Effects of Misgurnus anguillicaudatus and Cipangopaludina cathayensis on Pollutant Removal and Microbial Community in Constructed Wetlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Benthic Animal Selection
2.2. CWs Microcosm Set-Up
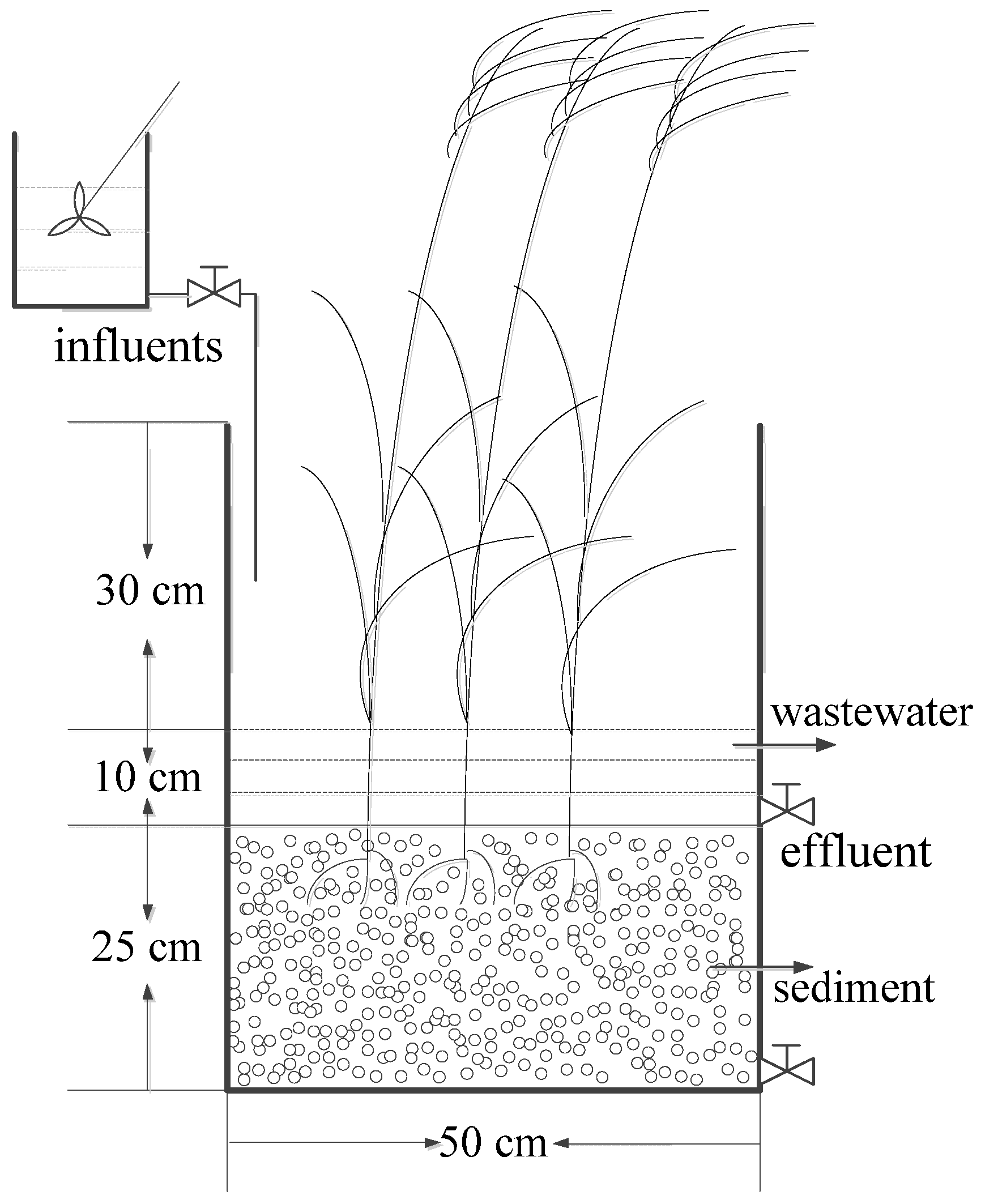
| Parameter | Mean Concentration | Standard Deviation |
|---|---|---|
| COD (mg/L) | 61.21 | 3.24 |
| TN (mg/L) | 21.53 | 1.12 |
| NH4-N (mg/L) | 7.94 | 0.32 |
| TP (mg/L) | 1.22 | 0.14 |
| DO (mg/L) | 7.15 | 1.35 |
| pH | 7.56 | 0.31 |
2.3. Experimental Design and Sampling
| Group | Benthic Animals | Density (g/m2) |
|---|---|---|
| Control | - | - |
| Snail | M. anguillicaudatus | 149.43 ± 3.34 |
| Loach | C. cathayensis | 150.62 ± 5.42 |
| Mixture | M. anguillicaudatus | 75.21 ± 2.31 |
| C. cathayensis | 76.13 ± 3.53 |
2.4. DNA Extraction and Pyrosequencing
2.5. Statistical Analysis
3. Results and Discussion
3.1. Pollutant Removal in Water
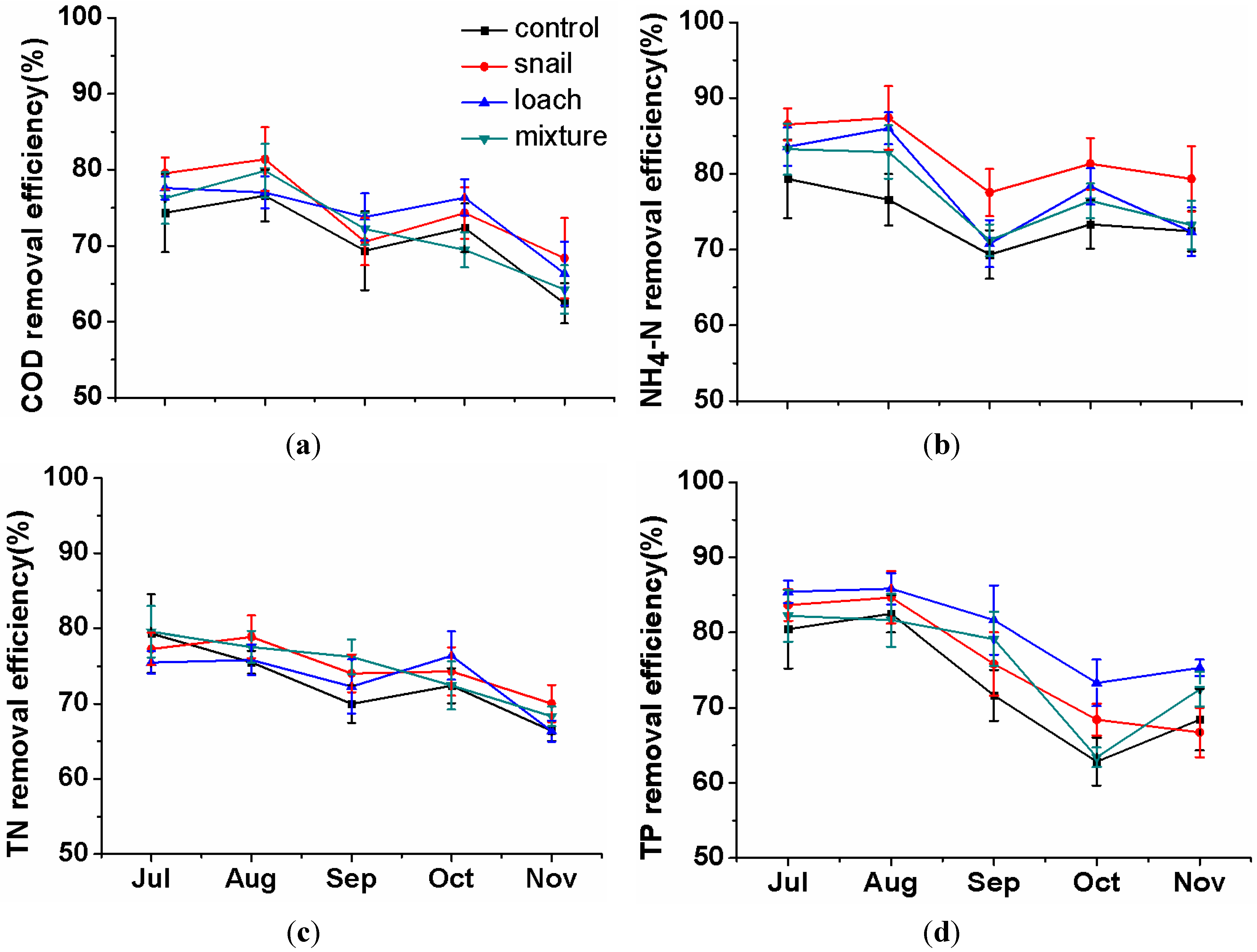

3.2. Nutrient Changes in the Sediment
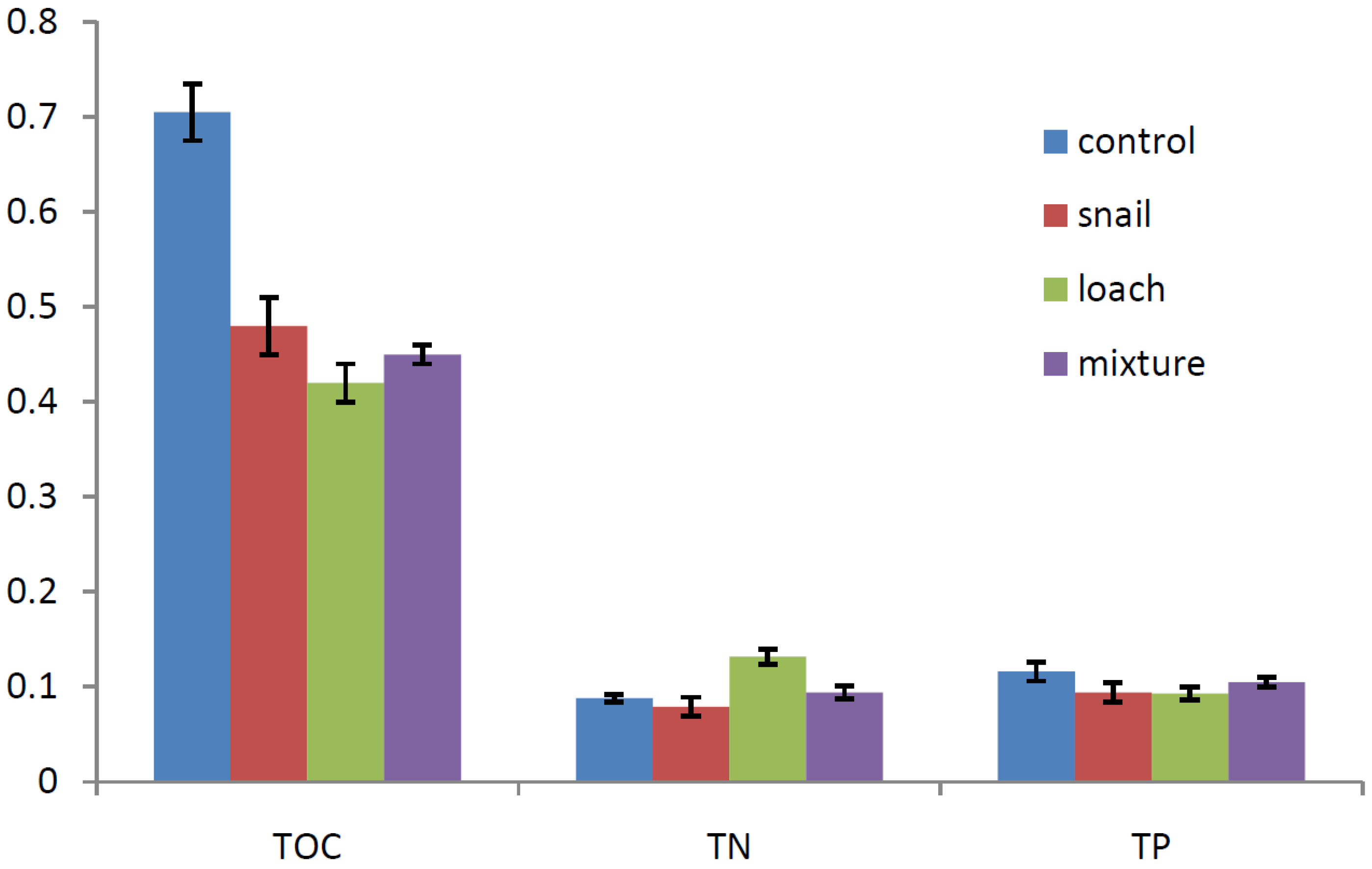
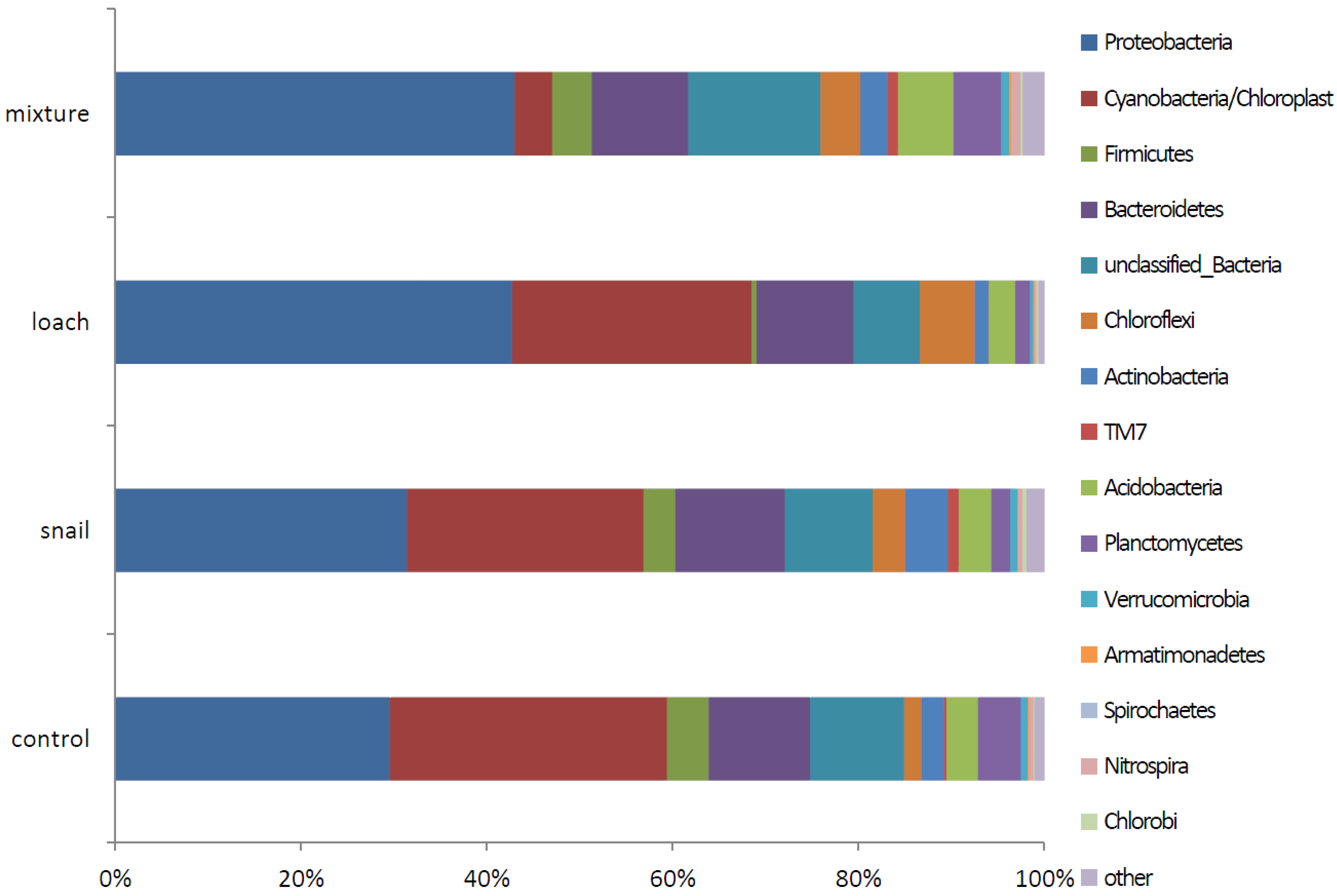
3.3. Microbial Communities
| Group | Cutoff | OTUs | ACE | Chao 1 | Shannon | Simpson (×10−3) | Coverage (%) |
|---|---|---|---|---|---|---|---|
| control | 0.03 | 4,216 | 9,417.33 | 7,367.66 | 6.03 | 41.62 | 94.73 |
