Plant Trait Assembly in Species-Rich Forests at Varying Elevations in the Northwest Andes of Colombia
Abstract
:1. Introduction
2. Materials and Methods
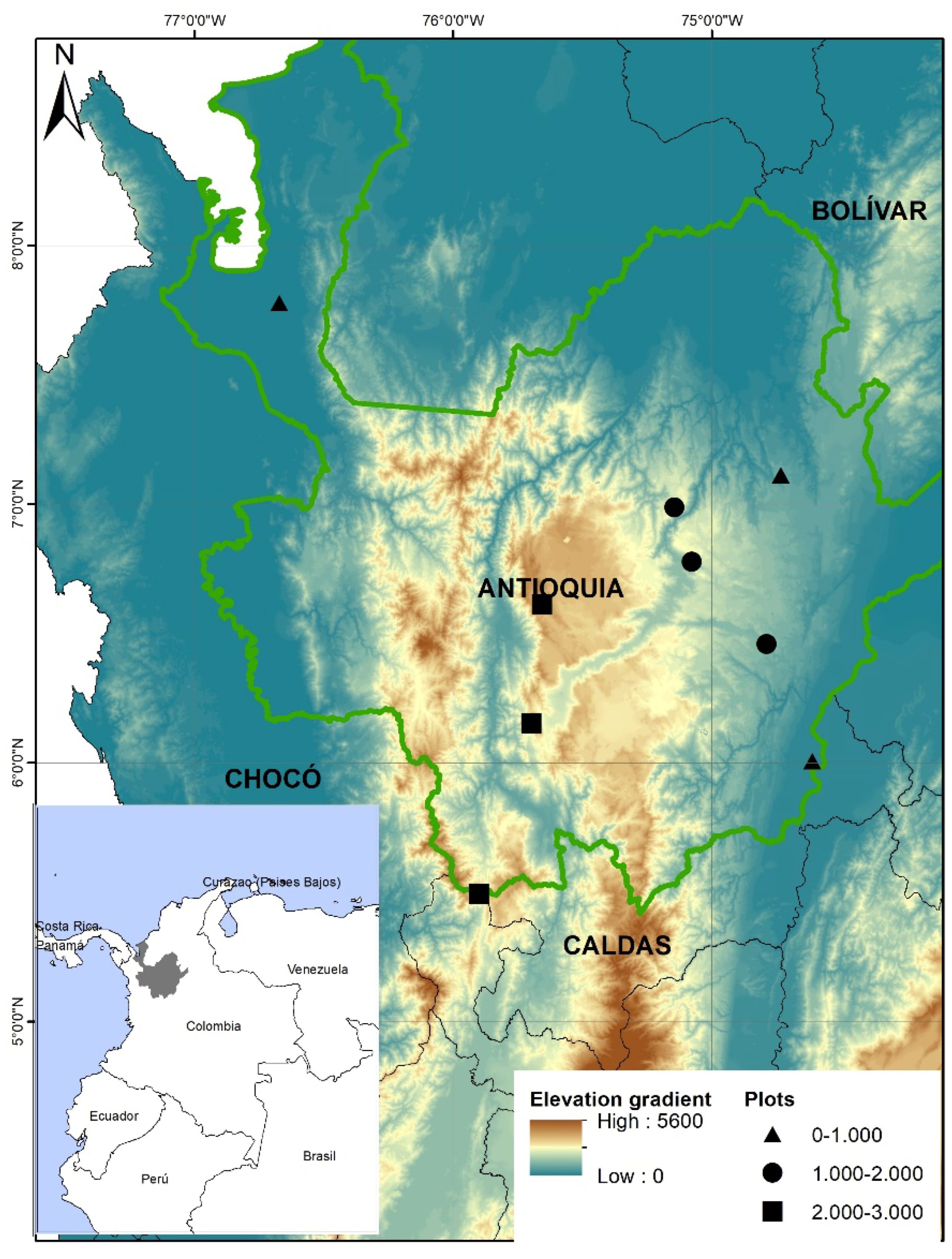
2.1. Study Area
2.2. Tree Species Abundance (L Matrix)
2.3. Environmental Variables (R Matrix)
2.4. Trait Sampling (Q Matrix)
2.5. Data Analysis
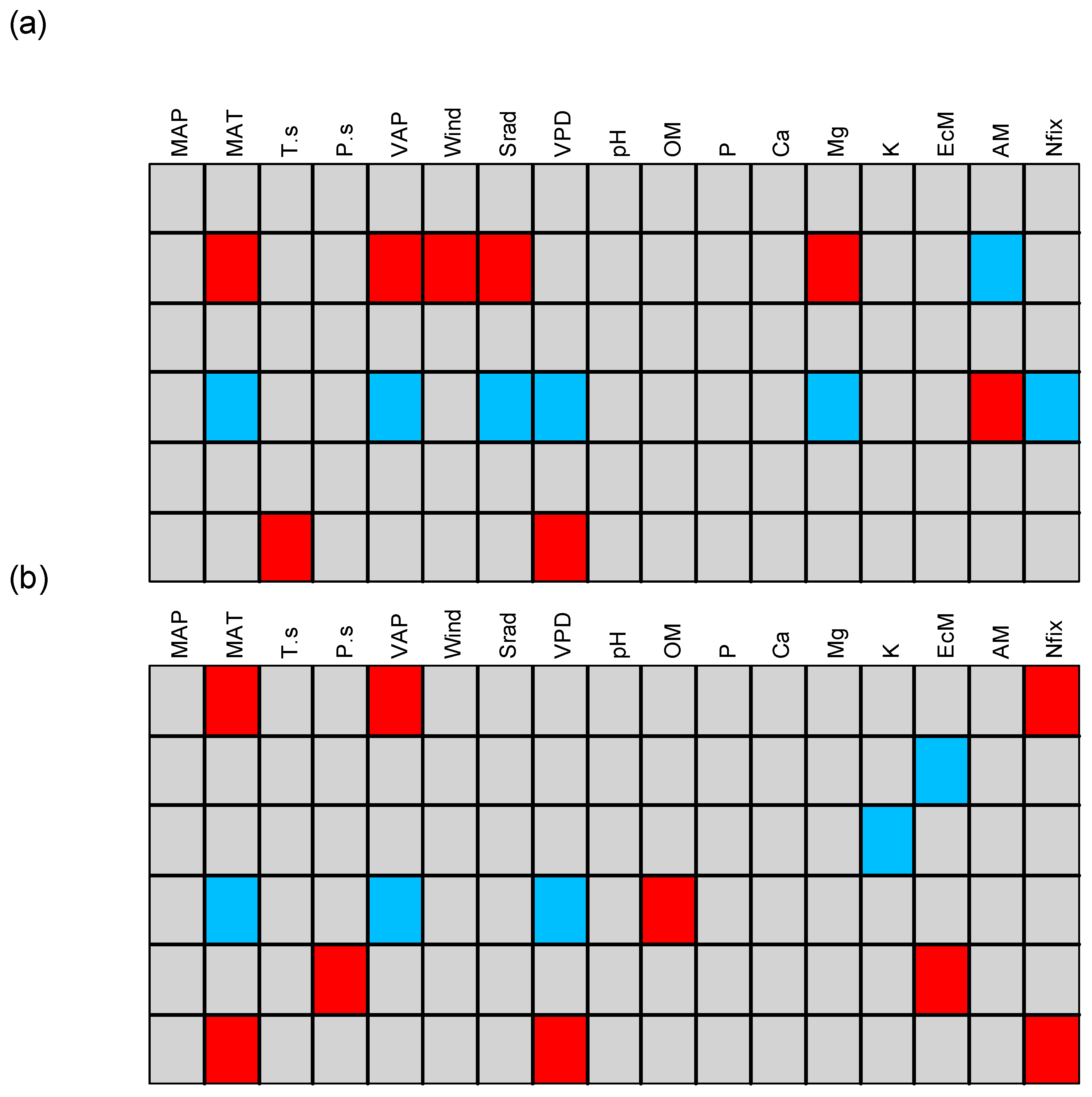
3. Results
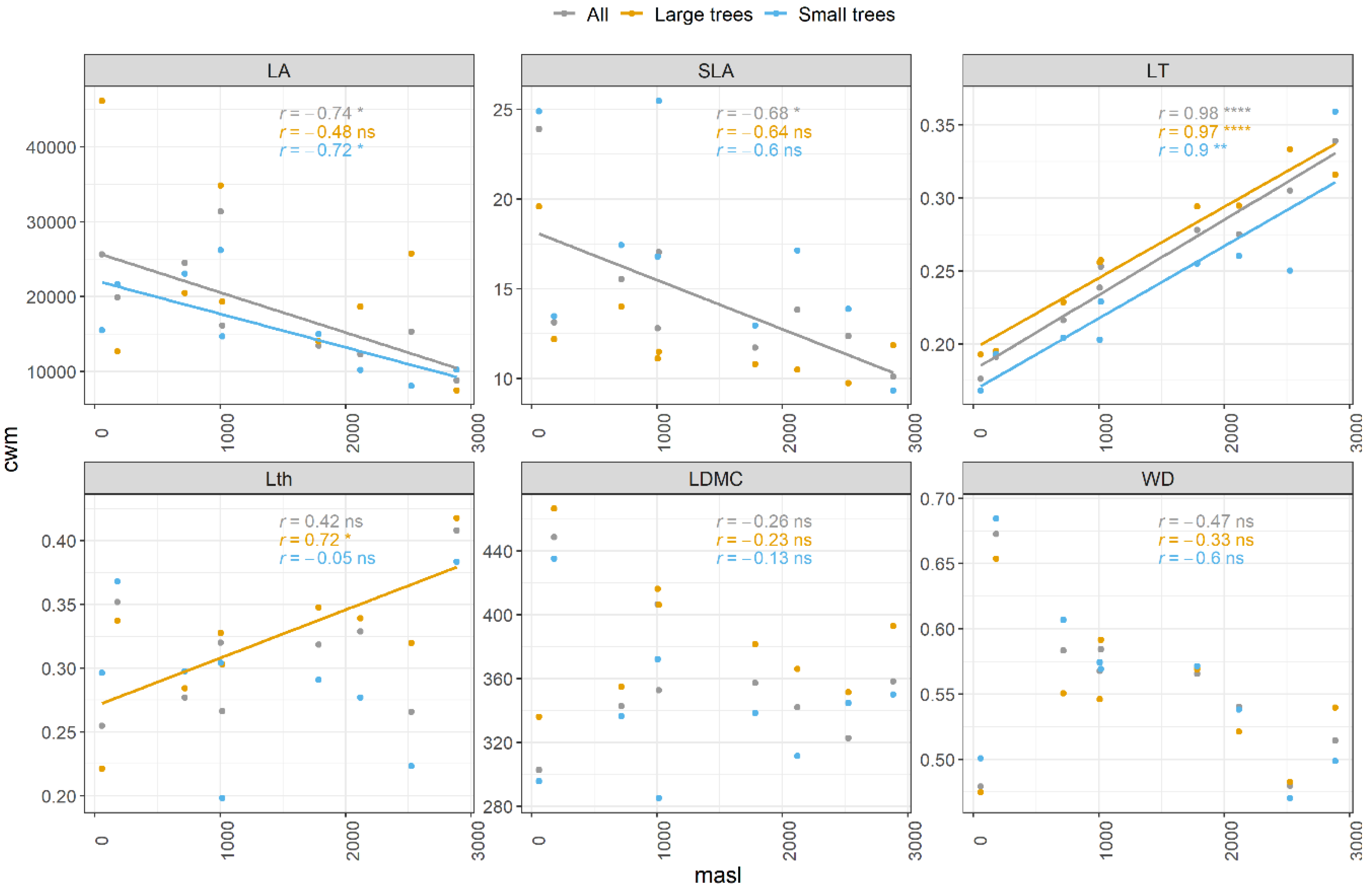
3.1. Patterns of Change with Elevation
3.2. Definition of Functional Groups
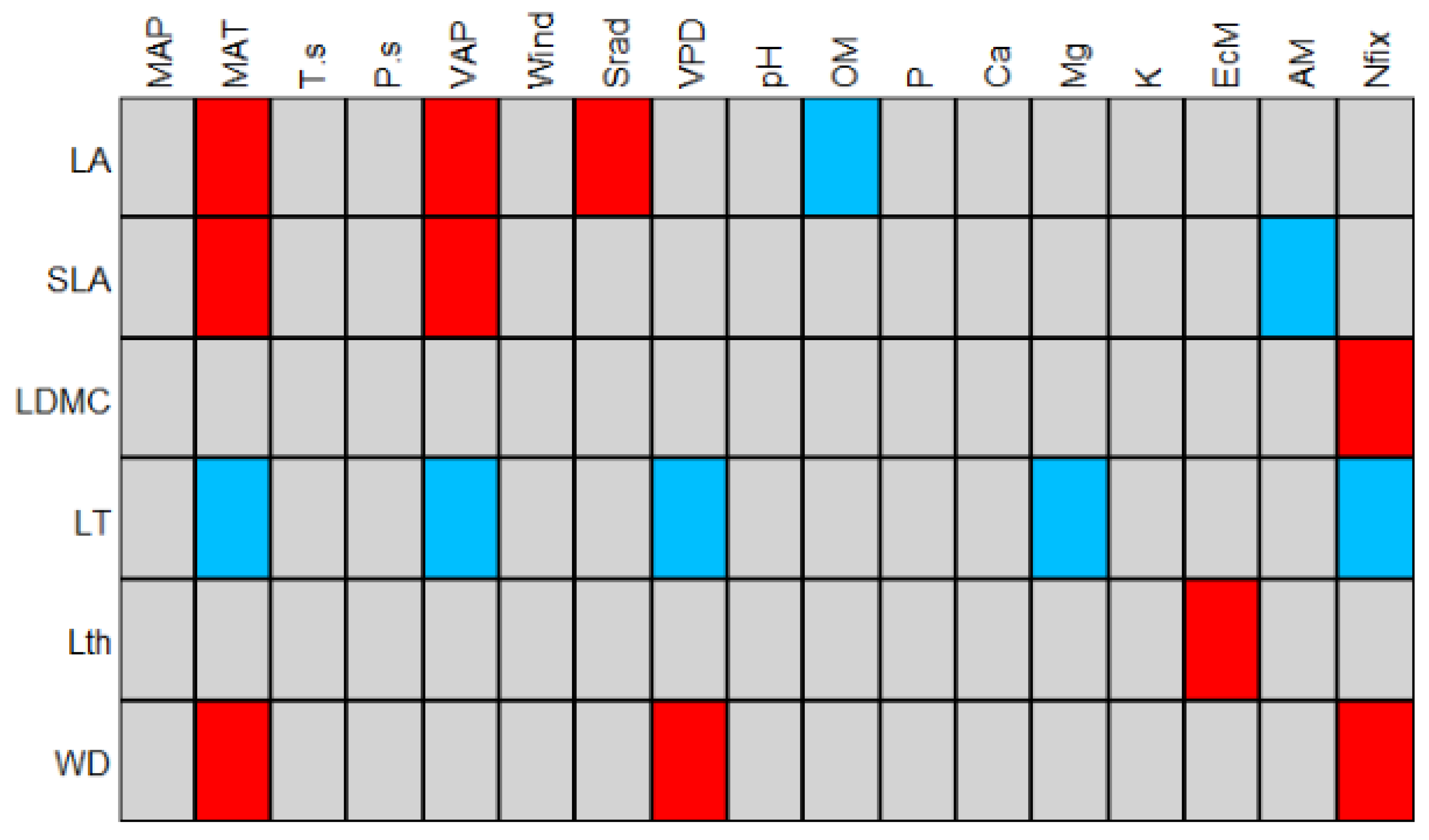
3.3. Traits–Environment Relationship for Different Cutoff Tree Sizes
4. Discussion
4.1. Functional Groups
4.2. Drivers of Plant Trait Assembly
4.3. Traits–Environment Relationship for Different Tree Size Cutoff
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.; Mittermeier, C.; da Fonseca, G.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Orme, C.D.L.; Davies, R.G.; Burgess, M.; Eigenbrod, F.; Pickup, N.; Olson, V.A.; Webster, A.J.; Ding, T.S.; Rasmussen, P.C.; Ridgely, R.S.; et al. Global hotspots of species richness are not congruent with endemism or threat. Nature 2005, 436, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Aide, T.M.; Clark, M.L.; Grau, H.R.; López-Carr, D.; Levy, M.A.; Redo, D.; Bonilla-Moheno, M.; Riner, G.; Andrade-Núñez, M.J.; Muñiz, M. Deforestation and reforestation of Latin America and the Caribbean (2001–2010). Biotropica 2013, 45, 262–271. [Google Scholar] [CrossRef]
- Duque, A.; Peña, M.A.; Cuesta, F.; González-Caro, S.; Kennedy, P.; Phillips, O.L.; Calderón-Loor, M.; Blundo, C.; Carilla, J.; Cayola, L.; et al. Mature Andean forests as globally important carbon sinks and future carbon refuges. Nat. Commun. 2021, 12, 2138. [Google Scholar] [CrossRef]
- Rodríguez, N.; Armenteras, D.; Morales, M.; Romero, M. Ecosistemas de los Andes Colombianos, 2nd ed.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2006; ISBN 9588151309. [Google Scholar]
- Sánchez-Cuervo, A.M.; Aide, T.M. Consequences of the armed conflict, forced human displacement, and land abandonment on forest cover change in Colombia: A multi-scaled analysis. Ecosystems 2013, 16, 1052–1070. [Google Scholar] [CrossRef]
