Toward Best Management Practices for Ecological Corridors
Abstract
1. Introduction
2. Methods
3. Recommendations for Human-Created Linear Barriers Crossing Corridors
3.1. Influence of Roads
3.2. Influence of Canals on Connectivity and Ecosystems
3.3. Influence of Border Security Structures on Connectivity and Ecosystems
3.4. Influence of Other Transmission Lines and Livestock Fences on Connectivity
3.5. Avoid Building Roads in Corridors
3.6. Minimize Artificial Lighting on Roads That Pass through the Linkage Design
3.7. Reduce Vehicle Speeds on Roads in Corridors
3.8. Raise the Highway Bed above the Surrounding Terrain to Minimize Road Kill and Direct Animals toward Crossing Structures
3.9. Design Highway and Canal Crossing Structures Specifically to Provide for Animal Movement
3.10. Build Multiple Types of Crossing Structures Spanning Highways and Canals to Provide Connectivity for All Species, Preferably with Appropriate Structures No More Than One Home Range Width Apart
3.11. Maintain Suitable Habitat on Both Sides of Highway and Canal Crossing Structures
3.12. Use Fencing along Highways and Canals to Keep Animals off the Roadway and Funnel Them toward Crossing Structures
3.13. Provide Alternative Water Sources near Canals and Escape Structures along Unfenced Canals
3.14. Use Wildlife-Friendly Fencing
4. Recommendations for Streams and Riparian Zones in Corridors
4.1. Support Native Riparian Ecosystems along Streams
4.2. Create and Protect Buffer Regions of Natural Habitat Beyond the Riparian Zone
4.3. Maintain Biotic/Abiotic Interactions
4.4. Eradicate or Control Invasive Riparian Plants
4.5. Enforce Existing Regulations
5. Recommendations for Urban and Suburban Development in Corridors
5.1. Avoid and Minimize Urbanization in or Adjacent to Corridors
5.2. Encourage People Residing in or Adjacent to the Linkage to Be Proud Stewards
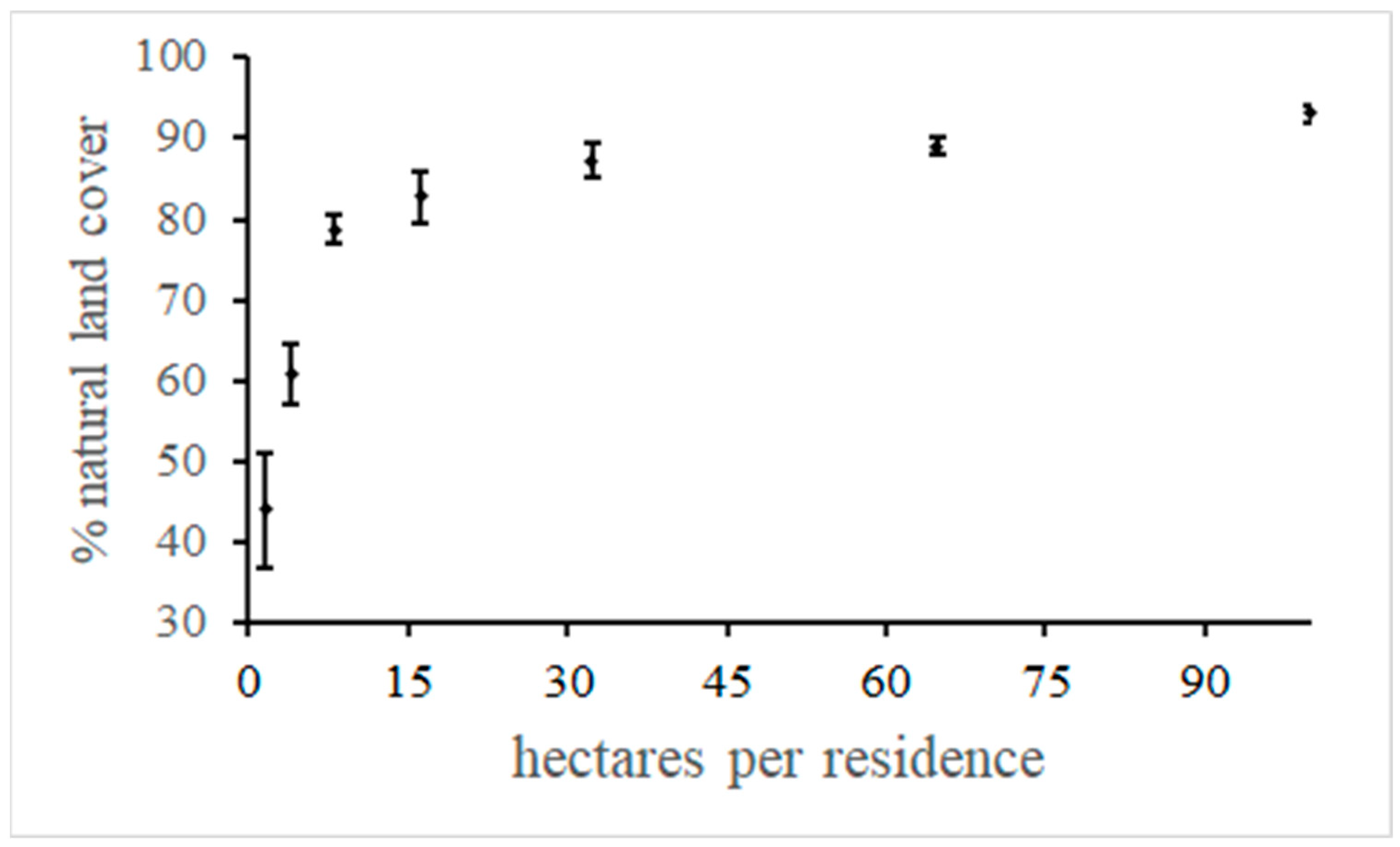
5.3. Encourage Small Building Footprints on Large Parcels with a Minimal Road Network
5.4. Regulate Access by Recreational Users
5.5. Discourage Residents and Visitors from Feeding Wildlife
5.6. Take Special Steps to Protect Species That Might Be Perceived as Undesirable
6. Recommendations for Agricultural Development in Corridors
6.1. Encourage Farmers to be Good Stewards of the Corridor
6.2. Encourage Farmers to Reduce the Amount of Herbicide, Pesticide, and Fertilizer They Use and Provide Information about No-Till Farming Practices
6.3. Encourage Controlled Burns or Mechanical Removal of Debris along Corridor and Adjacent Lands That Most Closely Mimic Natural Fire Regimes
