Effects of Climate Change on 2-Methylisoborneol Production in Two Cyanobacterial Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Cell Culture
2.2. Odorant Quantification
2.3. Experimental Procedures
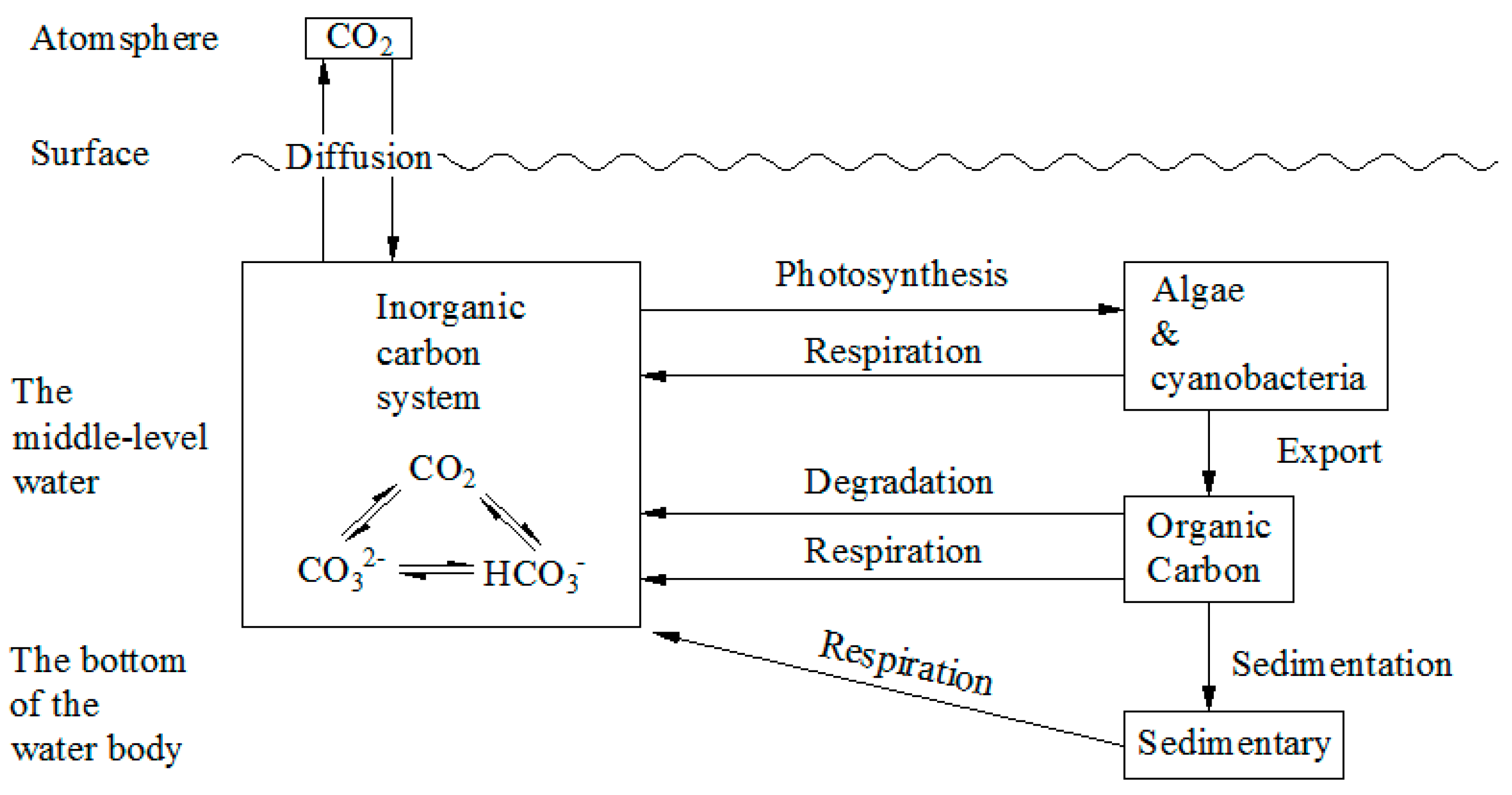
2.3.1. The Technical Pathway
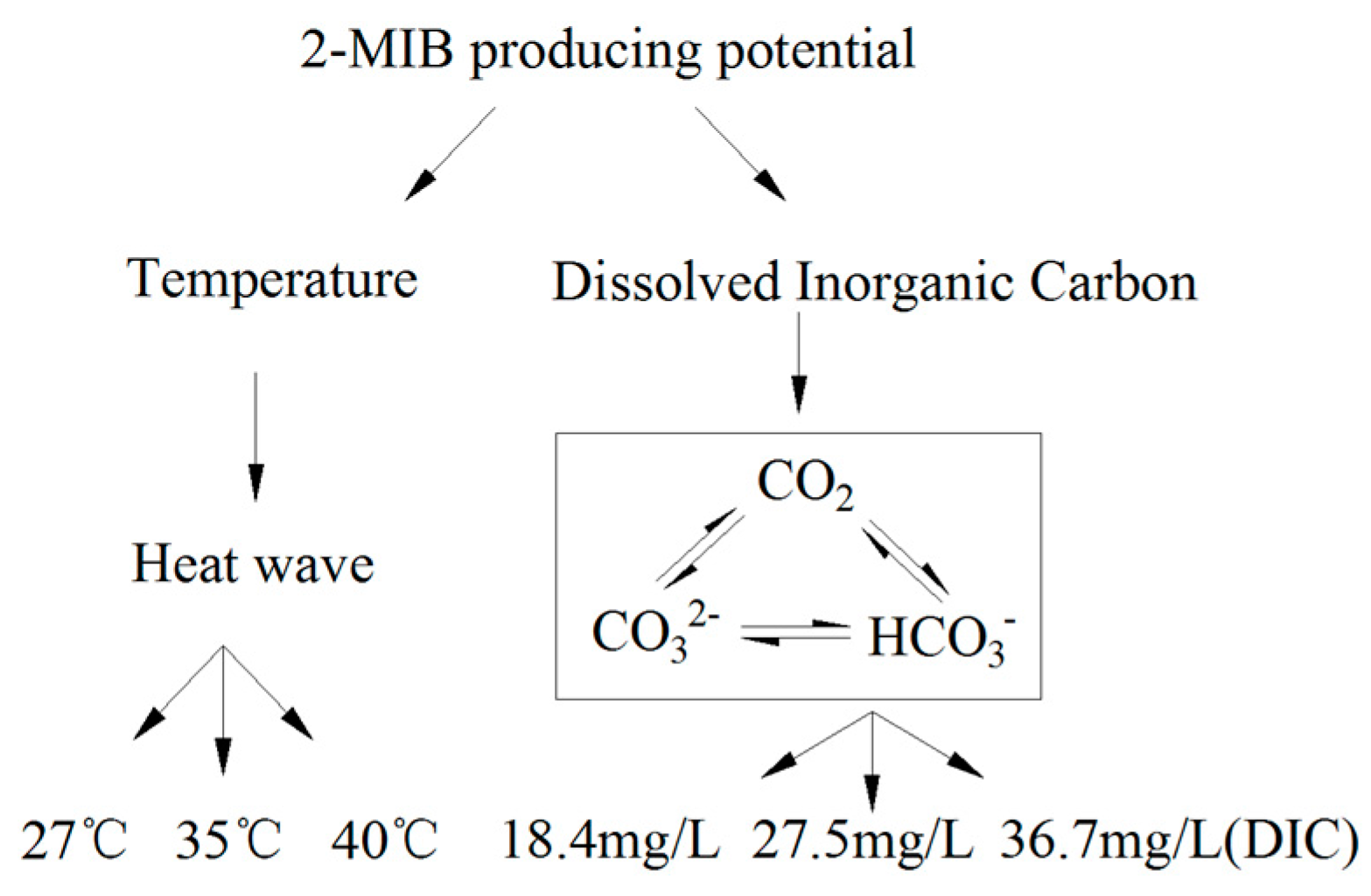
2.3.2. Culture Design
2.3.3. Determination
2.3.4. Statistical Analysis
3. Results
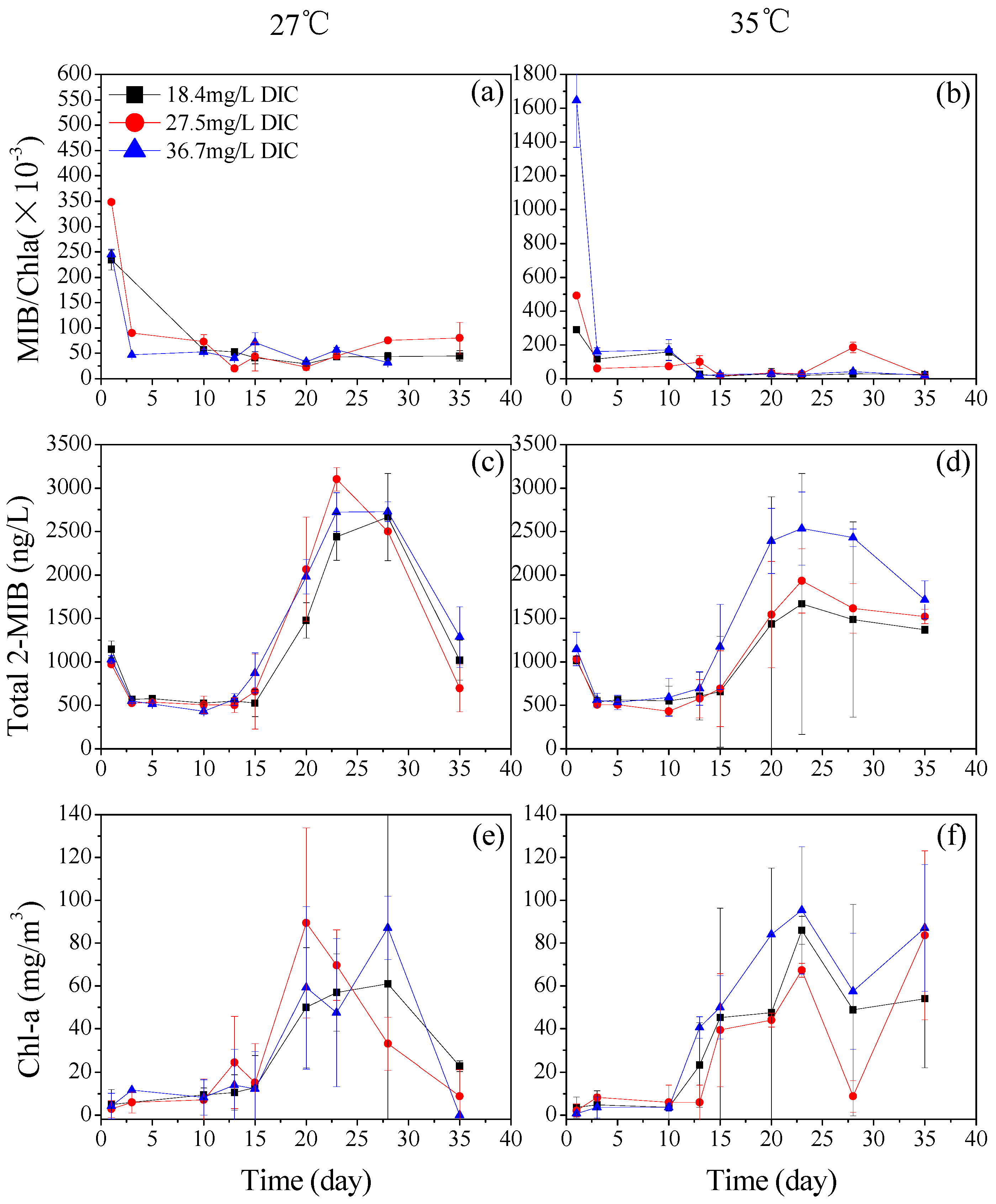