- Martínez-Villa, J.A.; González-Caro, S.; Duque, Á. The importance of grain and cut-off size in shaping tree beta diversity along an elevational gradient in the northwest of Colombia. For. Ecosyst. 2020, 7, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Booth, B.D.; Swanton, C.J. Assembly theory applied to weed communities. Weed Sci. 2002, 50, 2–13. [Google Scholar] [CrossRef]
- Keddy, P.A. A pragmatic approach to functional ecology. Funct. Ecol. 1992, 6, 621–626. [Google Scholar] [CrossRef]
- Mcgill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Malizia, A.; Blundo, C.; Carilla, J.; Acosta, O.O.; Cuesta, F.; Duque, A.; Aguirre, N.; Aguirre, Z.; Ataroff, M.; Baez, S.; et al. Elevation and latitude drives structure and tree species composition in Andean forests: Results from a large-scale plot network. PLoS ONE 2020, 14, e0231553. [Google Scholar] [CrossRef] [Green Version]
- Agudelo, C.M.; Benavides, A.M.; Taylor, T.; Feeley, K.J.; Duque, A. Functional composition of epiphyte communities in the Colombian Andes. Ecology 2019, 100, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Blonder, B.; Salinas, N.; Bentley, L.P.; Shenkin, A.; Chambi Porroa, P.O.; Valdez Tejeira, Y.; Cyrille, V.; Fyllas, N.M.; Goldsmith, G.R.; Martin, R.E.; et al. Predicting trait-environment relationships for venation networks along an Andes-Amazon elevation gradient. Ecology 2017, 98, 1239–1255. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Zang, R.; Lu, X.; Huang, J.; Xu, Y. The effect of environmental filtering on variation in functional diversity along a tropical elevational gradient. J. Veg. Sci. 2019, 30, 973–983. [Google Scholar] [CrossRef]
- Homeier, J.; Seeler, T.; Pierick, K.; Leuschner, C. Leaf trait variation in species—Rich tropical Andean forests. Sci. Rep. 2021, 11, 9993. [Google Scholar] [CrossRef] [PubMed]
- van de Weg, M.J.; Meir, P.; Grace, J.; Atkin, O.K. Altitudinal variation in leaf mass per unit area, leaf tissue density and foliar nitrogen and phosphorus content along an Amazon-Andes gradient in Peru. Plant Ecol. Divers. 2009, 2, 243–254. [Google Scholar] [CrossRef]
