Microbiological Confinement of Two Adjacent Water Wells in Lake Karla Basin, Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Molecular Analysis
2.3. Data Processing and Analysis
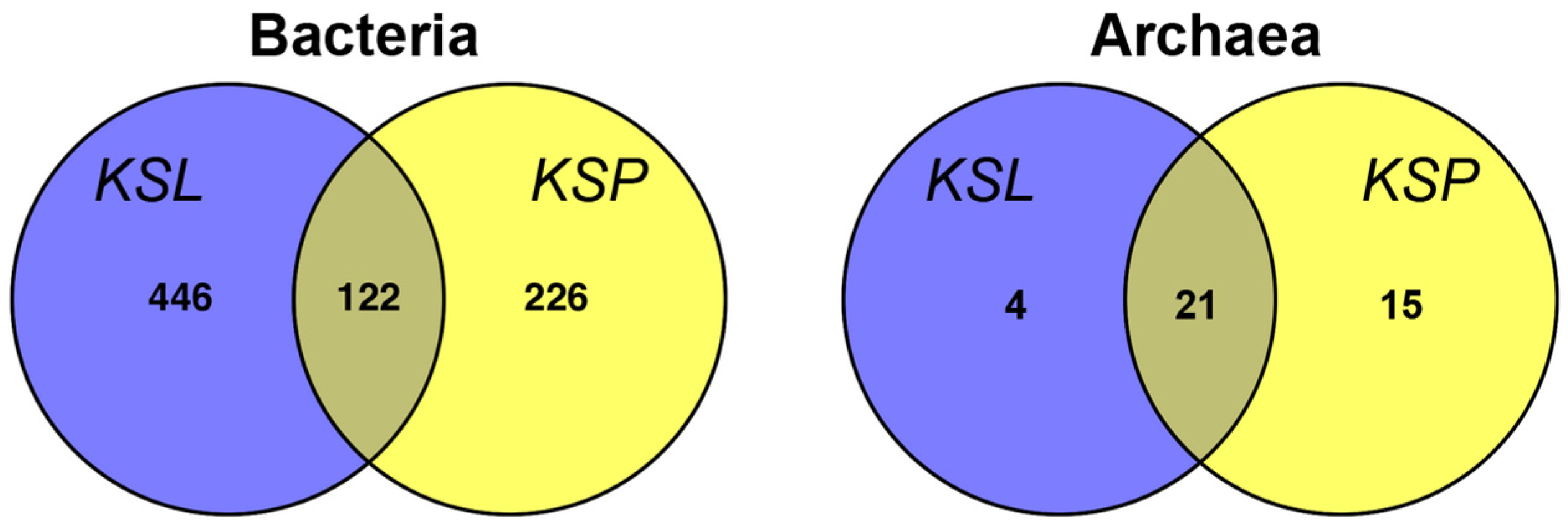
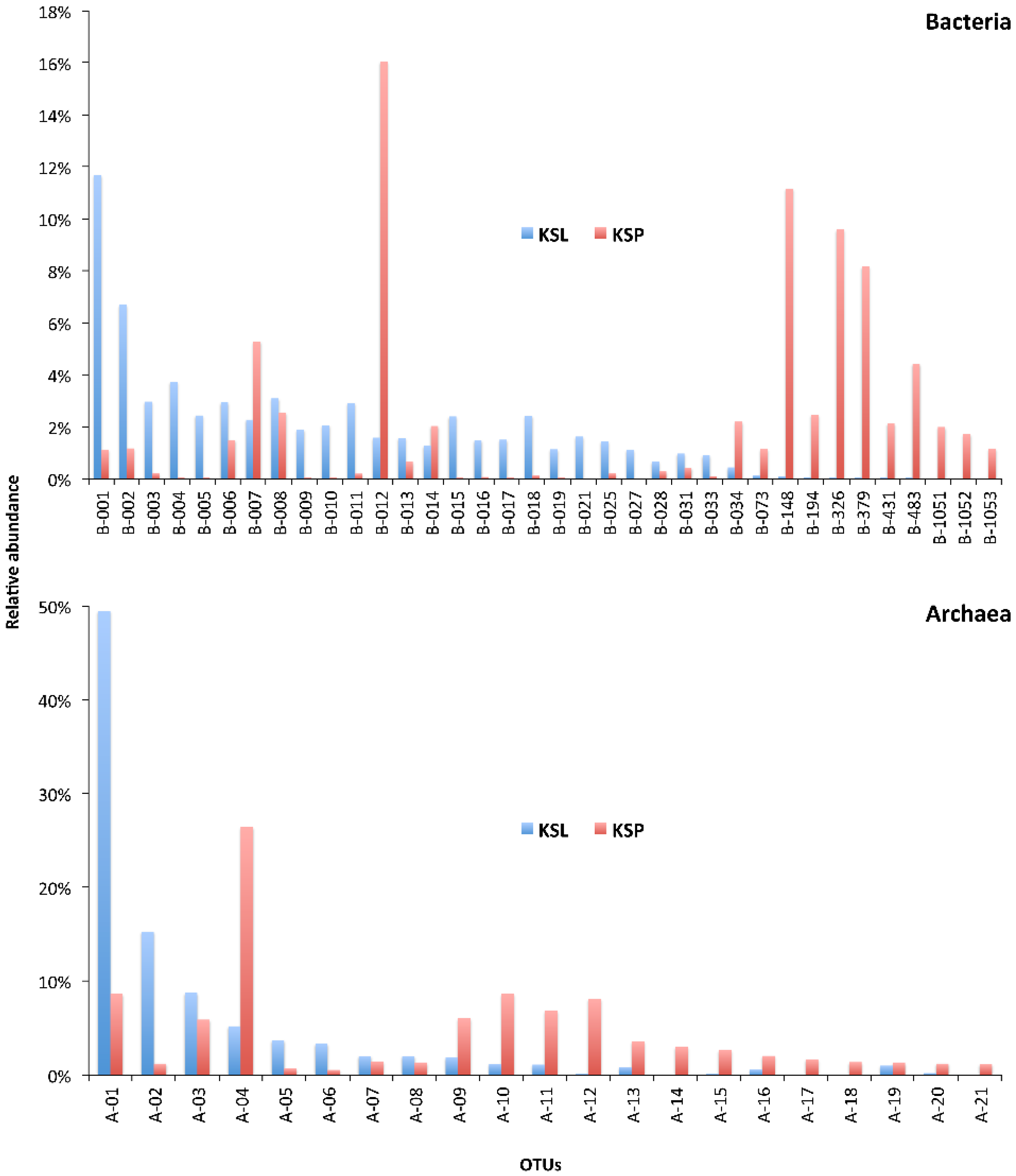
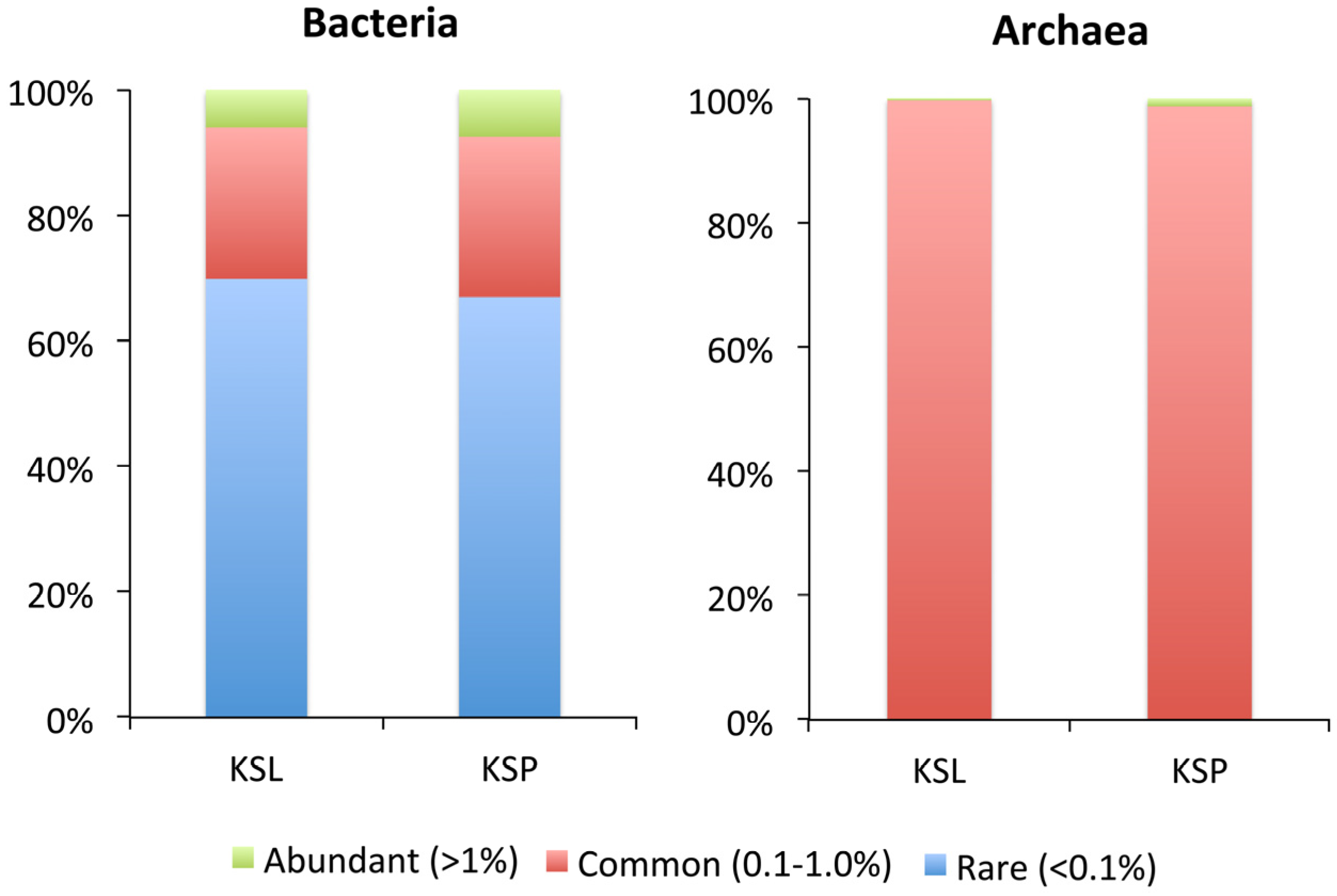
3. Results and Discussion
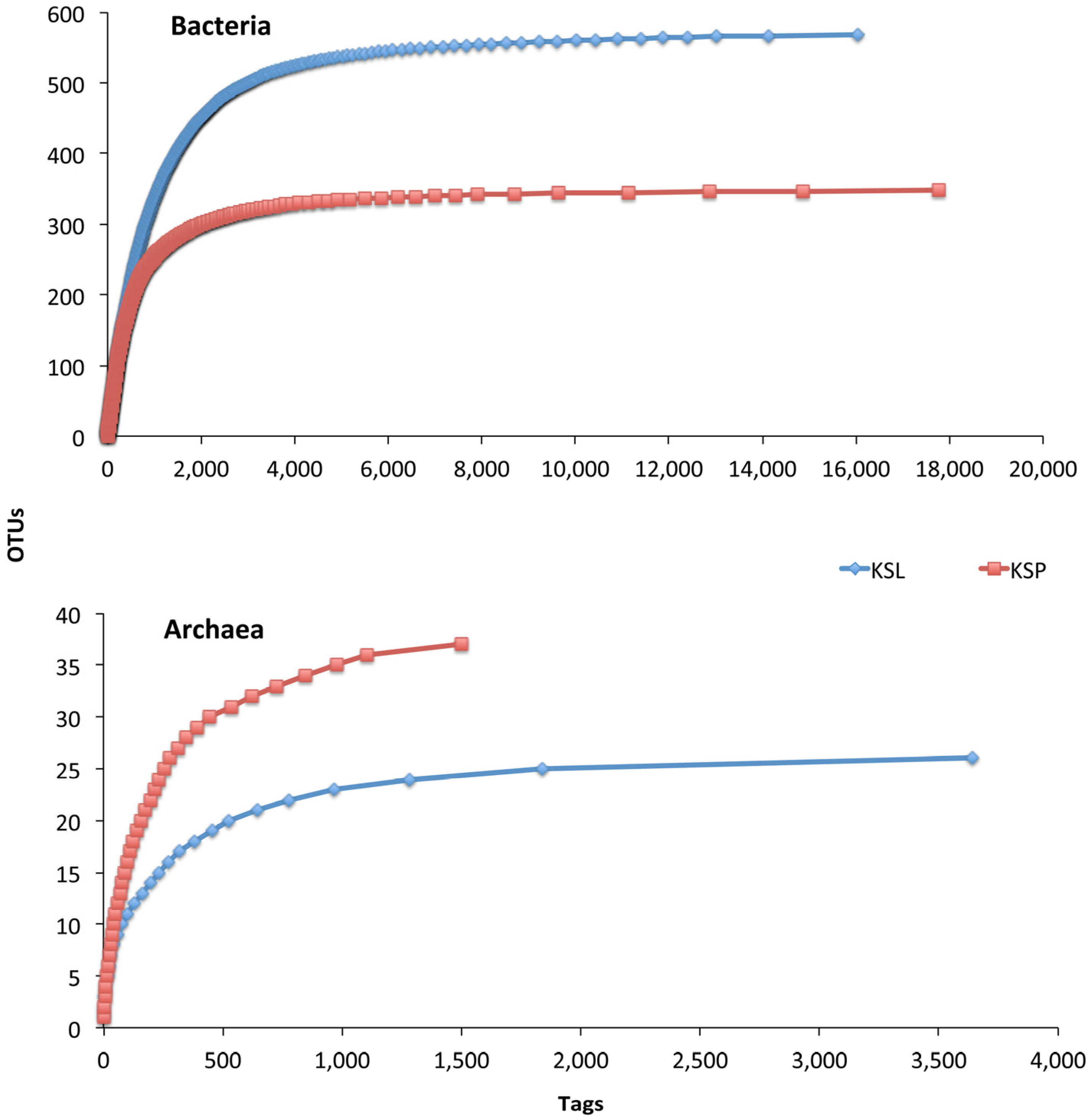
| Sample | Reads | OTUs 1 | Coverage (SChao) | Dominance of the Most Abundant OTU | No. of Most Dominant OTUs (Cumulative Relative Dominance) |
|---|---|---|---|---|---|
| Bacteria | |||||
| KSL | 16,529 | 568 | 91.8% | 11.7% | 29 (66.0%) |
| KSP | 18,056 | 348 | 92.6% | 16.0% | 11 (66.0%) |
| Archaea | |||||
| KSL | 3,637 | 25 | 99.4% | 49.5% | 3 (73.4%) |
| KSP | 1,500 | 36 | 93.9% | 26.4% | 8 (73.9%) |

4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zalidis, G.C.; Takavakoglou, V.; Panoras, A.; Bilas, G.; Katsavouni, S. Re-establishing a sustainable wetland at former Lake Karla, Greece, using Ramsar restoration guidelines. Environ. Manag. 2004, 34, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulos, P.; Mylopoulos, N.; Loukas, A. Optimal management of an overexploited aquifer under climate change: The lake Karla case. Water Resour. Manag. 2013, 27, 1635–1649. [Google Scholar] [CrossRef]
- Sidiropoulos, P. Simulation and Management of Groundwater Reservoir of Lake Karla with Modflow 2000. Bachelor’s Thesis, University of Thessaly, Karditsa, Greece, June 2004. [Google Scholar]
- Griebler, C.; Lueders, T. Microbial biodiversity in groundwater ecosystems. Freshw. Biol. 2009, 54, 649–677. [Google Scholar] [CrossRef]