3.1. The Effect of DIC on the Production of 2-MIB by Planktothrix sp.
3.2. The Effects of Temperature on Dolichospermum spiroides and Planktothrix sp.
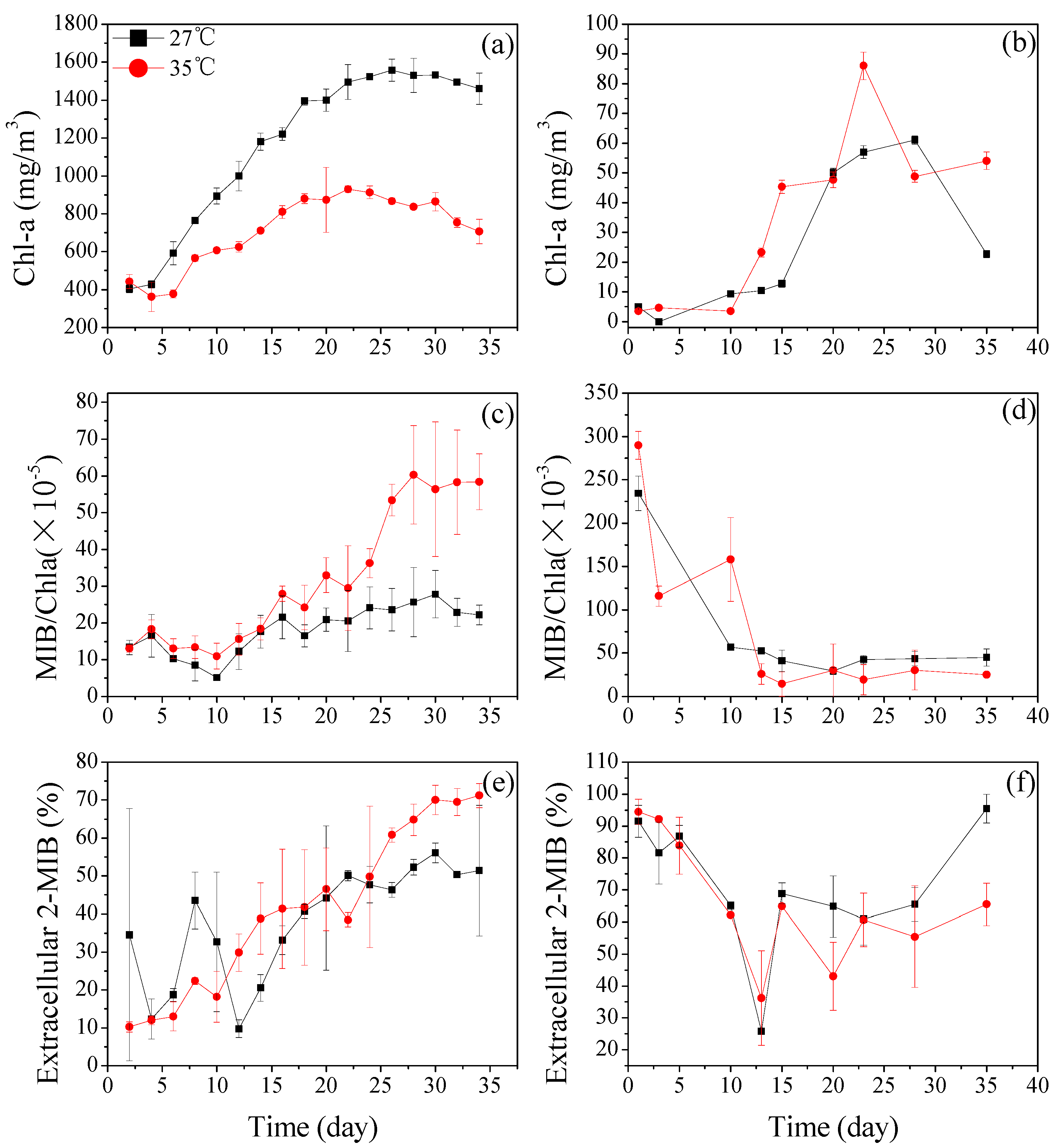
3.2.1. Dolichospermum spiroides and Planktothrix sp. Cell Growth at Different Temperatures
3.2.2. The 2-MIB-Producing Potentials of Dolichospermum spiroides and Planktothrix sp. Under Different Temperature Conditions
