Abstract
This research was conducted to study the anaerobic sludge filtration capacity regarding helminth egg removal in upflow anaerobic sludge blanket (UASB) reactors. Two 25 L lab-scale UASB reactors were operated at an ambient temperature which varied between 17.1 and 28.6 °C. Ascaris suum egg was selected as the model egg considering its similarity in terms of size and morphology to Ascaris lumbricoides, a human pathogen. Ascaris suum eggs were obtained from female parasites of infected pigs. The anaerobic sludge filtration capacity was performed applying upflow velocities between 0.09 and 0.68 m·h−1. Three sludge bed heights in the range of 0.30–0.40 m, 0.50–0.60 m and 0.60–0.70 m were applied. These sludge bed heights corresponded to 19%–25%, 31%–38% and 38%–44% of the total reactor height, respectively. Under the mentioned conditions, the average helminth egg removal efficiency was reciprocally correlated to the imposed upflow velocity. The studied lab-scale reactors reported an average helminth egg removal between 34%–100%, 30%–91% and 34%–56%, when the sludge bed in the UASB reactor was 19%–25%, 31%–38% and 38%–44% of the total reactor height, respectively. The decreased filtration capacity at increasing sludge bed heights might be likely related to biogas production and channeling formation. The average helminth egg removal efficiency in the control experiments performed without any sludge bed, by plain sedimentation, varied between 44% and 66%.
1. Introduction
When treated wastewater is intended to be used for agricultural purposes, the presence of pathogens may limit its application potential [1,2]. Due to their shell resistance, helminth eggs are the most persistent pathogens to inactivation [3,4]. Particularly in developing countries, high concentrations of helminth eggs are present in domestic wastewater, which cause parasitic diseases like ascariasis, taeniasis and trichuriasis [5,6,7]. The prevailing symptoms caused by these diseases include diarrhea, effects on mental development, and anemia [8,9,10]. Within the group of pathogenic organisms, helminth eggs are infective agents which range in size from 10 μm to more than 100 μm [1,11,12].
Most literature regarding removal of helminth eggs is related to inactivation of helminth eggs contained in excess sludge [4,13,14,15,16] and physical removal from wastewater [1,17,18]. Technologies to inactivate helminth eggs in sludge are aimed at destroying the structure of the egg (mainly damages in its lipid layer) which prevents further development and survival of the eggs [4,19,20]. The best technologies for inactivation of helminth eggs present in sludge are thermal treatment at 108 °C [16], irradiation at 3500 Gy [14,15], pasteurization at 70 °C [13,21] or chemical treatment using sulfuric, hydrochloric, propionic, acetic or peracetic acid [19]. Processes like alkaline pre- and post-stabilization, by, for example, adding lime or other alkaline compound to the sludge, and thermophilic anaerobic digestion have shown high residual concentrations of worm eggs, i.e., more than 1 egg·g−1 TS, and 0.99–1.1 egg·g−1 TS in the sludge, respectively [3,4]. These values are higher than the restrictive limit in developing countries, where the use of treated waste and wastewater in (irrigated) agriculture is commonly applied. [1,8,18,22]. Maya et al. [4] reported that four genera of helminth eggs, i.e., Ascaris lumbricoides, Ascaris suum, Toxocara canis and Trichuris trichiura, are sensitive to environmental conditions in the larval state in the sludge. Furthermore, a proper combination of pH, dryness and contact time with temperatures above 60 °C can be applied to inactivate the eggs, efficiently [4,23]. Unfortunately, external energy and chemical-dependent technologies are in general not feasible for developing countries because they are complex, not sustainable and expensive in terms of investment and operation and maintenance costs [4,17,18,24].
Within the group of technologies applied to physical helminth egg removal (not inactivation) from wastewater, land-based post-treatment technologies such as sand filtration, wetlands and polishing ponds are reported to achieve helminth egg removal of 90%–99%, 100% and 100%, respectively [1,25,26]. In addition, Jimenez [19] reported that grit removal followed by a coagulation flocculation process in what is known as advanced primary treatment (APT), combined with an upflow sand filtration, reduced the amount of helminth eggs from 1.2 to 0.2 egg·g−1. Additionally, a study using APT followed by a sand filter combined with a synthetic medium reduced the amount of helminth eggs in average from 26 to 1.2 egg·g−1. Furthermore, APT followed by a multimedia filter and inclined parallel plates reduced the concentration from 27.0 to 1.2 egg·g−1 [19].
Limited research is executed on physical helminth egg removal in UASB reactors [1,3,25]. Filtration and sedimentation has been considered the main mechanism of helminth egg removal in UASB reactors [1,17,25]. During filtration and sedimentation, helminth eggs are respectively accumulated in the sludge bed and on the bottom of the reactor [1,27].
The removal of helminth eggs in UASB reactors has been reported to amount to 60%–90% [1]. UASB reactor technology is relatively cheap and compact and could contribute to domestic wastewater treatment in a sustainable way to improve environmental protection, resource recovery and public health protection [18,26,28,29,30,31]. However, the effect of different operational conditions of UASB reactors on helminth egg removal has not been evaluated thus far. Helminth egg removal through sedimentation and filtration would give an added value to UASB reactors. Mahmoud et al. [32] described the sludge bed filtration of UASB reactors as a mechanism for solids removal in domestic wastewater. Similar processes might affect the removal of helminth eggs in UASB reactors.
Pig helminths like Ascaris suum, Trichuris suis and Oesophagostomum spp. are often used in research as model organisms for human intestinal parasites, because they are very similar in morphology and size to the corresponding human parasite eggs and are relative easy to obtain in high numbers from infected pigs [33]. Maya et al. [4] reported that no significant differences were found between Ascaris lumbricoides and Ascaris suum regarding the inactivation conditions. In addition, Ascaris eggs were found to be the most resistant helminth egg genus to inactivation, combining unfavorable pH, dryness and temperature conditions, in comparison with Taenia sp. and Toxocara sp., Trichuris sp. and Hymennolepis [4]. In previous work [34,35], it has been shown that in the municipal wastewater in Peru, Ascaris lumbricoides was the predominant specie. Therefore, this research was conducted using Ascaris suum as helminth eggs as surrogate for the human parasite.
Mature Ascaris sp. eggs have an ovoid shape with average sizes of 40–70 μm [1,36]. This helminth egg is very resistant to inactivation under different environmental conditions [1,4]. This resistance is related to their four-layered shell composed of a lipid layer with a total thickness of about 4.5 μm, a mechanically rigid chitinous layer, a vitelline membrane and an external coat [3,36,37]. The shell is sensitive to lipid solvents and shows reduced surfaces and ridges. This mammillated layer is bile-stained to a golden brown color, and its high hydration makes it limp in the natural environment [4,37]. Microorganisms present in anaerobic sludge may play a role in degrading nematode eggs, though limited research results are available. For example, it has been reported that Duddingtonia flagrans and Angiostrongylus Cantonensis (nematofagous fungi) feed on free-living nematodes at the larval stage at 27 °C [38,39,40]. These fungi could survive in the digestive tract of different animal species and kill parasite larvae as they develop in the feces. Evidence exists that they are able to degrade the eggshell enzymatically and infect the helminth eggs [41,42].
Sludge bed density, extracellular polymeric substances [43,44,45,46], stability [47] and methanogenic conversion capacity [47,48] are some of the parameters that may impact the sludge bed filtration capacity for helminth eggs. Depending on the applied solids retention time (SRT) and the concentration of helminth eggs in the influent, long-term filtration may lead to saturation of the sludge bed, possibly lowering the filtration capacity. According to reviewed literature [1,18,19,49,50,51,52,53], no studies have been done thus far to characterize the sludge bed capacity for helminth egg filtration. Therefore, the main aim of this research was to study the sludge bed filtration capacity of UASB reactors with respect to the physical retention of helminth eggs under different upflow velocities at the prevailing subtropical temperatures. Filtration capacity is defined in this research as the physical process to retain helminth eggs using anaerobic sludge as a filtration medium.
2. Materials and Methods
2.1. Influent
The research was carried using raw wastewater from two urban villages called El Angel and El Milagro located in Lima (Peru). This wastewater was fed into a pilot plant located at the Research Center for Wastewater Treatment and Hazardous Wastes (CITRAR) at the campus of the National University of Engineering (Lima, Peru). The main characteristics of the wastewater are shown in Table 1.

Table 1.
Influent wastewater characteristics from two urban villages called El Angel and El Milagro located in Lima (Peru), used for this research.
| Parameter | Units | Average | n |
|---|---|---|---|
| Chemical Oxygen Demand | mg·L−1 | 723.2 ± 320.3 | 90 |
| Suspended Solids | mg·L−1 | 126.5 ± 28.5 | 36 |
| Oils and Grease | mg·L−1 | 30.8 ± 14.1 | 36 |
| Total Phosphorous—P | mg·L−1 | 6.6 ± 2 | 35 |
| Total Kjeldahl nitrogen—TKN | mg·L−1 | 16.2 ± 6.5 | 36 |
| Dissolved Oxygen | mg·L−1 | 6.8 ± 0.4 | 36 |
| Temperature | °C | 22.8 ± 4.1 | 233 |
| pH | -- | 7.1 ± 0.3 | 233 |
| Fecal Coliforms | MPN/100 mL | 9.67 × 108 ± 1.89 × 108 | 36 |
| Helminth eggs | egg·g−1 | 2.4 ± 1.4 | 90 |
Note: Where n is a number of grab analyzed samples.
