Diversity Is Not Everything
Abstract
:1. Introduction
2. Methods
2.1. Site Descriptions and Study Design
2.2. Vegetation
2.3. Soil
2.4. Statistics
3. Results
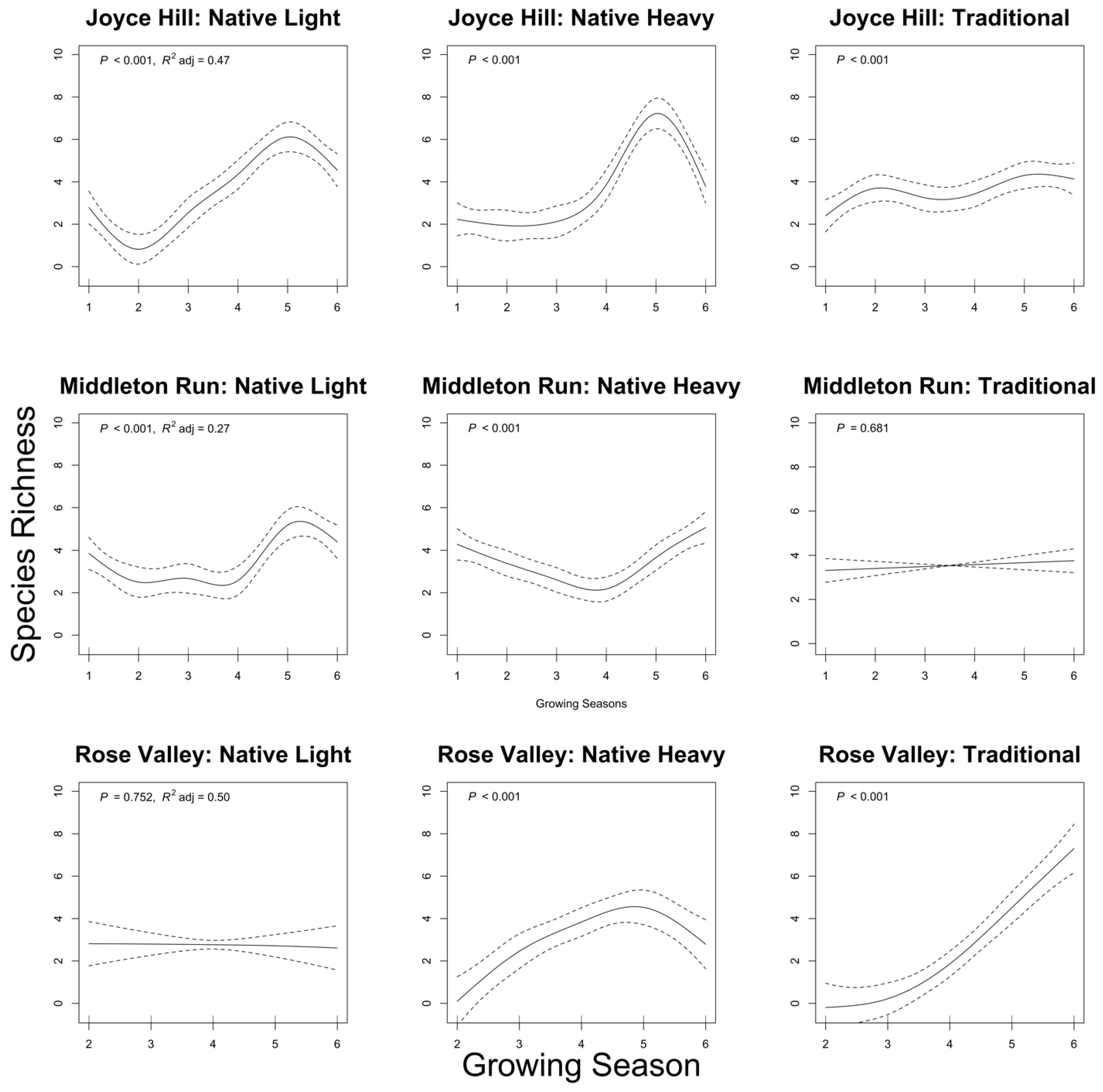
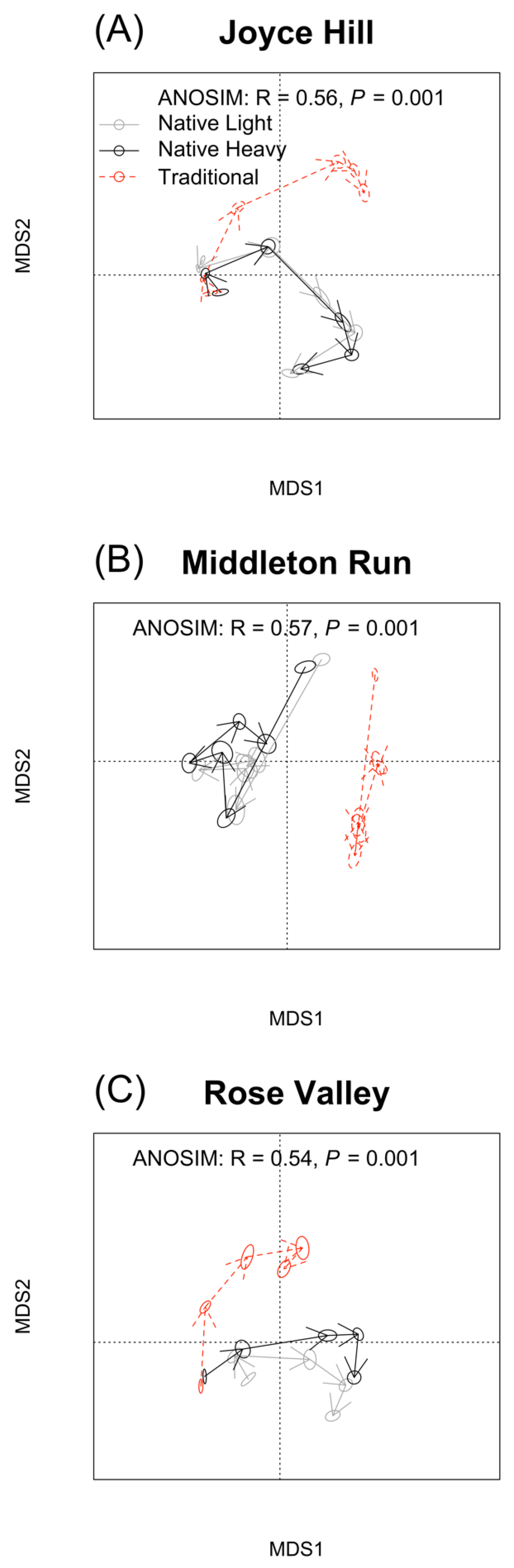
3.1. Vegetation
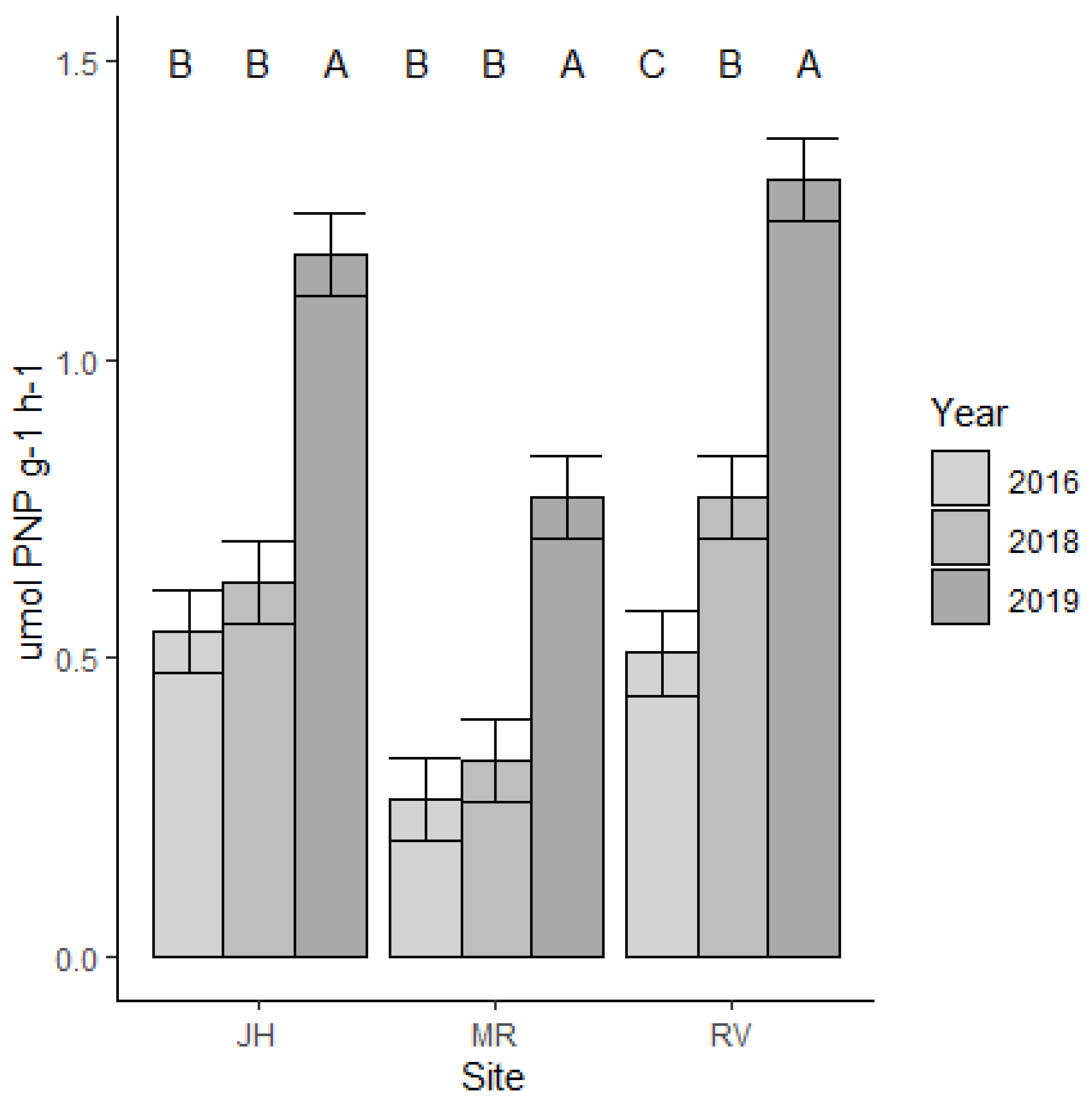
3.2. Soil
4. Discussion
4.1. Vegetation Change over Time
4.2. Soil Microbiology and Chemistry Changes over Time
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skousen, J.; Gorman, J.; Pena-Yewtukhiw, E.; King, J.; Stewart, J.; Emerson, P.; DeLong, C. Hardwood Tree Survival in Heavy Ground Cover on Reclaimed Land in West Virginia: Mowing and Ripping Effects. J. Environ. Qual. 2009, 38, 1400–1409. [Google Scholar] [CrossRef] [Green Version]
- Burger, J.A. Sustainable Mined Land Reclamation in the Eastern U.S. Coalfields: A Case for an Ecosystem Reclamation Approach. JASMR 2011, 15, 113–141. [Google Scholar] [CrossRef]
- Skousen, J.; Zipper, C.E. Coal Mining and Reclamation in Appalachia. In Appalachia’s Coal-Mined Landscapes; Zipper, C.E., Skousen, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 55–83. [Google Scholar]
- Angel, H.Z.; Stoval, J.P.; Williams, H.M.; Farrish, K.W.; Oswald, B.P.; Young, J.L. Surface and Subsurface Tillage Effects on Mine Soil Properties and Vegetative Response. Soil Sci. Soc. Am. J. 2018, 82, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Skousen, J.; Daniels, W.L.; Zipper, C.E. Soils on Appalachian Coal-Mined Lands. In Appalachia’s Coal-Mined Landscapes; Zipper, C.E., Skousen, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 85–109. [Google Scholar]
- Shrestha, R.K.; Lal, R. Changes in Physical and Chemical Properties of Soil after Surface Mining and Reclamation. Geoderma 2011, 161, 168–176. [Google Scholar] [CrossRef]
- Swab, R.M.; Lorenz, N.; Byrd, S.; Dick, R. Native Vegetation in Reclamation: Improving Habitat and Ecosystem Function through Using Prairie Species in Mine Land Reclamation. Ecol. Eng. 2017, 108, 525–536. [Google Scholar] [CrossRef]