3.2.3. The Extracellular 2-MIB Portion of Dolichospermum spiroides and Planktothrix sp.
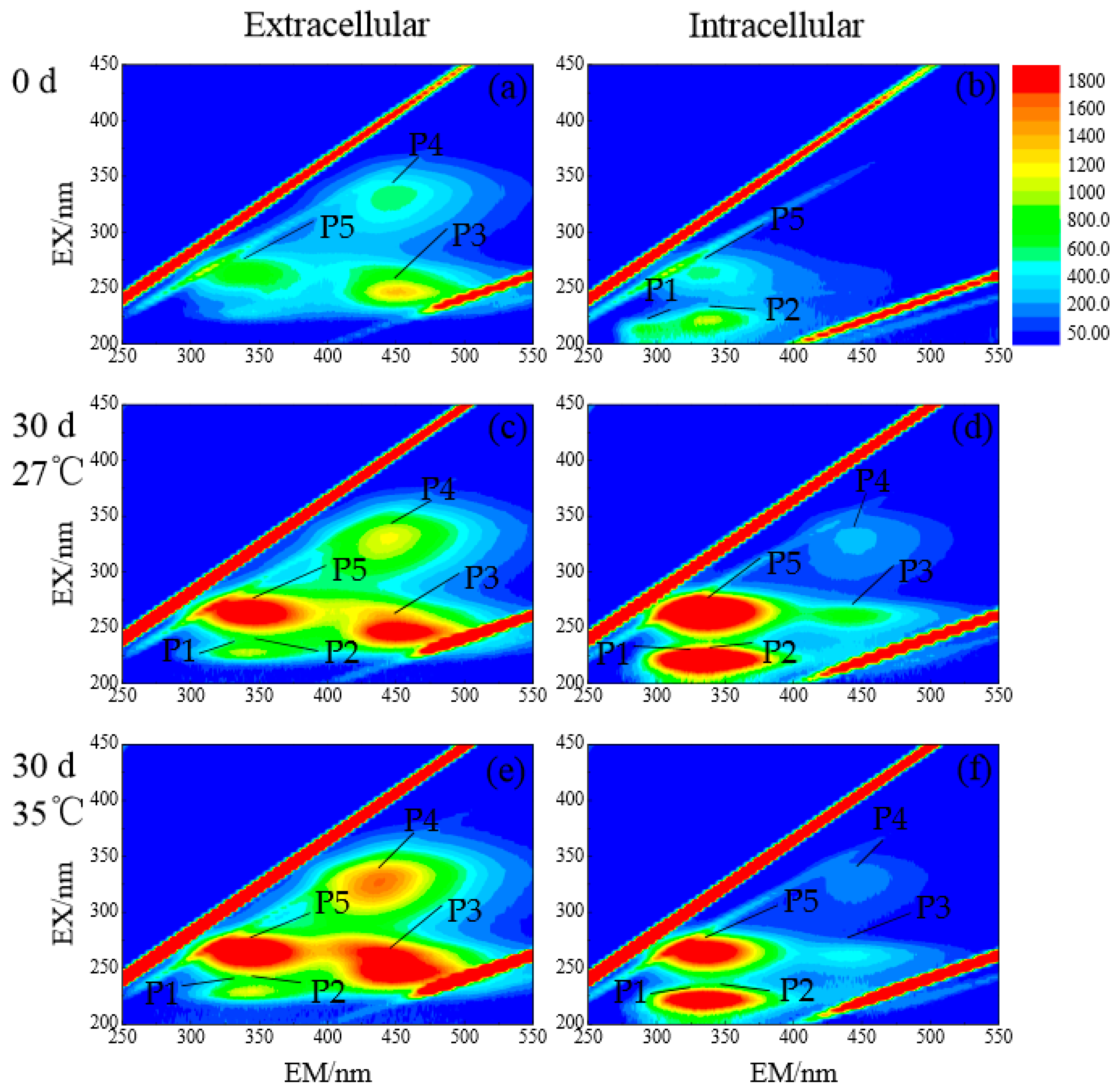
3.2.4. DOM Composition of Dolichospermum spiroides at Different Temperatures
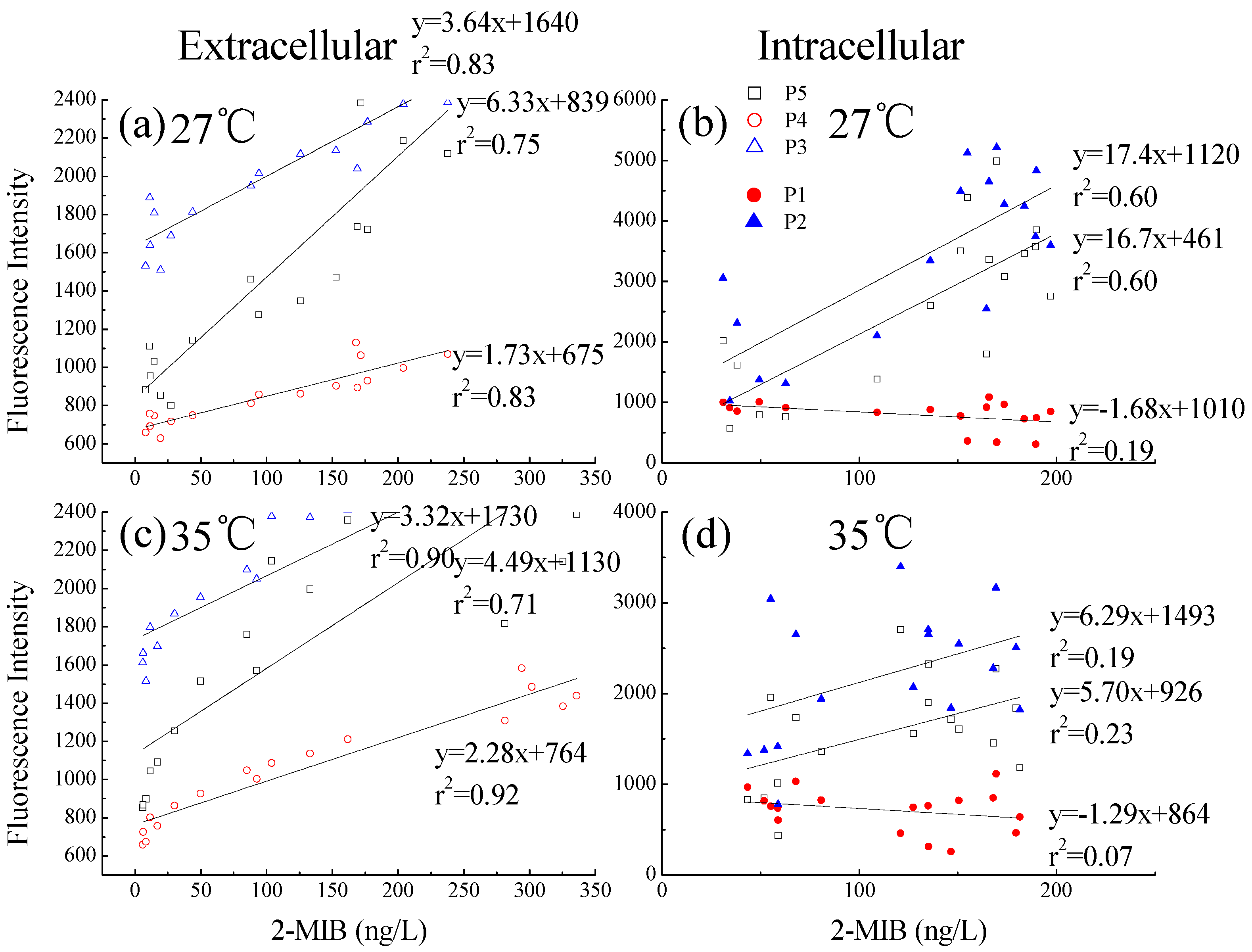
3.2.5. Relationship between Dolichospermum spiroides 2-MIB, and Intra- and Extracellular DOMs
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Working Group I Contribution to the IPCC Fifth Assessment Report Climate Change 2013: The Physical Science Basis; IPCC: Geneva, Switzerland, 2013. [Google Scholar]
- Shen, Y.; Guoya, W. Key findings and assessment results of IPCC Work Group I fifth assessment report. J. Glaciol. Geocryol. 2013, 35, 1068–1076. [Google Scholar]
- Raven, J.; Beardall, J. CO2 concentrating mechanisms and environmental change. Aquat. Bot. 2014, 118, 24–37. [Google Scholar] [CrossRef]
- Cao, L.; Caldeira, K. Atmospheric CO2 stabilization and ocean acidification. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Krüger, G.H.J.; Eloff, J.N. Effect of CO2 and HCO3− on Photosynthetic Oxygen Evolution by Microcystis aeruginosa. Z. Pflanzenphysiol. 1983, 112, 231–236. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Yu, L.; Kong, F.; Shi, X.; Yang, Z.; Zhang, M.; Yu, Y. Effects of elevated CO2 on dynamics of microcystin-producing and non-microcystin-producing strains during Microcystis blooms. J. Environ. Sci. 2015, 27, 251–258. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Winck, F.V.; Melo, D.O.P.; Barrios, A.F.G. Carbon acquisition and accumulation in microalgae Chlamydomonas: Insights from “omics” approaches. J. Proteom. 2013, 94, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Andersen, F.Ø. Effects of CO2 concentration on growth of filamentous algae and Littorella uniflora in a Danish softwater lake. Aquat. Bot. 2006, 84, 267–271. [Google Scholar] [CrossRef]