The wastewater was pumped daily into a 200 L tank. The tank was filled with fresh wastewater every morning for all cases except when the upflow velocity of 0.68 m·h−1 was tested. For the latter situation, it was filled again in the afternoon when the remaining volume of the wastewater was 20 L. After filling the tank, the wastewater was mixed using a mechanical stirrer (18 RPM) with a stock solution containing Ascaris suum. The helminth egg concentration in the tank varied between 20–50 egg·g−1. The tank was kept at ambient temperatures and its content was used to continuously feed the UASB reactors. The pH and temperature of the wastewater was measured daily at 9:00, 12:00 and 16:00. The setup of the experiments is shown in Figure 1.
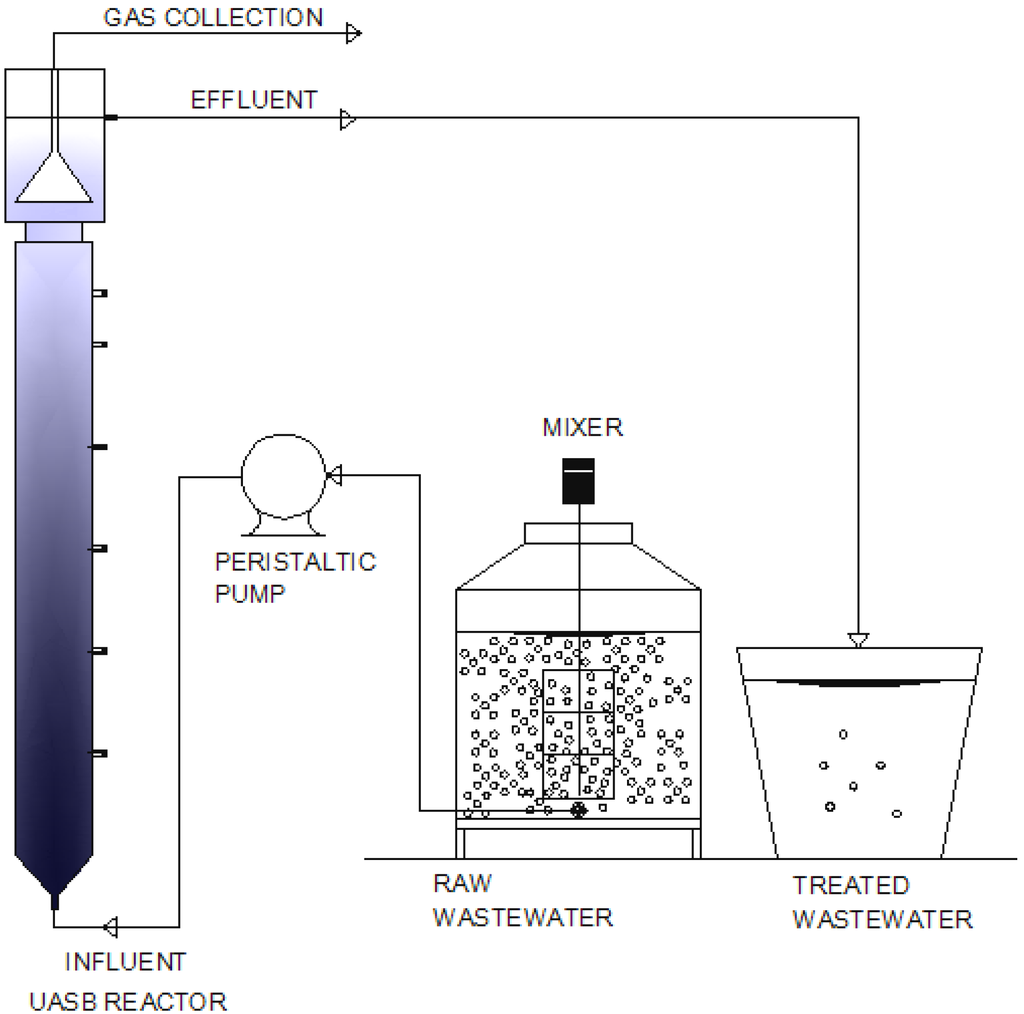
Figure 1.
Set up of the filtration experiments in UASB (upflow anaerobic sludge blanket) reactors using wastewater inoculated with Ascaris suum eggs.
2.2. Upflow Anaerobic Sludge Blanket (UASB) Reactors
Two 25 L identical acrylic cylindrical lab-scale UASB reactors with a total height of 1.60 m and a diameter of 0.15 m were used separately in parallel. They were located at CITRAR. The experiments were performed from January 2010 to August 2013.
2.3. Inoculum
The inoculum was anaerobic flocculent sludge sampled from the 536 m3 pilot-scale UASB reactor located at CITRAR. The inoculum was taken at a height of 1.5 m from the bottom of this reactor (total height of the reactor was 6.0 m). The total solids and volatile solids concentration of inoculum was 163 ± 37 and 106 ± 44 g·L−1 respectively.
2.4. Helminth Eggs
The experiment was conducted using Ascaris suum as helminth egg surrogate for the human parasite. The Ascaris suum helminth eggs were collected from female parasites of infected pigs (Sus crofa domesticus). In order to collect helminth eggs, dissections of the female parasite were performed according to Diawara et al. [54] by means of a longitudinal incision to obtain the reproductive system (womb and ovary). The womb and ovary were placed in 50 mL of physiological whey solution where they were opened to extract the helminth eggs. The optimal morphology and viability of the eggs of Ascaris suum were verified by microscopic observation according to Johnson et al. [55] and by using the staining procedure applied by de Victorica and Galván [56], respectively. Helminth eggs were added to the 200 L wastewater tank, which was fed to the UASB reactors.
2.5. Helminth Egg Counting
A multi-step methodology using local materials was developed from the modified Bailenger method [57,58]. This method was chosen due to its simplicity and the low cost of materials, in addition to the fact that it allows recovery of a wide range of helminths from the sample. The detailed methodology consists of collection of a 1 L sample, followed by settling for 24 h in a 1 L clear borosilicate glass bottle with graduations to concentrate the helminth eggs and to remove 90% of the supernatant (900 mL) by using a siphon. Then, 60 mL of the sediment are transferred to six centrifuge tubes of 10 mL each. Afterwards, the tubes are centrifuged at 1000 g for 15 min, and 70% of the supernatant (7 mL) is removed without shaking the tubes to avoid mixing the pellet with the supernatant. The remaining 40 mL of sediment is distributed over the same centrifuge tubes until the tubes are filled with 10 mL. Next, the bottle is rinsed two or more times with 10 mL of distilled water until it is completely clean. The corresponding rinse water is spread over the same centrifuge tubes or in new tubes. Distilled water is used to complete the remaining volume to fill 10 mL of water in each centrifuge tubes. Again, the centrifugation step is repeated. Subsequently, 2 mL of saturated sodium chloride solution with a specific gravity of 1.18 is added as flotation solution and, the tubes are shaken vigorously laterally. Afterwards, it is controlled whether all solids are located in the liquid phase. After 10 min, two phases are distinguished in the tubes. Finally, the top phase (1.5 mL) formed in the tubes is transferred to glass slides to be observed under the microscope (objectives lens 4× and 10×) and to count the eggs.
2.6. Physicochemical and Bacteriological Analysis
Total chemical oxygen demand (COD), suspended solid, volatile solids, oil/grease, pH, temperature, biochemical oxygen demand [59] and fecal coliform analysis were determined following standard methods [60]. Gravimetric and extractive-gravimetric methods carried out with hexane as a solvent were executed for solids and oil/grease determination, respectively. COD analysis was executed using high range Hach’s COD digestion vials as well as a digester reactor DR 200, and program 17 from colorimeter DR 890. Dissolved oxygen, total nitrogen Kjeldahl and total phosphorous were measured according to Method HACH 10360, 8038 and 8048, correspondingly [61]. Microscopic views were performed with an optical microscope ZEISS Primo Star Serial number 3122001719.
2.7. UASB Operational Conditions
In order to study the influence of different upflow velocities and sludge bed heights in the UASB reactors, four experiments were carried out as indicated in Table 2. Each experiment was performed in duplicate (two reactors).
In order to facilitate the statistical interpretation of the results, it is assumed that at an upflow velocity near to zero, all helminth eggs are removed in the UASB reactor in experiment 1, 2 and 3. This assumption is in line with the results described in previous research [34,35].

Table 2.
Setup of experiments in lab-scale UASB reactors to test the sludge filtration capacity to remove helminth eggs.
| Experiment | SB Height Variation | SBp | Upflow Velocities |
|---|---|---|---|
| (m) | (%) | (m·h−1) | |
| 1 | 0.30 to 0.40 | 19 to 25 | 0.09, 0.17, 0.23, 0.34 and 0.68 |
| 2 | 0.50 to 0.60 | 31 to 38 | 0.09, 0.11, 0.17, 0.23, 0.34 and 0.68 |
| 3 | 0.60 to 0.70 | 38 to 44 | 0.09, 0.14, 0.17, 0.23, 0.34, 0.45 and 0.68 |
| 4 (blank experiment) | 0 | 0 | 0.09, 0.11, 0.14, 0.17, 0.23, 0.34, and 0.68 |
Notes: Where SB means sludge bed and SBp is the sludge bed expressed as a percentage of the total reactor height. The upflow velocities of 0.09, 0.11, 0.14, 0.17, 0.23, 0.34, 0.45 and 0.68 m·h−1 correspond to an hydraulic retention time (HRT) of 15, 12, 10, 8, 6, 4, 3 and 2 h, respectively. Each experiment was repeated three times.
The startup of the UASB reactors was performed at an upflow velocity of 0.34 m·h−1 and hydraulic retention time (HRT) of 4 h. Each upflow velocity for every experiment was applied during seven days and samples were taken on the last day. The samples were taken after an elapsed time equivalent to one HRT, after introducing a known wastewater corresponding in the influent tank. The effluent of UASB reactors was collected separately from each reactor in order to be able to take separate samples. For every upflow velocity, six samples were analyzed for COD and helminth egg content and temperature in the influent and effluent. A total of six samples were performed per upflow velocity, which were collected respectively from three measurements in each UASB reactor.