- Pernthaler, J. Freshwater microbial communities. In The Prokaryotes; Rosenberg, E., DeLong, E., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Bourlat, S.J.; Borja, A.; Gilbert, J.; Taylor, M.I.; Davies, N.; Weisberg, S.B.; Griffith, J.F.; Lettieri, T.; Field, D.; Benzie, J.; et al. Genomics in marine monitoring: New opportunities for assessing marine health status. Marine Pollut. Bull. 2013, 74, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Meziti, A.; Kormas, K.A.; Moustaka-Gouni, M.; Karayanni, H. Spatially uniform but temporally variable bacterioplankton in a semi-enclosed coastal area. Syst. Appl. Microbiol. 2015, 38, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Pachiadaki, M.G.; Kormas, K.A. Interconnectivity vs. Isolation of prokaryotic communities in European deep-sea mud volcanoes. Biogeosciences 2013, 10, 2821–2831. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Wu, Y.; Tu, C.; Soininen, J.; Stegen, J.C.; He, J.; Liu, X.; Zhang, L.; Zhang, E. Phylogenetic beta diversity in bacterial assemblages across ecosystems: Deterministic versus stochastic processes. ISME J. 2013, 7, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, Y.; Gorby, Y.; Nealson, K.; Pan, Y. Integrating niche-based process and spatial process in biogeography of magnetotactic bacteria. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Zinger, L.; Amaral-Zettler, L.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Huse, S.M.; Welch, D.B.M.; Martiny, J.B.H.; Sogin, M.; Boetius, A.; Ramette, A. Global patterns of bacterial beta-diversity in seafloor and seawater ecosystems. PLoS ONE 2011, 6, e24570. [Google Scholar] [CrossRef] [PubMed]
- Dowd, S.; Callaway, T.; Wolcott, R.; Sun, Y.; McKeehan, T.; Hagevoort, R.; Edrington, T. Evaluation of the bacterial diversity in the feces of cattle using 16s rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing MOTHUR: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Quince, C.; Lanzen, A.; Curtis, T.P.; Davenport, R.J.; Hall, N.; Head, I.M.; Read, L.F.; Sloan, W.T. Accurate determination of microbial diversity from 454 pyrosequencing data. Nat. Meth. 2009, 6, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R. Search and clustering orders of magnitude faster than blast. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Reeder, J.; Knight, R. The rare biosphere: A reality check. Nat. Rev. Microbiol. 2009, 6, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Kunin, V.; Engelbrektson, A.; Ochman, H.; Hugenholtz, P. Wrinkles in the rare biosphere: Pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ. Microbiol. 2010, 12, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.; Ludwig, W.; Peplies, J.; Glöckner, F. Silva: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, G.; Granitsiotis, M.S.; Engel, M.; Lueders, T. Testing the limits of 454 pyrotag sequencing: Reproducibility, quantitative assessment and comparison to T-RFLP fingerprinting of aquifer microbes. PLoS ONE 2012, 7, e40467. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [PubMed]
- Smeti, E.; Kormas, K.A.; Spatharis, S. A non-phylogenetic alpha diversity approach on prokaryotic community structure in aquatic systems. Ecol. Indic. 2013, 29, 361–366. [Google Scholar] [CrossRef]
- Valentine, D.L. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat. Rev. Microbiol. 2007, 5, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Shabarova, T.; Pernthaler, J. Karst pools in subsurface environments: Collectors of microbial diversity or temporary residence between habitat types. Environ. Microbiol. 2010, 12, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Iwatsuki, T.; Naganuma, T. Phylogenetic characterization of 16s rRNA gene clones from deep-groundwater microorganisms that pass through 0.2-micrometer-pore-size filters. Appl. Environ. Microbiol. 