Sorption of Emerging Organic Wastewater Contaminants to Four Soils
Abstract
:1. Introduction
2. Materials and Methods (or Experimental)
2.1. Chemicals and Soil
| Chemical | Source | CASRN | Retention Time (min) | Qualifying Ions (m/z) |
|---|---|---|---|---|
| Triclosan | KIC Chem, New Paltz, NY, USA | 3380-34-5 | 16.682 | 289.9 (218.0, 146.0) |
| 4-Nonylphenol | Aldrich, St. Louis, MO, USA | 25154-52-3 | 8.22–12.0 | 135.1 (220.2, 107.1, 121.1) |
| Bisphenol A | Aldrich, St. Louis, MO, USA | 80-05-7 | 17.610 | 213.1 (228.1, 119.0) |
| Estrone | Aldrich, St. Louis, MO, USA | 53-16-7 | 23.931 | 270.2 (146.1, 185.1, 172.1) |
| 17b-Estradiol | Aldrich, St. Louis, MO, USA | 50-28-2 | 24.106 | 272.2 (160.1, 213.1) |
| 17a-Ethynylestradiol | Aldrich, St. Louis, MO, USA | 57-63-6 | 24.993 | 213.1 (296.2, 160.1, 133.1) |
| 4-normal-nonylphenol | Ehrenstorfer, Augsburg, Germany | 104-40-5 | 12.785 | 107.05 (220.2, 77.0) |
| d6 Bisphenol A | CIL, Tewksbury, MA, USA | 86588-58-1 | 17.551 | 216.1 (234.1, 121.1) |
| d4 17β-estradiol | CIL, Tewksbury, MA, USA | 66789-03-5 | 24.084 | 276.2 (161.1, 147.1, 214.1) |
| Characteristic | Sand | Loamy Sand | Sandy Loam | Loam |
|---|---|---|---|---|