Experiment 1 was performed after 85 days of the start of the UASB reactor. Before starting experiment 2, reactors were operated for approximately 30 days and continuously fed with domestic wastewater containing an average helminth egg concentration of 2.4 egg·g−1 and an HRT of 4 h. The two reactors were fed with exactly the same influent using two peristaltic pumps (2 Masterflex, Oldham, UK). Some samples from the effluent in experiment 2 were taken for each upflow velocity in order to do microscopic observations. Experiment 3 started immediately after finishing experiment 2. Experiment 4 (control experiment) was performed without sludge in the acrylic UASB reactor 7 days after all experiments were finished. All experiments were performed at ambient temperatures. Sludge was removed in each UASB reactor in order to maintain the established sludge bed height variation according to Table 2.
3. Results and Discussion
A summary of the results of experiments 1, 2, 3 and 4 is listed in Table 3. The sludge filtration capacity at ambient temperatures and sludge bed heights in the ranges of 0.30–0.40 m and 0.50–0.60 m, showed a reciprocal correlation between the average helminth egg removal efficiency and upflow velocity with a coefficient of determination of 0.94 and 0.91, respectively. When the sludge bed height increased to 0.60–0.70 m, the reciprocal correlation is still present but the coefficient of determination decreased to 0.57.

Table 3.
Results of helminth egg removal and chemical oxygen demand (COD) removal efficiencies at applied upflow velocities and wastewater temperatures. Each upflow velocity was applied three times in each UASB reactor. Then a total of six samples per upflow velocity was analyzed.
| Experiment | Upflow Velocity (m·h−1) | Temperature (°C) | Helminth Egg removal (%) | COD (%) |
|---|---|---|---|---|
| Experiment 1 | 0.09 | 24.6 ± 2.4 | 93 ± 5 | 71.9 ± 7.1 |
| 0.17 | 28.6 ± 2 | 77 ± 4 | 66.4 ± 8.2 | |
| 0.23 | 25.6 ± 3.5 | 61 ± 7 | 63.1 ± 8.6 | |
| 0.34 | 23 ± 3.3 | 52 ± 9 | 60.3 ± 6.4 | |
| 0.68 | 26.5 ± 2 | 26 ± 7 | 45.4 ± 6.3 | |
| Experiment 2 | 0.09 | 22 ± 6 | 91 ± 3 | 71.6 ± 10.3 |
| 0.11 | 22.5 ± 5.3 | 75 ± 10 | 71.6 ± 2.2 | |
| 0.17 | 24.2 ± 1.5 | 71 ± 11 | 66.2 ± 12.7 | |
| 0.23 | 23.2 ± 3.1 | 61 ± 10 | 65 ± 7.4 | |
| 0.34 | 26.1 ± 0.5 | 51 ± 7 | 63.7 ± 15.1 | |
| 0.68 | 25.5 ± 3 | 30 ± 15 | 63 ± 19.1 | |
| Experiment 3 | 0.09 | 23.3 ± 0.9 | 55 ± 1 | 80.3 ± 2.4 |
| 0.14 | 21.4 ± 2.9 | 53 ± 5 | 80.2 ± 15.5 | |
| 0.17 | 27.1 ± 0.5 | 56 ± 7 | 80.2 ± 8.1 | |
| 0.23 | 23.3 ± 5.7 | 56 ± 8 | 79.3 ± 0.8 | |
| 0.34 | 22.1 ± 4.2 | 55 ± 11 | 69.5 ± 14.8 | |
| 0.45 | 28.5 ± 2 | 46 ± 8 | 60.5 ± 0.4 | |
| 0.68 | 26.2 ± 2.3 | 34 ± 8 | 45.3 ± 3.4 | |
| Experiment 4 | 0.09 | 16.9 ± 1 | 66 ± 3 | 77.6 ± 2.4 |
| 0.11 | 16.9 ± 0.5 | 48 ± 3 | 44.8 ± 9.8 | |
| 0.14 | 17.3 ± 1 | 57 ± 3 | 84 ± 1.9 | |
| 0.17 | 17.3 ± 2 | 44 ± 3 | 50.7 ± 5.7 | |
| 0.23 | 16.9 ± 0.8 | 53 ± 3 | 64.9 ± 5 | |
| 0.34 | 18.1 ± 1 | 52 ± 10 | 71.1 ± 3.7 | |
| 0.68 | 17.7 ± 1 | 54 ± 8 | 55.2 ± 7.3 |
3.1. Experiment 1: Upflow Velocity between 0.09 and 0.68 m·h−1 and Sludge Bed Height between 0.30 and 0.40 m (19% to 25% of the Total Reactor Height)
The efficiencies of helminth egg removal as a function of the upflow velocity, applying a sludge bed height between 0.30 and 0.40 m in two reactors, are shown in Figure 2, both operated at five upflow velocities of 0.09, 0.17, 0.23, 0.34 and 0.68 m·h−1. Results show a decreasing trend for helminth egg removal efficiency at an increasing upflow velocity. A reciprocal linear relationship was observed between upflow velocity and helminth egg removal with a high coefficient of determination (R2 = 0.92). The current results of the experiment applying a low sludge bed height of 19%–25% show that an increment of the upflow velocity leads to a decrease of the sludge filtration capacity. The latter statement could be explained because as soon as the wastewater upflow velocity increases, the associated sludge viscosity probably decreases [44]. Analogous to the removal of helminth eggs, the COD removal efficiency is decreasing at an increasing upflow velocity (Figure 3). A reciprocal linear relationship was observed between upflow velocity and COD removal with a high coefficient of determination (R2 = 0.99). Average ambient temperature varied between 23 and 28.6 °C in both UASB reactors.
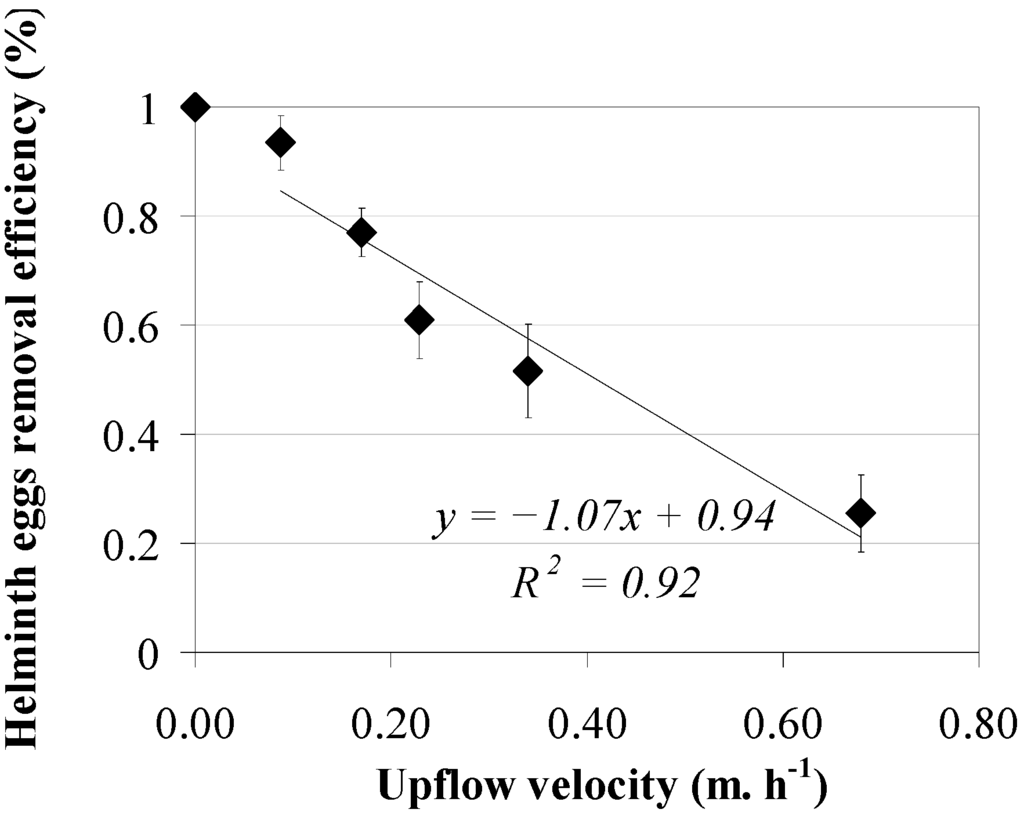
Figure 2.
Helminth egg removal efficiencies (♦) versus upflow velocity at a sludge bed height between 0.30 and 0.40 m.
