Pseudomonas aeruginosa Psl Exopolysaccharide Interacts with the Antimicrobial Peptide LG21
Abstract
1. Introduction
2. Results and Discussion
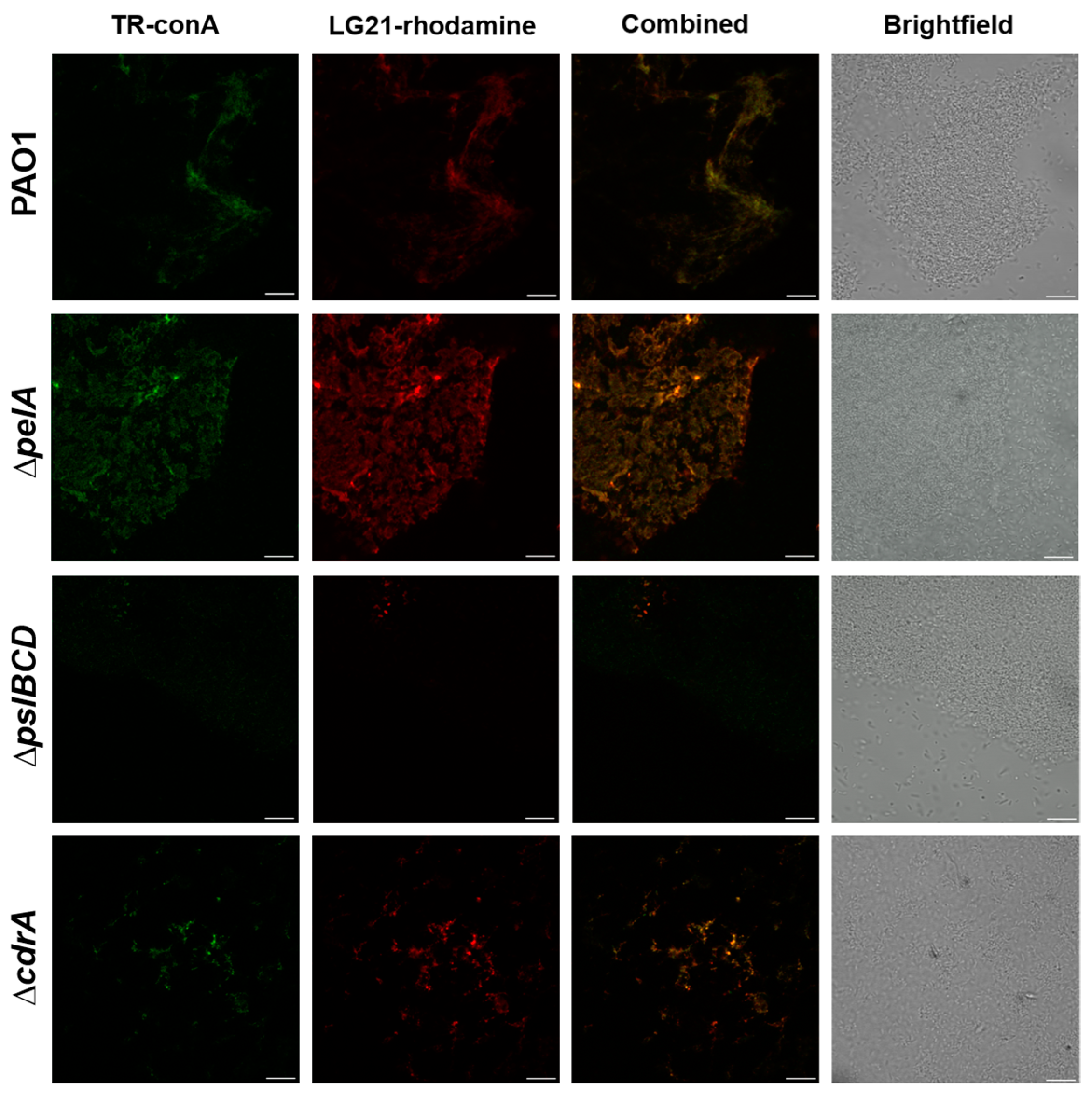
2.1. LG21 Stains Psl Positive P. Aeruginosa Biofilms
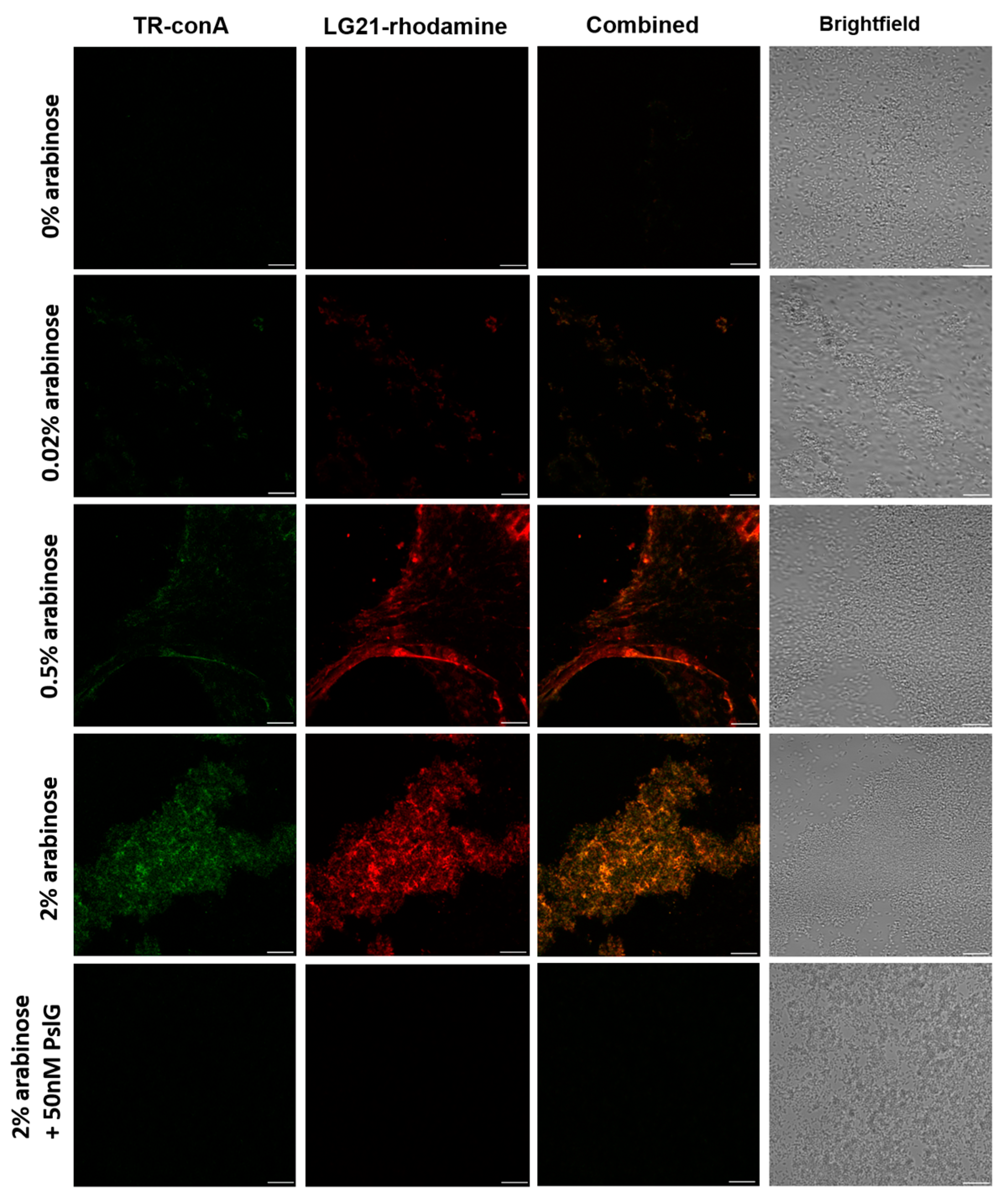
2.2. PslG Treatment Abolishes Binding of LG21 to Psl+ Biofilms
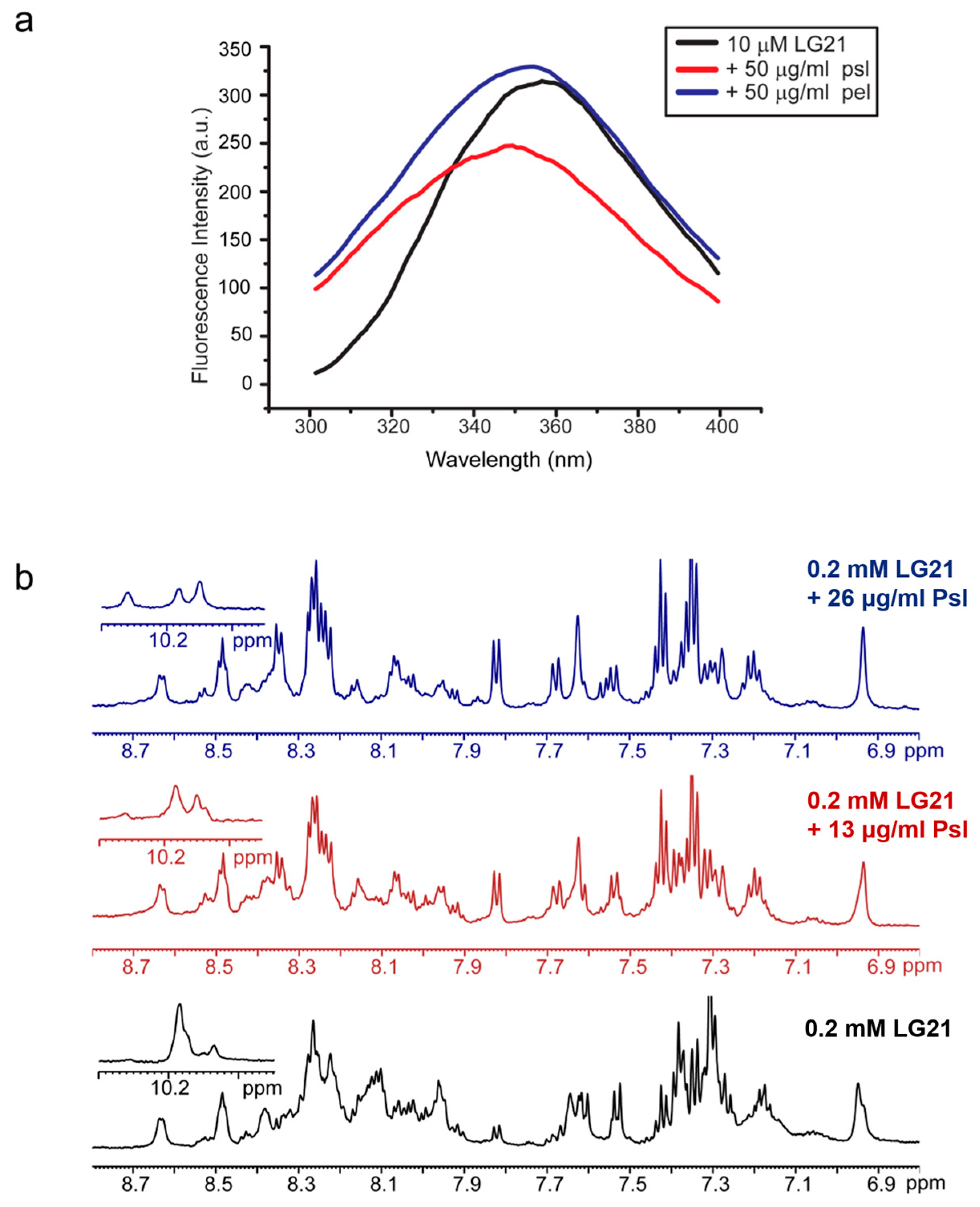
2.3. LG21 Interacts with Crude Extracted Psl Exopolysaccharide
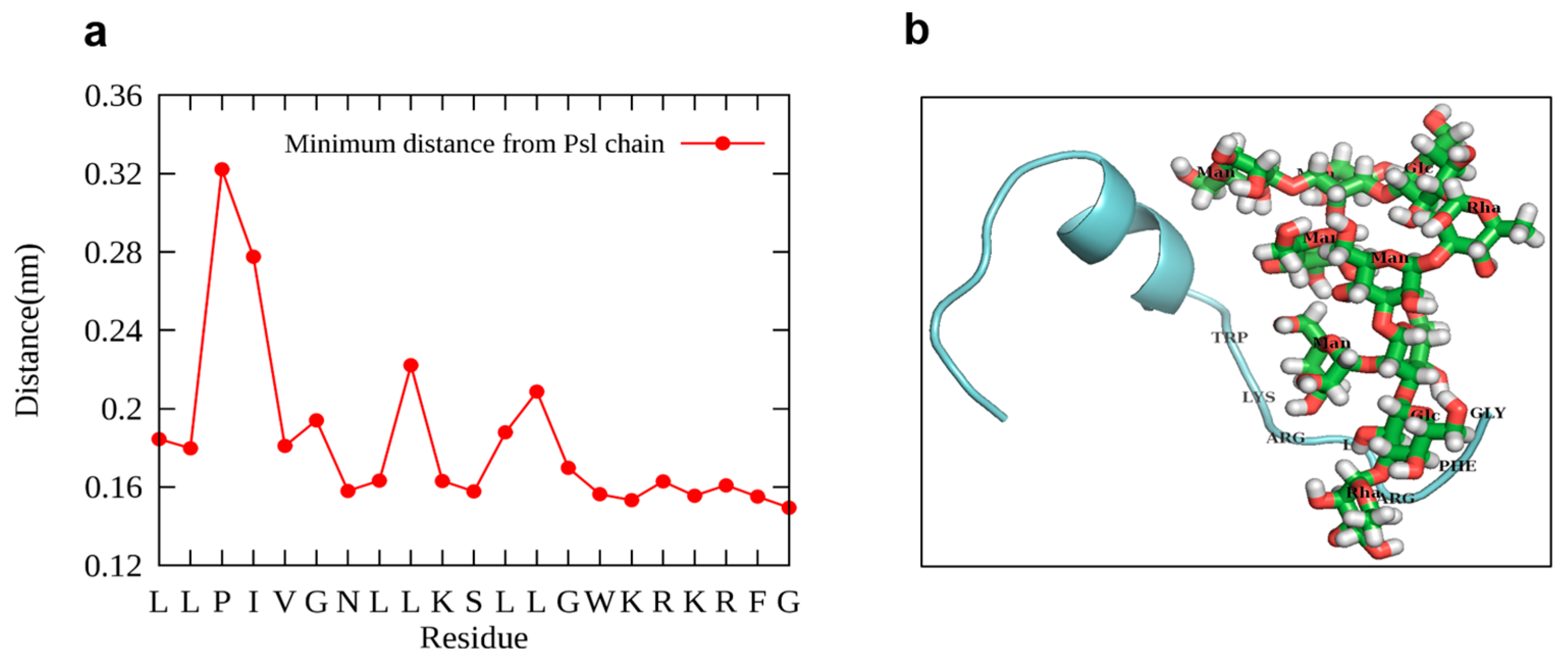
2.4. Molecular Dynamics (MD) Simulation of Psl-LG21 Binding Mode
3. Conclusions
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, Media and Growth Conditions
4.2. Screening of Psl-Binding AMPs
4.3. Binding of LG21 to Psl Overproducing Strain before and after PslG Treatment
4.4. Crude Extraction of Psl and Pel Exopolysaccharides from P. Aeruginosa
4.5. Tryptophan Fluorescence Spectroscopy
4.6. NMR Analysis
4.7. Molecular Dynamics (MD) Simulation of the Interaction between LG21 and Psl Chain
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, Y.; Shai, Y. Lipopolysaccharide (endotoxin)-host defense antibacterial peptides interactions: Role in bacterial resistance and prevention of sepsis. Biochim. Biophys. Acta 2006, 1758, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Ye, Y.; Kozlowska, J.; Lam, J.K.; Drake, A.F.; Mason, A.J. Structural contributions to the intracellular targeting strategies of antimicrobial peptides. Biochim. Biophys. Acta 2010, 1798, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Alalwani, S.M.; Sierigk, J.; Herr, C.; Pinkenburg, O.; Gallo, R.; Vogelmeier, C.; Bals, R. The antimicrobial peptide ll-37 modulates the inflammatory and host defense response of human neutrophils. Eur. J. Immunol. 