edition; Chelsea Green Publishing: White River Junction, VT, USA, 2016. [Google Scholar]
- IPCC. Climate Change and Land: An IPCC Special Report on Chlimate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC Climate Change and Land: Geneva, Switzerland, 2019. [Google Scholar]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Baah-Acheamfour, M.; Carlyle, C.N.; Lim, S.-S.; Bork, E.W.; Chang, S.X. Forest and grassland cover types reduce net greenhouse gas emissions from agricultural soils. Sci. Total Environ. 2016, 571, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK; New York, NY, USA, 2015. [Google Scholar]
- Brenzinger, K.; Drost, S.M.; Korthals, G.; Bodelier, P.L.E. Organic residue amendments to modulate greenhouse gas emissions from agricultural soils. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Chang, S.X.; Bork, E.W.; Carlyle, C.N. Enrichment planting and soil amendments enhance carbon sequestration and reduce greenhouse gas emissions in agroforestry systems: A review. Forests 2018, 9, 369. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Soil Res. 2010, 48, 516. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. Chapter 2-A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Hillel, D.; Rosenzweig, C. Handbook of Climate Change and Agroecosystems: Impacts, Adaptation, And Mitigation; Imperial College Press: London, UK, 2010. [Google Scholar]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732. [Google Scholar] [CrossRef]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; Kammann, C.; Abalos, D. Biochar effects on methane emissions from soils: A meta-analysis. Soil Biol. Biochem. 2016, 101, 251–258. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and tce adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.-H.; Islam, M.S.; Wang, S.; Messele, S.A.; Naeth, M.A.; El-Din, M.G.; Chang, S.X. Biochar properties and lead(II) adsorption capacity depend on feedstock type, pyrolysis temperature, and steam activation. Chemosphere 2019, 231, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Kwak, J.-H.; Ok, Y.S.; Chang, S.X. Pine sawdust biochar reduces GHG emission by decreasing microbial and enzyme activities in forest and grassland soils in a laboratory experiment. Sci. Total Environ. 2018, 625, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Laird, D.A.; Ahmedna, M.A.; Niandou, M.A.S. Short-term CO2 mineralization after additions of biochar and switchgrass to a typic kandiudult. Geoderma 2010, 154, 281–288. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef]
- Li, J.; Kwak, J.-H.; Chen, J.; An, Z.; Gong, X.; Chang, S.X. Canola straw biochars produced under different pyrolysis temperatures and nitrapyrin independently affected cropland soil nitrous oxide emissions. Biol. Fertil. Soils 2021, 57, 319–328. [Google Scholar] [CrossRef]
