Soil Amendments Alter Ammonia-Oxidizing Archaea and Bacteria Communities in Rain-Fed Maize Field in Semi-Arid Loess Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Site
2.2. Experimental Design and Soil Sampling
2.3. Soil Chemical Properties
2.4. DNA Isolation and Quantitative PCR Analysis
2.5. High-Throughput Sequencing and Bioinformatic Analysis
2.6. Data Analysis
2.7. Network, SEM, and Random Forest Analysis
3. Results
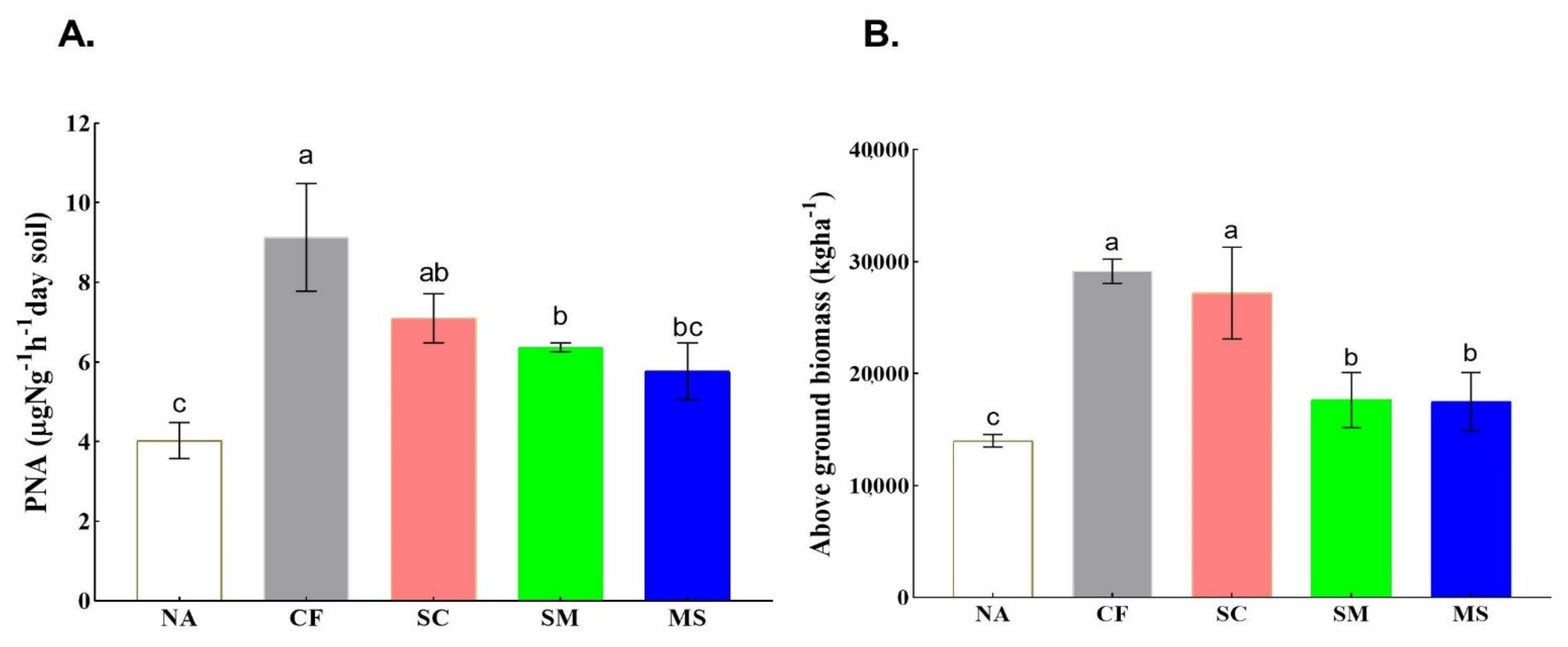
3.1. Soil Physicochemical Properties and Aboveground Biomass
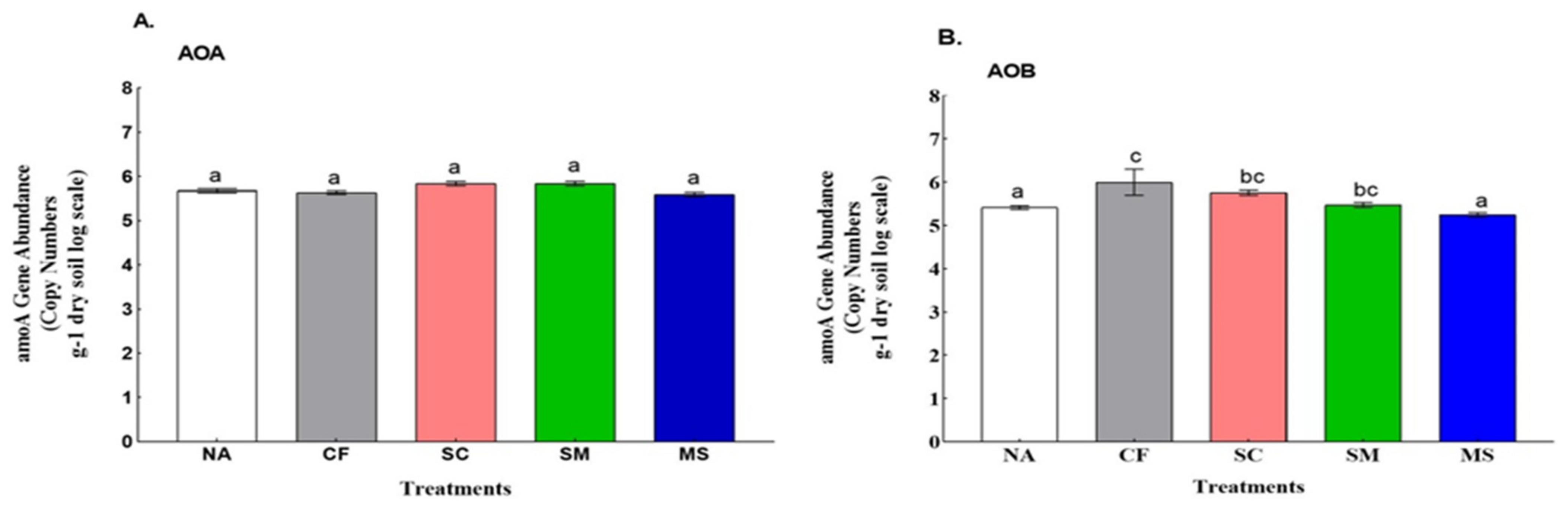
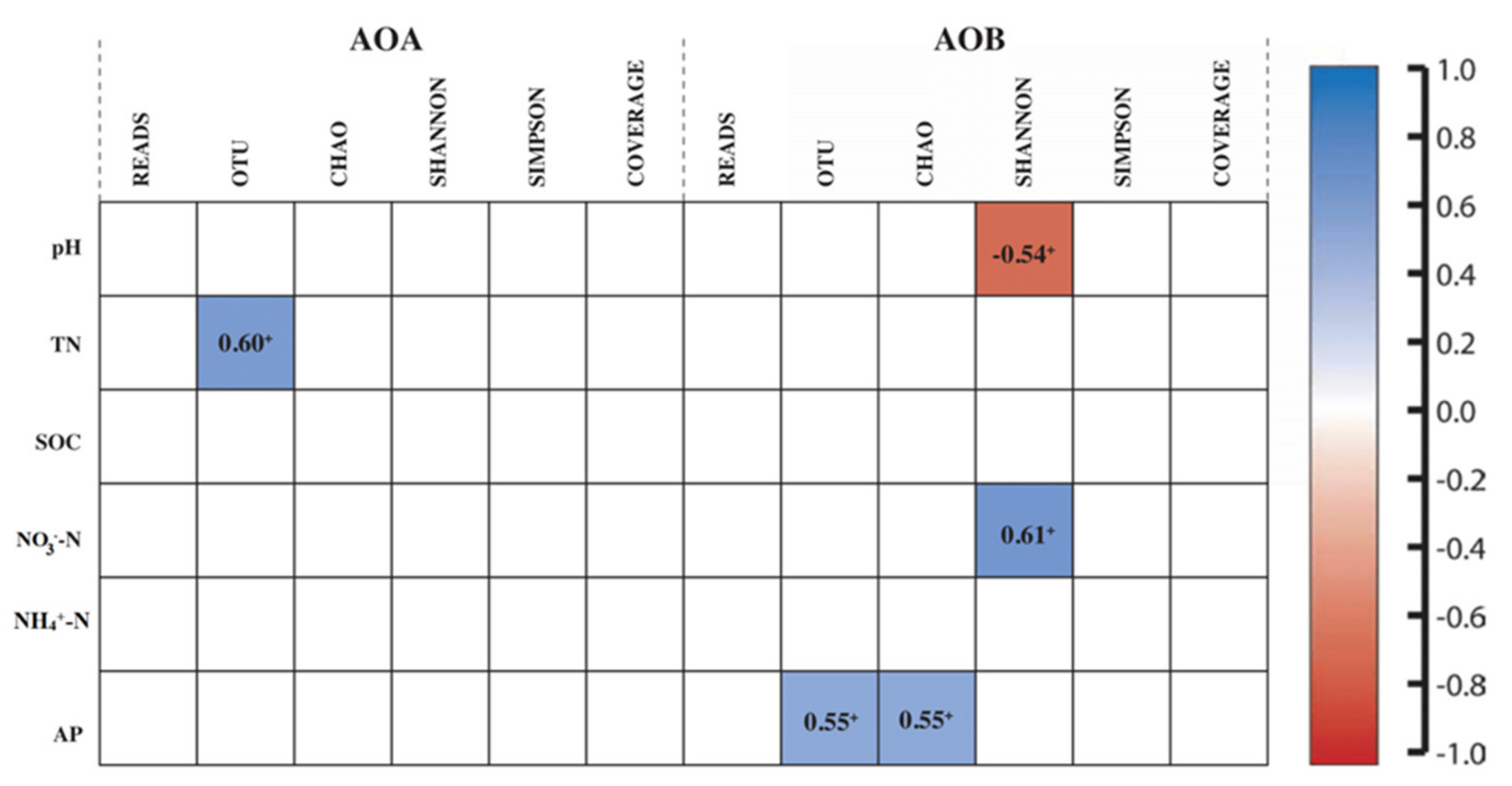
3.2. Abundance and Diversity of Ammonia Oxidizers with Its Association to Soil Physiochemical Properties
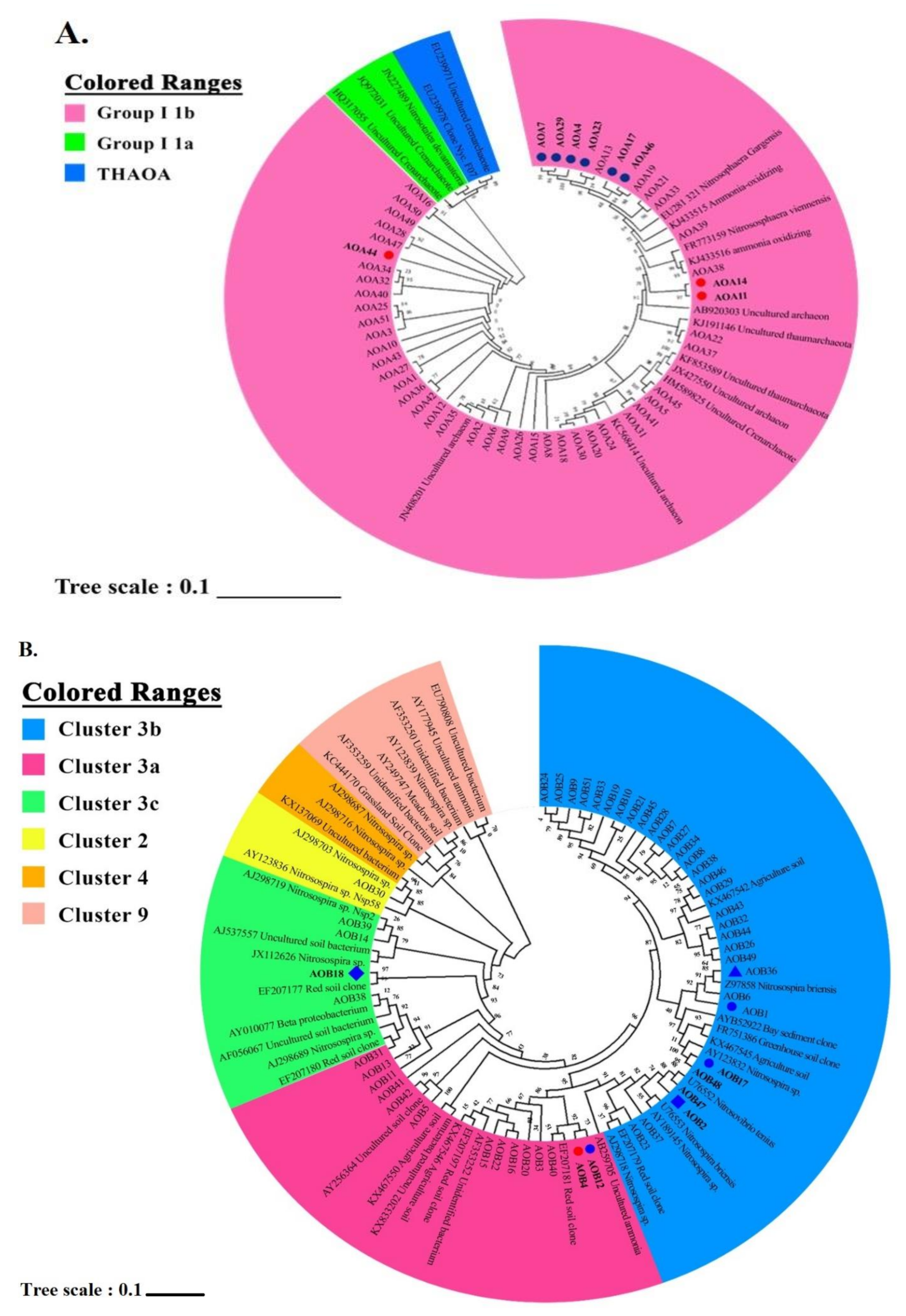
3.3. The Compositions of Ammonia Oxidizers
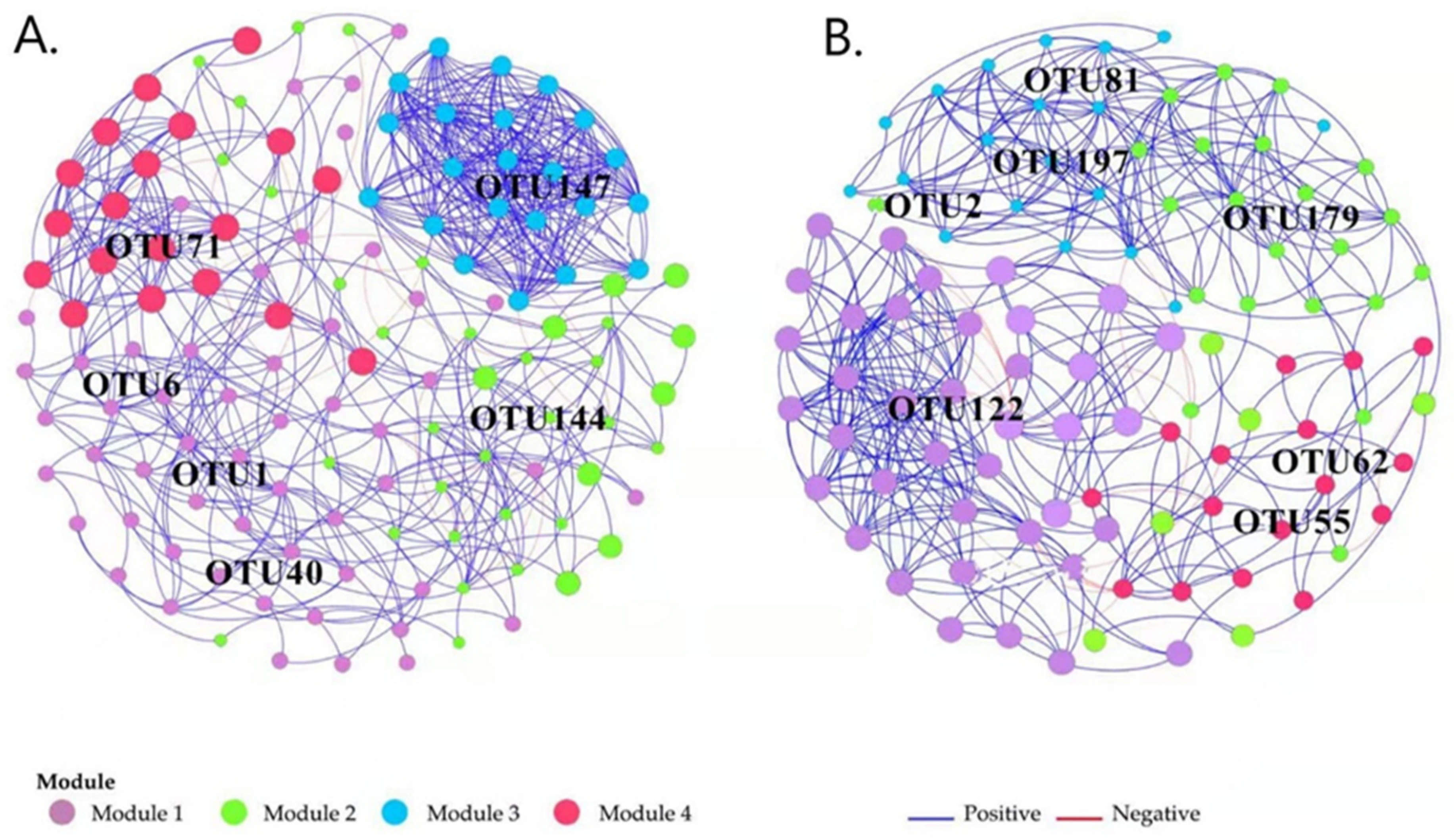
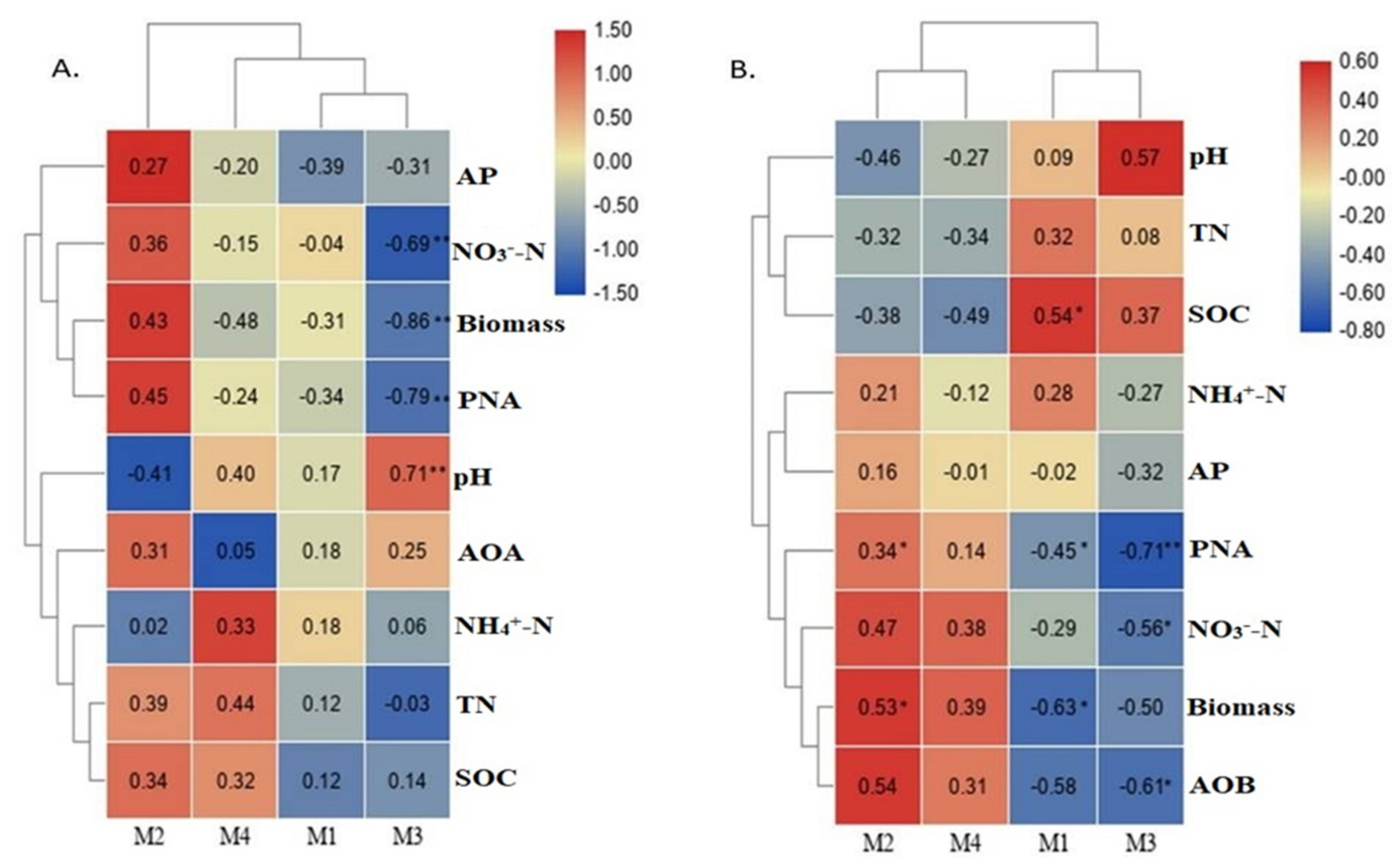
3.4. The Co-Occurrence Network of Ammonia Oxidizers and Its Association of Major Modules with Environmental Variables
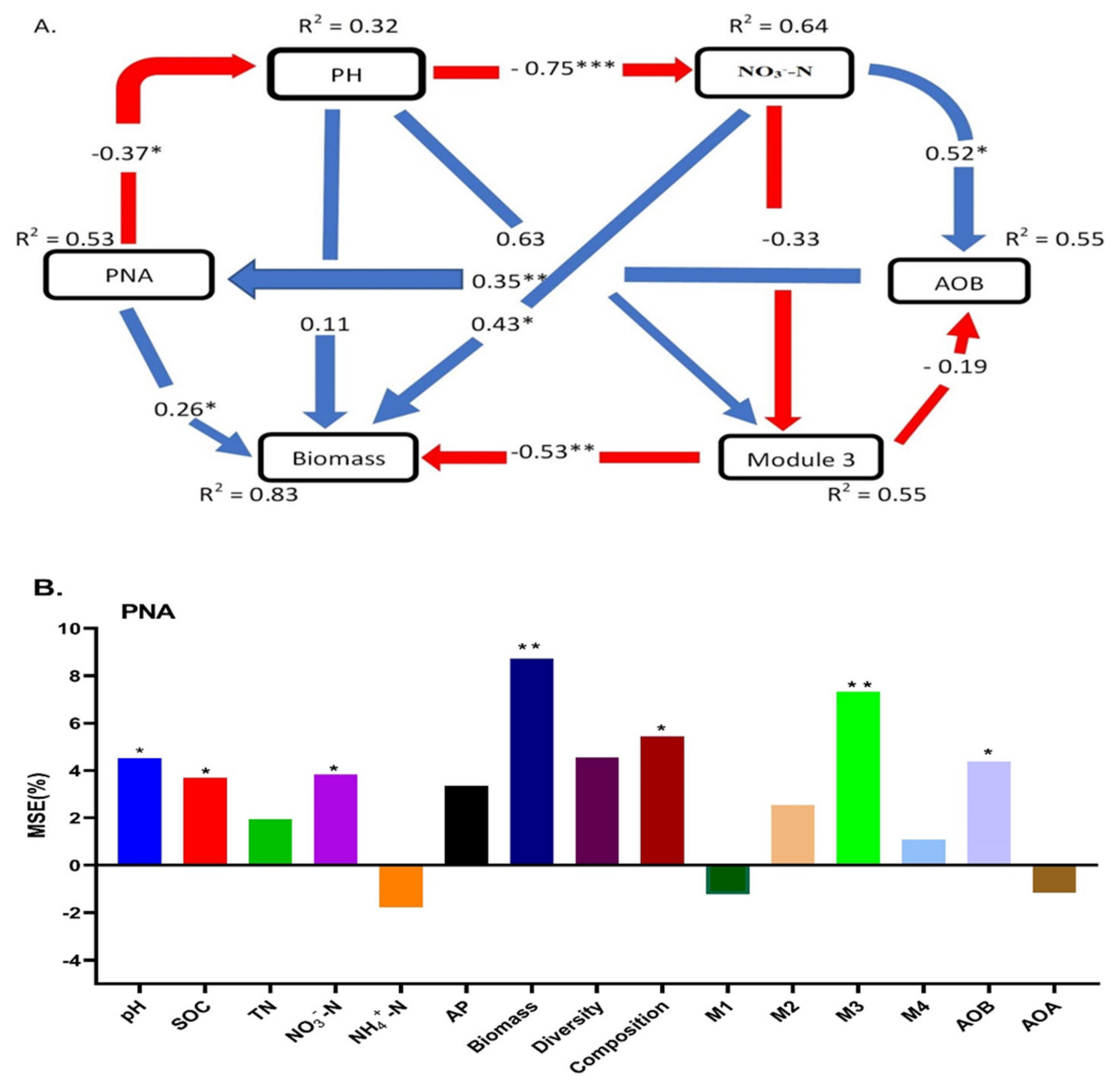
3.5. Soil Properties and Ammonia Oxidizers Regulated the PNA and Crop Productivity (Yield)
4. Discussion
4.1. The Impact of Fertilizer Treatments in Calcareous Soils on the Abundance and Diversity of AOA and AOB
4.2. The Changes in the Co-Occurrence Network of AOA and AOB Communities among Soil Amendments
4.3. Relative Contributions of AOA and AOB Community to Soil Nitrification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tao, R.; Wakelin, S.; Liang, Y.; Chu, G. Response of ammonia-oxidizing archaea and bacteria in calcareous soil to mineral and organic fertilizer application and their relative contribution to nitrification. Soil Biol. Biochem. 2017, 114, 20–30. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.; Qi, J.; Li, F.-M. A regional evaluation of plastic film mulching for improving crop yields on the Loess Plateau of China. Agric. For. Meteorol. 2018, 248, 458–468. [Google Scholar] [CrossRef]
- Bu, L.-D.; Liu, J.-L.; Zhu, L.; Luo, S.-S.; Chen, X.-P.; Li, S.-Q. Attainable yield achieved for plastic film-mulched maize in response to nitrogen deficit. Eur. J. Agron. 2014, 55, 53–62. [Google Scholar] [CrossRef]