2005, 71, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Jeffries, T.C.; Roudnew, B.; Fitch, A.J.; Seymour, J.R.; Delpin, M.W.; Newton, K.; Brown, M.H.; Mitchell, J.G. Metagenomic comparison of microbial communities inhabiting confined and unconfined aquifer ecosystems. Environ. Microbiol. 2012, 14, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.; Steinberg, P.; Rusch, D.; Kjelleberg, S.; Thomas, T. Bacterial community assembly based on functional genes rather than species. Proc. Natl. Acad. Sci. 2011, 108, 14288–14293. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A. What is microbial community ecology? ISME J. 2009, 3, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Schut, F.; de Vries, E.J.; Gottschal, J.C.; Robertson, B.R.; Harder, W.; Prins, R.A.; Button, D.K. Isolation of typical marine bacteria by dilution culture: Growth, maintenance, and characteristics of isolates under laboratory conditions. Appl. Environ. Microbiol. 1993, 59, 2150–2160. [Google Scholar] [PubMed]
- Lauro, F.M.; McDougald, D.; Thomas, T.; Williams, T.J.; Egan, S.; Rice, S.; DeMaere, M.Z.; Ting, L.; Ertan, H.; Johnson, J.; et al. The genomic basis of trophic strategy in marine bacteria. Proc. Natl. Acad. Sci. 2009, 106, 15527–15533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lai, Q.; Wang, W.; Li, S.; Shao, Z. Thioclava dalianensis sp. nov., isolated from surface seawater. Int. J. Syst. Evolut. Microbiol. 2013, 63, 2981–2985. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Tourova, T.P.; Spiridonova, E.M.; Rainey, F.A.; Muyzer, G. Thioclava pacifica gen. nov., sp. nov., a novel facultatively autotrophic, marine, sulfur-oxidizing bacterium from a near-shore sulfidic hydrothermal area. Int. J. Syst. Evolut. Microbiol. 