- Swab, R.M.; Lorenz, N.; Lee, N.R.; Culman, S.W.; Dick, R.P. From the Ground Up: Prairies on Reclaimed Mine Land—Impacts on Soil and Vegetation. Land 2020, 9, 455. [Google Scholar] [CrossRef]
- Howard, J.L.; Amos, D.F.; Daniels, W.L. Phosphorus and Potassium Relationships in Southwestern Virginia Coal-Mine Spoils. J. Environ. Qual. 1988, 17, 695–700. [Google Scholar] [CrossRef]
- Li, R.S.; Daniels, W.L. Nitrogen Accumulation and Form over Time in Young Mine Soils. J. Environ. Qual. 1994, 23, 166–172. [Google Scholar] [CrossRef]
- Nippgen, F.; Ross, M.R.V.; Bernhardt, E.S.; McGlynn, B.L. Creating a More Perennial Problem? Mountaintop Removal Coal Mining Enhances and Sustains Saline Baseflows of Appalachian Watersheds. Environ. Sci. Technol. 2017, 51, 8324–8334. [Google Scholar] [CrossRef]
- Lindsay, R.E.; Nawrot, J.R. Evaluation of Natural Revegetation of Problem Spoilbanks; Southern Illinois University: Carbondale, IL, USA, 1981. [Google Scholar]
- Corbett, E.A.; Anderson, R.C.; Rodgers, C.S. Prairie Revegetation of a Strip Mine in Illinois: Fifteen Years after Establishment. Restor Ecol. 1996, 4, 346–354. [Google Scholar] [CrossRef]
- Zipper, C.E.; Burger, J.A.; Skousen, J.G.; Angel, P.N.; Barton, C.D.; Davis, V.; Franklin, J.A. Restoring Forests and Associated Ecosystem Services on Appalachian Coal Surface Mines. Environ. Manag. 2011, 47, 751–765. [Google Scholar] [CrossRef]
- Wilson-Kokes, L.; DeLong, C.; Thomas, C.; Emerson, P.; O’Dell, K.; Skousen, J. Hardwood Tree Growth on Amended Mine Soils in West Virginia. J. Environ. Qual. 2013, 42, 1363–1371. [Google Scholar] [CrossRef]
- Lima, A.T.; Mitchell, K.; O’Connell, D.W.; Verhoeven, J.; Van Cappellen, P. The Legacy of Surface Mining: Remediation, Restoration, Reclamation and Rehabilitation. Environ. Sci. Policy 2016, 66, 227–233. [Google Scholar] [CrossRef]
- Ingold, D.J. Use of a Reclaimed Stripmine by Grassland Nesting Birds in East-Central Ohio. Ohio J. Sci. 2002, 102, 56–62. [Google Scholar]