- Wessén, E.; Nyberg, K.; Jansson, J.; Hallin, S. Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Appl. Soil Ecol. 2010, 45, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Y.; Norton, J.M.; Stark, J.M.; Reeve, J.R.; Habteselassie, M.Y. Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil. Soil Biol. Biochem. 2016, 96, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Levičnik-Höfferle, Š.; Nicol, G.W.; Ausec, L.; Mandić-Mulec, I.; Prosser, J.I. Stimulation of thaumarchaeal ammonia oxidation by ammonia derived from organic nitrogen but not added inorganic nitrogen. FEMS Microbiol. Ecol. 2012, 80, 114–123. [Google Scholar] [CrossRef]
- Jia, Z.; Conrad, R. Bacteriarather than Archaeadominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, Y.; Lang, M.; Christie, P.; Bei, S.; Zhang, J. Dynamics of ammonia oxidizers in response to different fertilization inputs in intensively managed agricultural soils. Appl. Soil Ecol. 2021, 157, 103729. [Google Scholar] [CrossRef]
- Eshen, J.-P.; Ezhang, L.-M.; Di, H.J.; He, J.-Z. A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front. Microbiol. 2012, 3, 296. [Google Scholar] [CrossRef] [Green Version]
- Erguder, T.H.; Boon, N.; Wittebolle, L.; Marzorati, M.; Verstraete, W. Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol. Rev. 2009, 33, 855–869. [Google Scholar] [CrossRef] [Green Version]
- Hink, L.; Gubry-Rangin, C.; Nicol, G.; Prosser, J.I. The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J. 2018, 12, 1084–1093. [Google Scholar] [CrossRef]
- Kong, Y.; Ling, N.; Xue, C.; Chen, H.; Ruan, Y.; Guo, J.; Zhu, C.; Wang, M.; Shen, Q.; Guo, S. Long-term fertilization regimes change soil nitrification potential by impacting active autotrophic ammonia oxidizers and nitrite oxidizers as assessed by DNA stable isotope probing. Environ. Microbiol. 2019, 21, 1224–1240. [Google Scholar] [CrossRef] [PubMed]