2005, 55, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Könneke, M.; Bernhard, A.E.; de la Torre, J.R.; Walker, C.B.; Waterbury, J.B.; Stahl, D.A. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 2005, 437, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Mosier, A.C.; Allen, E.E.; Kim, M.; Ferriera, S.; Francis, C.A. Genome sequence of “Candidatus Nitrosopumilus salaria” BD31, an ammonia-oxidizing archaeon from the San Francisco Bay estuary. J. Bacteriol. 2012, 194, 2121–2122. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Kim, J.-G.; Jung, M.-Y.; Kim, S.-J.; Cha, I.-T.; Kwon, K.; Lee, J.-H.; Rhee, S.-K. Draft genome sequence of an ammonia-oxidizing archaeon, “Candidatus Nitrosopumilus koreensis” AR1, from marine sediment. J. Bacteriol. 2012, 194, 6940–6941. [Google Scholar] [CrossRef] [PubMed]
- Mylopoulos, N.; Kolokytha, E.; Loukas, A.; Mylopoulos, Y. Agricultural and water resources development in Thessaly, Greece in the framework of new European union policies. Int. J. River Basin Manag. 2009, 7, 73–89. [Google Scholar] [CrossRef]
- Sidiropoulos, P. Management of Groundwater Resources under Uncertainty Conditions the Value of Information in Environmentally Degraded Aquifers. Ph.D. Thesis, University of Thessaly, Karditsa, Greece, November 2014. [Google Scholar]
- Sebilo, M.; Mayer, B.; Nicolardot, B.; Pinay, G.; Mariotti, A. Long-term fate of nitrate fertilizer in agricultural soils. Proc. Natl. Acad. Sci. 2013, 110, 18185–18189. [Google Scholar] [CrossRef] [PubMed]
- Worrall, F.; Howden, N.J.K.; Burt, T.P. Evidence for nitrogen accumulation: The total nitrogen budget of the terrestrial biosphere of a lowland agricultural catchment. Biogeochemistry 2015, 123, 411–428. [Google Scholar] [CrossRef]