- Read, Q.D.; Moorhead, L.C.; Swenson, N.G.; Bailey, J.K.; Sanders, N.J. Convergent effects of elevation on functional leaf traits within and among species. Funct. Ecol. 2014, 28, 37–45. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. N. Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [Green Version]
- Wieczynski, D.J.; Boyle, B.; Buzzard, V.; Duran, S.M.; Henderson, A.N.; Hulshof, C.M.; Kerkhoff, A.J.; McCarthy, M.C.; Michaletz, S.T.; Swenson, N.G.; et al. Climate shapes and shifts functional biodiversity in forests worldwide. Proc. Natl. Acad. Sci. USA 2019, 116, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Reich, P.; Wright, I.; Bares, J.C.; Craine, J.M.; Oleksyn, J.; Walters, M.B.; Reich, P.B.; Wright, I.J.; Craine, J.M.; Oleksyn, J.; et al. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, 143–164. [Google Scholar] [CrossRef]
- Worthy, S.J.; Swenson, N.G. Functional perspectives on tropical tree demography and forest dynamics. Ecol. Process. 2019, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Poorter, L.; Wright, S.J.; Paz, H.; Ackerly, D.; Condit, R.; Ibarra-Manríquez, G.; Harms, K.E.; Licona, J.; Martínez-Ramos, M.; Mazer, S.J.; et al. Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecol. Res. 2008, 89, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Díaz, S.; et al. Functional traits and the growth—Mortality trade-off in tropical trees. Ecography 2010, 91, 3664–3674. [Google Scholar] [CrossRef] [PubMed]
- Baraloto, C.; Paine, C.E.T.; Poorter, L.; Beauchene, J.; Bonal, D.; Domenach, A.-M.; Hérault, B.; Patiño, S.; Roggy, J.-C.; Chave, J. Decoupled leaf and stem economics in rain forest trees. Ecol. Lett. 2010, 13, 1338–1347. [Google Scholar] [CrossRef]