| Snail | 0.03 | 5,191 | 13,097.03 | 9,955.64 | 6.43 | 18.50 | 93.43 |
| Loach | 0.03 | 3,501 | 9,009.30 | 6,852.16 | 5.66 | 31.29 | 95.03 |
| mixture | 0.03 | 4,233 | 10,029.95 | 7,917.18 | 6.85 | 3.645 | 93.62 |
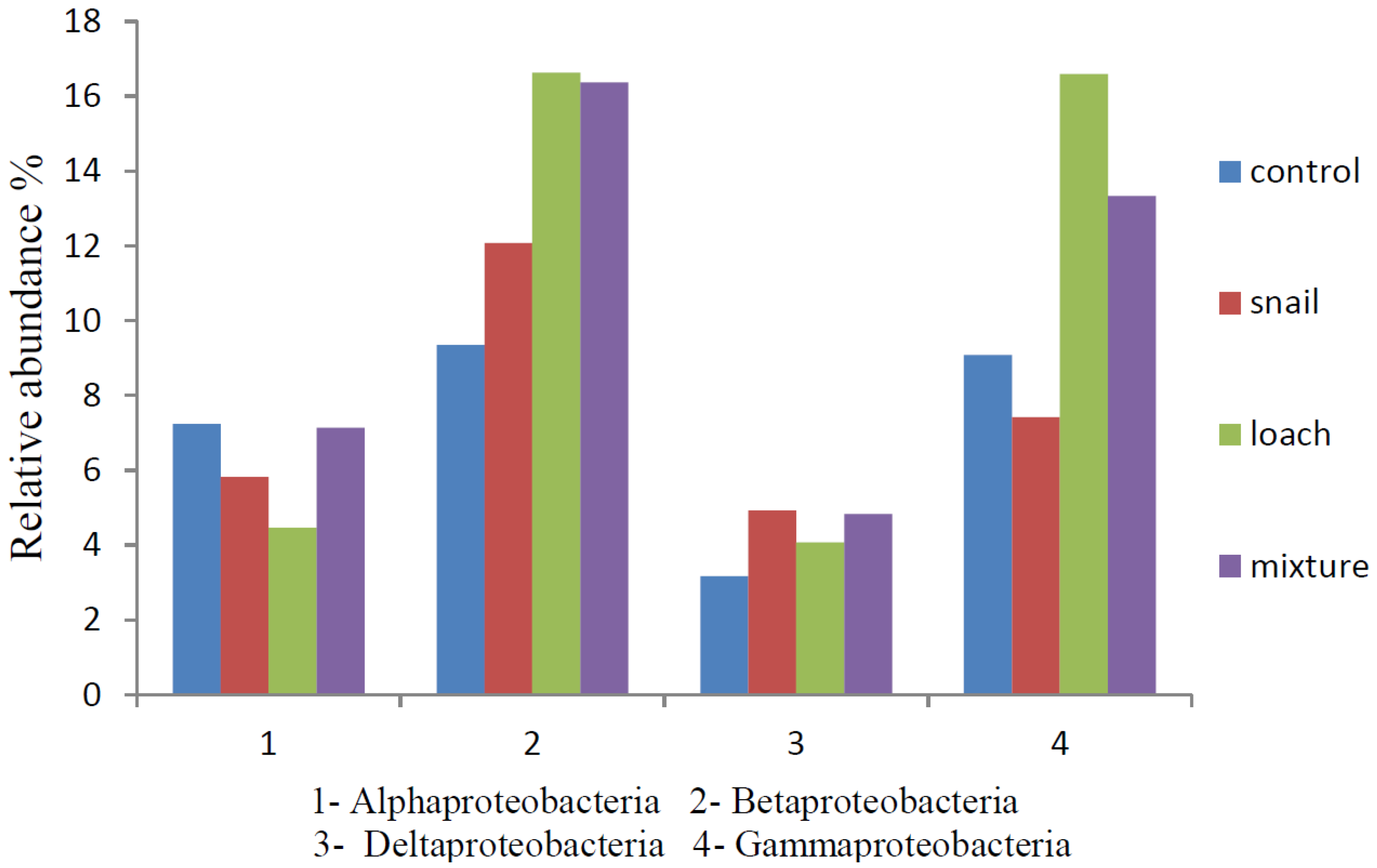
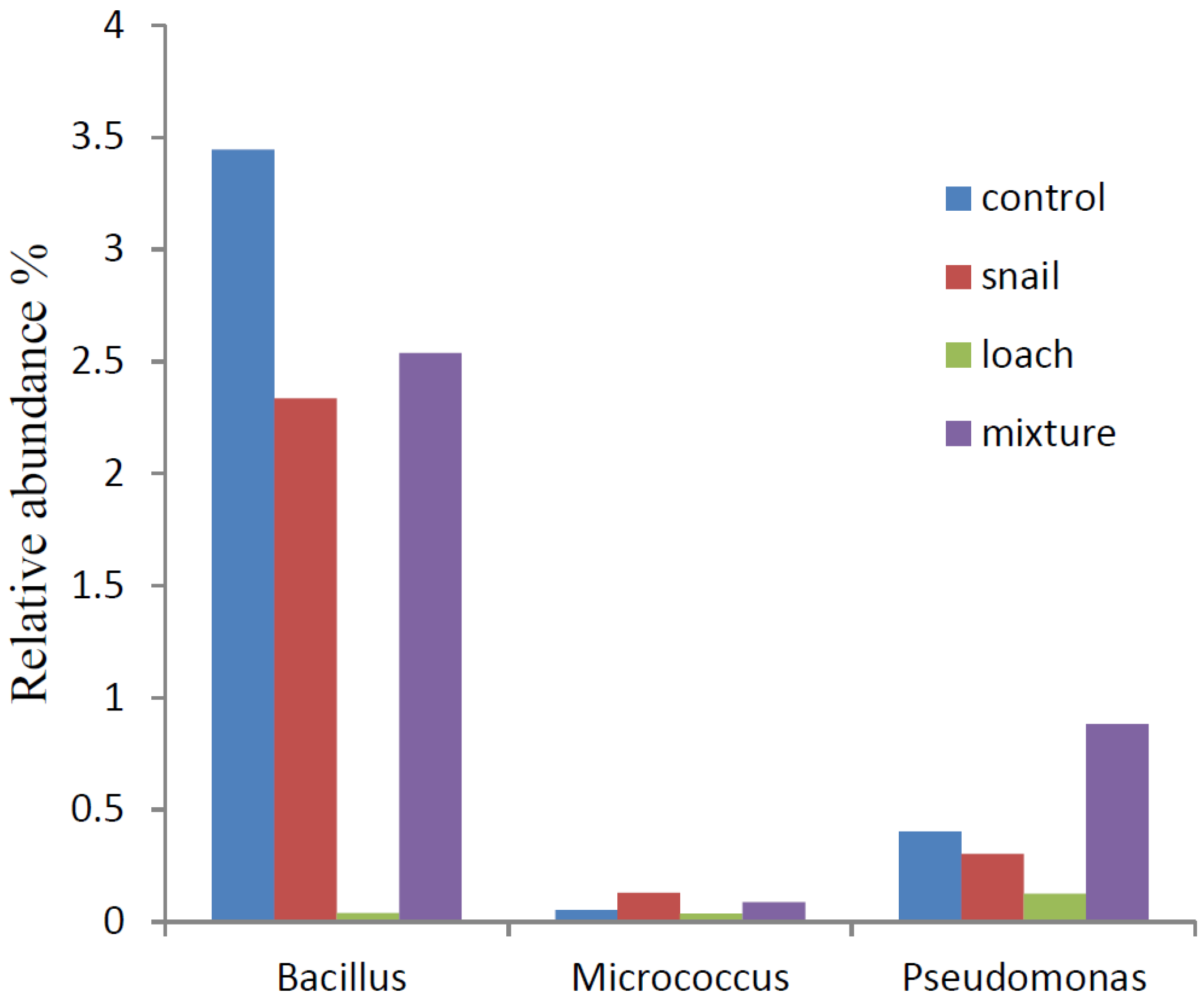
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thullen, J.S.; Sartoris, J.J.; Nelson, S.M. Managing vegetation in surface-flow wastewater-treatment wetlands for optimal treatment performance. Ecol. Eng. 2005, 25, 583–593. [Google Scholar] [CrossRef]
- Nahlik, A.M.; Mitsch, W.J. Tropical treatment wetlands dominated by free-floating macrophytes for water quality improvement in Costa Rica. Ecol. Eng. 2006, 28, 246–257. [Google Scholar] [CrossRef]
- Ávila, C.; Reyes, C.; Bayona, J.M.; García, J. Emerging organic contaminant removal depending on primary treatment and operational strategy in horizontal subsurface flow constructed wetlands: Influence of redox. Water Res. 2013, 47, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Spieles, D.J.; Mitsch, W.J. Macroinvertebrate community structure in high-and low-nutrient constructed wetlands. Wetlands 2000, 20, 716–729. [Google Scholar] [CrossRef]