| Organic Carbon (%) | 0.1 | 2.1 | 3.1 | 5.4 |
| Soil pH | 10.4 | 5.8 | 6.7 | 6.9 |
| CEC (cmol(+)/kg) | 6.0 | 12.0 | 11.3 | 26.4 |
| Ca (% of cations) | 86.6 | 54.2 | 73.3 | 66.2 |
| Mg (% of cations) | 10.9 | 12.5 | 19.6 | 16.7 |
| K (% of cations) | 1.0 | 10.2 | 6.6 | 5.4 |
| Na (% of cations) | 1.4 | 1 | 0.5 | 0.4 |
| % Sand | 98 | 86 | 65 | 30 |
| % Silt | 0.8 | 6 | 15 | 44 |
| % Clay | 1.2 | 8 | 20 | 26 |
2.2. Experimental Setup
2.3. Aqueous Phase OWC Analysis
2.4. Sorption Coefficients
3. Results and Discussion
3.1. Sorption of OWCs on Different Soils
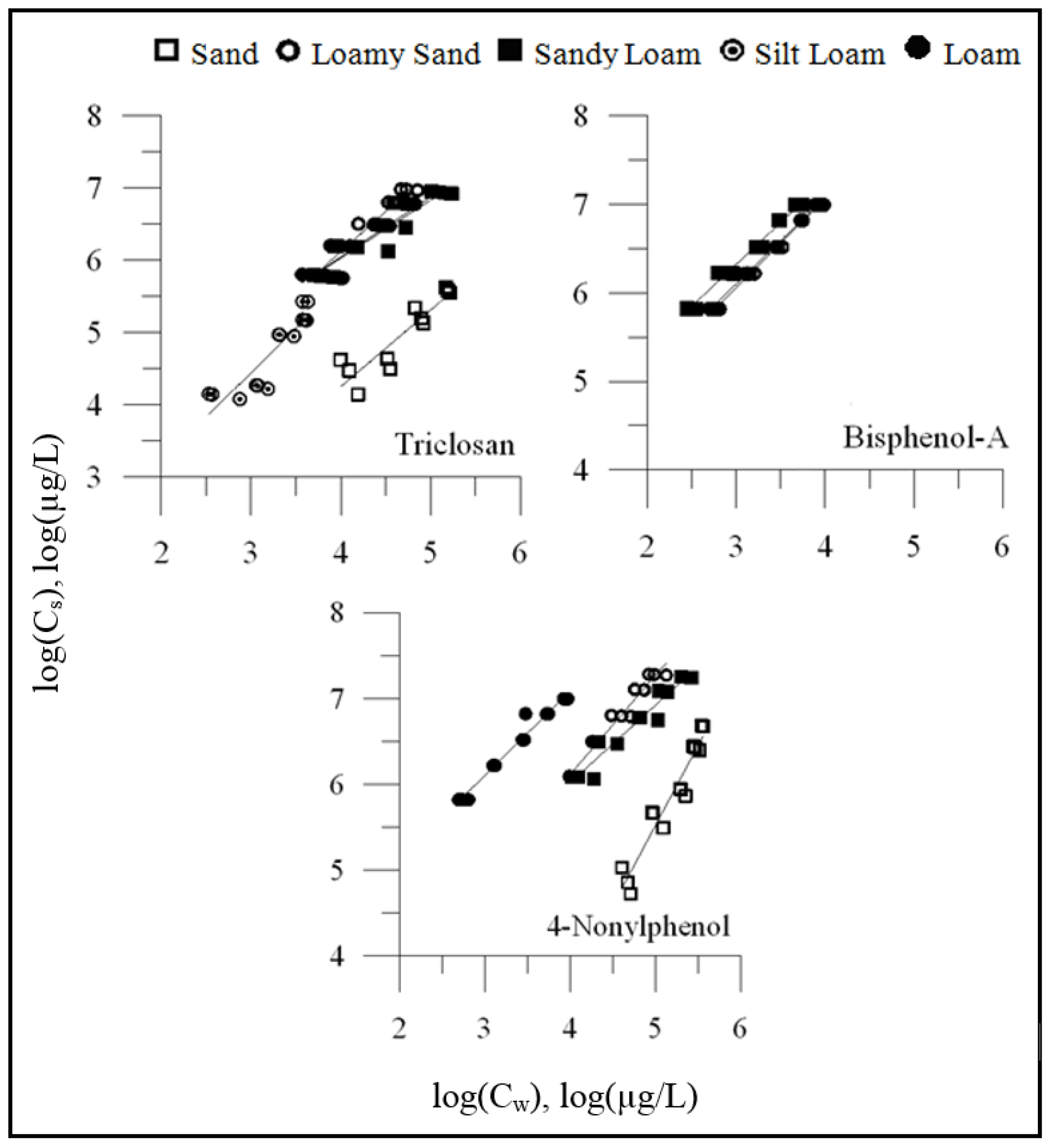
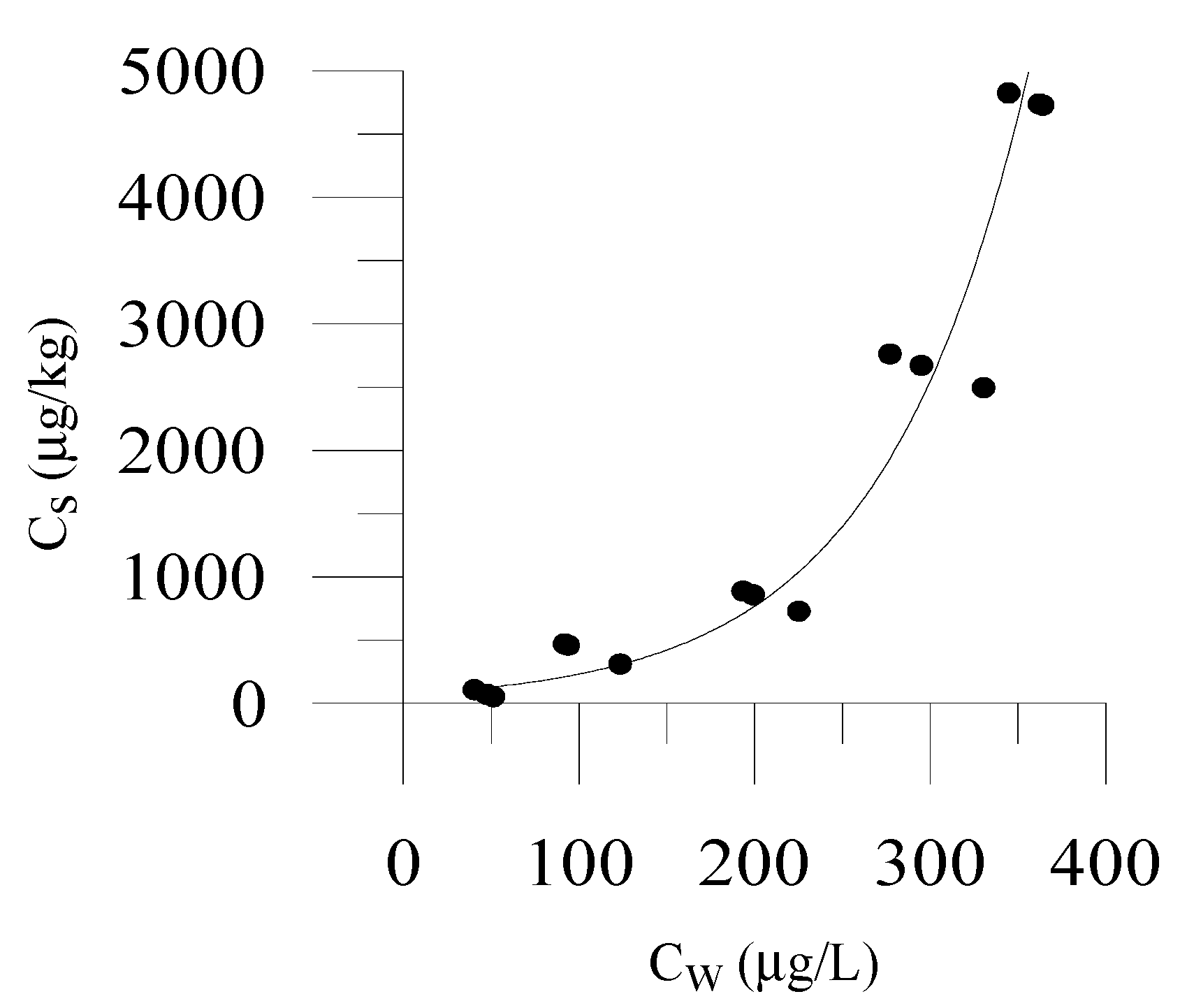
3.2. Relationship between OWC Sorption and Fraction of Organic Carbon
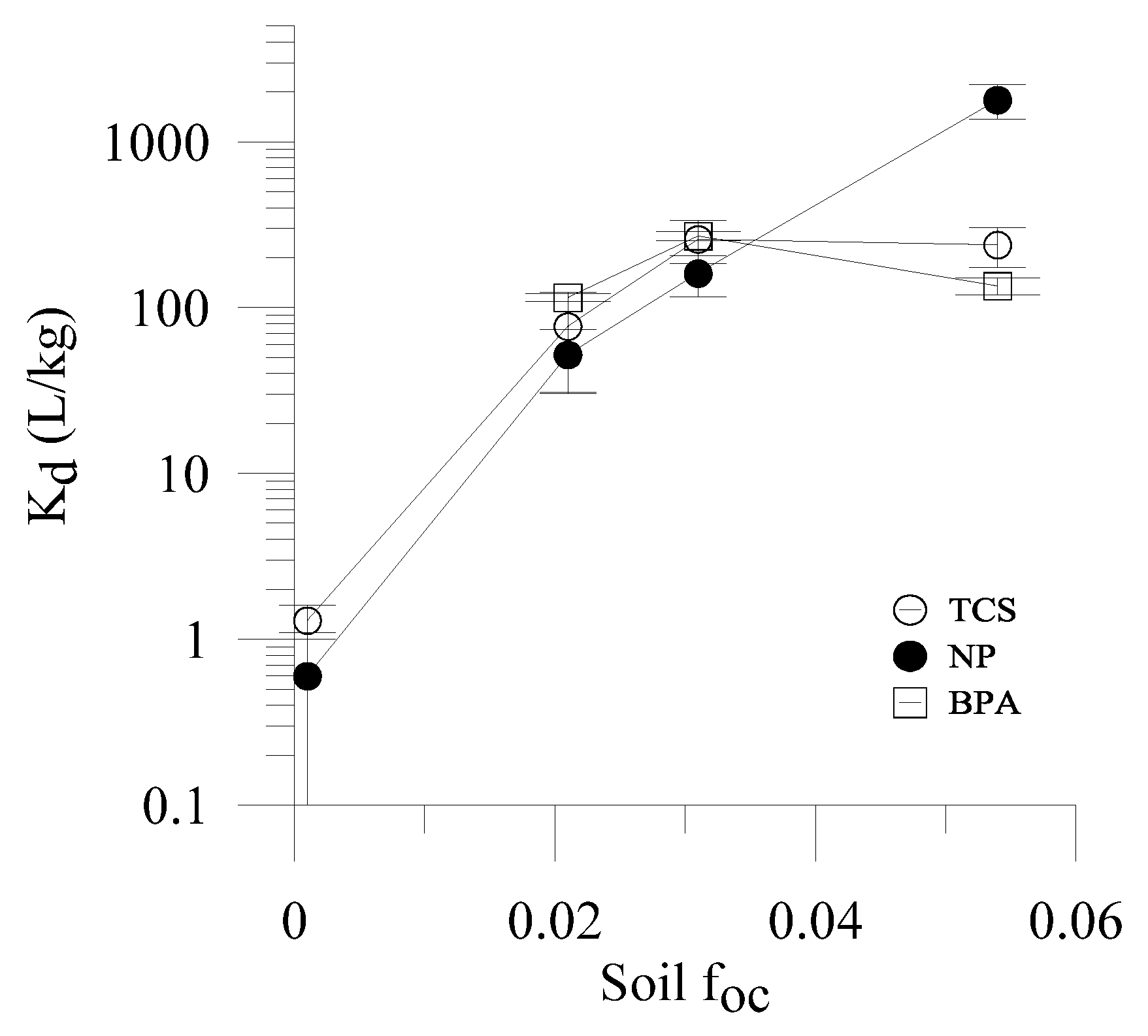
3.3. Influence of pH on Sorption of Anionic forms of Aqueous OWCs

 is the sorption coefficient for neutral species. This relationship assumes that the deprotonated (negatively charged) OWC species do not significantly contribute to sorption of the chemical [35,36].
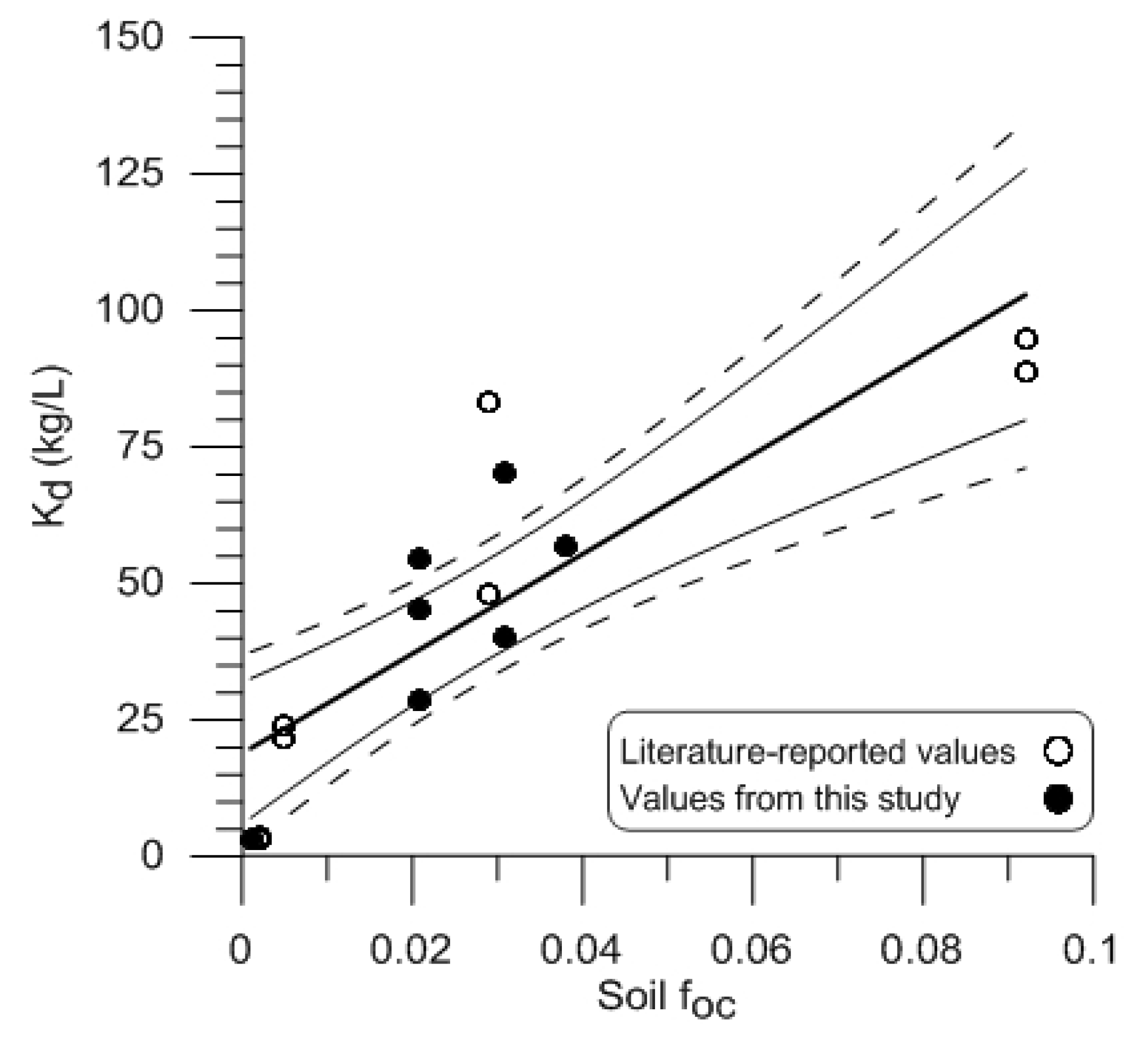
is the sorption coefficient for neutral species. This relationship assumes that the deprotonated (negatively charged) OWC species do not significantly contribute to sorption of the chemical [35,36].3.4. Comparison of Experimentally Determined and Literature Reported Koc Values