- Fisk, L.M.; Maccarone, L.D.; Barton, L.; Murphy, D.V. Nitrapyrin decreased nitrification of nitrogen released from soil organic matter but not amoA gene abundance at high soil temperature. Soil Biol. Biochem. 2015, 88, 214–223. [Google Scholar] [CrossRef]
- Dawar, K.; Khan, H.; Zaman, M.; Muller, C.; Alam, S.S.; Fahad, S.; Alwahibi, M.S.; Alkahtani, J.; Saeed, B.; Saud, S.; et al. The effect of biochar and nitrogen inhibitor on ammonia and nitrous oxide emissions and wheat productivity. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Sheikhi, J.; Mirsyed, H.H.; Etesami, H.; Majidi, A. Biochar counteracts nitrification inhibitor DMPP–mediated negative effect on Spinach (Spinacia Oleracea, L.) growth. Ecotox. Environ. Safe 2020, 191, 110243. [Google Scholar] [CrossRef]
- Li, B.; Fan, C.H.; Xiong, Z.Q.; Li, Q.L.; Zhang, M. The combined effects of nitrification inhibitor and biochar incorporation on yield-scaled N2O emissions from an intensively managed vegetable field in southeastern China. Biogeosciences 2015, 12, 2003–2017. [Google Scholar] [CrossRef]
- Soil Classification Working Group. The Canadian System of Soil Classification; NRC Research Press: Ottawa, OT, Canada, 1998; Volume 3. [Google Scholar]
- Islam, A.; Chen, D.; White, R.E. Developing a technique to quantify heterotrophic and autotrophic nitrification in acidic pasture soils. Commun. Soil Sci. Plan. 2007, 38, 2309–2321. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Brookes, P.C.; Powlson, D.S. Measuring soil microbial biomass. Soil Biol. Biochem. 2004, 36, 5–7. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Han, P.W.Y.; Xu, J.M.; Yi, X.Y.; Lin, Y.D. Net and gross nitrification in tea soils of varying productivity and their adjacent forest and vegetable soils. Soil Sci. Plant. Nutr. 2012, 58, 173–182. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Song, X.; Pan, G.; Zhang, C.; Zhang, L.; Wang, H. Effects of biochar application on fluxes of three biogenic greenhouse gases: A meta-analysis. Ecosyst. Health Sust. 2016, 2, e01202. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1489. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Deenik, J.L.; McClellan, T.; Uehara, G.; Antal, M.J.; Campbell, S. Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci. Soc. Am. J. 2010, 74, 1259–1270. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhu, L.; Cheng, H.; Yue, S.; Li, S. Effects of biochar application on CO2 emissions from a cultivated soil under semiarid climate conditions in northwest China. Sustainability 2017, 9, 1482. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Wang, Y.; Huang, H.; Chen, Y. Influence of pyrolysis temperature and holding time on properties of biochar derived from medicinal herb (Radix Isatidis) residue and its effect on soil CO2 emission. J. Anal. Appl. Pyrol. 2014, 110, 277–284. [Google Scholar] [CrossRef]