6.4. Encourage Conservation Easements or Acquisition of Conservation Land
6.5. Use National and Local Agricultural Programs
6.6. Help Ranchers Reduce Depredation Losses
6.7. Concentrate Supplemental Feeding and Water Sites Away from Natural Areas
6.8. Prohibit Farmed Wildlife within or Adjacent to Corridors
6.9. Limit the Number of Landowners Whose Property Is Either within or Adjacent to the Corridor
7. Knowledge Gaps, Summary, and Conclusions
7.1. What Are Critical Dimensions of Corridors?
7.2. Is There a Critical Threshold in Intensity of Land Use?
7.3. How do Landscape Traits of Corridors Affect Different Species?
7.4. How Far Away from the Linkage Edge Do We Need to Manage Human Uses?
7.5. How Do Time Lags Affect Corridor Use?
7.6. Which Governance and Management Activities Are Most Acceptable to People Living in or near Corridors and Which Policies Are Beneficial for Corridors?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diamond, J.M. Island Biogeography and conservation: Strategies and limitations. Science 1976, 193, 1027–1029. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G. Linkages in Practice: A Review of their Conservation Value; IUCN: Gland, Switzerland, 2004. [Google Scholar]
- Hilty, J.L.; Lidicker, W.M.; Merenlender, A.M. Corridor Ecology: The Science and Practice of Linking Landscapes for Biodiversity Conservation; Island Press, Inc.: Washington, DC, USA, 2006. [Google Scholar]
- Beier, P.; Majka, D.R.; Spencer, W.D. Forks in the road: Choices in procedures for designing wildland linkages. Conserv. Biol. 2008, 22, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Beier, P.; Gregory, A.J. Desperately seeking sTable 50-year-old landscapes with patches and long, wide corridors. PLoS Biol. 2012, 10, 1001253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Resasco, J. Meta-analysis on a Decade of Testing Corridor Efficacy: What New Have We Learned? Curr. Landsc. Ecol. Rep. 2019, 4, 61–69. [Google Scholar] [CrossRef]
- Hilty, J.; Worboys, G.L.; Keeley, A.; Woodley, S.; Lausche, B.; Locke, H.; Carr, M.; Pulsford, I.; Pittock, J.; White, J.W.; et al. Guidelines for Conserving Connectivity through Ecological Networks and Corridors; Best Practice Protected Area Guidelines Series No. 30; IUCN: Gland, Switzerland, 2020. [Google Scholar]
- Beier, P.; Spencer, W.D.; Baldwin, R.; McRae, B.H. Toward best practices for developing regional connectivity maps. Conserv. Biol. 2011, 25, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Cushman, S.A.; McRae, B.H.; Adriaensen, F.; Beier, P.; Shirley, M.; Zeller, K. Biological corridors. In Key Topics in Conservation Biology; Macdonald, D.W., Willis, K., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; Volume 2, pp. 384–404. [Google Scholar]
- Rudnick, D.A.; Ryan, S.J.; Beier, P.; Cushman, S.A.; Dieffenbach, F.; Epps, C.W.; Gerber, L.R.; Hartter, J.; Jenness, J.S.; Kintsch, J.; et al. Emerging principles in understanding landscape connectivity: Practical tools for conservation decision-making. Issues Ecol. 2012, 16, 1–19. [Google Scholar]
- Keeley, A.T.H.; Beier, P.; Creech, T.; Jones, K.; Jongman, R.H.G.; Stonecipher, G.; Tabor, G.M. Thirty years of connectivity conservation planning: An assessment of factors influencing plan implementation. Environ. Res. Lett. 2019, 14, 103001. [Google Scholar] [CrossRef]
- Fahrig, L.; Arroyo-Rodríguez, V.; Bennett, J.R.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.G.; et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; Volume 1. [Google Scholar]
- Diamond, J.M. The island dilemma: Lessons of modern biogeographic studies for the design of natural reserves. Biol. Conserv. 1975, 7, 129–146. [Google Scholar] [CrossRef]
- Gilbert-Norton, L.; Wilson, R.; Stevens, J.R.; Beard, K.H. A meta-analytic review of corridor effectiveness. Conserv. Biol. 2010, 24, 660–668. [Google Scholar] [CrossRef]
- Gregory, A.J.; Beier, P. Response variables for evaluation of the effectiveness of conservation corridors. Conserv. Biol. 2014, 28, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Beier, P.; Majka, D.; Bayless, T. Arizona Missing Linkages: Eight Linkage Designs. 2007. Available online: www.corridordesign.org (accessed on 17 April 2020).
- Beier, P.; Garding, E.; Majka, D. Arizona Missing Linkages: Eight Linkage Designs in Urban Settings. 2008. Available online: www.corridordesign.org (accessed on 24 January 2021).
- Penrod, K.; Cabañero, C.; Beier, P.; Luke, C.; Spencer, W.; Rubin, E. 2003–2006 (11 Reports). South Coast Missing Linkages Project: 11 Linkage Designs. Available online: www.scwildlands.org (accessed on 24 January 2021).
- Ford, A.T.; Sunter, E.J.; Fauvelle, C.; Bradshaw, J.L.; Ford, B.; Hutchen, J.; Phillipow, N.; Teichman, K.J. Effective corridor width: Linking the spatial ecology of wildlife with land use policy. Eur. J. Wildl. Res. 2020, 66, 69. [Google Scholar] [CrossRef]
- Beckman, J.P.; Clevenger, A.P.; Huijser, M.P.; Hilty, J.A. Safe Passage; Island Press: Washington, DC, USA, 2010. [Google Scholar]
- Popowski, R.J.; Krausman, P.R. Use of crossings over the Tucson aqueduct by selected mammals. Southwest. Nat. 2002, 47, 363–371. [Google Scholar] [CrossRef]
- Young, A.G.; Clarke, G.M. Genetics, Demography, and Viability of Fragmented Populations; Cambridge University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Peris, S.; Morales, J. Use of passages across a canal by wild mammals and related mortality. Eur. J. Wildl. Res. 2004, 50, 67–72. [Google Scholar] [CrossRef]
- McGregor, R.L.; Bender, D.J.; Fahrig, L. Do small mammals avoid roads because of the traffic? J. Appl. Ecol. 2008, 45, 117–123. [Google Scholar] [CrossRef]
- Schwab, A.C.; Zandbergen, P.A. Vehicle-related mortality and road crossing behavior of the Florida panther. Appl. Geogr. 2011, 31, 859–870. [Google Scholar] [CrossRef]
- Rao, R.S.P.; Girish, M.K.S. Road kills: Assessing insect casualties using flagship taxon. Curr. Sci. 2007, 92, 830–837. [Google Scholar]
- Haines, A.M.; Tewes, M.E.; Laack, L.L. Survival and sources of mortality in ocelots. J. Wildl. Manag. 2005, 69, 255–263. [Google Scholar] [CrossRef]
- Rosen, P.C.; Lowe, C.H. Highway mortality of snakes in the Sonoran Desert of southern Arizona. Biol. Conserv. 1994, 68, 143–148. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Sperling, D.; Bissonette, J.A.; Clevenger, A.P.; Cutshall, C.D.; Dale, V.H.; Fahrig, L.; France, R.L.; Goldman, C.R.; Heanue, K. Road Ecology: Science and Solutions; Island Press: Washington, DC, USA, 2003. [Google Scholar]
- Loss, S.; Will, T.; Marra, P. Estimation of bird-vehicle collision mortality on US roads. J. Wildl. Manag. 2014, 78, 763–771. [Google Scholar] [CrossRef]