- Muscarella, R.; Uriarte, M.; Erickson, D.L.; Swenson, N.G.; Kress, W.J.; Zimmerman, J.K. Variation of tropical forest assembly processes across regional environmental gradients. Perspect. Plant Ecol. Evol. Syst. 2016, 23, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Pinho, B.X.; Tabarelli, M.; Engelbrecht, B.M.J.; Sfair, J.; Melo, F.P.L. Plant functional assembly is mediated by rainfall and soil conditions in a seasonally dry tropical forest. Basic Appl. Ecol. 2019, 40, 1–11. [Google Scholar] [CrossRef]
- Poorter, L.; Mcdonald, I.; Alarco, A.; Fichtler, E.; Licona, J.; Marielos, P.-C.; Sterck, F.; Villegas, Z.; Sass-klaassen, U. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. N. Phytol. 2010, 185, 481–492. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Lian, J.; Shen, H.; Cao, H.-L.; Lu, H.-P.; Ye, W.-H. Inferring community assembly processes from trait diversity across environmental gradients. J. Trop. Ecol. 2016, 32, 1–10. [Google Scholar] [CrossRef]
- Capers, R.S.; Chazdon, R.L. Rapid assessment of understory light availability in a wet tropical forest. Agric. For. Meteorol. 2004, 123, 177–185. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Chazdon, R.L. Forest structure, canopy architecture, and light transmittance in Tropical Wet Forests. Ecology 2001, 82, 2707–2718. [Google Scholar] [CrossRef]
- Poorter, L. Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. N. Phytol. 2009, 181, 890–900. [Google Scholar] [CrossRef]
- Salgado-Luarte, C.; Gianoli, E. Herbivores modify selection on plant functional traits in a temperate rainforest understory. Am. Nat. 2012, 180, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhang, M.; Yang, W.; Liu, Y.; Wang, W.; Zheng, J.; An, S. Leaf functional traits and trait relationships of tropical woody vegetation in relation to successional stage: Shifts in understory and canopy layers leaf functional traits and trait relationships of tropical woody vegetation in relation to successional. Ecoscience 2012, 19, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Dantas de Paula, M.; Forrest, M.; Langan, L.; Bendix, J.; Homeier, J.; Velescu, A.; Wilcke, W.; Hickler, T. Nutrient cycling drives plant community trait assembly and ecosystem functioning in a tropical mountain biodiversity hotspot. N. Phytol. 2021, 232, 551–566. [Google Scholar] [CrossRef]
