Peracetic Acid (PAA) Disinfection: Inactivation of Microbial Indicators and Pathogenic Bacteria in a Municipal Wastewater Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Media
2.2. Sampling
2.3. Microbiological Analyses for Pathogen Detection
2.4. Microbiological Analyses for the Detection of Microbial Indicators
2.5. Statistical Analyses
3. Results and Discussion
Author Contributions
Conflicts of Interest
References
- Veschetti, E.; Cutilli, D.; Bonadonna, L.; Briancesco, R.; Martini, C.; Cecchini, G.; Anastasi, P.; Ottaviani, M. Pilot-plant comparative study of peracetic acid and sodium hypochlorite wastewater disinfection. Water Res. 2003, 37, 78–94. [Google Scholar] [CrossRef]
- Chhetri, R.K.; Klupsch, E.; Andersen, H.R.; Jensen, P.E. Treatment of Arctic wastewater by chemical coagulation, UV and peracetic acid disinfection Environ. Sci. Pollut. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, J.; Heinonen-Tanski, H. Peracetic acid (PAA) disinfection of primary, secondary and tertiary treated municipal wastewaters. Water Res. 2005, 39, 4445–4453. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.K.; Kauppinen, A.; Martikainen, K.; Pitkänen, T.; Kusnetsov, J.; Miettinen, I.T.; Pessi, M.; Poutiainen, H.; Heinonen-Tanski, H. Microbial reduction in wastewater treatment using Fe3+ and Al3+ coagulants and PAA disinfectant. J. Water Health 2013, 11, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Varela, A.R.; Manaia, C.M. Human health implications of clinically relevant bacteria in wastewater habitats. Environ. Sci. Pollut. Res. 2013, 20, 3550–3569. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, S.; Pignata, C.; Lorenzi, E.; De Ceglia, M.; Meucci, L.; Bonetta, S.; Gilli, G.; Carraro, E. Detection of pathogenic Campylobacter, E. coli O157:H7 and Salmonella spp. in wastewater by PCR assay. Environ. Sci. Pollut. Res. 2016, 23, 15302–15309. [Google Scholar] [CrossRef] [PubMed]
- Levantesi, C.; La Mantia, R.; Masciopinto, C.; Bockelmann, U.; Ayuso-Gabella, M.N.; Salgot, M.; Tandoi, V.; Van Houtte, E.; Wintgens, T.; Grohmann, E. Quantification of pathogenic microorganisms and microbial indicators in three wastewater reclamation and managed aquifer recharge facilities in Europe. Sci. Total Environ. 2010, 408, 4923–4930. [Google Scholar] [CrossRef] [PubMed]
- De Souza, B.J.; Valdez, F.Q.; Jeranoski, R.F.; De Sousa Vidal, C.M.; Cavallini, G.S. Water and wastewater disinfection with peracetic acid and UV radiation and using advanced oxidative process PAA/UV. Int. J. Photoenergy 2015. [Google Scholar] [CrossRef]
- Luukkonen, T.; Teeriniemi, J.; Prokkola, H.; Rämö, J.; Lassi, U. Chemical aspects of peracetic acid based wastewater disinfection. Water SA 2014, 40, 73–80. [Google Scholar] [CrossRef]
- Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- De Luca, G.; Sacchetti, R.; Zanetti, F.; Leoni, E. Comparative study on the efficiency of peracetic acid and chlorine dioxide at low doses in the disinfection of urban wastewaters. Ann. Agric. Environ. Med. 2008, 15, 217–224. [Google Scholar] [PubMed]
- Antonelli, M.; Turolla, A.; Mezzanotte, V.; Nurizzo, C. Peracetic acid for secondary effluent disinfection: A comprehensive performance assessment. Water Sci. Technol. 2013, 68, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.B.; Rodgher, S.; Daniel, L.A.; Espindola, E.L.G. Toxicity on aquatic organisms exposed to secondary effluent disinfected with chlorine, peracetic acid, ozone and UV radiation. Ecotoxicology 2014, 23, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, F.; De Luca, G.; Sacchetti, R.; Stampi, S. Disinfection Efficiency of Peracetic Acid (PAA): Inactivation of Coliphages and Bacterial Indicators in a Municipal Wastewater Plant. Environ. Technol. 2007, 28, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, S.; Borelli, E.; Bonetta, S.; Conio, O.; Palumbo, F.; Carraro, E. Development of a PCR protocol for the detection of Escherichia coli O157:H7 and Salmonella spp. in surface water. Environ. Monit. Assess. 2011, 177, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità (ISS). Metodi Analitici di Riferimento per la Valutazione Microbiologica dei Fanghi di Depurazione e di Matrici ad esso Assimilabili; Rapporti ISTISAN, 14/18; Bonadonna, L., Musmeci, L., Eds.; Istituto Superiore di Sanità: Roma, Italia, 2014. [Google Scholar]