- Bei, S.; Zhang, Y.; Li, T.; Christie, P.; Li, X.; Zhang, J. Response of the soil microbial community to different fertilizer inputs in a wheat-maize rotation on a calcareous soil. Agric. Ecosyst. Environ. 2018, 260, 58–69. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Xu, M.; Tong, X.; Sun, F.; Wang, J.; Huang, S.; Zhu, P.; He, X. Long-term combined chemical and manure fertilizations increase soil organic carbon and total nitrogen in aggregate fractions at three typical cropland soils in China. Sci. Total Environ. 2015, 532, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, X.; Zhang, L.; Zeng, L.; Liu, Y.; Wang, X.; He, P.; Li, S.; Liang, G.; Zhou, W.; et al. The stronger impact of inorganic nitrogen fertilization on soil bacterial community than organic fertilization in short-term condition. Geoderma 2021, 382, 114752. [Google Scholar] [CrossRef]
- Pan, Y.; Cassman, N.; de Hollander, M.; Mendes, L.W.; Korevaar, H.; Geerts, R.H.; van Veen, J.A.; Kuramae, E.E. Impact of long-term N, P, K, and NPK fertilization on the composition and potential functions of the bacterial community in grassland soil. FEMS Microbiol. Ecol. 2014, 90, 195–205. [Google Scholar] [CrossRef]
- Muema, E.; Cadisch, G.; Röhl, C.; Vanlauwe, B.; Rasche, F. Response of ammonia-oxidizing bacteria and archaea to biochemical quality of organic inputs combined with mineral nitrogen fertilizer in an arable soil. Appl. Soil Ecol. 2015, 95, 128–139. [Google Scholar] [CrossRef]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [Green Version]
- Goberna, M.; Verdú, M. Phylogenetic-scale disparities in the soil microbial diversity–ecosystem functioning relationship. ISME J. 2018, 12, 2152–2162. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.; Zhang, X.; Zhu, C.; Zhao, J.; Zhu, P.; Peng, C.; Ling, N.; Shen, Q. Quantitative and compositional responses of ammonia-oxidizing archaea and bacteria to long-term field fertilization. Sci. Rep. 2016, 6, 28981. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Miao, Y.; Zhang, L.; Chen, Y.; Gan, Y.; Liu, N.; Dong, L.; Dai, J.; Chen, W. The Structure and Diversity of Nitrogen Functional Groups from Different Cropping Systems in Yellow River Delta. Microorganisms 2020, 8, 424. [Google Scholar] [CrossRef] [Green Version]