- Jurado, G.B.; Johnson, J.; Feeley, H.; Harrington, R.; Kelly-Quinn, M. The potential of integrated constructed wetlands (ICWs) to enhance macroinvertebrate diversity in agricultural landscapes. Wetlands 2010, 30, 393–404. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant diversity, soil microbial communities, and ecosystem function: Are there any links? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Fanjul, E.; Escapa, M.; Montemayor, D.; Addino, M.; Alvarez, M.F.; Grela, M.A.; Iribarne, O. Effect of crab bioturbation on organic matter processing in South West Atlantic intertidal sediments. J. Sea Res. 2014, 95, 206–216. [Google Scholar] [CrossRef]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrate species in freshwater ecosystems: Zoobenthic species influence energy flows and nutrient cycling. Bioscience 1999, 49, 119–127. [Google Scholar] [CrossRef]
- Sims, A.; Zhang, Y.; Gajaraj, S.; Brown, P.B.; Hu, Z. Toward the development of microbial indicators for wetland assessment. Water Res. 2013, 47, 1711–1725. [Google Scholar] [CrossRef] [PubMed]
- Adrados, B.; Sánchez, O.; Arias, C.A.; Becares, E.; Garrido, L.; Mas, J.; Brix, H.; Morató, J. Microbial communities from different types of natural wastewater treatment systems: Vertical and horizontal flow constructed wetlands and biofilters. Water Res. 2014, 55, 304–312. [Google Scholar] [CrossRef] [PubMed]