- Zipper, C.E.; Burger, J.A.; McGrath, J.M.; Rodrigue, J.A.; Holtzman, G.I. Forest Restoration Potentials of Coal-Mined Lands in the Eastern United States. J. Environ. Qual. 2011, 40, 1567–1577. [Google Scholar] [CrossRef]
- Cavender, N.; Byrd, S.; Bechtoldt, C.L.; Bauman, J.M. Vegetation Communities of a Coal Reclamation Site in Southeastern Ohio. Northeast. Nat. 2014, 21, 31–46. [Google Scholar] [CrossRef]
- Wood, P.B.; Williams, J.M. Terrestrial Salamander Abundance on Reclaimed Mountaintop Removal Mines: Salamanders on Surface Mines. Wildl. Soc. Bull. 2013, 37, 815–823. [Google Scholar] [CrossRef]
- Baer, S.G.; Kitchen, D.J.; Blair, J.M.; Rice, C.W. Changes in Ecosystem Structure and Function along a Chronosequence of Restored Grasslands. Ecol. Appl. 2002, 12, 1688–1701. [Google Scholar] [CrossRef]
- Baer, S.G.; Meyer, C.K.; Bach, E.M.; Klopf, R.P.; Six, J. Contrasting Ecosystem Recovery on Two Soil Textures: Implications for Carbon Mitigation and Grassland Conservation. Ecosphere 2010, 1, 1–22. [Google Scholar] [CrossRef]
- Baer, S.G.; Heneghan, L.; Eviner, V.T. Applying Soil Ecological Knowledge to Restore Ecosystem Services. In Soil Ecology and Ecosystem Services; Wall, D.H., Bardgett, R.D., Behan-Pelletier, V., Herrick, J.E., Jones, H., Ritz, K., Six, J., Strong, D.R., van der Putten, W.H., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 377–393. [Google Scholar]
- McLauchlan, K.K.; Hobbie, S.E.; Post, W.M. Conversion from Agriculture to Grassland Builds Soil Organic Matter on Decadal Timescales. Ecol. Appl. 2006, 16, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Hurisso, T.T.; Norton, J.B.; Norton, U. Soil Profile Carbon and Nitrogen in Prairie, Perennial Grass–Legume Mixture and Wheat-Fallow Production in the Central High Plains, USA. Agric. Ecosyst. Environ. 2013, 181, 179–187. [Google Scholar] [CrossRef]
- Baer, S.G.; Bach, E.M.; Meyer, C.K.; Du Preez, C.C.; Six, J. Belowground Ecosystem Recovery During Grassland Restoration: South African Highveld Compared to US Tallgrass Prairie. Ecosystems 2015, 18, 390–403. [Google Scholar] [CrossRef]