- ISO 9308-2:2014. Water Quality. Enumeration of Escherichia coli and Coliform Bacteria. Most Probable Number Method; European Committee for Standardization: Brussels, Belgium, 2014. [Google Scholar]
- Koivunen, J.; Siitonen, A.; Heinonen-Tanski, H. Elimination of enteric bacteria in biological-chemical wastewater treatment and tertiary filtration units. Water Res. 2003, 37, 690–698. [Google Scholar] [CrossRef]
- Wery, N.; Lhoutellier, C.; Ducray, F.; Delgenes, J.; Godon, J. Behaviour of pathogenic and indicator bacteria during urban wastewater treatment and sludge composting, as revealed by quantitative PCR. Water Res. 2008, 42, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Teklehaimanot, G.Z.; Genthe, B.; Kamika, I.; Momba, M.N.B. Prevalence of enteropathogenic bacteria in treated effluents and receiving water bodies and their potential health risks. Sci. Total Environ. 2015, 518–519, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Ostoich, M.; Aimo, E.; Vazzoler, M.; Stradella, S.; Osti, P. Integrated approach for microbiological impact assessment of public wastewater treatment plants. Chem. Ecol. 2007, 23, 43–62. [Google Scholar] [CrossRef]
- Koivunen, J.; Heinonen-Tanski, H. Inactivation of enteric microorganisms with chemical disinfectants, UV irradiation and combined chemical/UV treatments. Water Res. 2005, 39, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Dumke, R.; Schroter-Bobsin, U.; Jacobs, E.; Roske, I. Detection of phages carrying the Shiga toxin 1 and 2 genes in waste water and river water samples. Lett. Appl. Microbiol. 2006, 4, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Muniesa, M.; Jofre, J.; Garcia-Aliaro, C.; Blanch, A.R. Occurrence of Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli in the environment. Environ. Sci. Technol. 2006, 40, 7141–7149. [Google Scholar] [CrossRef] [PubMed]
- James, C.E.; Stanley, K.N.; Allison, H.E.; Flint, H.J.; Stewart, C.S.; Sharp, R.J.; Saunders, J.R.; McCarthy, A.J. Lytic and lysogenic infection of diverse Escherichia coli and Shigella strains with a verocytotoxigenic bacteriophage. Appl. Environ. Microbiol. 2001, 67, 4335–4337. [Google Scholar] [CrossRef] [PubMed]
- Strauch, E.; Hammerl, J.A.; Konietzny, A.; Schneiker-Bekel, S.; Arnold, W.; Goesmann, A.; Pühler, A.; Beutin, L. Bacteriophage 2851 is a prototype phage for dissemination of the Shiga toxin variant gene 2c in Escherichia coli O157:H7. Infect. Immun. 2008, 76, 5466–5477. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Castillo, A.; Allue-Guardia, A.; Dahbi, G.; Blanco, J.; Creuzburg, K.; Schmidt, H.; Muniesa, M. Type III effector genes and other virulence factors of Shiga toxin-encoding Escherichia coli isolated from wastewater. Environ. Microbiol. Rep. 2012, 4, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, C.; Bissa, M.; Illiano, E.; Mezzanotte, V.; Marazzi, F.; Turolla, A.; Antonelli, M.; De Giuli Morghen, C.; Radaelli, A. Identification of antibiotic-resistant Escherichia coli isolated from a municipal waste water treatment plant. Chemosphere 2016, 164, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Gehr, R.; Wagner, M.; Veerasubramanian, P.; Payment, P. Disinfection efficiency of peracetic acid, UV and ozone after enhanced primary treatment of municipal wastewater. Water Res. 2003, 37, 4573–4586. [Google Scholar] [CrossRef]
- Provincia di Torino 21.04.2012. n. 572-33167. Autorizzazione allo Scarico di Acque Reflue Urbane in Corpo Idrico Superficiale/Approvazione del Piano di Prevenzione e Gestione Delle Acque di Prima Pioggia e di Lavaggio Delle aree Esterne; Provincia di Torino: Torino, Italia, 2012. [Google Scholar]
- Decreto Ministero dell’Ambiente e della Tutela del Territorio. Norme Tecniche per il Riutilizzo delle Acque Reflue, ai Sensi Dell’articolo 99, Comma 1, del Decreto Legislativo 3 Aprile 2006, n. 152; Ministero dell’Ambiente e Tutela del Territorio: Roma, Italia, 2006. [Google Scholar]
- World Health Organization (WHO). Guidelines for the Safe Use of Wastewater, Excreta and Greywater; WHO: Geneva, Swizerland, 2006; Volume 2. [Google Scholar]
- Environmental Protection Agency (EPA). Guidelines for Water Reuse; EPA/600/R-12/618; USAID: Washington, DC, USA, 2012.