- Rost, B.; Riebesell, U.; Sültemeyer, D. Carbon acquisition of marine phytoplankton: Effect of the photoperiodic length. Limnol. Oceanogr. 2006, 51, 12–20. [Google Scholar] [CrossRef]
- Francis, G. Poisonous Australian Lake. Nature 1878, 18, 11–12. [Google Scholar] [CrossRef]
- Gkelis, S.; Papadimitriou, T.; Zaoutsos, N.; Leonardos, I. Anthropogenic and climate-induced change favors toxic cyanobacteria blooms: Evidence from monitoring a highly eutrophic, urban Mediterranean lake. Harmful Algae 2014, 39, 322–333. [Google Scholar] [CrossRef]
- Dzialowski, A.R.; Smith, V.H.; Huggins, D.G.; deNoyelles, F.; Lim, N.-C.; Baker, D.S.; Beury, J.H. Development of predictive models for geosmin-related taste and odor in Kansas, USA, drinking water reservoirs. Water Res. 2009, 43, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.K.; Chislock, M.F.; Wilson, A.E. Eutrophication mediates a common off-flavor compound, 2-methylisoborneol, in a drinking water reservoir. Water Res. 2016, 92, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Watson, S.; Charlton, M.; Rao, Y.; Howell, T.; Ridal, J.; Brownlee, B.; Marvin, C.; Millard, S. Off flavours in large waterbodies: Physics, chemistry and biology in synchrony. Water Sci. Technol. 2007, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yu, J.; Zhang, J.; Chen, H.; An, W.; Vogt, R.D.; Andersen, T.; Jia, D.; Wang, J.; Yang, M. MIB-producing cyanobacteria (Planktothrix sp.) in a drinking water reservoir: Distribution and odor producing potential. Water Res. 2015, 68, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, Y.; Tuji, A.; Takemoto, K.; Ichise, S. Pseudanabaena foetida sp. nov. and P. subfoetida sp. nov.(Cyanophyta/Cya-nobacteria) producing 2-methylisoborneol from Japan. Fottea 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, L.; Li, L.; Song, L. 2-Methylisoborneol production characteristics of Pseudanabaena sp. FACHB 1277 isolated from Xionghe Reservoir, China. J. Appl. Phycol. 2016, 28, 3353–3362. [Google Scholar] [CrossRef]