2010, 40, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.W.; Sanowar, S.; Daley, M.E.; Schneider, A.R.; Cho, U.; Xu, W.; Klevit, R.E.; Le Moual, H.; Miller, S.I. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 2005, 122, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lai, Y.; Villaruz, A.E.; Cha, D.J.; Sturdevant, D.E.; Otto, M. Gram-positive three-component antimicrobial peptide-sensing system. Proc. Natl. Acad. Sci. USA 2007, 104, 9469–9474. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Jenssen, H.; Bains, M.; Wiegand, I.; Gooderham, W.J.; Hancock, R.E. The two-component system cprrs senses cationic peptides and triggers adaptive resistance in pseudomonas aeruginosa independently of parrs. Antimicrob. Agents Chemother. 2012, 56, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Thwaite, J.E.; Hibbs, S.; Titball, R.W.; Atkins, T.P. Proteolytic degradation of human antimicrobial peptide ll-37 by bacillus anthracis may contribute to virulence. Antimicrob. Agents Chemother. 2006, 50, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Ulvatne, H.; Haukland, H.H.; Samuelsen, O.; Kramer, M.; Vorland, L.H. Proteases in Escherichia coli and Staphylococcus aureus confer reduced susceptibility to lactoferricin b. J. Antimicrob. Chemother. 2002, 50, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.L.; Yam, J.K.; Hao, P.; Adav, S.S.; Salido, M.M.; Liu, Y.; Givskov, M.; Sze, S.K.; Tolker-Nielsen, T.; Yang, L. Selective labelling and eradication of antibiotic-tolerant bacterial populations in pseudomonas aeruginosa biofilms. Nat. Commun. 2016, 7, 10750. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Seviour, T.; Hansen, S.H.; Yang, L.; Yau, Y.H.; Wang, V.B.; Stenvang, M.R.; Christiansen, G.; Marsili, E.; Givskov, M.; Chen, Y.; et al. Functional amyloids keep quorum-sensing molecules in check. J. Biol. Chem. 2015, 290, 6457–6469. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, M.; Mohanty, A.; Yam, J.K.; Liu, Y.; Chua, S.L.; Nielsen, T.E.; Tolker-Nielsen, T.; Givskov, M.; Cao, B.; et al. Multiple diguanylate cyclase-coordinated regulation of pyoverdine synthesis in pseudomonas aeruginosa. Environ. Microbiol. Rep. 2015, 7, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.A.; Brody, S.L.; Kollef, M.H. The epidemiology, pathogenesis and treatment of pseudomonas aeruginosa infections. Drugs 2007, 67, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Tolker-Nielsen, T. Pseudomonas aeruginosa biofilm infections: From molecular biofilm biology to new treatment possibilities. APMIS 2014, 122, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hu, Y.; Liu, Y.; Zhang, J.; Ulstrup, J.; Molin, S. Distinct roles of extracellular polymeric substances in pseudomonas aeruginosa biofilm development. Environ. Microbiol. 2011, 13, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Kundukad, B.; Seviour, T.; van der Maarel, J.R.; Yang, L.; Rice, S.A.; Doyle, P.; Kjelleberg, S. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. MBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and development of the pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009, 5, e1000354. [Google Scholar] [CrossRef] [PubMed]
- Haussler, S.; Tummler, B.; Weissbrodt, H.; Rohde, M.; Steinmetz, I. Small-colony variants of pseudomonas aeruginosa in cystic fibrosis. Clin. Infect. Dis. 1999, 29, 621–625. [Google Scholar] [PubMed]