- Mamet, S.D.; Redlick, E.; Brabant, M.; Lamb, E.G.; Helgason, B.L.; Stanley, K.; Siciliano, S. Structural equation modeling of a winnowed soil microbiome identifies how invasive plants re-structure microbial networks. ISME J. 2019, 13, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guan, Y.; Bello, A.; Wu, Y.; Ding, J.; Wang, L.; Ren, Y.; Chen, G.; Yang, W. Dynamics of ammonia oxidizers and denitrifiers in response to compost addition in black soil, Northeast China. PeerJ 2020, 8, e8844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Y.; Evans, S.E.; Friesen, M.; Tiemann, L.K. Effect of nitrogen fertilization on the abundance of nitrogen cycling genes in agricultural soils: A meta-analysis of field studies. Soil Biol. Biochem. 2018, 127, 71–78. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Wei, Y.; Su, F.; Guo, H.; Guo, J.; Wang, Y.; Zhang, Y.; Hu, S. Responses of soil ammonia-oxidizing bacteria and archaea to short-term warming and nitrogen input in a semi-arid grassland on the Loess Plateau. Eur. J. Soil Biol. 2021, 102, 103267. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, X.; Chen, J.; Zhang, G. Origin and development of soil science in ancient China. Geoderma 2003, 115, 3–13. [Google Scholar] [CrossRef]
- FAO. Soil Map of the World: Revised Legend; World Soil Resources Report 60; Food and Agriculture Organization of the United Nations: Rome, Italy, 1990. [Google Scholar]
- Lamptey, S.; Xie, J.; Li, L.; Coulter, J.A.; Jagadabhi, P.S.; Li, X. Influence of Organic Amendment on Soil Respiration and Maize Productivity in a Semi-Arid Environment. Agronomy 2019, 9, 611. [Google Scholar] [CrossRef] [Green Version]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis: Part 2—Chemical and Microbiological Properties; Agronomy Monograph 9; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Nelson, D.W.; Sommers, L.W. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Part 2, Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy and Soil Science: Madison, WI, USA, 1982; pp. 301–312. [Google Scholar]