- Oikonomou, A.; Katsiapi, M.; Karayanni, H.; Moustaka-Gouni, M.; Kormas, K.A. Plankton microorganisms coinciding with two consecutive mass fish kills in a newly reconstructed lake. Sci. World J. 2012, 2012, 504135. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. 1998, 95, 6578–6583. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K. The deep subterranean biosphere. Earth Sci. Rev. 1993, 34, 243–260. [Google Scholar] [CrossRef]
- Krumholz, L.R. Microbial communities in the deep subsurface. Hydrogeol. J. 2000, 8, 4–10. [Google Scholar]
- Griebler, C.; Avramov, M. Groundwater ecosystem services: A review. Freshw. Sci. 2015, 34, 355–367. [Google Scholar] [CrossRef]
- Rinke, C.; Schwientek, P.; Sczyrba, A.; Ivanova, N.N.; Anderson, I.J.; Cheng, J.-F.; Darling, A.; Malfatti, S.; Swan, B.K.; Gies, E.A.; et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature 2013, 499, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, M.; Hugenholtz, P.; Skarshewski, A.; Nielsen, K.L.; Tyson, G.W.; Nielsen, P.H. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat. Biotech. 2013, 31, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Sogin, M.L.; Morrison, H.G.; Huber, J.A.; Welch, D.M.; Huse, S.M.; Neal, P.R.; Arrieta, J.M.; Herndl, G.J. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl. Acad. Sci. USA 2006, 103, 12115–12120. [Google Scholar] [CrossRef] [PubMed]
- Pedrós-Alió, C. The rare bacterial biosphere. Ann. Rev. Marine Sci. 2012, 4, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R.; Castenholz, R.W. The Archaea and the Deeply Branching and Phototrophic Bacteria; Springer: Berlin, Germany, 2001. [Google Scholar]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T. The Proteobacteria; Springer: Berilin, Germany, 2001. [Google Scholar]