- Decreto Legislativo 3.04.2006, n. 152: Norme in Materia Ambientale; GU Repubblica Italiana n. 88 del 14.04.2006; Ministero della Giustizia: Roma, Italia, 2006.
| Season | Sample | pH | TSS mg/L | BOD mg/L | COD mg/L | NH4+ mg/L | NO2− mg/L N | NO3− mg/L N | N Total mg/L | P Total mg/L |
|---|---|---|---|---|---|---|---|---|---|---|
| Summer | I | 7.7 | 122 | 162 | 341 | 34.8 | <0.025 | <1.30 | 32.4 | 4.36 |
| DE | 7.8 | <5 | <5 | <15 | 3.2 | 0.084 | <1.30 | 4.3 | 0.62 | |
| Autumn | I | 7.8 | 144 | 206 | 550 | 44.8 | <0.025 | <1.30 | 45.0 | 6.47 |
| DE | 7.9 | <5 | <5 | <15 | <2 | <0.025 | 2.69 | 3.1 | 0.25 | |
| Winter | I | 7.2 | 71 | 45 | 320 | 38.4 | 0.067 | <1.30 | 36.9 | 4.93 |
| DE | 7.4 | <5 | <5 | <15 | <2 | 0.29 | 3.43 | 4.4 | 0.31 | |
| Spring | I | 7.5 | 105 | 97 | 295 | 30.1 | <0.025 | <1.30 | 25.8 | 3.07 |
| DE | 7.5 | 10.9 | <5 | 22.9 | <2 | 0.037 | 1.6 | 3.9 | 0.36 |
| Sampling Period | Sampling Points | Cultural Analysis | Molecular Analysis | PAA Dose (mg/L) |
|---|---|---|---|---|
| Summer–July 2015 | I, E, DE | E.coli, Faecal Coliforms (FC), Enterococci, C. perfringens, Salmonella spp. | E.coli O157:H7, Shiga toxin I, Shiga toxin II, Intimin, Campylobacter spp., Campylobacter coli, Campylobacter jejuni, Salmonella spp. | 0.99 |
| Autumn–November 2015 | I, E, DE | 1.10 | ||
| Winter–January 2016 | I, E, DE | 1.06 | ||
| Spring–April 2016 | I, E, DE | 2.10 |
| Salmonella | E. coli O157:H7 | Campylobacter | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Genus | O157 | H7 | Intimin (eae) | SLT-I (stx1) | SLT-II (stx2) | Genus | C. jejuni | C. coli | |
| Summer | |||||||||
| I | + | − | + | − | + | − | − | − | − |
| E | + | − | + | − | − | − | − | − | − |
| DE | + | − | + | − | − | − | − | − | − |
| Autumn | |||||||||
| I | + | − | + | − | + | − | − | − | − |
| E | + | − | + | − | + | − | + | − | + |
| DE | − | − | + | + | − | − | − | − | − |
| Winter | |||||||||