- Bremner, J.M.; Shaw, K. Denitrification in soil. I. Methods of investigation. J. Agric. Sci. 1958, 51, 22–39. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular 939; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Eds.; America Society of Agronomy, Inc.: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Kurola, J.; Salkinoja-Salonen, M.; Aarnio, T.; Hultman, J.; Romantschuk, M. Activity, diversity and population size of ammonia-oxidising bacteria in oil-contaminated landfarming soil. FEMS Microbiol. Lett. 2005, 250, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [Green Version]
- Rotthauwe, J.; Witzel, K. The Ammonia Monooxygenase Structural Gene amoA as a Functional Marker: Molecular Fine-Scale Analysis of Natural Ammonia-Oxidizing Populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high- throughput community sequencing data Intensity normalization improves color calling in SOLiD sequencing. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fish, J.A.; Chai, B.; Wang, Q.; Sun, Y.; Brown, C.T.; Tiedje, J.M.; Cole, J.R. FunGene: The functional gene pipeline and repository. Front. Microbiol. 2013, 4, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Ling, N.; Chen, H.; Zhu, C.; Kong, Y.; Wang, M.; Shen, Q.; Guo, S. Distinct drivers of activity, abundance, diversity and composition of ammonia-oxidizers: Evidence from a long-term field experiment. Soil Biol. Biochem. 2017, 115, 403–414. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Liu, Y.; Shi, P.; Wei, G. Co-occurrence patterns of soybean rhizosphere microbiome at a continental scale. Soil Biol. Biochem. 2018, 118, 178–186. [Google Scholar] [CrossRef]
- Benjamani, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Newman, M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef] [Green Version]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the Third International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009; pp. 361–362. [Google Scholar]
- Langfelder, P.; Horvath, S. Eigengene networks for studying the relationships between co-expression modules. BMC Syst. Biol. 2007, 1, 54. [Google Scholar] [CrossRef] [Green Version]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 3, 18–22. [Google Scholar]