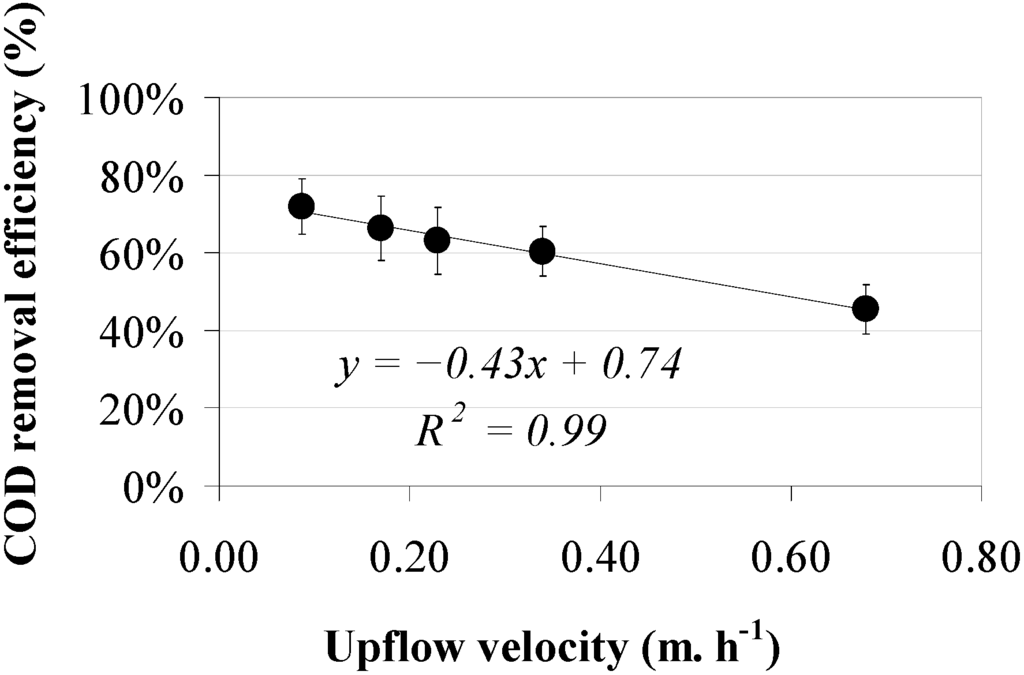
Figure 3.
Total COD removal efficiencies in two UASB reactors, characterized by a sludge bed height between 0.30 and 0.40 m.
3.2. Experiment 2: Upflow Velocity between 0.09 and 0.68 m·h−1 and Sludge Bed Height between 0.50 and 0.60 m (31% to 38% of the Total Reactor Height)
The efficiencies of helminth egg removal as a function of the upflow velocity, applying a sludge bed height between 0.50 and 0.60 m in two reactors, are shown in Figure 4, both operated six different upflow velocities: 0.09, 0.11, 0.17, 0.23, 0.34 and 0.68 m·h−1. Results show a decreasing trend for helminth egg removal efficiency at an increasing upflow velocity (Figure 4). A reciprocal linear relationship was observed between upflow velocity and helminth egg removal with a high coefficient of determination (R2 = 0.91). The observed results applying a sludge bed height of 31%–38% were similar to those at a sludge bed height of 19%–25%.
In contrast, the COD removal efficiency did not show a clear trend at an increasing upflow velocity (Figure 5) when applying a sludge bed height of 0.50–0.60 m. Average ambient temperature varied from 22.0 to 26.1 °C.
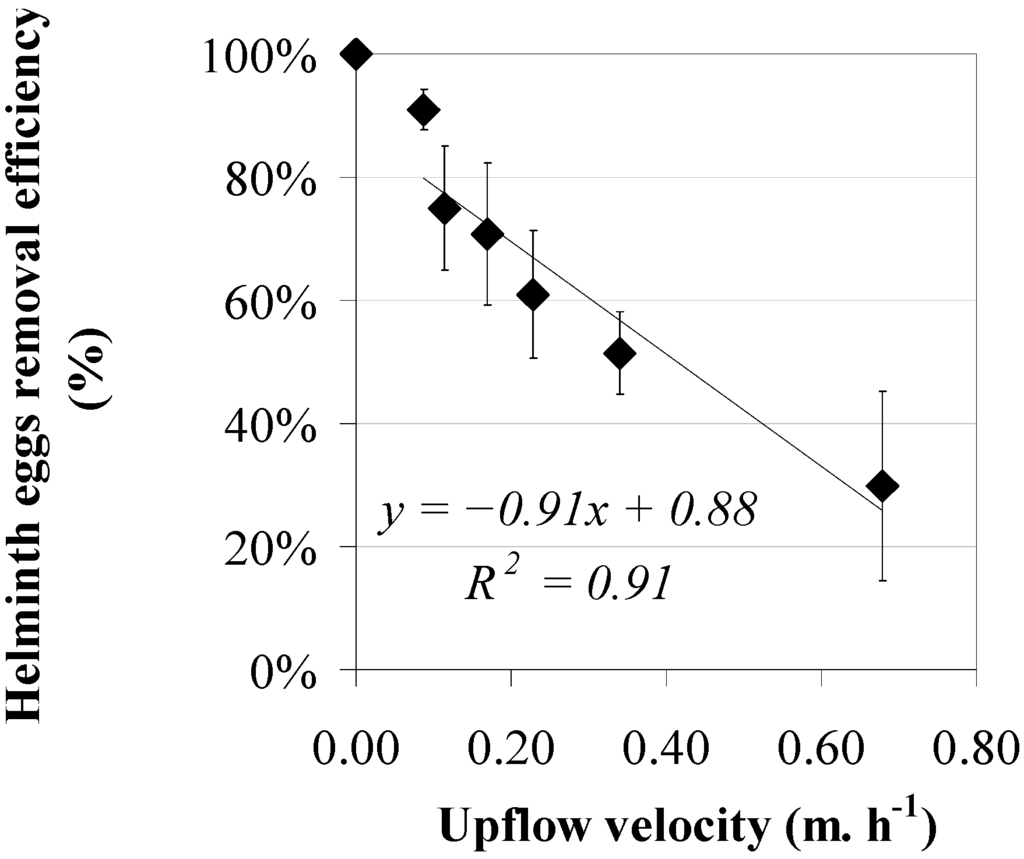
Figure 4.
Helminth egg removal efficiencies (♦) versus upflow velocity using a sludge bed height between 0.50 and 0.60 m.
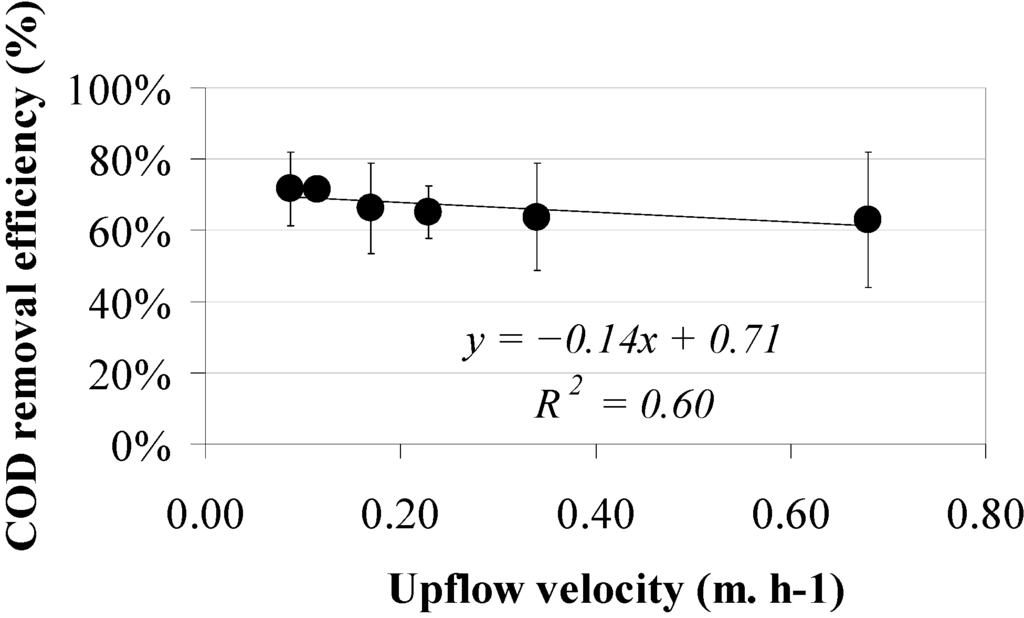
Figure 5.
Total COD removal efficiencies versus upflow velocity using a sludge bed height between 0.50 and 0.60 m.
3.3. Experiment 3: Upflow Velocity between 0.09 and 0.68 m·h−1 and Sludge Bed Height between 0.60 and 0.70 m (38% to 44% of the Total Reactor Height)
Results of the helminth egg removal as a function of the upflow velocity at a sludge bed height between 0.60 and 0.70 m, in two UASB reactors operated at 0.09, 0.14, 0.17, 0.23, 0.34, 0.45 and 0.68 m·h−1, are shown in Figure 6. Though a slightly decreasing trend is shown with increasing upflow velocity, the coefficient of determination is low (R2 = 0.83). Moreover, standard deviations are large.
Results on COD removal efficiency show a decreasing trend at an increasing upflow velocity (Figure 7). A reciprocal linear relationship was observed between upflow velocity and COD removal with a high coefficient of determination (R2 = 0.97). Average ambient temperature varied from 21.4 to 28.5 °C.
Although counterintuitive, the decreasing trend in helminth egg removal efficiency at an increasing sludge bed height in the studied lab-scale reactor might be explained by an increase in turbulence, created by the biogas production and formation of channels through the sludge bed [62,63] during all studied velocities. The possible saturation with helminth eggs during previous experiments could also have influenced the stability of the system with respect to helminth egg removal. Since none of the eggs are spherical [1,3,36,37], it is likely that they settle with different, but unknown orientations [64].