- Chou, W.K.W.; Gould, C.A.; Cane, D.E. Incubation of 2-methylisoborneol synthase with the intermediate analog 2-methylneryl diphosphate. J. Antibiot. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dickschat, J.S.; Nawrath, T.; Thiel, V.; Kunze, B.; Müller, R.; Schulz, S. Biosynthesis of the Off-Flavor 2-Methylisoborneol by the Myxobacterium Nannocystis exedens. Angew. Chem. Int. Ed. 2007, 46, 8287–8290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, R. Effects of light and temperature on the odor production of 2-methylisoborneol-producing Pseudanabaena sp. and geosmin-producing Anabaena ucrainica (cyanobacteria). Biochem. Syst. Ecol. 2015, 58, 219–226. [Google Scholar] [CrossRef]
- Lin, T.-F.; Wong, J.-Y.; Kao, H.-P. Correlation of musty odor and 2-MIB in two drinking water treatment plants in South Taiwan. Sci. Total Environ. 2002, 289, 225–235. [Google Scholar] [CrossRef]
- Wert, E.C.; Korak, J.A.; Trenholm, R.A.; Rosario-Ortiz, F.L. Effect of oxidant exposure on the release of intracellular microcystin, MIB, and geosmin from three cyanobacteria species. Water Res. 2014, 52, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, D.M.; Wang, P.; Cui, F.Y. Formation of THMs, Microcystin-LR and Odors from Microcystis aeruginosa-Derived Organic Matter. Adv. Mater. Res. 2014, 1051, 348–352. [Google Scholar] [CrossRef]
- Ho, L.; Newcombe, G.; Croué, J.-P. Influence of the character of NOM on the ozonation of MIB and geosmin. Water Res. 2002, 36, 511–518. [Google Scholar] [CrossRef]
- Li, L.; Gao, N.; Deng, Y.; Yao, J.; Zhang, K. Characterization of intracellular & extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor & taste compounds. Water Res. 2012, 46, 1233–1240. [Google Scholar] [PubMed]