- De Vos, P.; Garrity, G.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.-H.; Whitman, W.B. The Firmicutes; Springer: Berlin, Germany, 2009. [Google Scholar]
- Goodfellow, M.; Kämpfer, P.; Busse, H.-J.; Trujillo, M.E.; Suzuki, K.-I.; Ludwig, W.; Whitman, W.B. The Actinobacteria; Springer: Berlin, Germany, 2012. [Google Scholar]
- Rosenberg, E.; DeLong, E.; Lory, S.; Stackebrandt, E.; Thompson, F. The Prokaryotes: Prokaryotic Biology and Symbiotic Associations; Springer: Berlin, Germany, 2013. [Google Scholar]
- Rosenberg, E.; DeLong, E.; Lory, S.; Stackebrandt, E.; Thompson, F. The Prokaryotes: Prokaryotic Communities and Ecophysiology; Springer: Berlin, Germany, 2013. [Google Scholar]
- Rosenberg, E.; DeLong, E.; Lory, S.; Stackebrandt, E.; Thompson, F. The Prokaryotes: Prokaryotic Physiology and Biochemistry; Springer: Berlin, Germany, 2013. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kormas, K.A.; Meziti, A.; Papadimitriou, T. Microbiological Confinement of Two Adjacent Water Wells in Lake Karla Basin, Greece. Water 2015, 7, 5272-5283. https://doi.org/10.3390/w7105272
Kormas KA, Meziti A, Papadimitriou T. Microbiological Confinement of Two Adjacent Water Wells in Lake Karla Basin, Greece. Water. 2015; 7(10):5272-5283. https://doi.org/10.3390/w7105272
Chicago/Turabian StyleKormas, Konstantinos Ar., Alexandra Meziti, and Theodoti Papadimitriou. 2015. "Microbiological Confinement of Two Adjacent Water Wells in Lake Karla Basin, Greece" Water 7, no. 10: 5272-5283. https://doi.org/10.3390/w7105272
APA StyleKormas, K. A., Meziti, A., & Papadimitriou, T. (2015). Microbiological Confinement of Two Adjacent Water Wells in Lake Karla Basin, Greece. Water, 7(10), 5272-5283. https://doi.org/10.3390/w7105272