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil Texture Affects Soil Microbial and Structural Recovery during Grassland Restoration. Soil Biol. Biochem. 2010, 42, 2182–2191. [Google Scholar] [CrossRef]
- Scott, D.A.; Baer, S.G.; Blair, J.M. Recovery and Relative Influence of Root, Microbial, and Structural Properties of Soil on Physically Sequestered Carbon Stocks in Restored Grassland. Soil Sci. Soc. Am. J. 2017, 81, 50–60. [Google Scholar] [CrossRef]
- Sluis, W.J. Patterns of Species Richness and Composition in Re-Created Grassland. Restor. Ecol. 2002, 10, 677–684. [Google Scholar] [CrossRef]
- Camill, P.; McKone, M.J.; Sturges, S.T.; Severud, W.J.; Ellis, E.; Limmer, J.; Martin, C.B.; Navratil, R.T.; Purdie, A.J.; Sandel, B.S.; et al. Community- and Ecosystem-Level Changes in a Species-Rich Tallgrass Prairie Restoration. Ecol. Appl. 2004, 14, 1680–1694. [Google Scholar] [CrossRef]
- Myers, J.A.; Harms, K.E. Seed Arrival and Ecological Filters Interact to Assemble High-Diversity Plant Communities. Ecology 2011, 92, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.J.; Gibson, D.J. Use of Multiple Criteria in an Ecological Assessment of a Prairie Restoration Chronosequence. Appl. Veg. Sci. 2014, 17, 63–73. [Google Scholar] [CrossRef]
- Baer, S.G.; Blair, J.M.; Collins, S.L. Environmental Heterogeneity Has a Weak Effect on Diversity during Community Assembly in Tallgrass Prairie. Ecol. Monogr. 2016, 86, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Grman, E.; Zirbel, C.R.; Bauer, J.T.; Groves, A.M.; Bassett, T.; Brudvig, L.A. Super-abundant C 4 Grasses Are a Mixed Blessing in Restored Prairies. Restor. Ecol. 2021, 29. [Google Scholar] [CrossRef]
- Baer, S.G.; Blair, J.M.; Collins, S.L.; Knapp, A.K. Plant Community Responses to Resource Availability and Heterogeneity during Restoration. Oecologia 2004, 139, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Faber, S.; Markham, J. Biotic and Abiotic Effects of Remnant and Restoration Soils on the Performance of Tallgrass Prairie Species. Ecol. Rest. 2012, 30, 106–115. [Google Scholar] [CrossRef]