- Haussler, S.; Ziegler, I.; Lottel, A.; von Gotz, F.; Rohde, M.; Wehmhohner, D.; Saravanamuthu, S.; Tummler, B.; Steinmetz, I. Highly adherent small-colony variants of pseudomonas aeruginosa in cystic fibrosis lung infection. J. Med. Microbiol. 2003, 52, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Byrd, M.S.; Sergeant, S.; Azad, A.K.; Parsek, M.R.; McPhail, L.; Schlesinger, L.S.; Wozniak, D.J. Pseudomonas aeruginosa psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell. Microbiol. 2012, 14, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Byrd, M.S.; Sadovskaya, I.; Vinogradov, E.; Lu, H.; Sprinkle, A.B.; Richardson, S.H.; Ma, L.; Ralston, B.; Parsek, M.R.; Anderson, E.M.; et al. Genetic and biochemical analyses of the pseudomonas aeruginosa psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in psl and lps production. Mol. Microbiol. 2009, 73, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Kocharova, N.A.; Knirel, Y.A.; Shashkov, A.S.; Kochetkov, N.K.; Pier, G.B. Structure of an extracellular cross-reactive polysaccharide from pseudomonas aeruginosa immunotype 4. J. Biol. Chem. 1988, 263, 11291–11295. [Google Scholar] [PubMed]
- Tran, C.S.; Rangel, S.M.; Almblad, H.; Kierbel, A.; Givskov, M.; Tolker-Nielsen, T.; Hauser, A.R.; Engel, J.N. The pseudomonas aeruginosa type iii translocon is required for biofilm formation at the epithelial barrier. PLoS Pathog. 2014, 10, e1004479. [Google Scholar] [CrossRef] [PubMed]
- Borlee, B.R.; Goldman, A.D.; Murakami, K.; Samudrala, R.; Wozniak, D.J.; Parsek, M.R. Pseudomonas aeruginosa uses a cyclic-di-gmp-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 2010, 75, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Su, T.; Wu, H.; Liu, S.; Wang, D.; Zhao, T.; Jin, Z.; Du, W.; Zhu, M.J.; Chua, S.L.; et al. Pslg, a self-produced glycosyl hydrolase, triggers biofilm disassembly by disrupting exopolysaccharide matrix. Cell Res. 2015, 25, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