| I | + | − | + | − | + | − | − | − | − |
| E | + | − | + | − | + | − | − | − | − |
| DE | + | − | + | − | + | − | − | − | − |
| Spring | |||||||||
| I | + | − | + | − | − | − | − | − | − |
| E | + | − | + | − | − | − | − | − | − |
| DE | − | − | + | − | − | − | − | − | − |
| Season | Sample | Faecal Coliforms MPN/100 mL | E. coli MPN/100 mL | Enterococci MPN/100 mL | C. perfringens Spore CFU/100 mL | Salmonella spp. |
|---|---|---|---|---|---|---|
| Summer | I | 9.66 × 106 | 7.91 × 106 | 1.09 × 106 | 2.61 × 105 | + |
| E | 1.35 × 104 | 8.00 × 103 | 4.36 × 103 | 1.92 × 103 | + | |
| DE | 2.84 × 103 | 2.52 × 103 | 7.56 × 102 | 1.41 × 103 | − | |
| Autumn | I | 2.10 × 107 | 8.63 × 106 | 7.95 × 105 | 1.42 × 105 | + |
| E | 2.64 × 104 | 6.27 × 103 | 1.55 × 103 | 1.68 × 103 | + | |
| DE | 5.42 × 103 | 4.09 × 102 | 8.45 × 102 | 1.84 × 103 | − | |
| Winter | I | 2.33 × 107 | 5.93 × 106 | 2.17 × 106 | 2.26 × 105 | + |
| E | 5.48 × 105 | 2.61 × 105 | 6.17 × 104 | 1.61 × 104 | + | |
| DE | 3.87 × 104 | 1.10 × 104 | 1.26 × 103 | 1.63 × 103 | + | |
| Spring | I | 2.37 × 107 | 1.05 × 107 | 1.28 × 106 | 1.77 × 105 | + |
| E | 9.20 × 103 | 8.23 × 102 | 1.21 × 103 | 7.30 × 103 | + | |
| DE | 2.95 × 102 | 1.21 × 101 | 5.20 | 1.84 × 103 | − |
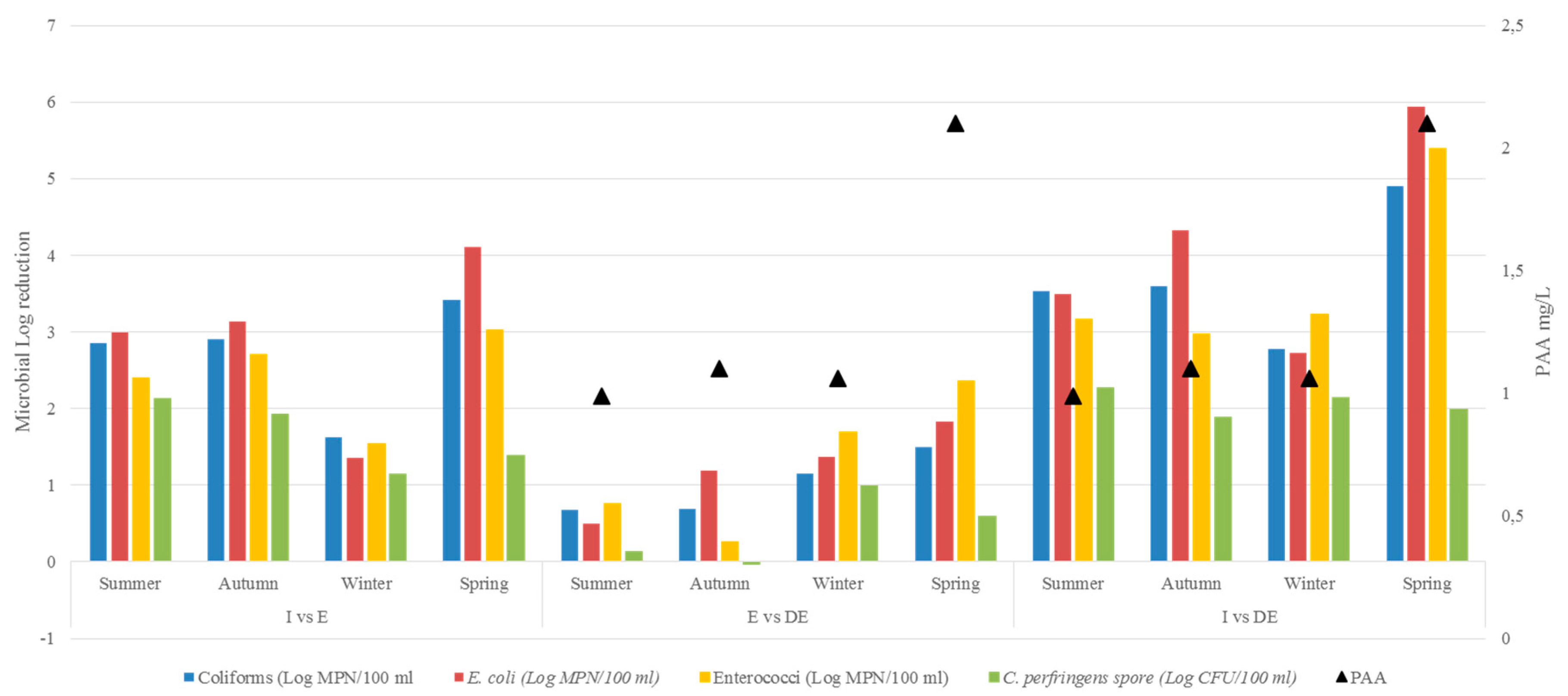
| Indicators | ANOVA p | Mean Abatement (Log) | Post-Hoc p | |
|---|---|---|---|---|
| Faecal coliform | 0.0005 | I vs E | 2.670 | 0.001 |
| I vs DE | 3.701 | 0.0005 | ||
| E vs DE | 1.001 | 0.158 | ||
| E. coli | 0.001 | I vs E | 2.899 | 0.005 |
| I vs DE | 4.123 | 0.0005 | ||
| E vs DE | 1.224 | 0.218 | ||
| Enterococci | 0.0005 | I vs E | 2.420 | 0.005 |
| I vs DE | 3.690 | 0.0005 | ||
| E vs DE | 1.270 | 0.117 | ||
| C. perfringens (spore) | 0.0005 | I vs E | 1.648 | 0.0005 |
| I vs DE | 2.070 | 0.0005 | ||
| E vs DE | 0.422 | 0.142 |
| Effluent Before PAA Disinfection | PAA-Treated Effluents | Reference | |
|---|---|---|---|
| Irrigation reuse | |||