- İnceoğlu, Ö.; Al-Soud, W.A.; Salles, J.F.; Semenov, A.V.; van Elsas, J.D. Comparative analysis of bacterial communities in a potato field as determined by pyrosequencing. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Alonso, Á.; Camargo, J.A. Nitrate causes deleterious effects on the behaviour and reproduction of the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Environ. Sci. Pollut. Res. 2013, 20, 5388–5396. [Google Scholar] [CrossRef]
- Gonçalves, A.F.; Castro, L.F.C.; Pereira-Wilson, C.; Coimbra, J.; Wilson, J.M. Is there a compromise between nutrient uptake and gas exchange in the gut of Misgurnus anguillicaudatus, an intestinal air-breathing fish? Comp. Biochem. Physiol. Part D Genomics Proteomics 2007, 2, 345–355. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
- Wu, H.; Zhang, J.; Li, P.; Zhang, J.; Xie, H.; Zhang, B. Nutrient removal in constructed microcosm wetlands for treating polluted river water in northern China. Ecol. Eng. 2011, 37, 560–568. [Google Scholar] [CrossRef]
- Wu, S.Q.; Chang, J.J.; Dai, Y.; Wu, Z.B.; Liang, W. Treatment performance and microorganism community structure of integrated vertical-flow constructed wetland plots for domestic wastewater. Environ. Sci. Pollut. Res. 2013, 206, 3789–3798. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jing, S.; Lee, D.; Wang, T. Nutrient removal from aquaculture wastewater using a constructed wetlands system. Aquaculture 2002, 209, 169–184. [Google Scholar] [CrossRef]