- Ulu, F.; Barışçı, S.; Kobya, M.; Sillanpää, M. An evaluation on different origins of natural organic matters using various anodes by electrocoagulation. Chemosphere 2015, 125, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Her, N.; Amy, G.; McKnight, D.; Sohn, J.; Yoon, Y. Characterization of DOM as a function of MW by fluorescence EEM and HPLC-SEC using UVA, DOC, and fluorescence detection. Water Res. 2003, 37, 4295–4303. [Google Scholar] [CrossRef]
- Tang, Z.; Yu, G.; Liu, D.; Xu, D.; Shen, Q. Different analysis techniques for fluorescence excitation–emission matrix spectroscopy to assess compost maturity. Chemosphere 2011, 82, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Dong, B. Study on the Characteristics of Algogenic Organnic Metters. Environ. Sci. Technol. 2016, 39, 144–149. [Google Scholar]
- Ghoshal, D.; Husic, H.D.; Goyal, A. Dissolved inorganic carbon concentration mechanism in Chlamydomonas moewusii. Plant Physiol. Biochem. 2002, 40, 299–305. [Google Scholar] [CrossRef]
- Visser, P.M.; Verspagen, J.M.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, L.; Qiu, L.; Wang, X.; Meng, X.; You, Y.; Yu, J.; Ma, W. Effects of Climate Change on 2-Methylisoborneol Production in Two Cyanobacterial Species. Water 2017, 9, 859. https://doi.org/10.3390/w9110859
Zhang J, Li L, Qiu L, Wang X, Meng X, You Y, Yu J, Ma W. Effects of Climate Change on 2-Methylisoborneol Production in Two Cyanobacterial Species. Water. 2017; 9(11):859. https://doi.org/10.3390/w9110859
Chicago/Turabian StyleZhang, Junzhi, Luwei Li, Lijia Qiu, Xiaoting Wang, Xuanyi Meng, Yu You, Jianwei Yu, and Wenlin Ma. 2017. "Effects of Climate Change on 2-Methylisoborneol Production in Two Cyanobacterial Species" Water 9, no. 11: 859. https://doi.org/10.3390/w9110859
APA StyleZhang, J., Li, L., Qiu, L., Wang, X., Meng, X., You, Y., Yu, J., & Ma, W. (2017). Effects of Climate Change on 2-Methylisoborneol Production in Two Cyanobacterial Species. Water, 9(11), 859. https://doi.org/10.3390/w9110859