| Chemical | Measured Log(Koc) Values | Literature Reported Log(Koc) Values | Chemical Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| Ave ± SD (N) | Range | Range | Predicted | Measured–Single Point | Measured–Isotherm | pKa | Log (Kow) | |
| 4 Nonylphenol | 3.61 ± 0.73 (4) | 2.74–4.52 | 3.9–5.4 | ND | 4.7–5.6 d, 5.3 e, 4.5–5.2 f, 4.7 e | 3.9 j, 4.6 k | 10.28 o | 4.48 t |
| Triclosan | 3.56 ± 0.34 (5) | 3.12–3.92 | 3.2–4.6 | 3.8–4.0 a, 3.2 b | 4.6 g | ND | 7.9 p | 4.76 p |
| Bisphenol–A | 3.69 ± 0.27 (3) | 3.40–3.94 | 2.8–3.9 | 3.2 c, 2.8 b | ND | 3.5 i, 3.8 l, 2.9 m, 3.9 n | 9.6–11.3 q | 3.40 q |
| Estrone | 3.50 ± 0.16 (3) | 3.34–3.65 | 3.2–3.6 | 3.6 b | 3.2 h, 3.3 i | 3.3 l | 10.4 r | 2.95 u |
| 17β-Estradiol | 3.23 ± 0.16 (3) | 3.11–3.42 | 3.3–3.6 | 3.6 b | 3.3 i | 3.3 l | 10.2 s | 3.86 u |
| 17α-Ethynylestradiol | 3.25 ± 0.16 (2) | 3.13–3.36 | 2.9–3.3 | ND | 3.2 h, 3.3 i | 2.9 l | 10.2 s | 3.67 u |
| Chemical | Sand foc = 0.001 | Loamy Sand foc = 0.021 | Sandy Loam foc = 0.031 | Loam foc = 0.054 | ||||
|---|---|---|---|---|---|---|---|---|