- Pruett, C.L.; Patten, M.A.; Wolfe, D.H. Avoidance behavior by prairie grouse: Implications for development of wind energy. Conserv. Biol. 2009, 23, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Beier, P. Determining minimum habitat areas and habitat corridors for cougars. Conserv. Biol. 1993, 7, 94–108. [Google Scholar] [CrossRef]
- Ferreras, P.; Aldama, J.J.; Beltrán, J.F.; Delibes, M. Rates and causes of mortality in a fragmented population of Iberian lynx Felis pardina Temminck, 1824. Biol. Conserv. 1992, 6, 197–202. [Google Scholar] [CrossRef]
- Vickers, T.W.; Sanchez, J.N.; Johnson, C.K.; Morrison, S.A.; Botta, R.; Smith, T.; Cohen, B.S.; Huber, P.R.; Ernest, H.B.; Boyce, W.M. Survival and mortality of pumas (Puma concolor) in a fragmented, urbanizing landscape. PLoS ONE 2015, 10, e0131490. [Google Scholar] [CrossRef]
- Benson, J.F.; Mahoney, P.J.; Vickers, T.W.; Sikich, J.A.; Beier, P.; Riley, S.P.D.; Ernest, H.B.; Boyce, W.M. Extinction vortex dynamics of top predators isolated by urbanization. Ecol. Appl. 2019, 29, e01868. [Google Scholar] [CrossRef]
- Van der Zee, F.F.; Wiertz, J.; Ter Braak, C.J.F.; van Apeldoorn, R.C. Landscape change as a possible cause of the badger (Meles meles) decline in the Netherlands. Biol. Conserv. 1992, 61, 17–22. [Google Scholar] [CrossRef]
- Marsh, D. Edge effects of gated and ungated roads on terrestrial salamanders. J. Wildl. Manag. 2007, 71, 389–394. [Google Scholar] [CrossRef]
- Hetherington, T. Role of the opercularis muscle in seismic sensitivity in the bullfrog Rana catesbeiana. J. Exp. Zool. 2005, 235, 27–34. [Google Scholar] [CrossRef]
- Findlay, C.S.; Houlahan, J. Anthropogenic correlates of species richness in southeastern Ontario wetlands. Conserv. Biol. 1997, 11, 1000–1009. [Google Scholar] [CrossRef]
- Slabekoorn, H.; Ripmeester, E.A. Birdsong and anthropogenic noise: Implications and applications for conservation. Mol. Ecol. 2008, 17, 72–83. [Google Scholar] [CrossRef]
- Goodwin, S.E.; Shriver, G.W. Effects of traffic noise on occupancy patterns of forest birds. Conserv. Biol. 2010, 25, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, J.; Hoopes, M.F.; Marchett, M.P. Invasion Ecology; Wiley-Blackwell Publishers: West Sussex, UK, 2007. [Google Scholar]
- Collinge, S. Ecology of Fragmented Landscapes; The John Hopkins University Press: Baltimore, MD USA, 2009. [Google Scholar]
- Von der Lippe, M.; Kowarik, I. Long-distance dispersal of plants by vehicles as a driver of plant invasions. Conserv. Biol. 2007, 21, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Rich, C.; Longcore, T. Ecological Effects of Artificial Night Lighting; Island Press: Washington, DC, USA, 2006. [Google Scholar]
- Rautenstrauch, K.R.; Krausman, P.R. Preventing mule deer drowning in the Mohawk canal, Arizona. Wildl. Soc. Bull. 1989, 17, 281–286. [Google Scholar]
- Terry, A.; Ullrich, K.; Riecken, U. The Green Belt of Europe: FromVision to Reality; IUCN: Gland, Switzerland; Cambridge, UK, 2006; ISBN 978-2-8317-0945-1. [Google Scholar]
- Pahalwan, A. Fenced in, Kashmir’s Leopard, Bears Stalk Villages. Reuters. Available online: http://www.reuters.com/article/2006/11/23/us-environment-kashmir-idUSDEL17197120061123 (accessed on 21 June 2013).
- Flesch, A.D.; Epps, C.W.; Cain, J.W., III; Clark, M.; Krausman, P.R.; Morgart, J.R. Potential effects of the United States-Mexico border fence on wildlife. Conserv. Biol. 2009, 24, 171–181. [Google Scholar] [CrossRef]
- Haddad, C.C.; Kim, Y.; Garcia, M.C. Border Security: Barriers along the US International Border; Congressional Research Service: Washington, DC, USA, 2009. [Google Scholar]
- Manville, A.M. Avian Mortality at Communication Towers: Background and Overview; Evans, W.R., Manville, A.M., II, Eds.; 2000; pp. 1–5. Available online: http:migratorybirds.fws.gov/issues/towers/agenda.html (accessed on 9 July 2011).
- Wolfe, D.H.; Patten, M.A.; Shochat, E.; Pruett, C.L.; Sherod, S.K. Causes and patterns of mortality in lesser prairie-chickens Tympanuchus pallidicinctus and implications for management. Wildl. Biol. 2007, 13, 95–104. [Google Scholar] [CrossRef]
- Lopucki, R.; Klich, D.; Gielarek, S. Do terrestrial animals avoid areas close to turbines in functioning wind farms in agricultural landscapes? Environ. Monit. Assess. 2017, 189, 343. [Google Scholar] [CrossRef]
- Gehring, T.M.; Swihart, R.K. Home range and movements of long-tailed weasels in a landscape fragmented by agriculture. J. Mammal. 2004, 85, 138–145. [Google Scholar] [CrossRef]
- Mladenoff, D.J.; Sickley, T.A.; Haight, R.G.; Wydeven, A.P. A regional landscape analysis and prediction of favorable grey wolf habitat in the northern Great Lakes region. Conserv. Biol. 1995, 9, 279–294. [Google Scholar] [CrossRef]
- Rowland, M.M.; Wisdom, M.J.; Johnson, B.K.; Penninger, M.A. Effects of roads on elk: Implications for management in forested ecosystems. In Proceedings of the Transactions of the 69th North American Wildlife and Natural Resources Conference, Spokane, WA, USA, 16–20 March 2004; pp. 491–508. [Google Scholar]
- Boone, R.B.; Hobbs, T.N. Lines around fragments: Effects of fencing on large herbivores. Afr. J. Range Forage Sci. 2004, 21, 147–158. [Google Scholar] [CrossRef]
- Gaston, K.J.; Davies, T.W.; Nedelec, S.L.; Holt, L.A. Impacts of artificial light at night on biological timings. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 49–68. [Google Scholar] [CrossRef]
- Khalilikhah, M.; Heaslip, K. Improvement of the performance of animal crossing warning signs. J. Saf. Res. 2017, 62, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.K.; Smith, D.J.; Noss, R.F. Reducing the threat of wildlife-vehicle collisions during peak tourism periods using a roadside animal detection system. Accid. Anal. Prev. 2017, 109, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, A.P.; Chruszcz, B.; Gunson, K.E. Spatial patterns and factors influencing small vertebrate fauna road-kill aggregations. Biol. Conserv. 2003, 109, 15–26. [Google Scholar] [CrossRef]
- Clevenger, A.P.; Chruszcz, B.; Gunson, K. Drainage culverts as habitat linkages and factors affecting passage by mammals. J. Appl. Ecol. 2001, 38, 1340–1349. [Google Scholar] [CrossRef]
- Clevenger, A.P.; Huijser, M.P. Handbook for Design and Evaluation of Wildlife Crossing Structures; Western Transportation Institute, Bozeman Montana, and Federal Highways Administration: Washington, DC, USA, 2009. [Google Scholar]
- Clevenger, A.P.; Waltho, N. Performance indices to identify attributes of highway crossing structures facilitating movement of large mammals. Biol. Conserv. 2005, 121, 453–464. [Google Scholar] [CrossRef]