- Bremner, J.M. Sources of nitrous oxide in soils. Nutr. Cycl. Agroecosyst. 1997, 49, 7–16. [Google Scholar] [CrossRef]
- Groffman, P.M.; Altabet, M.A.; Böhlke, J.K.; Butterbach-Bahl, K.; David, M.B.; Firestone, M.K.; Giblin, A.E.; Kana, T.M.; Nielsen, L.P.; Voytek, M.A. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecol. Appl. 2006, 16, 2091–2122. [Google Scholar] [CrossRef]
- Wrage-Mönnig, N.; Horn, M.A.; Well, R.; Müller, C.; Velthof, G.; Oenema, O. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 2018, 123, A3–A16. [Google Scholar] [CrossRef]
- Fan, C.; Duan, P.; Zhang, X.; Shen, H.; Chen, M.; Xiong, Z. Mechanisms underlying the mitigation of both N2O and NO emissions with field-aged biochar in an anthrosol. Geoderma 2020, 364, 114178. [Google Scholar] [CrossRef]
- Spokas, K.A.; Reicosky, D.C. Impacts of sixteen different biochars on soil greenhouse gas production. Ann. Environ. Sci. 2009, 3, 179–193. [Google Scholar]
- Ji, C.; Li, S.; Geng, Y.; Yuan, Y.; Zhi, J.; Yu, K.; Han, Z.; Wu, S.; Liu, S.; Zou, J. Decreased N2O and NO emissions associated with stimulated denitrification following biochar amendment in subtropical tea plantations. Geoderma 2020, 365, 114223. [Google Scholar] [CrossRef]
- Xu, X.; He, C.; Yuan, X.; Zhang, Q.; Wang, S.; Wang, B.; Guo, X.; Zhang, L. Rice straw biochar mitigated more N2O emissions from fertilized paddy soil with higher water content than that derived from ex situ biowaste. Environ. Pollut. 2020, 263, 114477. [Google Scholar] [CrossRef] [PubMed]
- Weier, K.L.; Doran, J.W.; Power, J.F.; Walters, D.T. Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Sc. Soc. Am. J. 1993, 57, 66–72. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.; Zheng, X.; Wang, Y.; Xu, X. Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- van Zwieten, L.; Singh, B.; Joseph, S.; Kimber, S.; Cowie, A.; and Chan, K.Y. Biochar and emissions of non-CO2 greenhouse gases from soil. In Biochar Environmental Management Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 227–250. [Google Scholar]
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R727–R732. [Google Scholar] [CrossRef]