- Scott, D.A.; Baer, S.G. Degraded Soil Increases the Performance of a Dominant Grass, Andropogon Gerardii (Big Bluestem). Plant Ecol. 2018, 219, 901–911. [Google Scholar] [CrossRef]
- Allison, V.J.; Miller, R.M.; Jastrow, J.D.; Matamala, R.; Zak, D.R. Changes in Soil Microbial Community Structure in a Tallgrass Prairie Chronosequence. Soil Sci. Soc. Am. J. 2005, 69, 1412–1421. [Google Scholar] [CrossRef] [Green Version]
- Matamala, R.; Jastrow, J.D.; Miller, R.M.; Garten, C.T. Temporal changes in C and N stocks of restored prairie: Implications for C sequestration strategies. Ecol. Appl. 2008, 18, 1470–1488. [Google Scholar] [CrossRef]
- Galbraith, J.; Shaw, R.K. Human-Altered and Human-Transported Soils. In Soil Science Division Staff. Soil Survey Manual; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; USDA Handbook 18; Government Printing Office: Washington, DC, USA, 2017. [Google Scholar]
- Rossiter, D.G. Classification of Urban and Industrial Soils in the World Reference Base for Soil Resources. J. Soils Sediments 2007, 7, 96–100. [Google Scholar] [CrossRef]
- Daubenmire, R. A Canopy-Coverage Method of Vegetational Analysis. Northwest Sci. 1959, 33, 43–64. [Google Scholar]
- Schutter, M.E.; Dick, R.P. Comparison of Fatty Acid Methyl Ester (FAME) Methods for Characterizing Microbial Communities. Soil Sci. Soc. Am. J. 2000, 64, 1659–1668. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of microbial phospholipids and lipopolysaccharides. In Methods of Soil Biology; Schinner, F., Ed.; Springer: Berlin, Germany, 1996; pp. 80–92. [Google Scholar]
- Zelles, L. Fatty Acid Patterns of Phospholipids and Lipopolysaccharides in the Characterisation of Microbial Communities in Soil: A Review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Kaur, A.; Chaudhary, A.; Kaur, A.; Choudhary, R.; Kaushik, R. Phospholipid Fatty Acid—A Bioindicator of Environment Monitoring and Assessment in Soil Ecosystem. Curr. Sci. 2005, 89, 1103–1112. [Google Scholar]