- Ng, S.J.; Dole, J.W.; Sauvajot, R.M.; Riley, S.P.D.; Valone, T.J. Use of highway undercrossings by wildlife in southern California. Biol. Conserv. 2004, 115, 499–507. [Google Scholar] [CrossRef]
- Brudin, C.O., III. Wildlife use of existing culverts and bridges in north central Pennsylvania. In Proceedings of the 2003 International Conference on Ecology and Transportation, Lake Placid, NY, USA, 24–29 August 2003; Center for Transportation and the Environment, North Carolina State University: Raleigh, NC, USA, 2003; pp. 344–352. [Google Scholar]
- Gagnon, J.W.; Theimer, T.C.; Dodd, N.L.; Manzo, A.L.; Schweinsburg, R.E. Effects of traffic on elk use of wildlife underpasses in Arizona. J. Wildl. Manag. 2007, 71, 2324–2328. [Google Scholar] [CrossRef]
- Dodd, N.L.; Gagnon, J.W.; Manzo, A.L.; Schweinsburg, R.E. Video surveillance to assess highway underpass use by elk in Arizona. J. Wildl. Manag. 2007, 71, 637–645. [Google Scholar] [CrossRef]
- MacDonald, D.; Crabtree, J.R.; Weisinger, G.; Dax, Y.; Stamou, N.; Fleury, P.; Guttierez, L.; Gibon, A. Agricultural abandonment in mountain areas of Europe: Environmental consequences and policy response. J. Environ. Manag. 2000, 59, 47–69. [Google Scholar] [CrossRef]
- Yanes, M.; Velasco, J.M.; Suárez, F. Permeability of roads and railways to vertebrates: The importance of culverts. Biol. Conserv. 1995, 71, 217–222. [Google Scholar] [CrossRef]
- Dodd, C.K.; Barichivich, W.J.; Smith, L.L. Effectiveness of a barrier wall and culverts in reducing wildlife mortality on a heavily traveled highway in Florida. Biol. Conserv. 2004, 118, 619–631. [Google Scholar] [CrossRef]
- Cain, A.T.; Tuovila, V.R.; Hewitt, D.G.; Tewes, M.E. Effects of a highway and mitigation projects on bobcats in Southern Texas. Biol. Conserv. 2003, 114, 189–197. [Google Scholar] [CrossRef]
- Mata, C.; Hervas, I.; Herranz, J.; Suarez, F.; Malo, J.E. Complementary use by vertebrates of crossing structures along a fences Spanish motorway. Biol. Conserv. 2005, 124, 397–405. [Google Scholar] [CrossRef]
- Little, S.J. The influence of predator-prey relationships on wildlife passage evaluation. In Proceedings of the 2003 International Conference on Ecology and Transportation, Lake Placid, NY, USA, 24–29 August 2003. [Google Scholar]
- Evink, G.L. Interaction between Roadways and Wildlife Ecology; National Academy Press: Washington, DC, USA, 2002. [Google Scholar]
- Clevenger, A.P.; Wierzchowski, J. Maintaining and restoring connectivity in landscapes fragmented by roads. In Connectivity Conservation; Crooks, K.R., Sanjayan, M.A., Eds.; Cambridge University Press: New York, NY, USA, 2006; pp. 502–535. [Google Scholar]
- Ruediger, B. High, wide, and handsome: Designing more effective wildlife and fish crossings for roads and highways. In Proceedings of the 2001 International Conference on Ecology and Transportation, Keystone, CO, USA, 24–28 September 2001. [Google Scholar]
- Barnum, S.A. Identifying the Best Locations along Highways to Provide Safe Crossing Opportunities for Wildlife: A Handbook for Highway Planners and Designers; Colorado Department of Transportation: Denver, CO, USA, 2003. [Google Scholar]
- Malo, J.E.; Suarez, F.; Diez, A. Can we mitigate animal-vehicle accidents using predictive models. J. Appl. Ecol. 2004, 41, 701–710. [Google Scholar] [CrossRef]
- Davidson-Nelson, S.J.; Gehring, T.M. Testing flaydry as a nonlethal management tool for wolves and coyotes in Michigan. Hum. Wildl. Interact. 2010, 4, 87–94. [Google Scholar]
- Fremier, A.K.; Kiparsky, M.; Gmur, S.; Aycrigg, J.; Craig, R.K.; Svancara, L.K.; Goble, D.D.; Cosens, B.; Davis, F.W.; Scott, J.M. A riparian conservation network for ecological resilience. Biol. Conserv. 2015, 191, 29–37. [Google Scholar] [CrossRef]
- Stromberg, J. Parts 1-3: Restoration of Riparian Vegetation in the Arid Southwest: Challenges and Opportunities. In Arizona Riparian Council Newsletter Volume 13 No. 1–3; Arizona Riparian Council: Tempe, AZ, USA, 2000; Available online: http://azriparian.asu.edu/newsletters.htm (accessed on 24 January 2021).
- Semlitsch, R.D.; Bodie, R.J. Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv. Biol. 2003, 17, 1219–1228. [Google Scholar] [CrossRef]
- Lyons, J.; Trimble, S.W.; Paine, L.K. Grass verses trees: Managing riparian areas to benefit streams of central North America. J. Am. Water Resour. Assoc. 2000, 36, 19–930. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Blaustein, A.R. Effects of introduced Bullfrogs and smallmouth bass on microhabitat use, growth, and survival of native red-legged frogs (Rana aurora). Conserv. Biol. 1998, 12, 776–787. [Google Scholar] [CrossRef]
- Maret, T.J.; Snyder, J.D.; Collins, J.P. Altered drying regime controls distribution of endangered salamanders and introduced predators. Biol. Conserv. 2006, 127, 129–138. [Google Scholar] [CrossRef]
- Stromberg, J.C.; Lite, S.J.; Marler, R.; Paradzick, C.; Shafroth, P.B.; Shorrock, D.; White, J.M.; White, M.S. Altered stream-flow regimes and invasive plant species: The Tamarix case. Global Ecol. Biogeogr. 2007, 16, 381–393. [Google Scholar] [CrossRef]
- Fisher, R.A.; Fischenich, J.C. Design Recommendations for Riparian Corridors and Vegetated Buffer Strips. U.S. Army Engineer Research and Development Center. ERDC-TN-EMRRPSR-24. 2000. Available online: www.elkhornsloughctp.org/uploads/files/1381443282Fischer%20and%20Fischenich%202000%20buffer%20design.pdf (accessed on 24 January 2021).
- Parkyn, S. Review of Riparian Buffer Zone Effectiveness; MAF Technical Paper No: 2004/05; 2004; Available online: www.maf.govt.nz/publications (accessed on 24 January 2021).
- Lee, P.; Smyth, C.; Boutin, S. Quantitative review of riparian buffer width guidelines from Canada and the United States. J. Environ. Manag. 2004, 70, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Wenger, S.J. A Review of the Scientific Literature on Riparian Buffer Width, Extent and Vegetation; University of Georgia, Public Service & Outreach, Institute of Ecology: Athens, GA, USA, 1999; 59p. [Google Scholar]
- Wenger, S.J.; Fowler, L. Protecting Stream and River Corridors; Policy Notes, Public Policy Research Series Volume 1, No. 1; Carl Vinson Institute of Government, University of Georgia: Athens, GA, USA, 2000; Available online: http://www.cviog.uga.edu/publications/pprs/96.pdf (accessed on 2 January 2021).
- Environmental Law Institute. Conservation Thresholds for Land Use Planners; Environmental Law Institute: Washington DC, USA, 2003; Available online: www.elistore.org (accessed on 21 January 2021).