- Gong, L.J.; Yang, X.F.; Xiong, B.X.; Li, G.P.; Chen, X.L. Study on Nitrogen, Phosphor and Chemical Oxygen Demand of Differnt Categories of Aquaculture Lakes by Means of Principal Component Analysis, Factor Analysis and Cluster Analysis. Adv. Mater. Res. 2012, 340, 369–377. [Google Scholar] [CrossRef]
- Xu, D.; Xu, J.; Wu, J.; Muhammad, A. Studies on the phosphorus sorption capacity of substrates used in constructed wetland systems. Chemosphere 2006, 63, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Bachand, P.A.; Horne, A.J. Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecol. Eng. 1999, 14, 17–32. [Google Scholar] [CrossRef]
- Poach, M.E.; Hunt, P.G.; Reddy, G.B.; Stone, K.C.; Johnson, M.H.; Grubbs, A. Swine wastewater treatment by marsh–pond–marsh constructed wetlands under varying nitrogen loads. Ecol. Eng. 2004, 23, 165–175. [Google Scholar] [CrossRef]
- Blair, J.M.; Parmelee, R.W.; Allen, M.F.; McCartney, D.A.; Stinner, B.R. Changes in soil N pools in response to earthworm population manipulations in agroecosystems with different N sources. Soil Biol. Biochem. 1997, 29, 361–367. [Google Scholar] [CrossRef]