- Olsson, P.A.; Bååth, E.; Jakobsen, I.; Söderström, B. The Use of Phospholipid and Neutral Lipid Fatty Acids to Estimate Biomass of Arbuscular Mycorrhizal Fungi in Soil. Mycol. Res. 1995, 99, 623–629. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and Galactosidases in Soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 Soil Test Extractant: A Modification of Mehlich 2 Extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Lorenz, N.; Gardener, B.B.M.; Lee, N.R.; Ramsier, C.; Dick, R.P. Soil Enzyme Activities Associated with Differential Outcomes of Contrasting Approaches to Soil Fertility Management in Corn and Soybean Fields. Appl. Ecol. Environ. Sci. 2020, 8, 517–525. [Google Scholar] [CrossRef]
- Culman, S.W.; Mann, M.; Sharma, S.; Saeed, M.T.; Fulford, A.M.; Lindsey, L.E.; Brooker, A.; Dayton, E.; Warden, R.; Joern, B. Calibration of Mehlich-3 with Bray P1 and Ammonium Acetate in the Tri-State Region of Ohio, Indiana and Michigan. Commun. Soil Sci. Plant Anal. 2020, 51, 86–97. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 9 October 2021).
- Wood, S.N. Thin Plate Regression Splines. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models: Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, E.J.; Miller, D.L.; Simpson, G.L.; Ross, N. Hierarchical Generalized Additive Models in Ecology: An Introduction with mgcv. PeerJ 2019, 7, e6876. [Google Scholar] [CrossRef] [Green Version]
- Maindonald, J. Gamclass: Functions and Data for a Course on Modern Regression and Classification. R Package Version 0.62.3. 2020. Available online: https://CRAN.R-project.org/package=gamclass (accessed on 12 August 2020).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Ordination Methods, Diversity Analysis and Other Functions for Community and Vegetation Ecologists; The Comprehensive R Archive Network: Berkeley, CA, USA, 2016. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. R Core Team nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-147. 2020. Available online: https://CRAN.R-project.org/package=nlme/ (accessed on 12 August 2020).
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 12 August 2020).