- Ficetola, G.F.; Padoa-Schippa, E.; de Bernardi, R. Influence of landscape elements in riparian fubbers on the conservation of semi-aquatic amphibians. Conserv. Biol. 2009, 23, 114–123. [Google Scholar] [CrossRef]
- Naiman, R.J.; Décamps, H. The ecology of interfaces: The riparian zone. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Allen, D.J. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Nilsson, C.; Berggren, K. Alterations of riparian ecosystems caused by river regulation. BioScience 2000, 50, 783–792. [Google Scholar] [CrossRef]
- Carter, J. Tamarix Ramossima Whole Plant and Leaf Level Physiological Response to Increasing Salinity. Master’s Thesis, Division of Biology, Kansas State university, Manhattan, KS, USA, 2010. [Google Scholar]
- Zavaleta, E. The economic value of controlling an invasive shrub. AMBIO 2000, 29, 462–467. [Google Scholar] [CrossRef]
- Belsky, A.J.; Matzke, A.; Uselman, S. Survey of livestock influences on stream and riparian ecosystems in the western United States. J. Soil Water Conserv. 1999, 54, 419–431. [Google Scholar]
- Savage, M. Community Monitoring for Restoration Projects in Southwestern Riparian Communities; National Community Forestry Center Southwest Working Paper No. 16; Forest Guild: Santa Fe, NM, USA, 2004. [Google Scholar]
- D’Antonio, C.; Meyerson, L.A. Exotic plant species as problems and solutions in ecological restoration: A synthesis. Restor. Ecol. 2002, 10, 703–713. [Google Scholar] [CrossRef]
- Balbi, M.; Corci, S.; Petit, E.J.; Butet, A.; Georges, R.; Madec, L.; Caudal, J.-P.; Ernooult, A. Least=cost path analysis for urban greenways planning: A test with moths and birds across two habitats and two cities. J. Appl. Ecol. 2020. [Google Scholar] [CrossRef]
- Yu, D.; Xun, B.; Shi, P.; Shao, H.; Liu, Y. Ecological restoration planning based on connectivity in an urban area. Ecol. Eng. 2012, 46, 24–33. [Google Scholar] [CrossRef]
- Adams, C.E.; Lindsey, K.J. Urban Wildlife Management; CRC Press, Inc.: Boca Raton, FL, USA, 2010. [Google Scholar]
- [UNDESA] United Nations Department for Economic and Social Affairs. World Urbanization Prospects: 2018; UNDESA: Nairobi, Kenya, 2018. [Google Scholar]
- Maestas, J.D.; Knight, R.L.; Bilgert, W.C. Biodiversity across a rural land-use gradient. Conserv. Biol. 2003, 17, 1425–1434. [Google Scholar] [CrossRef]
- Wagner, M. Land Fragmentation in Texas: Meeting the Challenge; Technical Guidance Document PWD LF W7000-1155; Texas Wildlife and Parks: Austin, TX, USA, 2017. [Google Scholar]
- Germaine, S.S.; Rosenstock, S.S.; Schweinsburg, R.E.; Richardson, W.S. Relationships among breeding birds, habitat, and residential development in greater Tucson, Arizona. Ecol. Appl. 1998, 8, 680–691. [Google Scholar] [CrossRef]
- Germaine, S.S.; Wakeling, B.F. Lizard species distributions and habitat occupation along an urban gradient in Tucson, Arizona, USA. Biol. Conserv. 2001, 97, 229–237. [Google Scholar] [CrossRef]
- Blair, R.B. Land use and avian species diversity along an urban gradient. Ecol. Appl. 1996, 6, 506–519. [Google Scholar] [CrossRef]
- Blair, R.B.; Launer, A.E. Butterfly diversity and human land use: Species assemblages along an urban gradient. Biol. Conserv. 1997, 80, 113–125. [Google Scholar] [CrossRef]
- Blair, R.B. Birds and butterflies along an urban gradient: Surrogate taxa for assessing biodiversity? Ecol. Appl. 1999, 9, 164–170. [Google Scholar] [CrossRef]
- Stralberg, D.; Williams, B. Effects of Residential Development and Landscape Composition on the Breeding Birds of Placer County’s Foothill Oak Woodlands; Gen. Tech. Rep. PSW-GTR-184; USDA Forest Service: Washington, DC, USA, 2002. [Google Scholar]
- Donnelly, R.; Marzluff, J.M. Importance of reserve size and landscape context to urban bird conservation. Conserv. Biol. 2004, 18, 733–745. [Google Scholar] [CrossRef]
- Odell, E.A.; Knight, R.L. Songbird and medium-sized mammal communities associated with exurban development in Pitkin County, Colorado. Conserv. Biol. 2001, 15, 1143–1150. [Google Scholar] [CrossRef]
- Friesen, L.E.; Eagles, P.F.J.; Mackay, R.J. Effects of residential development on forest-dwelling neotropical migrant songbirds. Conserv. Biol. 1995, 9, 1408–1414. [Google Scholar] [CrossRef]
- Courchamp, F.; Sugihara, G. Biological control of introduced predator populations to protect native island species from extinction. Ecol. Appl. 1999, 9, 112–123. [Google Scholar] [CrossRef]
- May, S.A.; Norton, T.W. Influence of fragmentation and disturbance on the potential impact of feral predators on native fauna in Australian forest ecosystems. Wildl. Res. 1996, 15, 387–400. [Google Scholar] [CrossRef]
- Maestas, J.D.; Knight, R.L.; Gilgert, W.C. Cows condos or neither: What’s best for rangeland ecosystems. Rangelands 2003, 24, 36–42. [Google Scholar] [CrossRef][Green Version]
- Crooks, K.R.; Soule, M.E. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 1999, 400, 563–566. [Google Scholar] [CrossRef]
- Conservation Biology Institute. Analysis of General Plan-2020 San Diego County; Conservation Biology Institute: Encinitas, CA, USA, 2005; 27p. [Google Scholar]
- Woodroffe, R.; Frank, L.G. Lethal control of African lions (Panthera leo): Local and regional population impacts. Anim. Conserv. 2005, 8. [Google Scholar] [CrossRef]
- Orlando, A.M. Impacts of Rural Development on Puma Ecology in California’s Sierra Nevada. Ph.D. Thesis, University of California, Davis, CA, USA, 2008. [Google Scholar]
- Haines, J. FWP moves bears out of town. Bozeman (Montana) Daily Chronicle, 8 October 2006. [Google Scholar]
- McMillion, S. Bears in town seeking food. Bozeman (Montana) Daily Chronicle, 15 February 2004. [Google Scholar]
- Riley, S.P.D.; Bromley, C.; Poppenga, R.H.; Uzal, F.A.; Whited, L.; Sauvajot, R.M. Anticoagulant exposure and notoedric mange in bobcats and mountain lions in urban Southern California. J. Wildl. Manag. 2007, 71, 1874–1884. [Google Scholar] [CrossRef]