| Italy, Ministry Decree 2 May 2006 a | [31] | ||
| % of sample <10 E. coli/100 mL | 0 (0/4) | 0 (0/4) | |
| % of sample <100 E. coli/100 mL | 0 (0/4) | 25 (1/4) | |
| % of sample Salmonella absent | 0 (0/4) | 75 (3/4) | |
| WHO, 2006 | [32] | ||
| % of sample <1000 FC/100 mL | 0 (0/4) | 0 (0/4) | |
| EPA, 2012 | [33] | ||
| % of sample with 0 FC/100 mL | 0 (0/4) | 0 (0/4) | |
| Discharge into surface waters | |||
| Italy, Legislative Decree n. 152/2006 | [34] | ||
| % of sample <5000 E. coli/100 mL | 0 (0/4) | 50 (2/4) | |
| Italy, Autorization Province Torino, 2012 | [30] | ||
| % of sample <11,000 E. coli/100 mL | 75 (3/4) | 100 (4/4) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetta, S.; Pignata, C.; Lorenzi, E.; De Ceglia, M.; Meucci, L.; Bonetta, S.; Gilli, G.; Carraro, E. Peracetic Acid (PAA) Disinfection: Inactivation of Microbial Indicators and Pathogenic Bacteria in a Municipal Wastewater Plant. Water 2017, 9, 427. https://doi.org/10.3390/w9060427
Bonetta S, Pignata C, Lorenzi E, De Ceglia M, Meucci L, Bonetta S, Gilli G, Carraro E. Peracetic Acid (PAA) Disinfection: Inactivation of Microbial Indicators and Pathogenic Bacteria in a Municipal Wastewater Plant. Water. 2017; 9(6):427. https://doi.org/10.3390/w9060427
Chicago/Turabian StyleBonetta, Silvia, Cristina Pignata, Eugenio Lorenzi, Margherita De Ceglia, Lorenza Meucci, Sara Bonetta, Giorgio Gilli, and Elisabetta Carraro. 2017. "Peracetic Acid (PAA) Disinfection: Inactivation of Microbial Indicators and Pathogenic Bacteria in a Municipal Wastewater Plant" Water 9, no. 6: 427. https://doi.org/10.3390/w9060427
APA StyleBonetta, S., Pignata, C., Lorenzi, E., De Ceglia, M., Meucci, L., Bonetta, S., Gilli, G., & Carraro, E. (2017). Peracetic Acid (PAA) Disinfection: Inactivation of Microbial Indicators and Pathogenic Bacteria in a Municipal Wastewater Plant. Water, 9(6), 427. https://doi.org/10.3390/w9060427