- Van Dyke, F.; Van Kley, S.E.; Page, C.E.; Van Beek, J.G. Restoration Efforts for Plant and Bird Communities in Tallgrass Prairies Using Prescribed Burning and Mowing. Restor. Ecol. 2004, 12, 575–585. [Google Scholar] [CrossRef]
- Rooney, T.P.; Leach, M.K. Replacing Hay-Mowing with Prescribed Fire Restores Species Diversity and Conservation Value in a Tallgrass Prairie Sampled Thrice: A 59-Year Study. Am. Midl. Nat. 2010, 164, 311–321. [Google Scholar] [CrossRef]
- Weir, J.R.; Scasta, J.D. Vegetation Responses to Season of Fire in Tallgrass Prairie: A 13-Year Case Study. Fire Ecol. 2017, 13, 137–142. [Google Scholar] [CrossRef]
- Howe, H.F. Dominance, Diversity and Grazing in Tallgrass Restoration: Ecology Has Much to Contribute to Debates over the Role of Grazing in Restoration—and Much to Learn from the Results of Experiments in Restorative Grazing. Ecol. Restor. N. Am. 1999, 17, 59–66. [Google Scholar] [CrossRef]
- Burger, J.; Graves, D.; Angel, P.; Davis, V.; Zipper, C. The Forestry Reclamation Approach. In The Forestry Reclamation Approach: Guide to Successful Reforestation of Mined Lands; Adams, M.B., Ed.; U.S. Department of Agriculture, Forest Service, Northern Research Station, FOREST SERVICE: Newtown Square, PA, USA, 2017; pp. 21–28. [Google Scholar]
- Hobbs, R.J.; Higgs, E.; Hall, C.M. Novel Ecosystems: Intervening in the New Ecological World Order; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 9781118354209. [Google Scholar]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- McCain, K.N.S.; Baer, S.G.; Blair, J.M.; Wilson, G.W.T. Dominant Grasses Suppress Local Diversity in Restored Tallgrass Prairie. Restor. Ecol. 2010, 18, 40–49. [Google Scholar] [CrossRef]
- Dickson, T.L.; Busby, W.H. Forb Species Establishment Increases with Decreased Grass Seeding Density and with Increased Forb Seeding Density in a Northeast Kansas, USA, Experimental Prairie Restoration. Restor. Ecol. 2009, 17, 597–605. [Google Scholar] [CrossRef]
- Sexton, A.N.; Emery, S.M. Grassland Restorations Improve Pollinator Communities: A Meta-Analysis. J. Insect Conserv. 2020, 24, 719–726. [Google Scholar] [CrossRef]
- Kennedy, P.L.; DeBano, S.J.; Bartuszevige, A.M.; Lueders, A.S. Effects of Native and Non-Native Grassland Plant Communities on Breeding Passerine Birds: Implications for Restoration of Northwest Bunchgrass Prairie. Restor. Ecol. 2009, 17, 515–525. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil Carbon Sequestration Accelerated by Restoration of Grassland Biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.A.; Rosenzweig, S.T.; Baer, S.G.; Blair, J.M. Changes in Potential Nitrous Oxide Efflux during Grassland Restoration. J. Environ. Qual. 2019, 48, 1913–1917. [Google Scholar] [CrossRef]
- Jastrow, J.D. Soil Aggregate Formation and the Accrual of Particulate and Mineral-Associated Organic Matter. Soil Biol. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- Scott, D.A.; Bach, E.M.; Du Preez, C.C.; Six, J.; Baer, S.G. Mechanisms Influencing Physically Sequestered Soil Carbon in Temperate Restored Grasslands in South Africa and North America. Biogeochemistry 2021, 156, 131–143. [Google Scholar] [CrossRef]