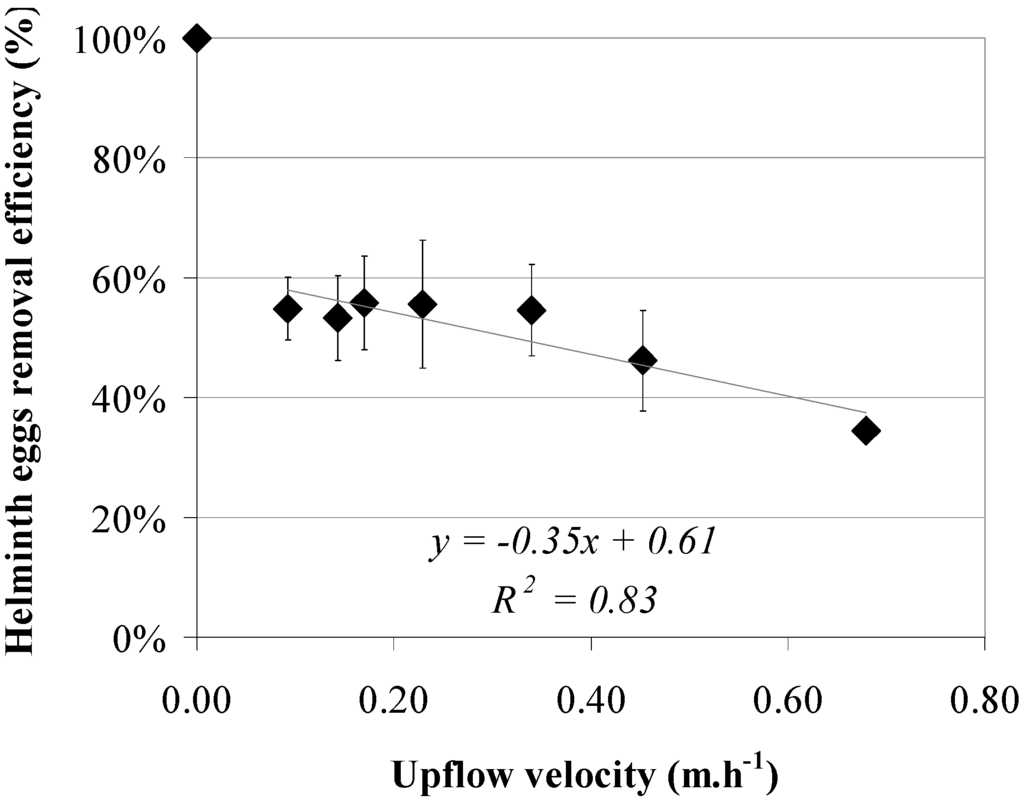
Figure 6.
Helminth egg removal efficiencies (♦) versus upflow velocity using a sludge bed height between 0.60 and 0.70 m.
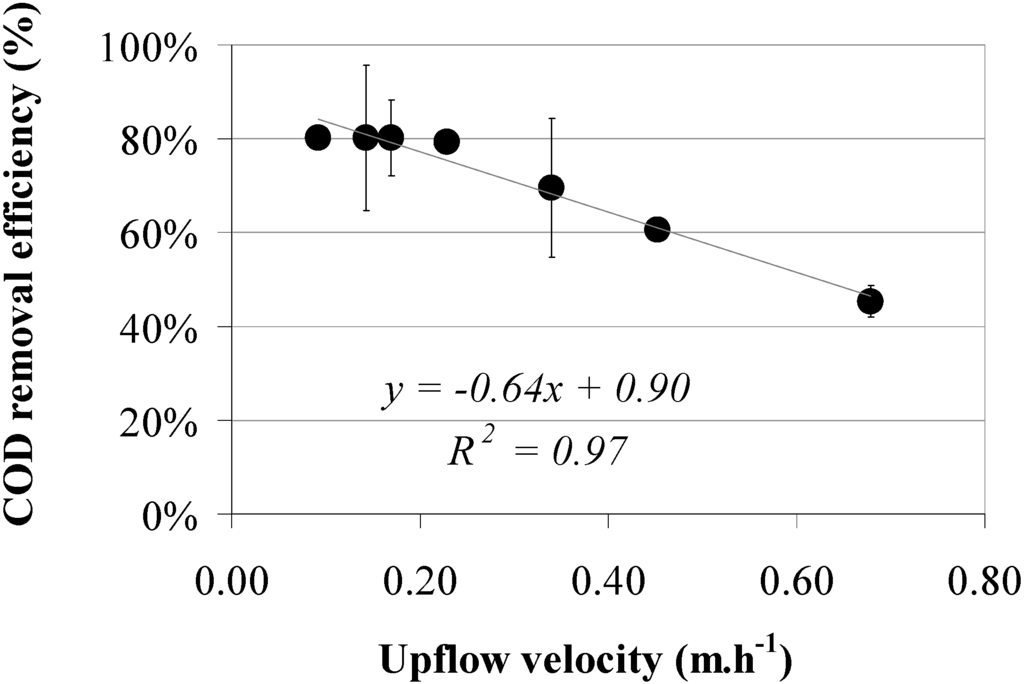
Figure 7.
Total COD removal efficiencies versus upflow velocity using a sludge bed height between 0.60 and 0.70 m. Results show the average of two reactors and standard deviation and three measurements per reactor.
3.4. Experiment 4: Blank Experiment Using Upflow Velocity between 0.09 and 0.68 m·h−1 and No Sludge Bed
The effect of the upflow velocity on the settling of helminth eggs is demonstrated by the results of the control experiment, applying a UASB reactor without sludge. Results for seven different upflow velocities, 0.09, 0.11, 0.14, 0.17, 0.23, 0.34, and 0.68 m·h−1, are shown in Figure 8 and Figure 9. Each point is the average of three samples in two reactors. Figure 8 indicates that the best efficiency for helminth egg removal was obtained at 0.09 m·h−1, when the removal efficiency reached 66% ± 3%. For upflow velocities higher than 0.09 m·h−1, the helminth egg efficiency removal was lower but always exceeded 44% ± 3%. It should be noted that standard deviations were large, so no significant differences were observed for helminth egg removal at an increasing upflow velocity. Figure 9 shows the trend for TSS and VSS removal. The best TSS and VSS removal efficiency (about 80%) was obtained at the lowest upflow velocities. For the higher upflow velocities the TSS and VSS removal efficiencies dropped to 40%–50% and 30%–40% for TSS and VSS, respectively.
The control experiment was executed in winter at a relatively low temperature and average ambient temperature varied from 16.9 to 18.1 °C. Latter temperatures were colder compared to previous experiments since experiment 4 was carried out coincidentally during winter.
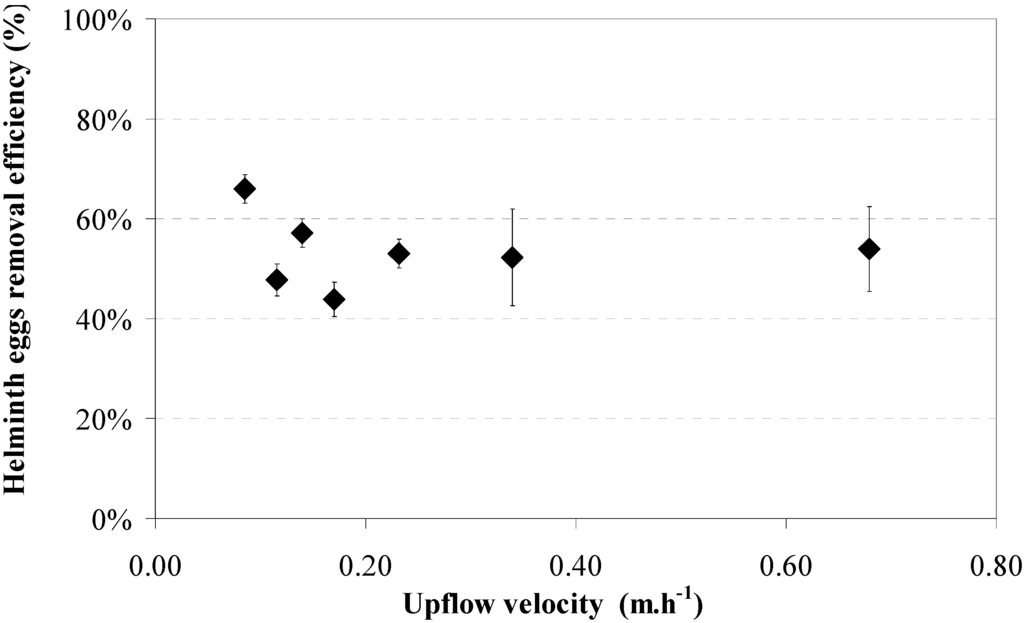
Figure 8.
Helminth egg removal efficiencies (♦) versus upflow velocity without sludge in the control experiment. Results shows the average of three samples and standard deviation; no sludge bed.
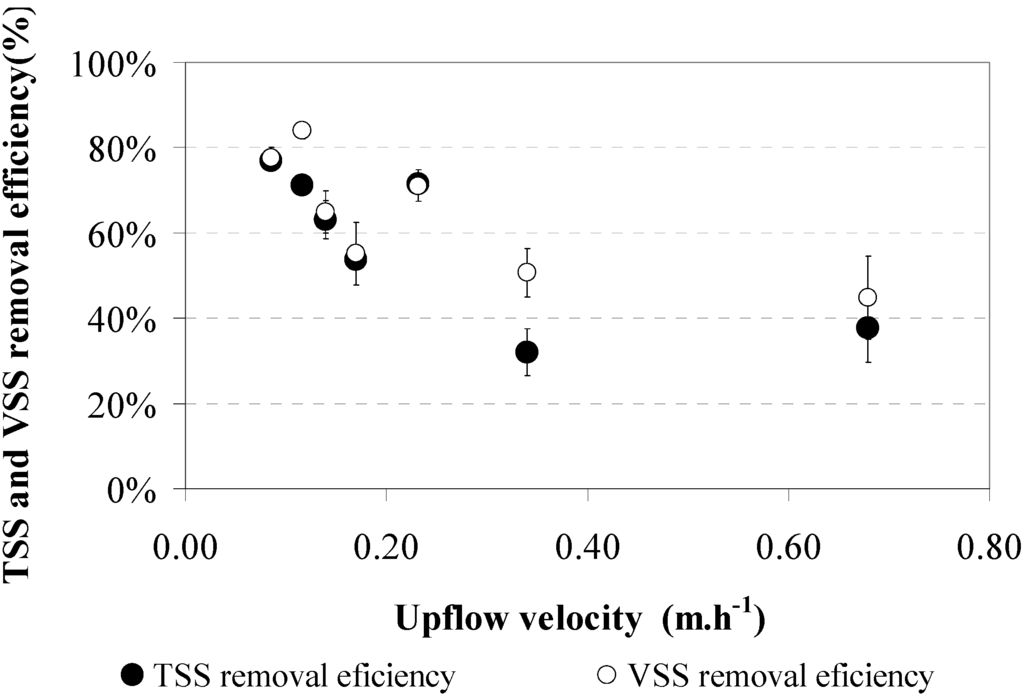
Figure 9.