- Dixon, R.; Rao, M.V.; Garg, V.K. Water relations and gas exchange of mycorrhizal leucaena leucocephala seedlings. J. Trop. For. Sci. 1994, 6, 542–552. [Google Scholar]
- Hernández-Vargas, G.; Perroni, Y.; López-Acosta, J.C.; Noa-Carrazana, J.C.; Sánchez-Velásquez, L.R. Do the distribution patterns of plant functional traits change during early secondary succession in tropical montane cloud forests? Acta Oecologica 2019, 95, 26–35. [Google Scholar] [CrossRef]
- Maherali, H. Mutualism as a plant functional trait: Linking variation in the mycorrhizal symbiosis to climatic tolerance, geographic range and population dynamics. Int. J. Plant Sci. 2020, 181, 1–30. [Google Scholar] [CrossRef]
- Shi, L.; Wang, J.; Liu, B.; Nara, K.; Lian, C.; Shen, Z.; Xia, Y.; Chen, Y. Ectomycorrhizal fungi reduce the light compensation point and promote carbon fixation of Pinus thunbergii seedlings to adapt to shade environments. Mycorrhiza 2017, 27, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Valladares, F.; Gianoli, E.; Gómez, J.M. Ecological limits to plant phenotypic plasticity. N. Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef]
- Etter, A.; McAlpine, C.; Possingham, H. Historical patterns and drivers of landscape change in Colombia since 1500: A regionalized spatial approach. Ann. Assoc. Am. Geogr. 2008, 98, 2–23. [Google Scholar] [CrossRef]
- Herault, M.R.-M.; Tanguy, A.; Piponiot, C.; Chave, J.; Herault, B. BIOMASS: An {R} package for estimating above-ground biomass and its uncertainty in tropical forests. Methods Ecol. Evol. 2017, 8, 1163–1167. [Google Scholar] [CrossRef]
- Fick, S.; Hijmans, R. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Steidinger, B.S.; Crowther, T.W.; Liang, J.; Van Nuland, M.E.; Werner, G.D.A.; Reich, P.B.; Nabuurs, G.; De-Miguel, S.; Zhou, M.; Picard, N.; et al. Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 2019, 569, 404–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maherali, H.; Oberle, B.; Stevens, P.F.; Cornwell, W.K.; McGlinn, D.J. Mutualism persistence and abandonment during the evolution of the mycorrhizal symbiosis. Am. Nat. 2016, 188, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Laanisto, L.; Rahimlou, S.; Toussaint, A.; Hallikma, T.; Pärtel, M. Global database of plants with root-symbiotic nitrogen fixation: NodDB. J. Veg. Sci. 2018, 29, 560–568. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; Urcelay, C.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H. A Handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–338. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Douzet, R.; Aubert, S.; Lavorel, S. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Funct. Ecol. 2010, 24, 1192–1201. [Google Scholar] [CrossRef]
- Kerkhoff, A.J.; Enquist, B.J. Multiplicative by nature: Why logarithmic transformation is necessary in allometry. J. Theor. Biol. 2009, 257, 519–521. [Google Scholar] [CrossRef]
- Dolédec, S.; Chessel, D.; Ter Braak, C.J.F.; Champely, S. Matching species traits to environmental variables: A new three-table ordination method. Environ. Ecol. Stat. 1996, 3, 143–166. [Google Scholar] [CrossRef]
- Legendre, P.; Galzin, R.G.; Harmelin-Vivien, M.L. Relating behavior to habitat: Solutions to the fourth-corner problem. Ecology 1997, 78, 547–562. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Cormont, A.; Dray, S. Improved testing of species traits–environment relationships in the fourth-corner problem. Ecology 2012, 93, 1525–1526. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Dray, S.; Legendre, P. Testing the species traits environment relationships: The fourth-corner problem revisited. Ecology 2008, 89, 3400–3412. [Google Scholar] [CrossRef]
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; Ter Braak, C.J.F. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Hartigan, J.A.; Wong, M.A. Algorithm AS 136: A K-means clustering algorithm. Appl. Stat. 1979, 28, 100–108. [Google Scholar] [CrossRef]
- Alboukadel, K.; Mundt, F.; Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2020. Available online: https://rpkgs.datanovia.com/factoextra/index.html (accessed on 7 October 2021).