- Fortmannroe, S. A3: Accurate, Adaptable, and Accessible Error Metrics for Predictive Models; R Package Version 1.0.0; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Archer, E. Rfpermute: Estimate Permutation p-Values for Importance Metrics; R package Version 2.1.5; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Coughlan, J.; Mullen, M. Structural Equation Modelling: Guidelines for Determining Model Structural Equation Modeling: Guidelines for Determining Model Fit. Electron. J. Bus. Res. Methods 2008, 6, 53–60. [Google Scholar]
- Xiang, X.; He, D.; He, J.-S.; Myrold, D.D.; Chu, H. Ammonia-oxidizing bacteria rather than archaea respond to short-term urea amendment in an alpine grassland. Soil Biol. Biochem. 2017, 107, 218–225. [Google Scholar] [CrossRef]
- Norman, J.; Barrett, J. Substrate availability drives spatial patterns in richness of ammonia-oxidizing bacteria and archaea in temperate forest soils. Soil Biol. Biochem. 2016, 94, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ni, L.; Song, Y.; Rhodes, G.; Li, J.; Huang, Q.; Shen, Q. Dynamic Response of Ammonia-Oxidizers to Four Fertilization Regimes across a Wheat-Rice Rotation System. Front. Microbiol. 2017, 8, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Cui, X.; Wang, S.; Wang, F.; Pang, Z.; Xu, N.; Zhao, G.; Wang, S. Changes in Biomass and Quality of Alpine Steppe in Response to N & P Fertilization in the Tibetan Plateau. PLoS ONE 2016, 11, e0156146. [Google Scholar] [CrossRef] [Green Version]
- Jalilian, J.; Modarres-Sanavy, S.A.M.; Saberali, S.F.; Sadat-Asilan, K. Effects of the combination of beneficial microbes and nitrogen on sunflower seed yields and seed quality traits under different irrigation regimes. Field Crop. Res. 2012, 127, 26–34. [Google Scholar] [CrossRef]
- Chu, H.; Fujii, T.; Morimoto, S.; Lin, X.; Yagi, K.; Hu, J.; Zhang, J. Community Structure of Ammonia-Oxidizing Bacteria under Long-Term Application of Mineral Fertilizer and Organic Manure in a Sandy Loam Soil. Appl. Environ. Microbiol. 2007, 73, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Schauss, K.; Focks, A.; Leininger, S.; Kotzerke, A.; Heuer, H.; Thiele-Bruhn, S.; Sharma, S.; Wilke, B.-M.; Matthies, M.; Smalla, K.; et al. Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environ. Microbiol. 2008, 11, 446–456. [Google Scholar] [CrossRef]