TSS (●) and VSS (○) removal efficiencies in the control experiment—No sludge bed. Results show the average of three samples and standard deviation.
Results of experiments 1, 2, 3 and 4 show that the sludge bed in a UASB reactor is an inappropriate and unreliable filter medium for helminth eggs. Therefore, for achieving a complete helminth egg removal, a UASB reactor must be followed by an adequate post-treatment unit like land-based settling units or a post-filtration step [18,26,53]. For the control experiment (without sludge), average helminth egg removal efficiency varied between 44% and 66% at upflow velocities between 0.09 m·h−1 and 0.68 m·h−1. Unexpectedly, these values exceed the removal efficiencies of the reactors filled with high volumes of sludge, particularly at the high upflow velocities. Previous research [34,35] showed that viscosity of the flocculent anaerobic sludge is approximately more than 50 times higher than the viscosity of the liquid water, thereby theoretically leading to a better retention of helminth eggs. The explanation in the control experiments for why the levels of helminth eggs removal were so high is not very clear. Likely, the better retention might be associated to the absence of biogas production and thus turbulence. Therefore, in the absence of turbulence, the wastewater flow is more homogeneous [65,66,67,68], and helminth eggs settling follow a discrete settling pattern. Another remarkable observation is that even at the lowest upflow velocity (0.09 m·h−1) helminth eggs do not settle completely nor are retained completely by the sludge bed. The wash out of helminth eggs under these conditions may indicate that either the egg density is much less than expected, or the flow distribution is far from laminar [47,68,69]. A higher degree of channeling, which is expected at higher volumes of sludge [47,62,70], will aggravate the extremes in the flow distribution patterns. The latter will certainly lead to poorer filtration performances, as was also observed in the conducted experiments.
COD removal efficiencies showed a similar trend to the helminth egg removal efficiency. An increased removal of COD with decreasing upflow velocity was shown by Mahmoud [67,71]. Though completely different in nature, helminth eggs are also particles that could be expected to behave similarly.
3.5. Microscopic Observations in the Effluent
Microscopic observations were performed only for experiment 2. The presence of helminth eggs was detected in the sludge samples. In addition, several damages have been microscopically observed in the morphology of helminth eggs in the effluent (Figure 10a,c,f) with respect to the influent (Figure 10b–e,g,h) of the UASB reactor. The observed damages in the internal morphological structure of the eggs might be related to a possible loss in egg viability. These damages could be possibly caused by the retention of the eggs in the sludge bed for the applied HRT prior to their washout. The indicated hypotheses need to be confirmed in further research. Figure 10c,d present some microscopic views of helminth eggs, respectively in the influent and effluent of the UASB at an applied upflow velocity of 0.09 m·h−1. There are some changes in the internal morphology like probable larval development but without progression to the next stage (Figure 10c–e). Figure 10g,h shows some observed damages in the structure of helminth eggs.
The percentage of damaged helminth eggs present in the effluent of the UASB reactor that operated under different conditions was not determined, but visually they only were present in the effluent and not in the influent.
Following microscopic observations of the sludge sampled at different upflow velocities in experiment 2, it is shown that damages of helminth eggs occurred in all applied upflow velocities. It is shown in Figure 10h that some helminth eggs formed clusters of eggs. The mechanisms behind this phenomenon are not known.
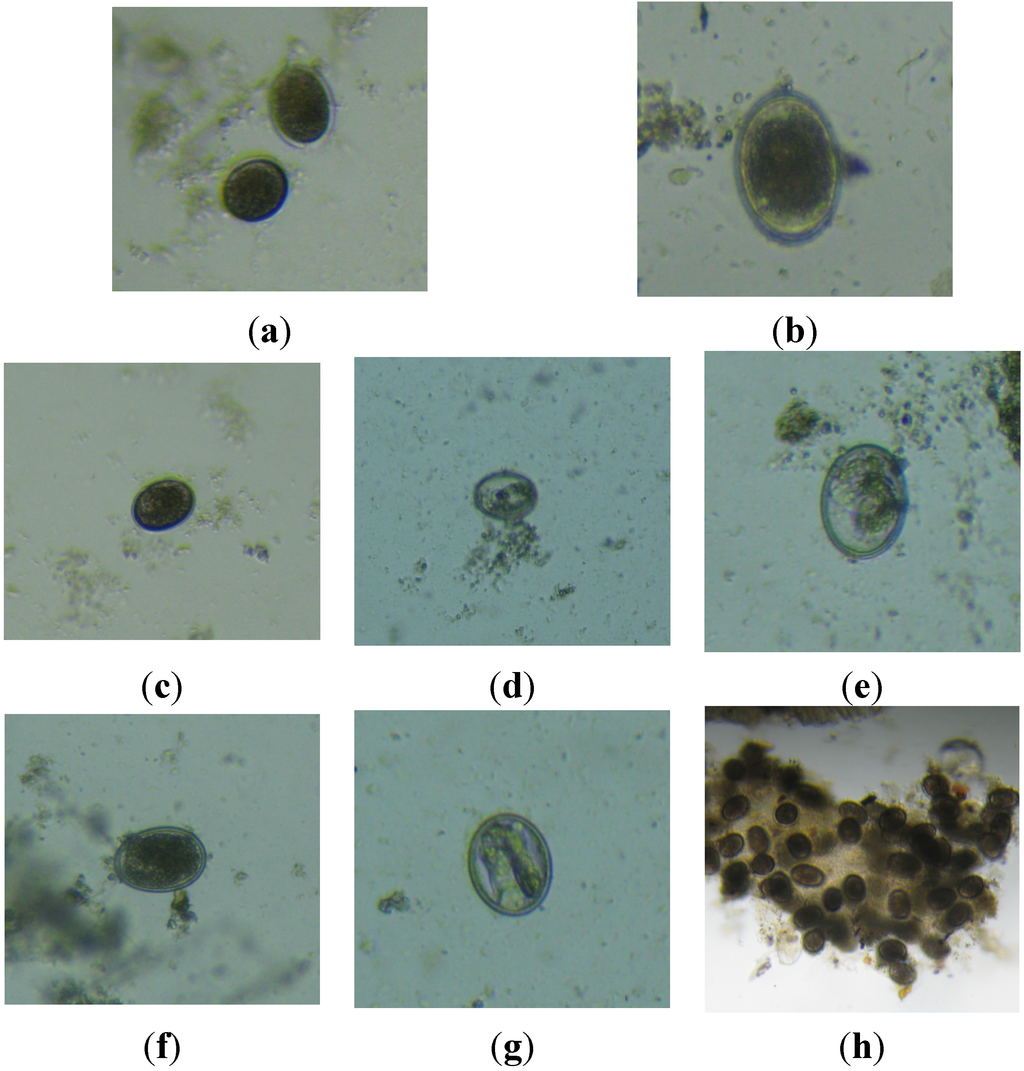
Figure 10.
Helminth eggs in the influent (a) and in the effluent (b) for an applied upflow velocity of 0.09 m·h−1. Helminth eggs in the influent (c) and in the effluent (d) and (e) for an upflow velocity of 0.34 m·h−1. Helminth eggs from the effluent show an internal morphology likely affected by the experimental conditions. Helminth eggs in the influent (f) and in the effluent (g) and (h) of lab-scale UASB reactor for an upflow velocity of 0.68 m·h−1. Helminth eggs from the effluent (g) show an apparently deteriorated semi-crystalline internal morphology (possible larval development but interrupted by the conditions of the experiment) and (h) group of attached helminth eggs.
The observed damages on Ascaris suum might be attributed to the prevailing physicochemical conditions in the direct vicinity of the eggs or to other microorganisms, which could be present in the anaerobic sludge like nematofagous fungi [38,39,40]. The relatively low helminth egg removal in the experiment with the highest sludge bed might also be related to a high percentage of damaged helminth eggs as a result of a long retention of helminth eggs in the sludge bed. Damaged helminth eggs might have a decreased density and thus a lowered settleability. The latter hypothesis could also explain why the removal of helminth eggs in the blank experiment (without sludge bed) is relatively high compared to the sludge bed reactors. This hypothesis needs to be verified in future research.
The results showed that helminth egg removal will not be sufficient for UASB systems operated with conventionally collected domestic wastewater where relatively high upflow velocities need to be applied as a result of the low COD concentration [72,73]. News trends in domestic wastewater collection and transport like uncoupling rainwater [74] and source separation [75,76,77] increase wastewater concentration and therefore reduce applied upflow velocities. The observed increased helminth egg removal at reduced upflow velocity might imply that application of UASB reactors, with similar loading rates in source separated domestic wastewater, leads to improved helminth egg removal.
4. Conclusions
This study demonstrated that with an increased sludge bed height there is a reduction in the sludge filtration capacity for helminth egg removal. If treated wastewater is used for irrigation purposes, the UASB reactor must be followed by an adequate post-treatment unit. The sludge filtration capacity at ambient temperatures and sludge bed height in the range of 0.30–0.40 m and 0.50–0.60 m, which agrees with 19%–25% and 31%–38% of the total height reactor, respectively, showed a reciprocal correlation between the average helminth egg removal efficiency and upflow velocity. This study reported an average helminth egg removal between 34%–100%, 30%–91% and 34%–56% when the sludge bed height was 19%–25%, 31%–38% and 38%–44%, respectively, of the total height in the UASB reactor at upflow velocities varying between 0.09 and 0.68 m·h−1. Several damages were observed during microscope observations in the morphology of helminth eggs present in the sludge and the effluent of UASB reactors at upflow velocities between 0.09 and 0.68 m·h−1.