- Tipping, E. Modeling the competition between alkaline earth cations and trace metal species for binding by humic substances. Environ. Sci. Technol. 1993, 27, 520–529. [Google Scholar] [CrossRef]
- Tchobanoglous, G. Constructed wetlands and aquatic plant systems: Research, design, operational, and monitoring issues. In Constructed Wetlands for Water Quality Improvement; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 23–34. [Google Scholar]
- Xu, D.; Li, Y.; Howard, A. Influence of earthworm Eiseniafetida on removal efficiency of N and P in vertical flow constructed wetland. Environ. Sci. Pollut. Res. 2013, 20, 5922–5929. [Google Scholar] [CrossRef]
- Kersters, K.; de Vos, P.; Gillis, M.; Swings, J.; Vandamme, P.; Stackebrandt, E. Introduction to the Proteobacteria. In The Prokaryotes: A Handbook on the Biology of Bacteria; Springer: New York, NY, USA, 2006; Volume 5, pp. 3–37. [Google Scholar]
- Wang, Y.; Sheng, H.; He, Y.; Wu, J.; Jiang, Y.; Tam, N.F.; Zhou, H. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef] [PubMed]
- Peralta, R.M.; Ahn, C.; Gillevet, P.M. Characterization of soil bacterial community structure and physicochemical properties in created and natural wetlands. Sci. Total Environ. 2013, 443, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Gorra, R.; Coci, M.; Ambrosoli, R.; Laanbroek, H.J. Effects of substratum on the diversity and stability of ammonia-oxidizing communities in a constructed wetland used for wastewater treatment. J. Appl. Microbiol. 