- Beier, P. Effects of artificial night lighting on terrestrial mammals. In Ecological Consequences of Artificial Night Lighting; Rich, C., Longcore, T., Eds.; Island Press: Covelo, CA, USA, 2006; pp. 19–42. [Google Scholar]
- Perry, G.; Fisher, R.N. Night Lights and Reptiles: Observed and Potential Effects. In Ecological Consequences of Artificial Night Lighting; Rich, C., Longcore, T., Eds.; Island Press: Covelo, CA, USA, 2006; pp. 169–191. [Google Scholar]
- Minton, S.A., Jr. The fate of amphibians and reptiles in a suburban area. J. Herpetol. 1968, 2, 113–116. [Google Scholar] [CrossRef]
- Liddle, M. Recreation Ecology: The Ecological Impact of Outdoor Recreation and Ecotourism; Chapman and Hall: New York, NY, USA, 1997; 639p. [Google Scholar]
- Blickley, J.L.; Patricelli, G.L. Potential acoustic masking of Greater Sage-Grouse (Centrocercus urophasianus) display components by chronic industrial noise. Ornithol. Monogr. 2012, 74, 23–35. [Google Scholar]
- Fuller, R.A.; Warren, P.H.; Gaston, K.J. Daytime noise predicts nocturnal singing in urban robins. Biol. Lett. 2007, 3, 368–370. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Dobkins, D.S.; Wheye, D. The Birders Handbook: A Field Guide to the Natural History of North American Birds; Simon and Schuster: New York, NY, USA, 1988. [Google Scholar]
- Hadidian, J. Wild Neighbors: The Humane Approach to Living with Wildlife; Humane Society: Washington, DC, USA, 2007. [Google Scholar]
- Whitaker, J.O. National Audubon Society Field Guide to Mammals; Knopf: New York, NY, USA, 1998. [Google Scholar]
- Curtis, P.; Sullivan, R. Tree Squirrels; Wildlife Damage Management Fact Sheet Series; Cornell Cooperative Extension Office: Ithaca NY, USA, 2001. [Google Scholar]
- Hennings, L.A.; Edge, W.D. Riparian bird community structure in Portland, OR: Habitat, urbanization, and spatial scale patterns. Condor 2003, 105, 288–302. [Google Scholar] [CrossRef]
- Dinkins, J.B.; Conover, M.R.; Kirol, C.P.; Beck, J.L.; Frey, S.N. Effects of common raven and coyote removal and temporal variation in climate on greater sage-grouse nesting success. Biol. Conserv. 2016, 202, 50–58. [Google Scholar] [CrossRef]
- Peirce, K.N.; vanDaele, L.J. Use of a garbage dump by brown bears in Dillingham Alaska. Ursus 2006, 17, 165–177. [Google Scholar] [CrossRef]
- Newsome, T.M.; van Eeden, L.M. The effects of food waste on wildlife and humans. Sustainability 2017, 9, 1269. [Google Scholar] [CrossRef]
- Viegas, D.X.; Allgöwer, B.; Koutsias, N.; Eftichidis, G. Fire spread and the wildland urban interface problem. In Proceedings of the International Scientific Workshop on Forest Fires in the Wildland-Urban Interface and Rural Areas in Europe: An Integral Planning and Management Challenge, Athens, Greece, 15–16 May 2003. [Google Scholar]
- Oregon Department of Forestry. The Oregon Forestland-Urban Interface Fire Protection Act. In Property Evaluation and Self-Certification Guide; Oregon Department of Forestry: Salem, MA, USA, 2006; 32p. [Google Scholar]
- Grings, E.E.; Heitschmidt, R.K.; Short, R.E.; Haferkamp, M.R. Intensive-early stocking for yearling cattle in the northern Great Plains. J. Range Manag. 2002, 55, 135–138. [Google Scholar] [CrossRef]
- Syphard, A.D.; Radeloff, V.C.; Keeley, J.E.; Hawbaker, T.J.; Clayton, M.K.; Stewart, S.I.; Hammer, R.B. Human influence on California fire regimes. Ecol. Appl. 2007, 17, 1388–1402. [Google Scholar] [CrossRef]
- Bergeron, Y.; Flannigan, M.; Gauthier, S.; Leduc, A.; Lefort, P. Past, current, and future fire frequency in the Canadian boreal forest: Implications for sustainable forest management. J. Hum. Environ. 2004, 33, 356–360. [Google Scholar] [CrossRef]
- King, J.W.; Savage, J.A. Effects of Conservation Reserve Program on wildlife in southeast Nebraska. Wildl. Soc. Bull. 1995, 23, 377–385. [Google Scholar]
- Taylor, M.W.; Wolfe, C.W.; Baxter, W.L. Land-use change and ring-necked pheasants in Nebraska. Wildl. Soc. Bull. 1978, 4, 11–15. [Google Scholar]
- Robbins, C.S.; Bystrak, D.; Geissler, P.H. The Breeding Bird Survey: Its First Fifteen Years, 1965–1979; Resource Publication #157; U.S. Fish and Wildlife Service: Washington, DC, USA, 1986; 196p. [Google Scholar]
- Casey, H. Origin and variation in nitrate nitrogen in the chalk springs, streams, and rivers in Dorset and its utilization by higher plants. Prog. Water Technol. 1977, 8, 225–235. [Google Scholar]
- McLaughlin, A.; Mineau, P. The impacts of agricultural practices on biodiversity. Agric. Ecosyst. Environ. 1995, 55, 201–212. [Google Scholar] [CrossRef]
- Horne, A.J.; Goldman, C.R. Limnology, 2nd ed.; McGraw Hill, Inc.: New York, USA, 1994; 520p. [Google Scholar]
- Freemark, K.; Boutin, C. Impacts of agricultural herbicide use on terrestrial wildlife in temperate landscapes: A review with special reference to North America. Agric. Ecosyst. Environ. 1995, 52, 67–91. [Google Scholar] [CrossRef]
- Friesen, L.E. A Literature Review on Wildlife Habitats in Agricultural Landscapes; Report No.: RES/MAN-009/94; Agriculture: Canada-Ontario Environmental Sustainability Accord (COESA); Research Branch, Agriculture and Agri-Food Canada Pest Management Research Centre: London, UK; Ontaria, CA, USA, 1994. [Google Scholar]
- Fry, G.L.A. The role of field margins in the landscape. BCPC Monograph. Field Margins Integr. Agric. Conserv. 1994, 58, 31–39. [Google Scholar]
- Kremen, C.; Ricketts, T. Global perspectives on pollination disruptions. Conserv. Biol. 2000, 14, 1226–1228. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Sponheim, D.; Smith, T.; Riessen, J. The 4 R Fact Sheet: 4R Plus Makes Agronomic and Economic Sense. The Nature Conservancy: Nutrient Management and Conservation for Healthier Soils: 4RPlus. 2018. Available online: https://4rplus.org/wp-content/uploads/4R-Plus-Handout_lores.pdf (accessed on 24 January 2021).