Acknowledgments
This work was coordinated by the Sub-Department of Environmental Technology of Wageningen University.
Author Contributions
Rosa Elena Yaya Beas developed the experimental strategy, performed the experiments, was the principal author of the the paper, and contributed to the paper revisions. Christian Ayala Limaylla contributed to the biological part of experiments concerning helminth eggs counting. Katarazyna Kujawa-Roeleveld and Grietje Zeeman contributed to the paper revisions and experimental strategy. Jules van Lier provided advice on the experimental set-up and draft manuscript; all authors have reviewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jimenez, B. Helminth ova removal from wastewater for agriculture and aquaculture reuse. Water Sci. Technol. 2007, 55, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Navarro, I.; Jiménez, B. Evaluation of the who helminth eggs criteria using a qmra approach for the safe reuse of wastewater and sludge in developing countries. Water Sci. Technol. 2011, 63, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, B. Helminthes (worms) eggs control in wastewater and sludge. In Proceedings of the International Symposium on New Directions in Urban Water Management, Paris, France, 12–14 September 2007; pp. 12–14.
- Maya, C.; Torner-Morales, F.; Lucario, E.; Hernández, E.; Jiménez, B. Viability of six species of larval and non-larval helminth eggs for different conditions of temperature, ph and dryness. Water Res. 2012, 46, 4770–4782. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, U.J.; Cifuentes, E.; Bennett, S.; Quigley, M.; Ruiz-Palacios, G. The risk of enteric infections associated with wastewater reuse: The effect of season and degree of storage of wastewater. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.J.; Chico, M.E.; Sandoval, C.; Espinel, I.; Guevara, A.; Kennedy, M.W.; Urban, J.F.; Griffin, G.E.; Nutman, T.B. Human infection with ascaris lumbricoides is associated with a polarized cytokine response. J. Infect. Dis. 2000, 182, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Cruz Toribio, L.I.F. Gastrointestinal Helminthiasis in Livestock Herding Dogs Puno Communities; Universidad Nacional Mayor de San Marcos: Lima-Peru, Spain, 2010. [Google Scholar]
- World Health Organization (WHO). Who Guidelines for the Safe Use of Wastewater, Excreta and Greywater Organization; World Health Organization: Geneva, Switzerland, 2006; Volume 2. [Google Scholar]
- De Bonilla, L.C. El problema de las parasitosis intestinales en venezuela. Investig. Clín. 1990, 31, 1–2. [Google Scholar]
- Santiso, R. Effects of chronic parasitosis on women’s health. Int. J. Gynecol. Obstet. 1997, 58, 129–136. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse; McGraw Hill: New York, NY, USA, 2003. [Google Scholar]
- Qadir, M.; Wichelns, D.; Raschid-Sally, L.; McCornick, P.; Drechsel, P.; Bahri, A.; Minhas, P. The challenges of wastewater irrigation in developing countries. Agric. Water Manag. 2010, 97, 561–568. [Google Scholar] [CrossRef]
- Keller, R.; Passamani-Franca, R.; Cassini, S.; Gonçalves, F. Disinfection of sludge using lime stabilisation and pasteurisation in a small wastewater treatment plant. Water Sci. Technol. 2004, 50, 13–17. [Google Scholar] [PubMed]
- Borrely, S.; Cruz, A.; del Mastro, N.; Sampa, M.; Somessari, E. Radiation processing of sewage and sludge. A review. Prog. Nucl. Energy 1998, 33, 3–21. [Google Scholar] [CrossRef]
- De Souza, G.S.; Rodrigues, L.A.; de Oliveira, W.J.; Chernicharo, C.A.; Guimarães, M.P.; Massara, C.L.; Grossi, P.A. Disinfection of domestic effluents by gamma radiation: Effects on the inactivation of ascaris lumbricoides eggs. Water Res. 2011, 45, 5523–5528. [Google Scholar] [CrossRef] [PubMed]
- Gantzer, C.; Gaspard, P.; Galvez, L.; Huyard, A.; Dumouthier, N.; Schwartzbrod, J. Monitoring of bacterial and parasitological contamination during various treatment of sludge. Water Res. 2001, 35, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Mara, D. Domestic Wastewater Treatment in Developing Countries; Earthscan: London, UK, 2003. [Google Scholar]
- Von Sperling, M.; Chernicharo, C.; Andreoli, C.V.; Fernandes, F. (Eds.) Biological Wastewater Treatment in Warm Climate Regions; IWA: London, UK, 2005; Volume 2.
- Jimenez, B.; Maya-Rendon, C.; Salgado-Velzquez, G. The elimination of helminth ova, faecal coliforms, salmonella and protozoan cysts by various physicochemical processes in wastewater and sludge. Water Sci. Technol. 2001, 43, 179–182. [Google Scholar] [PubMed]
- Koné, D.; Cofie, O.; Zurbrügg, C.; Gallizzi, K.; Moser, D.; Drescher, S.; Strauss, M. Helminth eggs inactivation efficiency by faecal sludge dewatering and co-composting in tropical climates. Water Res. 2007, 41, 4397–4402. [Google Scholar] [CrossRef] [PubMed]
- Cabaret, J.; Geerts, S.; Madeline, M.; Ballandonne, C.; Barbier, D. The use of urban sewage sludge on pastures: The cysticercosis threat. Vet. Res. 2002, 33, 575–597. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Health Guidelines for the Use of Wastewater in Agriculture and Aquaculture: Reports of a Who Scientific Group; Technical Report Series no. 778; World Health Organization: Geneva, Switzerland, 1989. [Google Scholar]
- Brownell, S.A.; Nelson, K.L. Inactivation of single-celled ascaris suum eggs by low-pressure uv radiation. Appl. Environ. Microbiol. 2006, 72, 2178–2184. [Google Scholar] [CrossRef] [PubMed]
- Von Sperling, M. Comparison among the most frequently used systems for wastewater treatment in developing countries. Water Sci. Technol. 1996, 33, 59–72. [Google Scholar] [CrossRef]
- Von Sperling, M.; Chernicharo, C.; Soares, A.; Zerbini, A. Coliform and helminth egg removal in a combined uasb reactor-baffled pond system in brazil: Performance evaluation and mathematical modelling. Water Sci. Technol. 2002, 45, 237. [Google Scholar] [PubMed]
- Chernicharo, C. Post-treatment options for the anaerobic treatment of domestic wastewater. Rev. Environ. Sci. Biotechnol. 2006, 5, 73–92. [Google Scholar] [CrossRef]
- Von Sperling, M.; Chernicharo, C.; Soares, A.; Zerbini, A. Evaluation and modelling of helminth egg removal in baffled and unbaffled ponds treating anaerobic effluent. Water Sci. Technol. 2003, 48, 113–120. [Google Scholar] [PubMed]
- Jorsaraei, A.; Gougol, M.; van Lier, J.B. A cost effective method for decentralized sewage treatment. Process Saf. Environ. Prot. 2013, 92, 815–821. [Google Scholar] [CrossRef]
- Uemura, S.; Harada, H. Treatment of sewage by a uasb reactor under moderate to low temperature conditions. Bioresour. Technol. 2000, 72, 275–282. [Google Scholar] [CrossRef]
- Van Lier, J.B.; Tilche, A.; Ahring, B.K.; Macarie, H.; Moletta, R.; Dohanyos, M.; Pol, L.W.; Lens, P.; Verstraete, W.; Management Committee of the IWA Anaerobic Digestion Specialised Group. New perspectives in anaerobic digestion. Water Sci. Technol. 2001, 43, 1–18. [Google Scholar] [PubMed]
- Van Lier, J.B.; Vashi, A.; van der Lubbe, J.; Heffernan, B.; Fang, H. (Eds.) Anaerobic Sewage Treatment Using Uasb Reactors: Engineering and Operational Aspects; Imperial College Press: London, UK, 2010.
- Mahmoud, N.; Zandvoort, M.; van Lier, J.; Zeeman, G. Development of sludge filterability test to assess the solids removal potential of a sludge bed. Bioresour. Technol. 2006, 97, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Boes, J.; Helwigh, A. Animal models of intestinal nematode infections of humans. Parasitology 2000, 121, 97–111. [Google Scholar] [CrossRef]
- Yaya-Beas, R.E.; Zeeman, G.; van Lier, J.B. Helminth ova removal using uasb reactors at 4 °C. In Proceedings of the 3rd International Congress Smallwat 11, Seville, Spain, 26 April 2010.