| n | Kd | n | Kd | n | Kd | n | Kd | |
| NP | 1.87 ± 0.32 | 0.6 ± 0.5 | 1.15 ± 0.15 | 52.0 ± 21.7 | 0.90 ± 0.14 | 161 ± 44.8 | 1.05 ± 0.21 | 1783 ± 419 |
| TCS | 1.06 ± 0.37 | 1.3 ± 0.3 | 1.11 ± 0.23 | 77.2 ± 46.5 | 0.82 ± 0.18 | 259 ± 75.5 | 0.83 ± 0.17 | 239 ± 63.9 |
| BPA | ND | ND | 0.99 ± 0.04 | 115 ± 5.9 | 0.95 ± 0.07 | 271 ± 16.9 | 0.98 ± 0.10 | 135 ± 15.3 |
| E1 | 1.22 ± 0.25 | 3.2 ± 0.6 | 0.90 ± 0.20 | 45.5 ± 23.6 | 0.89 ± 0.11 | 255 ± 48.0 | ND | ND |
| E2 | ND | ND | 0.87 ± 0.19 | 54.6 ± 0.6 | 0.93 ± 0.10 | 40.1 ± 10.9 | ND | ND |
| EE2 | ND | ND | 0.98 ± 0.22 | 28.6 ± 20.5 | 0.85 ± 0.08 | 70.3 ± 12.0 | ND | ND |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- U.S. Environmental Protection Agency. Onsite Wastewater Treatment Systems Manual; U.S. Environmental Protection Agency: Cincinnati, OH, US, 2002. [Google Scholar]
- Conn, K.E.; Barber, L.B.; Brown, G.K.; Siegrist, R.L. Occurrence and fate of organic contaminants during onsite wastewater treatment. Environ. Sci. Technol. 2006, 40, 7358–7366. [Google Scholar] [CrossRef]
- Matamoros, V.; Arias, C.; Brix, H.; Bayona, J.M. Preliminary screening of small-scale domestic wastewater treatment systems for removal of pharmaceutical and personal care products. Water Res. 2009, 43, 55–62. [Google Scholar] [CrossRef]