- Körner, C.; Neumayer, M.; Menendez-Riedl, S.P.; Smeets-Scheel, A. Functional morphology of mountain plants. Flora 1989, 182, 353–383. [Google Scholar] [CrossRef]
- Wright, I.; Reich, P.B.; Cornelissen, J.H.C.C.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. N. Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.G.; Montserrat-Martí, G.; Charles, M.; Jones, G.; Wilson, P.; Shipley, B.; Sharafi, M.; Cerabolini, B.E.L.; Cornelissen, J.H.C.; Band, S.R.; et al. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? Ann. Bot. 2011, 108, 1337–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. N. Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Jager, M.M.; Richardson, S.J.; Bellingham, P.J.; Clearwater, M.J.; Laughlin, D.C. Soil fertility induces coordinated responses of multiple independent functional traits. J. Ecol. 2015, 103, 374–385. [Google Scholar] [CrossRef]
- Zuleta, D.; Duque, A.; Cardenas, D.; Muller-Landau, H.; Davies, S. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Ecology 2017, 98, 2538–2546. [Google Scholar] [CrossRef]
- Martin, A.; Erickson, D.; Kress, J.; Thomas, S. Wood nitrogen concentrations in tropical trees: Phylogenetic patterns and ecological correlates. N. Phytol. 2014, 204, 484–495. [Google Scholar] [CrossRef]
- Asner, G.P.; Knapp, D.E.; Anderson, C.B.; Martin, R.E.; Vaughn, N. Large-scale climatic and geophysical controls on the leaf economics spectrum. Proc. Natl. Acad. Sci. USA 2016, 113, 4043–4051. [Google Scholar] [CrossRef] [Green Version]
- Swenson, N.G.; Enquist, B.J. Ecological and evolutionary determinants of a key plant functional trait: Wood density and its community-wide variation across latitude and elevation. Am. J. Bot. 2007, 94, 451–459. [Google Scholar] [CrossRef]
- Cárate-Tandalla, D.; Leuschner, C.; Homeier, J. Performance of seedlings of a shade-tolerant tropical tree species after moderate addition of N and P. Front. Earth Sci. 2015, 3, 75. [Google Scholar] [CrossRef] [Green Version]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wen, X.; Zhang, X.; Li, S.; Zhang, D.Y. Co-regulation of photosynthetic capacity by nitrogen, phosphorus and magnesium in a subtropical Karst forest in China. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.B.; Malhi, Y.; Torres, I.C.; Metcalfe, D.B.; van de Weg, M.J.; Meir, P.; Silva-Espejo, J.E.; Huasco, W.H. Nutrient limitation in rainforests and cloud forests along a 3000-m elevation gradient in the Peruvian Andes. Oecologia 2013, 172, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.V.J.; Vitousek, P.M.; Cuevas, E. Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology 1998, 79, 10–22. [Google Scholar] [CrossRef]