- Janado, M.; Yano, Y. Hydrophobic nature of sugars as evidenced by their differential affinity for polystyrene gel in aqueous-media. J. Solut. Chem. 1985, 14, 891–902. [Google Scholar] [CrossRef]
- Mohanram, H.; Bhattacharjya, S. Resurrecting inactive antimicrobial peptides from the lipopolysaccharide trap. Antimicrob. Agents Chemother. 2014, 58, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Mohanram, H.; Bhattacharjya, S. ‘Lollipop’-shaped helical structure of a hybrid antimicrobial peptide of temporin b-lipopolysaccharide binding motif and mapping cationic residues in antibacterial activity. Biochim. Biophys. Acta 2016, 1860, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Burrows, L.L.; Deber, C.M. Helix induction in antimicrobial peptides by alginate in biofilms. J. Biol. Chem. 2004, 279, 38749–38754. [Google Scholar] [CrossRef] [PubMed]
- Billings, N.; Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The extracellular matrix component psl provides fast-acting antibiotic defense in pseudomonas aeruginosa biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.L.; Ding, Y.; Liu, Y.; Cai, Z.; Zhou, J.; Swarup, S.; Drautz-Moses, D.I.; Schuster, S.C.; Kjelleberg, S.; Givskov, M.; et al. Reactive oxygen species drive evolution of pro-biofilm variants in pathogens by modulating cyclic-di-gmp levels. Open Biol. 2016, 6, 160162. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.L.; Hancock, R.E. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.L.; Liu, Y.; Yam, J.K.; Chen, Y.; Vejborg, R.M.; Tan, B.G.; Kjelleberg, S.; Tolker-Nielsen, T.; Givskov, M.; Yang, L. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat. Commun. 2014, 5, 4462. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jackson, K.D.; Landry, R.M.; Parsek, M.R.; Wozniak, D.J. Analysis of pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol. 2006, 188, 8213–8221. [Google Scholar] [CrossRef] [PubMed]