- Forman, R.T.T. Land Mosaics: The Ecology of Landscapes and Regions; Cambridge University Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Sekhar, N.U. Crop livestock depredation caused by wild animals in protected areas: The case of Sarisk Tiger Reserve, Rajasthan, India. Environ. Conserv. 1998, 25, 160–171. [Google Scholar] [CrossRef]
- MacGowan, B.J.; Humberg, L.A.; Beasley, J.C.; DeVault, T.L.; Retamosa, M.I.; Rhodes, O.E., Jr. Corn and Soybean Crop Depredation by Wildlife; Report FNR-265-W; Purdue University Extension: West Lafayette, IN, USA, 2006. [Google Scholar]
- Fritts, S.H.; Stephenson, R.O.; Hayes, R.D.; Boitani, L. Wolves and humans. In Wolves: Behavior, Ecology, and Conservation; Mech, L.D., Boitani, L., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 289–316. [Google Scholar]
- Hawley, J.E.; Gehring, T.M.; Schultz, R.N.; Rossler, S.T.; Wydeven, A.P. Assessment of shock collars as nonlethal management for wolves in Wisconsin. J. Wildl. Manag. 2009, 73, 518–525. [Google Scholar] [CrossRef]
- Van Tassell, L.W.; Yang, B.; Phillips, C. Depredation claim behavior and tolerance of wildlife in Wyoming. J. Agric. Appl. Econ. 2000, 32, 175–188. [Google Scholar] [CrossRef]
- VerCauteren, K.C.; Seward, N.; Hirchert, D.; Jones, M.; Beckerman, S. Dogs for reducing wildlife damage to organic crops: A case study. In Proceedings of the 11th Wildlife Damage Management Conference, Traverse City, MI, USA, 16–19 May 2005; University of Nebraska-Lincoln: Lincoln, NE, USA, 2005. [Google Scholar]
- Gehring, T.M.; VerCauteren, K.C.; Cellar, A.C. Good fences make good neighbors: Implementation of electric fencing for establishing effective livestock-protection dogs. Hum. Wildl. Interact. 2011, 5, 106–111. [Google Scholar]
- Woolhouse, M.E.J.; Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005, 11, 1842–1847. [Google Scholar] [CrossRef]
- Bohm, M.; Hutchings, M.R.; White, P.C.L. Contact networks in a wildlife-livestock host community: Identifying high-risk individuals in the transmission of bovine TB among badgers and cattle. PLoS ONE 2009, 4, e5016. [Google Scholar] [CrossRef]
- Atwood, T.C.; Deliberto, T.J.; Smith, H.J.; Stevenson, J.S.; Vercauteren, K.C. Spatial ecology of raccoons related to cattle and bovine tuberculosis in northeastern Michigan. J. Wildl. Manag. 2009, 73, 647–654. [Google Scholar] [CrossRef]
- Owensby, C.E.; Auen, L.M.; Berns, F.; Dhuyvetter, K.C. Grazing systems for yearling cattle in Tallgrass prairie. Rangel. Ecol. Manag. 2008, 61, 204–210. [Google Scholar] [CrossRef]
- Wilgers, D.J.; Horne, E.A.; Sandercock, B.K.; Volkman, A.W. Effects of rangeland management on community dynamics of the herpetofauna of the tallgrass prairie. Herpetologica 2006, 62, 378–388. [Google Scholar] [CrossRef]
- Hamilton, R.G. Restoring heterogeneity on the Tallgrass Prairie Preserve: Applying the fire-grazing interaction model. In Proceedings of the 23rd Tall Timbers Fire Ecology Conference: Fire in Grassland and Shrubland Ecosystems, Bartlesville, OK, USA, 17–20 October 2005; Tall Timbers Research Station: Tallahassee, FL, USA, 2007; pp. 163–169. [Google Scholar]
- Hirsch, K.; Kafka, V.; Tymstra, C.; McAlpine, R.; Hawkes, B.; Stegehuis, H.; Quintilio, S.; Gauthier, S.; Peck, K. Fire-smart forest management: A pragmatic approach to sustainable forest management in fire-dominated ecosystems. For. Chron. 2001, 77, 357–363. [Google Scholar] [CrossRef]
- Main, M.B.; Roka, F.M.; Noss, R.F. Evaluating the costs of conservation. Conserv. Biol. 1999, 13, 1262–1272. [Google Scholar] [CrossRef]
- McDonald, W.; St. Clair, C.C. Elements that promote highway crossing structure use by small mammals in Banff National Park. J. Appl. Ecol. 2004, 41, 82–93. [Google Scholar] [CrossRef]
- Wilcove, D.S.; Lee, J. Using economic and regulatory incentives to restore endangered species: Lessons learned from three new programs. Conserv. Biol. 2004, 18, 639–645. [Google Scholar] [CrossRef]
- Gray, R.L.; Teels, B.M. Wildlife and fish conservation through the Farm Bill. Wildl. Soc. Bull. 2006, 34, 906–913. [Google Scholar] [CrossRef]
- Heard, P.L.; Allen, A.W.; Best, L.B.; Brady, S.J.; Burger, W.; Esser, A.J.; Hackett, E.; Johnson, D.H.; Pederson, R.L.; Reynolds, R.E.; et al. A Comprehensive Review of Farm Bill Contributions to Wildlife Conservation, 1985–2000; Technical Report WHMI-200; U.S. Department of Agriculture, Natural Resources Conservation Service: Madison, MS, USA, 2000. [Google Scholar]
- Donald, P.F.; Pisano, G.; Rayment, M.D.; Pain, D.J. The common agricultural policy, EU enlargement and the conservation of Europe’s farmland birds. Agric. Ecosyst. Environ. 2002, 89, 167–182. [Google Scholar] [CrossRef]
- Bignal, E.M. Using an ecological understanding of farmland to reconcile nature conservation requirements, EU agriculture policy and world trade agreements. J. Appl. Ecol. 1998, 35, 949–954. [Google Scholar] [CrossRef]
- Newmark, W.D. Recommendations for Wildlife Corridors and the Extension and Management of Forest Resources in the Eastern Usambara Mountains, Tanzania; East Usambara Catchment Forest Project Technical Paper No. 4; Frontier-TanzaniaUniversity of Dar es SalaamSociety for Environmental Exploration: Helsinki, Finland, 1992. [Google Scholar]
- Newmark, W.D. The role and design of wildlife corridors with examples from Tanzania. Ambio 1993, 22, 500–504. [Google Scholar]
- Lentini, P.; Fischer, J.; Gibbons, P.; Lindenmayer, D.; Martin, T. Australia’s Stock Route Network: 2. Representation of fertile landscapes. Ecol. Manag. Restor. 2011, 12, 148–151. [Google Scholar] [CrossRef]
- VerCauteren, K.C.; Lavelle, M.J.; Seward, N.W.; Fischer, J.W.; Phillips, G.E. Fence-line contact between wild and farmed white-tail deer in Michigan: Potential for disease transmission. J. Wildl. Manag. 2007, 71, 1603–1606. [Google Scholar] [CrossRef]
- Mordecai, O.O.; Woodroff, R.; Oguge, N.O.; Frank, L.G. Limiting depredation by African carnivores: The role of livestock husbandry. Conserv. Biol. 2003, 17, 1521–1530. [Google Scholar]
- McMahan, C.A. Comparative food habits of deer and three classes of livestock. J. Wildl. Manag. 1964, 28, 798–808. [Google Scholar] [CrossRef]
- Joly, D.O.; Samuel, M.D.; Langenberg, J.A.; Blanchong, J.A.; Batha, C.A.; Rolley, R.E.; Keane, D.P.; Ribic, C.A. Spatial epidemiology of chronic wasting disease in Wisconsin white-tailed deer. J. Wildl. Dis. 2006, 42, 578–588. [Google Scholar] [CrossRef]
- Desmet, P.G. Using landscape fragmentation thresholds to determine ecological process targets in systematic conservation plans. Biol. Conserv. 2018, 221, 257–260. [Google Scholar] [CrossRef]
- M’Closkey, R.T.; Fieldwick, B. Ecological separation of sympatric rodents (Peromyscus and Microtus). J. Mammal. 1975, 56, 119–129. [Google Scholar] [CrossRef]
- Kantak, G.E. Behavioral, seed preference and habitat selection experiments with two sympatric Peromyscus species. Am. Midl. Nat. 1983, 109, 246–252. [Google Scholar] [CrossRef]
- Trautman, C.G. History, Ecology, and Management of the Ring-Necked Pheasant in South Dakota; Bulletin 7; South Dakota Department of Game, Fish, and Parks: Pierre, SD, USA, 1982. [Google Scholar]
- Brouat, C.; Sennedot, F.; Audiot, P.; Leblois, R.; Rasplus, J.-Y. Fine-scale genetic structure of two carabid species with contrasted levels of habitat specialization. Mol. Ecol. 2003, 12, 1731–1745. [Google Scholar] [CrossRef]
- Morgan, E.R.; Lundervold, M.; Medley, G.F.; Shaikenov, B.S.; Torgerson, P.R.; Milner-Gulland, E.J. Assessing the risks of disease transmission between wildlife and livestock: The saiga antelope as a case study. Biol. Conserv. 2006, 131, 244–254. [Google Scholar] [CrossRef]
- Patrick, C.; McCluney, K.E.; Thorp, J.; Sabo, J.; Ruhi, A.; Gregory, A.J. Multi-scale biodiversity drives temporal variability in macrosystems. Front. Ecol. Environ. 2021, in press. [Google Scholar]
- Kuussaari, M.; Bommarco, R.; Heikkinen, R.K.; Helm, A.; Krauss, J.; Lindborg, R.; Öckinger, E.; Pärtel, M.; Pino, J.; Rodà, F.; et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol. Evol. 2009, 24, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Graves, T. Spatial Ecology of Grizzly Bears in Northwestern Montana and Estimating Resistance to Gene Flow. Ph.D. Thesis, Northern Arizona University, School of Forestry, Flagstaff, AZ, USA, 2012. [Google Scholar]
- Liu, J.; Dietz, T.; Carpenter, S.R.; Folke, C.; Alberti, M.; Redman, C.L.; Schneider, S.H.; Ostrom, E.; Pell, A.N.; Lubchenco, J.; et al. Coupled Human and Natural Systems. AMBIO 2007, 36, 639–649. [Google Scholar] [CrossRef]
- Czech National Council. Act. No. 123/1998 Coll. on the Conservation of Nature and Landscape; Czech National Council: Prague, Czech Republic, 1992.