- Niu, Y.; Luo, J.; Liu, D.; Müller, C.; Zaman, M.; Lindsey, S.; Ding, W. Effect of biochar and nitrapyrin on nitrous oxide and nitric oxide emissions from a sandy loam soil cropped to maize. Biol. Fertil. Soils 2018, 54, 645–658. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Cai, Z.; Müller, C.; Zhang, J. Short-term effects of nitrapyrin, rice straw and its biochar application on N transformation in soils of humid subtropical China. Acta Agric. Scand. Sect. B Soil Plant. Sci. 2018, 68, 448–456. [Google Scholar] [CrossRef]
- Bremner, J.M.; Yeomans, J.C. Effects of nitrification inhibitors on denitrification of nitrate in soil. Biol. Fert. Soils 1986, 2, 173–179. [Google Scholar] [CrossRef]
- Topp, E.; Knowles, R. Effects of nitrapyrin [2-chloro-6-(trichloromethyl) pyridine] on the obligate methanotroph Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 1984, 47, 258–262. [Google Scholar] [CrossRef] [PubMed]
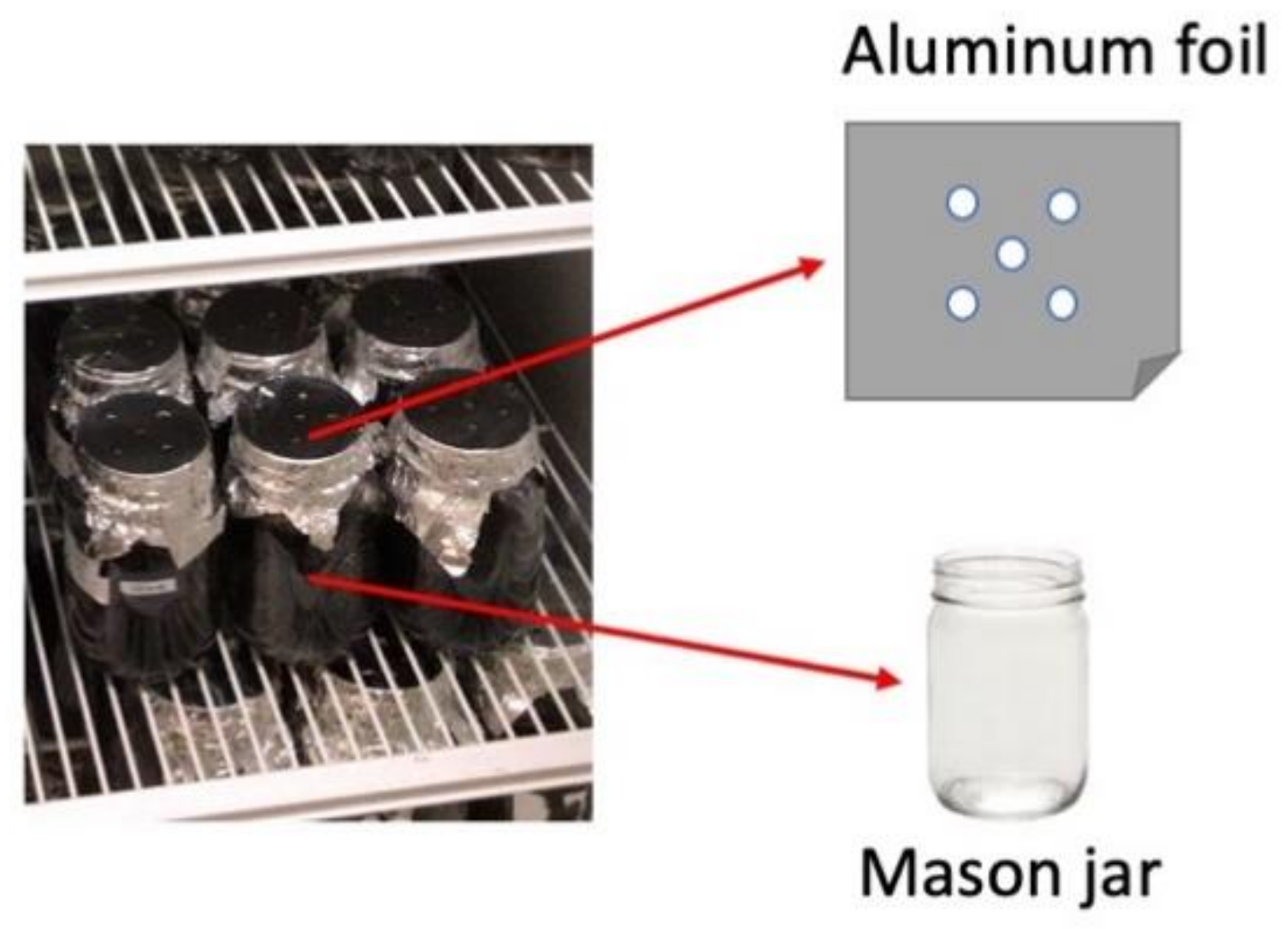
| Factor | CO2 | N2O | CH4 | GWP | pH | DOC | MBC | NNR |
|---|---|---|---|---|---|---|---|---|
| Biochar | 46.2 ** | 30.6 ** | 1.25 | 72.4 ** | 22.9 ** | 10.5 ** | 4.89 * | 2.27 |
| Nitrapyrin | 8.36 ** | 34.8 ** | 18.2 ** | 17.2 ** | 31.5 ** | 0.02 | 2.47 | 22.3 ** |
| Biochar × nitrapyrin | 1.30 | 0.16 | 0.36 | 1.41 | 0.06 | 0.06 | 0.78 | 0.54 |
| Treatment | CO2 (mg C kg−1) | N2O (µg N kg−1) | CH4 (µg C kg−1) | GWP (mg CO2-C kg−1) | pH | DOC (mg kg−1) | MBC (mg kg−1) | NNR (mg N kg−1 day−1) |
|---|---|---|---|---|---|---|---|---|
| CK 1 | 713.1 b | 19.5 2 b | −105.7 a | 718.8 b | 5.87 c | 653.1 b | 739.1 b | 0.49 a |
| BC300 | 905.2 a | 141.9 a | −101.4 a | 975.3 a | 6.03 b | 825.9 a | 989.5 a | 0.90 a |
| BC700 | 520.0 c | 23.2 b | −78.6 a | 526.9 c | 6.24 a | 769.8 a | 810.4 ab | 0.34 a |
| No nitrapyrin | 760.1 A | 78.8 A | −127.4 B | 803.7 A | 5.92 B | 747.5 A | 899.2 A | 1.17 A |
| Nitrapyrin | 665.5 B | 20.3 B | −63.1 A | 677.0 B | 6.17 A | 751.7 A | 793.5 A | −0.01 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Kwak, J.-H.; Chang, S.X.; Gong, X.; An, Z.; Chen, J. Greenhouse Gas Emissions from Forest Soils Reduced by Straw Biochar and Nitrapyrin Applications. Land 2021, 10, 189. https://doi.org/10.3390/land10020189
Li J, Kwak J-H, Chang SX, Gong X, An Z, Chen J. Greenhouse Gas Emissions from Forest Soils Reduced by Straw Biochar and Nitrapyrin Applications. Land. 2021; 10(2):189. https://doi.org/10.3390/land10020189
Chicago/Turabian StyleLi, Jinbiao, Jin-Hyeob Kwak, Scott X. Chang, Xiaoqiang Gong, Zhengfeng An, and Jinlin Chen. 2021. "Greenhouse Gas Emissions from Forest Soils Reduced by Straw Biochar and Nitrapyrin Applications" Land 10, no. 2: 189. https://doi.org/10.3390/land10020189
APA StyleLi, J., Kwak, J.-H., Chang, S. X., Gong, X., An, Z., & Chen, J. (2021). Greenhouse Gas Emissions from Forest Soils Reduced by Straw Biochar and Nitrapyrin Applications. Land, 10(2), 189. https://doi.org/10.3390/land10020189