- Yaya-Beas, R.E.; D’engremont, M.; Kujawa, K.; Zeeman, G.; van Lier, J.B. Filtration capacity of an anaerobic sludge bed for the removal of helminth eggs. Water Environ. J. submitted for publication. 2015. [Google Scholar]
- O’Lorcain, P.; Holland, C. The public health importance of ascaris lumbricoides. Parasitology 2000, 121, S51–S71. [Google Scholar] [CrossRef] [PubMed]
- Quilès, F.; Balandier, J.Y.; Capizzi-Banas, S. In situ characterisation of a microorganism surface by raman microspectroscopy: The shell of ascaris eggs. Anal. Bioanal. Chem. 2006, 386, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, D.G.; Araújo, F.B.; Molento, M.B.; DaMatta, R.A.; de Paula Santos, C. Kinetics of capture and infection of infective larvae of trichostrongylides and free-living nematodes panagrellus sp. By duddingtonia flagrans. Parasitol. Res. 2011, 109, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.S.; Suárez, J.; Cazapal-Monteiro, C.F.; Francisco, I.; López-Arellano, M.E.; Piñeiro, P.; Suárez, J.L.; Sánchez-Andrade, R.; Mendoza de Gives, P.; Paz-Silva, A. Trematodes enhance the development of the nematode-trapping fungus arthrobotrys (duddingtonia) flagran. Fungal Biol. 2013, 117, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Federica, S.M.; Alberto, F.L.; Emilia, I.L.; Carina, M.F.; Alfredo, S.C. Optimization of production of chlamydospores of the nematode-trapping fungus duddingtonia flagrans in solid culture media. Parasitol. Res. 2013, 112, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M. Prospects for controlling animal parasitic nematodes by predacious micro fungi. Parasitology 2000, 120, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Manzanilla-López, R.H.; Esteves, I.; Finetti-Sialer, M.M.; Hirsch, P.R.; Ward, E.; Devonshire, J.; Hidalgo-Díaz, L. Pochonia chlamydosporia: Advances and challenges to improve its performance as a biological control agent of sedentary endo-parasitic nematodes. J. Nematol. 2013, 45, 1. [Google Scholar] [PubMed]
- Seyssiecq, I.; Ferrasse, J.-H.; Roche, N. State-of-the-art: Rheological characterisation of wastewater treatment sludge. Biochem. Eng. J. 2003, 16, 41–56. [Google Scholar] [CrossRef]
- Pevere, A.; Guibaud, G.; van Hullebusch, E.; Lens, P.; Baudu, M. Viscosity evolution of anaerobic granular sludge. Biochem. Eng. J. 2006, 27, 315–322. [Google Scholar] [CrossRef]
- Mori, M.; Seyssiecq, I.; Roche, N. Rheological measurements of sewage sludge for various solids concentrations and geometry. Process Biochem. 2006, 41, 1656–1662. [Google Scholar] [CrossRef]
- Johansen, A.; Nielsen, H.B.; Hansen, C.M.; Andreasen, C.; Carlsgart, J.; Hauggard-Nielsen, H.; Roepstorff, A. Survival of weed seeds and animal parasites as affected by anaerobic digestion at meso-and thermophilic conditions. Waste Manag. 2013, 33, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Seghezzo, L. Anaerobic Treatment of Domestic Wastewater in Subtropical Regions. Ph.D. Thesis, Wageningen Universiteit, Wageningen, The Netherlands, 2004. [Google Scholar]
- De Graaff, M.S.; Temmink, H.; Zeeman, G.; Buisman, C.J. Anaerobic treatment of concentrated black water in a uasb reactor at a short hrt. Water 2010, 2, 101–119. [Google Scholar] [CrossRef]
- Mendez, J.; Jimenez, B.; Barrios, J. Improved alkaline stabilization of municipal wastewater sludge. Water Sci. Technol. 2002, 46, 139–146. [Google Scholar] [PubMed]
- Jiménez, B. Treatment technology and standards for agricultural wastewater reuse: A case study in mexico. Irrig. Drain. 2005, 54, S23–S33. [Google Scholar] [CrossRef]
- Jiménez, B.; Drechsel, P.; Koné, D.; Bahri, A.; Raschid-Sally, L.; Qadir, M. Wastewater, sludge and excreta use in developing countries: An overview. In Wastewater Irrigation and Health: Assessing and Mitigating Risk in Low-Income Countries; Taylor & Francis: London, UK, 2010; pp. 3–29. [Google Scholar]
- Jiménez, B.; Mara, D.; Carr, R.; Brissaud, F. Wastewater treatment for pathogen removal and nutrient conservation: Suitable systems for use in developing countries. In Wastewater Irrigation and Health. Assessing and Mitigating Risk in Low-Income Countries; Drechsel, P., Scott, C.A., Raschid-Sally, L., Redwood, M., Bahri, A., Eds.; International Water Management Institute and International Development Research Centre (IDRC): London, UK, 2010; pp. 149–169. [Google Scholar]
- Chernicharo, C.; da Silva Cota, R.; Zerbini, A.; von Sperling, M. Post-treatment of anaerobic effluents in an overland flow system. Water Sci. Technol. 2001, 44, 229–236. [Google Scholar] [PubMed]
- Diawara, A.; Drake, L.J.; Suswillo, R.R.; Kihara, J.; Bundy, D.A.; Scott, M.E.; Halpenny, C.; Stothard, J.R.; Prichard, R.K. Assays to detect β-tubulin codon 200 polymorphism in trichuris trichiura and ascaris lumbricoides. PLoS Negl. Trop. Dis. 2009, 3, e397. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Dixon, R.; Ross, A. An in vitro test for assessing the viability of ascaris suum eggs exposed to various sewage treatment processes. Int. J. Parasitol. 1998, 28, 627–633. [Google Scholar] [CrossRef] [PubMed]
- De Victorica, J.; Galván, M. Preliminary testing of a rapid coupled methodology for quantitation/viability determination of helminth eggs in raw and treated wastewater. Water Res. 2003, 37, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Ayres, R.M.; Mara, D.D. Analysis of Wastewater for Use in Agriculture. A Laboratory Manual of Parasitological and Bacteriological Techniques; World Health Organization: Geneva, Switzerland, 1996; Volume 1. [Google Scholar]
- Bailenger, J. Mechanisms of parasitological concentration in coprology and their practical consequences. J. Am. Med. Technol. 1979, 41, 65–71. [Google Scholar]
- Bodı́k, I.; Herdová, B.; Drtil, M. The use of upflow anaerobic filter and ansbr for wastewater treatment at ambient temperature. Water Res. 2002, 36, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; p. 1220. [Google Scholar]
- Hach Company. Hach Water Analysis Handbook, 5th ed.; Hach Company: Loveland, CO, USA, 2008. [Google Scholar]
- Lettinga, G.; Pol, L.H.; Koster, I.; Wiegant, W.; de Zeeuw, W.; Rinzema, A.; Grin, P.; Roersma, R.; Hobma, S. High-rate anaerobic waste-water treatment using the uasb reactor under a wide range of temperature conditions. Biotechnol. Genet. Eng. Rev. 1984, 2, 253–284. [Google Scholar] [CrossRef]
- Abdelgadir, A.; Chen, X.; Liu, J.; Xie, X.; Zhang, J.; Zhang, K.; Wang, H.; Liu, N. Characteristics, process parameters, and inner components of anaerobic bioreactors. BioMed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.E.; Thamsborg, S.M.; Andersen, T.J.; Olsen, A.; Dalsgaard, A. Sedimentation of helminth eggs in water. Water Res. 2011, 45, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Elmitwalli, T.A.; Zandvoort, M.H.; Zeeman, G.; Bruning, H.; Lettinga, G. Low temperature treatment of domestic sewage in upflow anaerobic sludge blanket and anaerobic hybrid reactors. Water Sci. Technol. 1999, 39, 177–185. [Google Scholar] [CrossRef]
- Lew, B.; Tarre, S.; Belavski, M.; Green, M. UASB reactor for domestic wastewater treatment at low temperatures: A comparison between a classical uasb and hybrid uasb-filter reactor. Water Sci. Technol. 2004, 49, 295–301. [Google Scholar] [PubMed]
- Mahmoud, N. Anaerobic Pre-treatment of Sewage under Low Temperature (15 °C) Conditions in an Integrated UASB-Digester System; Agricultural Wageningen University: Wageningen, The Netherlands, 2002. [Google Scholar]
- Bolle, W.; van Breugel, J.; van Eybergen, G.; Kossen, N.; Zoetemeyer, R. Modeling the liquid flow in up-flow anaerobic sludge blanket reactors. Biotechnol. Bioeng. 1986, 28, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Ojha, C.; Singh, R. Flow distribution parameters in relation to flow resistance in an upflow anaerobic sludge blanket reactor system. J. Environ. Eng. 2002, 128, 196–200. [Google Scholar] [CrossRef]
- Jeison, D.; Chamy, R. Comparison of the behaviour of expanded granular sludge bed (EGSB) and upflow anaerobic sludge blanket (UASB) reactors in dilute and concentrated wastewater treatment. Water Sci. Technol. 1999, 40, 91–97. [Google Scholar] [CrossRef]
- Mahmoud, N.; Zeeman, G.; Gijzen, H.; Lettinga, G. Solids removal in upflow anaerobic reactors, a review. Bioresour. Technol. 2003, 90, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Geng, Z.; Wang, Y. Expanded granular sludge bed (egsb) reactor treating actual domestic wastewater: Temperature influence. In Proceedings of the International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 26–28 July 2013.
- Ozgun, H.; Dereli, R.K.; Ersahin, M.E.; Kinaci, C.; Spanjers, H.; van Lier, J.B. A review of anaerobic membrane bioreactors for municipal wastewater treatment: Integration options, limitations and expectations. Sep. Purif. Technol. 2013, 118, 89–104. [Google Scholar] [CrossRef]
- Rulkens, W. Increasing the environmental sustainability of sewage treatment by mitigating pollutant pathways. Environ. Eng. Sci. 2006, 23, 650–665. [Google Scholar] [CrossRef]
- Zeeman, G.; Kujawa-Roeleveld, K. Anaerobic treatment of source-separated domestic wastewater. In Source Separation and Decentralization for Wastewater Treatment; Larsen, T.A., Udert, K.M., Lienert, J., Eds.; IWA: London, UK, 2013; pp. 307–319. [Google Scholar]
- Udert, K.M.; Lienert, J. Source Separation and Decentralization for Wastewater Management; IWA: London, UK, 2013. [Google Scholar]
- Kujawa-Roeleveld, K.; Zeeman, G. Anaerobic treatment in decentralised and source-separation-based sanitation concepts. Rev. Environ. Sci. Biotechnol. 2006, 5, 115–139. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).