- He, J.-Z.; Shen, J.-P.; Zhang, L.; Zhu, Y.-G.; Zheng, Y.-M.; Xu, M.-G.; Di, H. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 2007, 9, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-P.; Houlton, B.Z.; Field, C.B. A model of biogeochemical cycles of carbon, nitrogen, and phosphorus including symbiotic nitrogen fixation and phosphatase production. Glob. Biogeochem. Cycles 2007, 21, 1–15. [Google Scholar] [CrossRef]
- Tourna, M.; Stieglmeier, M.; Spang, A.; Könneke, M.; Schintlmeister, A.; Urich, T.; Engel, M.; Schloter, M.; Wagner, M.; Richter, A.; et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. USA 2011, 108, 8420–8425. [Google Scholar] [CrossRef] [Green Version]

| Soil Depth (cm) | Bulk Density (mg/m3) | pH | Total Nitrogen (g/kg) | Total Phosphorus (g/kg) | Organic C (g/kg) |
|---|---|---|---|---|---|
| 0–5 | 1.19 | 8.33 | 1.05 | 0.82 | 9.91 |
| 5–10 | 1.22 | 8.32 | 1.05 | 0.74 | 8.96 |
| 10–30 | 1.28 | 8.37 | 0.94 | 0.7 | 8.89 |
| Amendment | Organic Carbon | N | P | K | Ca | Mg |
|---|---|---|---|---|---|---|
| Corn stalk | 47.5 | 0.74 | 0.38 | 0.45 | 0.55 | 0.74 |
| Cow manure | 39.9 | 2.20 | 1.70 | 1.90 | 2.70 | 0.50 |
| Soil | pH | TN | SOC | NO3−-N | NH4+-N | AP |
|---|---|---|---|---|---|---|
| Amendment | (g/kg) | (g/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |
| NA | 8.66 ± 0.03 ab | 0.85 ± 0.01 b | 7.48 ± 0.18 c | 17.84 ± 1.04 c | 15.33 ± 1.63 a | 9.73 ± 1.41 c |
| CF | 8.32 ± 0.07 c | 0.93 ± 0.03 a | 7.93 ± 0.07 c | 30.81 ± 2.78 a | 16.07 ± 1.48 a | 16.70 ± 1.57 ab |
| SC | 8.44 ± 0.08 bc | 0.93 ± 0.02 a | 8.84 ± 0.20 b | 28.40 ± 3.57 ab | 14.87 ± 2.22 a | 18.32 ± 1.69 ab |
| SM | 8.45 ± 0.05 bc | 0.98 ± 0.03 a | 8.81 ± 0.33 b | 25.40 ± 3.41 abc | 15.81 ± 1.36 a | 19.81 ± 0.72 a |
| MS | 8.54 ± 0.06 b | 0.99 ± 0.002 a | 9.82 ± 0.19 a | 21.93 ± 1.81 bc | 16.53 ± 2.80 a | 15.14 ± 0.97 b |
| p-value | 0.013 | 0.001 | 0.036 | 0.024 | 0.107 | 0.002 |
| Treatment | Observed Species | OTUs | Chao1 | Shannon | Simpson | Goods Coverage |
|---|---|---|---|---|---|---|