- Kefa, C.A.; Lung, M.A.; Espira, A.; Gregory, A.J. Quantifying the rate of subsistence wood harvesting from Kakamega tropical rainforest in Kenya. Oryx 2017, 1–5. [Google Scholar] [CrossRef]
- Kefa, C.A.; Gregory, A.; Espira, A.; Lung, M. Does wood fuel gathering for households follow an optimality model? A study from Kakamega Forest, Western Kenya. Hum. Ecol. 2018, 46, 473–484. [Google Scholar] [CrossRef]
- Jongman, R.H.G.; Külvik, M.; Kristianen, I. European ecological networks and greenways. Landsc. Urban Plan. 2005, 68, 305–319. [Google Scholar] [CrossRef]
- Kwa, C.L. Mimicking Nature; University of Amsterdam: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Simberloff, D. Flagships, umbrellas, and keystones. Biol. Conserv. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Van Der Windt, H.J.; Swart, A.A. Ecological corridors, connecting science and politics: The case of the Green River in the Netherlands. J. Appl. Ecol. 2008, 45, 124–132. [Google Scholar] [CrossRef]
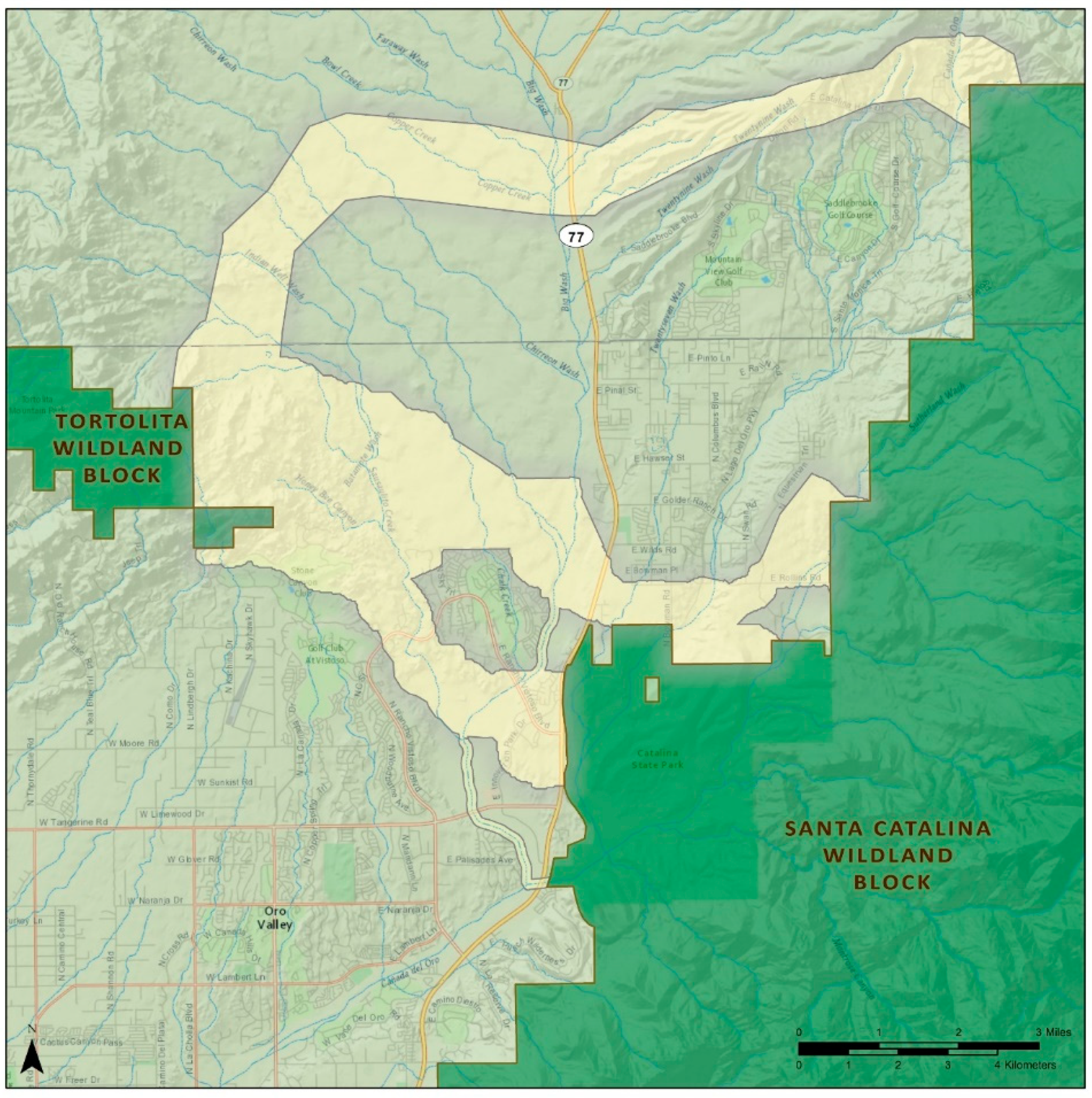

| Section 3. Recommendations for Human-Created Linear Barriers Crossing Corridors |
|
| Section 4. Recommendations for Streams and Riparian Zones |
|
| Section 5. Recommendations for Urban and Suburban Development in Corridors |
|
| Section 6. Recommendations for Agricultural Development Within Corridors |
|
| Section 7. Knowledge Gaps, Summary, and Conclusions |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregory, A.; Spence, E.; Beier, P.; Garding, E. Toward Best Management Practices for Ecological Corridors. Land 2021, 10, 140. https://doi.org/10.3390/land10020140
Gregory A, Spence E, Beier P, Garding E. Toward Best Management Practices for Ecological Corridors. Land. 2021; 10(2):140. https://doi.org/10.3390/land10020140
Chicago/Turabian StyleGregory, Andrew, Emma Spence, Paul Beier, and Emily Garding. 2021. "Toward Best Management Practices for Ecological Corridors" Land 10, no. 2: 140. https://doi.org/10.3390/land10020140
APA StyleGregory, A., Spence, E., Beier, P., & Garding, E. (2021). Toward Best Management Practices for Ecological Corridors. Land, 10(2), 140. https://doi.org/10.3390/land10020140