- Brown, A.R.; Riddle, A.M.; Cunningham, N.L.; Kedwards, T.J.; Shillabeer, N.; Hutchinson, T.H. Predicting the effects of endocrine disrupting chemicals on fish populations. Hum. Ecol. Risk Assess. 2003, 9, 761–788. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Chaudhry, M.I.; Arshad, M. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2002, 40, 65–81. [Google Scholar]
- Robertson, W.D. Chemical fate and transport in a domestic septic system: Site description and attenuation of dichlorobenzene. Environ. Toxicol. Chem. 1994, 13, 183–191. [Google Scholar] [CrossRef]
- Glaser, A. The Ubiquitous Triclosan: A Common Antibacterial Agent Exposed. Beyond Pesticides Fact Sheet. Available online: www.beyondpesticides.org/pesticides/factsheets/Triclosan%20cited.pdf (accessed on 24 March 2004).
- Singh, L. An Experiment to Identify Levels of Triclosan That are Harmful to Bacteria in Puget Sound; University of Washington: Seattle, WA, USA, 2007. [Google Scholar]
- Suarez, S.; Dodd, M.; Omil, F.; von Gunten, U. Kinetics of triclosan oxidation by aqueous ozone and consequent loss of antibacterial activity: Relevance to municipal wastewater ozonation. Water Res. 2007, 41, 2481–2490. [Google Scholar] [CrossRef]