| NA | 4865.1 ± 49.6 a | 518.1 ± 10.1 a | 629 ± 36.8 a | 0.9 ± 0.0 a | 2.9 ± 0.04 a | 0.21 ± 0.01 a |
| CF | 4297.9 ± 74.6 a | 524.3 ± 50.4 a | 641 ± 74.3 a | 0.9 ± 0.0 a | 3.2 ± 0.12 a | 0.17 ± 0.02 a |
| SC | 5331.2 ± 20.1 a | 557.2 ± 22.5 a | 643 ± 43.1 a | 0.9 ± 0.0 a | 2.98 ± 0.11 a | 0.12 ± 0.02 a |
| SM | 4434.3 ± 10.5 a | 551 ± 13.51 a | 631 ± 49.7 a | 0.9 ± 0.0 a | 3.04 ± 0.16 a | 0.19 ± 0.02 a |
| MS | 4329.4 ± 10.9 a | 521 ± 50.4 a | 618 a ± 38.2 a | 0.9 ± 0.1 a | 3.07 ± 0.33 a | 0.18 ± 0.01 a |
| p-value | <0.482 | 0.657 | 0.969 | 0.296 | 0.716 | 0.331 |
| Treatment | Observed Species | OTUs | Chao1 | Shannon | Simpson | Goods Coverage |
|---|---|---|---|---|---|---|
| NA | 4111.7 ± 83.2 a | 1296.3 ± 147.6 a | 1843 ± 27.3 a | 4.1 ± 0.25 a | 0.03 ± 0.01 ab | 0.98 ± 0.01 a |
| CF | 3980.7 ± 56.7 a | 1989.6 ± 81.5 b | 2691 ± 23.1 b | 5.0 ± 0.11 b | 0.02 ± 0.01 a | 0.99 ± 0.02 a |
| SC | 4405.5 ± 77.7 a | 2104 ± 33.6 b | 3141.7 ± 51.6 b | 4.8 ± 0.21 b | 0.02 ± 0.01 a | 0.98 ± 0.04 a |
| SM | 5039.5 ± 57.8 a | 1560 ± 30.7 ab | 2129.3 ± 35.6 a | 4.5 ± 0.56 ab | 0.03 ± 0.01 ab | 0.97 ± 0.01 a |
| MS | 2153.9 ± 30.3 a | 1359 ± 96.2 a | 1877 ± 17.1 b | 4.4 ± 0.01 ab | 0.04 ± 0.01 b | 0.98 ± 0.02 a |
| p-value | <0.471 | 0.003 | 0.001 | 0.049 | 0.13 | 0.205 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fudjoe, S.K.; Li, L.; Jiang, Y.; Karikari, B.; Xie, J.; Wang, L.; Anwar, S.; Wang, J. Soil Amendments Alter Ammonia-Oxidizing Archaea and Bacteria Communities in Rain-Fed Maize Field in Semi-Arid Loess Plateau. Land 2021, 10, 1039. https://doi.org/10.3390/land10101039
Fudjoe SK, Li L, Jiang Y, Karikari B, Xie J, Wang L, Anwar S, Wang J. Soil Amendments Alter Ammonia-Oxidizing Archaea and Bacteria Communities in Rain-Fed Maize Field in Semi-Arid Loess Plateau. Land. 2021; 10(10):1039. https://doi.org/10.3390/land10101039
Chicago/Turabian StyleFudjoe, Setor Kwami, Lingling Li, Yuji Jiang, Benjamin Karikari, Junhong Xie, Linlin Wang, Sumera Anwar, and Jinbin Wang. 2021. "Soil Amendments Alter Ammonia-Oxidizing Archaea and Bacteria Communities in Rain-Fed Maize Field in Semi-Arid Loess Plateau" Land 10, no. 10: 1039. https://doi.org/10.3390/land10101039