- Bandick, A.K.; Dick, R.P. Field Management Effects on Soil Enzyme Activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Ndiaye, E.L.; Sandeno, J.M.; McGrath, D.; Dick, R.P. Integrative Biological Indicators for Detecting Change in Soil Quality. Am. J. Altern. Agric. 2000, 15, 26–36. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Carreira, J.A.; García-Ruíz, R.; Dick, R.P. Soil Moisture Pre-Treatment Effects on Enzyme Activities as Indicators of Heavy Metal-Contaminated and Reclaimed Soils. Soil Biol. Biochem. 2004, 36, 1559–1568. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Pérez-Guzmán, L.; Johnson, J.M.F. Simultaneous Determination of β-Glucosidase, β-Glucosaminidase, Acid Phosphomonoesterase, and Arylsulfatase Activities in a Soil Sample for a Biogeochemical Cycling Index. Appl. Soil Ecol. 2019, 142, 72–80. [Google Scholar] [CrossRef]
- Mganga, K.Z.; Musimba, N.K.R.; Nyariki, D.M.; Nyangito, M.M.; Mwang’ombe, A.W. The Choice of Grass Species to Combat Desertification in Semi-Arid Kenyan Rangelands Is Greatly Influenced by Their Forage Value for Livestock. Grass Forage Sci. 2015, 70, 161–167. [Google Scholar] [CrossRef]
- Chaudhuri, S.; McDonald, L.M.; Skousen, J.; Pena-Yewtukhiw, E.M. Soil Organic Carbon Molecular Properties: Effects of Time Since Reclamation in a Minesoil Chronosequence. Land Degrad. Develop. 2015, 26, 237–248. [Google Scholar] [CrossRef]
- Conant, R.T.; Paustian, K.; Elliott, E.T. Grassland Management and Conversion into Grassland: Effects on Soil Carbon. Ecol. Appl. 2001, 11, 343–355. [Google Scholar] [CrossRef]
- de Almeida, R.F.; Naves, E.R.; da Mota, R.P. Soil Quality: Enzymatic Activity of Soil β-Glucosidase. Glob. J. Agric. Res. Rev. 2015, 3, 146–150. [Google Scholar]



| Site | Treatment | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|
| Joyce Hill 40.442189, −81.500585 | Heavy | 25 | 42 | 33 |
| Light | 21 | 46 | 33 | |
| Traditional | 24 | 44 | 32 | |
| Middleton Run 39.083297, −82.496459 | Heavy | 26 | 32 | 42 |
| Light | 21 | 35 | 44 | |
| Traditional | 47 | 21 | 32 | |
| Rose Valley 40.138537, −80.860425 | Heavy | 25 | 26 | 49 |
| Light | 21 | 27 | 53 | |
| Traditional | 21 | 26 | 53 |
| Site/Growing season | OM GS2 (2016) | OM GS6 (2020) |
|---|---|---|
| (%) | (%) | |
| Joyce Hill B | 1.64 (0.24) b | 3.13 (0.24) a |
| Middleton Run A | 2.06 (0.24) b | 5.01 (0.24) a |
| Rose Valley A | 2.48 (0.24) b | 4.63 (0.24) a |
| Site/Growing Season or Seed Mix | GS2 (2016) | GS 6 (2020) | Native Light | Native Heavy | Traditional |
|---|---|---|---|---|---|
| Joyce Hill B | 6.73 (0.09) A | 6.79 (0.09) A | 5.75 (0.11) b | 7.13 (0.11) a | 7.40 (0.11) a |
| Middleton Run C | 5.81 (0.09) A | 4.71 (0.09) B | 4.97 (0.11) b | 5.27 (0.11) ab | 5.55 (0.11) a |
| Rose Valley A | 7.49 (0.09) A | 7.91 (0.09) B | 7.73 (0.12) a | 7.68 (0.12) a | 7.68 (0.12) a |
| Site/Seed Mix | Native Light | Native Heavy | Traditional |
|---|---|---|---|
| Joyce Hill B | 12.37 (0.67) a | 11.98 (0.67) a | 12.28 (0.67) a |
| Middleton Run B | 16.93 (0.67) a | 12.33 (0.67) b | 9.73 (0.67) c |
| Rose Valley A | 19.97 (0.65) a | 20.40 (0.65) a | 20.90 (0.65) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, D.A.; Eckhoff, K.D.; Lorenz, N.; Dick, R.; Swab, R.M. Diversity Is Not Everything. Land 2021, 10, 1091. https://doi.org/10.3390/land10101091
Scott DA, Eckhoff KD, Lorenz N, Dick R, Swab RM. Diversity Is Not Everything. Land. 2021; 10(10):1091. https://doi.org/10.3390/land10101091
Chicago/Turabian StyleScott, Drew A., Kathryn D. Eckhoff, Nicola Lorenz, Richard Dick, and Rebecca M. Swab. 2021. "Diversity Is Not Everything" Land 10, no. 10: 1091. https://doi.org/10.3390/land10101091