- Camenzind, T.; Hättenschwiler, S.; Treseder, K.; Lehmann, A.; Rillig, M. Nutrient limitation of soil microbial processes in tropical forests. Ecol. Monogr. 2017, 88, 4–21. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Convergent elevation trends in canopy chemical traits of tropical forests. Glob. Chang. Biol. 2016, 22, 2216–2227. [Google Scholar] [CrossRef]
- Bunn, R.A.; Simpson, D.T.; Bullington, L.S.; Lekberg, Y.; Janos, D.P. Revisiting the ‘direct mineral cycling’ hypothesis: Arbuscular mycorrhizal fungi colonize leaf litter, but why? ISME J. 2019, 13, 1891–1898. [Google Scholar] [CrossRef] [Green Version]
- Nuccio, E.E.; Hodge, A.; Pett-Ridge, J.; Herman, D.J.; Weber, P.K.; Firestone, M.K. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ. Microbiol. 2013, 15, 1870–1881. [Google Scholar] [CrossRef]
- Phillips, R.P.; Brzostek, E.; Midgley, M.G. The mycorrhizal-associated nutrient economy: A new framework for predicting carbon-nutrient couplings in temperate forests. N. Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Ter Steege, H.; Lopez-Gonzalez, G.; Monteagudo Mendoza, A.; Brienen, R.; Feldpausch, T.R.; Pitman, N.; et al. Seasonal drought limits tree species across the Neotropics. Ecography 2017, 40, 618–629. [Google Scholar] [CrossRef] [Green Version]
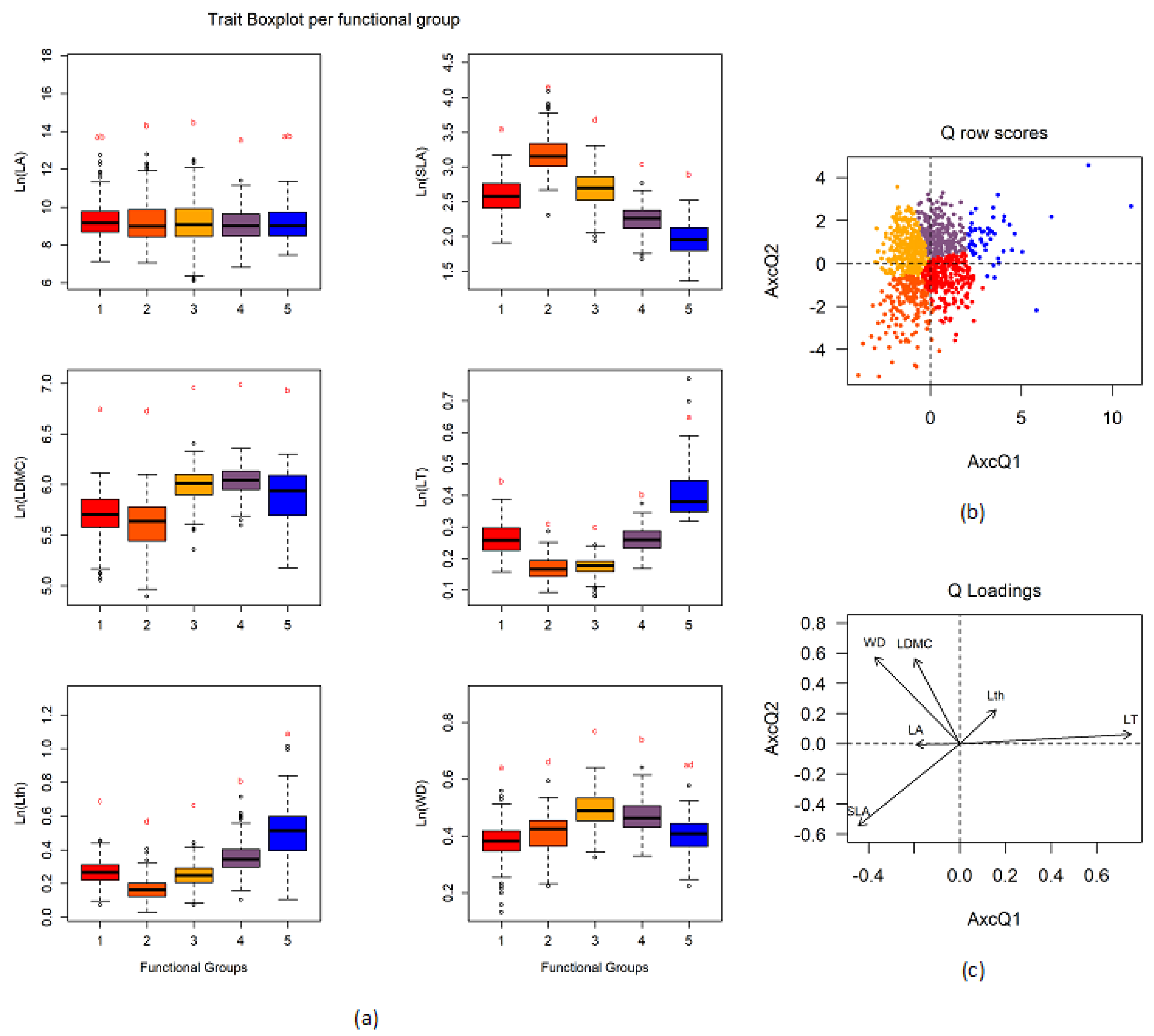
| Total Inertia: 4,16 | |||||
| A × 1 | A × 2 | A × 3 | A × 4 | A × 5 | |
| Eig | 3.23 | 0.75 | 0.13 | 0.03 | 0.01 |
| Eig % | 77.66 | 18.10 | 3.04 | 0.84 | 0.27 |
| Acumulative | 77.66 | 95.76 | 98.81 | 99.65 | 99.92 |
| Eigenvalues Decomposition | |||||
| Eig | covar | sdR | sdQ | corr | |
| 1 | 3.23 | 1.80 | 2.48 | 1.22 | 0.59 |
| 2 | 0.75 | 0.87 | 1.44 | 1.39 | 0.43 |
| Permutation Test (Randtest) | |||||
| Test | Obs | Std. Obs | Alter | p value | |
| 1 | Model 2 | 4.16 | 3.98 | greater | 0.00006 |
| 2 | Model 4 | 4.16 | 15.26 | greater | 0.00002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa-Beltrán, A.; Martínez-Villa, J.A.; Kennedy, P.G.; Salgado-Negret, B.; Duque, A. Plant Trait Assembly in Species-Rich Forests at Varying Elevations in the Northwest Andes of Colombia. Land 2021, 10, 1057. https://doi.org/10.3390/land10101057
Ochoa-Beltrán A, Martínez-Villa JA, Kennedy PG, Salgado-Negret B, Duque A. Plant Trait Assembly in Species-Rich Forests at Varying Elevations in the Northwest Andes of Colombia. Land. 2021; 10(10):1057. https://doi.org/10.3390/land10101057
Chicago/Turabian StyleOchoa-Beltrán, Angélica, Johanna Andrea Martínez-Villa, Peter G. Kennedy, Beatriz Salgado-Negret, and Alvaro Duque. 2021. "Plant Trait Assembly in Species-Rich Forests at Varying Elevations in the Northwest Andes of Colombia" Land 10, no. 10: 1057. https://doi.org/10.3390/land10101057
APA StyleOchoa-Beltrán, A., Martínez-Villa, J. A., Kennedy, P. G., Salgado-Negret, B., & Duque, A. (2021). Plant Trait Assembly in Species-Rich Forests at Varying Elevations in the Northwest Andes of Colombia. Land, 10(10), 1057. https://doi.org/10.3390/land10101057