2007, 103, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Gillevet, P.M.; Sikaroodi, M. Molecular characterization of microbial communities in treatment microcosm wetlands as influenced by macrophytes and phosphorus loading. Ecol. Indic. 2007, 7, 852–863. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Assessing bacterial diversity in soil. J. Soils Sediments 2008, 8, 379–388. [Google Scholar] [CrossRef]
- Sangwan, P.; Kovac, S.; Davis, K.E.; Sait, M.; Janssen, P.H. Detection and cultivation of soil Verrucomicrobia. Appl. Environ. Microbiol. 2005, 71, 8402–8410. [Google Scholar] [CrossRef] [PubMed]
- Chitra, A.V.; Lakshmanaperumalsamy, P. Biodegradation of nitrate in wastestreams from explosives manufacturing plants. Res. J. Microbiol. 2006, 1, 142–151. [Google Scholar] [CrossRef]
- Tiedje, J.M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Biol. Anaerob. Microorg. 1988, 717, 179–244. [Google Scholar]
- Ayyasamy, P.M.; Shanthi, K.; Lakshmanaperumalsamy, P.; Lee, S.; Choi, N.; Kim, D. Two-stage removal of nitrate from groundwater using biological and chemical treatments. J. Biosci. Bioeng. 2007, 104, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, S.; Ayyasamy, P.M.; Shanthi, K.; Thavamani, P.; Velmurugan, P.; Song, Y.C.; Lakshmanaperumalsamy, P. Nitrate removal efficiency of bacterial consortium (Pseudomonas sp. KW1 and Bacillus sp. YW4) in synthetic nitrate-rich water. J. Hazard. Mater. 2008, 157, 553–563. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhang, J.; Xie, H.; Hu, Z.; He, H.; Wang, W. Effects of Misgurnus anguillicaudatus and Cipangopaludina cathayensis on Pollutant Removal and Microbial Community in Constructed Wetlands. Water 2015, 7, 2422-2434. https://doi.org/10.3390/w7052422
Li P, Zhang J, Xie H, Hu Z, He H, Wang W. Effects of Misgurnus anguillicaudatus and Cipangopaludina cathayensis on Pollutant Removal and Microbial Community in Constructed Wetlands. Water. 2015; 7(5):2422-2434. https://doi.org/10.3390/w7052422
Chicago/Turabian StyleLi, Pengfei, Jian Zhang, Huijun Xie, Zhen Hu, Haiyan He, and Wenxing Wang. 2015. "Effects of Misgurnus anguillicaudatus and Cipangopaludina cathayensis on Pollutant Removal and Microbial Community in Constructed Wetlands" Water 7, no. 5: 2422-2434. https://doi.org/10.3390/w7052422
APA StyleLi, P., Zhang, J., Xie, H., Hu, Z., He, H., & Wang, W. (2015). Effects of Misgurnus anguillicaudatus and Cipangopaludina cathayensis on Pollutant Removal and Microbial Community in Constructed Wetlands. Water, 7(5), 2422-2434. https://doi.org/10.3390/w7052422