- Pronk, S.; Pall, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. Gromacs 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
| Coil | Bend | Turn | α-Helix | 3-Helix | |
|---|---|---|---|---|---|
| LG21 + Psl | 0.58 | 0.07 | 0.07 | 0.26 | 0.02 |
| LG21 only | 0.58 | 0.18 | 0.12 | 0.05 | 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, J.S.F.; Sinha, S.; Nalaparaju, A.; Yam, J.K.H.; Qin, Z.; Ma, L.; Liang, Z.-X.; Lu, L.; Bhattacharjya, S.; Yang, L. Pseudomonas aeruginosa Psl Exopolysaccharide Interacts with the Antimicrobial Peptide LG21. Water 2017, 9, 681. https://doi.org/10.3390/w9090681
Chin JSF, Sinha S, Nalaparaju A, Yam JKH, Qin Z, Ma L, Liang Z-X, Lu L, Bhattacharjya S, Yang L. Pseudomonas aeruginosa Psl Exopolysaccharide Interacts with the Antimicrobial Peptide LG21. Water. 2017; 9(9):681. https://doi.org/10.3390/w9090681
Chicago/Turabian StyleChin, Joyce Seow Fong, Sheetal Sinha, Anjaiah Nalaparaju, Joey Kuok Hoong Yam, Zhiqiang Qin, Luyan Ma, Zhao-Xun Liang, Lanyuan Lu, Surajit Bhattacharjya, and Liang Yang. 2017. "Pseudomonas aeruginosa Psl Exopolysaccharide Interacts with the Antimicrobial Peptide LG21" Water 9, no. 9: 681. https://doi.org/10.3390/w9090681
APA StyleChin, J. S. F., Sinha, S., Nalaparaju, A., Yam, J. K. H., Qin, Z., Ma, L., Liang, Z.-X., Lu, L., Bhattacharjya, S., & Yang, L. (2017). Pseudomonas aeruginosa Psl Exopolysaccharide Interacts with the Antimicrobial Peptide LG21. Water, 9(9), 681. https://doi.org/10.3390/w9090681