- Das, B.S.; Lee, L.S.; Rao, P.C.; Hultgren, R.P. Sorption and degradation of steroid hormones in soils during transport: Column studies and model evaluation. Environ. Sci. Technol. 2004, 38, 1460–1470. [Google Scholar] [CrossRef]
- Duering, R. Sorption behavior of nonylphenol in terrestrial soils. Environ. Sci. Technol. 2002, 36, 4052–4057. [Google Scholar] [CrossRef]
- Duran-Alverez, J.; Prado, B.; Ferroud, A.; Narcedalia, J.; Jimenez-Cisneros, B. Sorption, desorption and displacement of ibuprofen, estrone, and 17β-estradiol in wastewater irrigated and rain-fed agricultural soils. Sci. Total Environ. 2014, 473–474, 189–198. [Google Scholar]
- Lee, L.S.; Strock, T.J.; Sarmah, A.K.; Rao, P.C. Sorption and dissipation of testosterone, estrogens, and their primary transformation products in soils and sediment. Environ. Sci. Technol. 2003, 37, 4098–4105. [Google Scholar] [CrossRef]
- Scheytt, T.; Mersmann, P.; Lindstädt, R.; Heberer, T. Determination of sorption coefficients of pharmaceutically active substances carbamazepine, diclofenac, and ibuprofen, in sandy sediments. Chemosphere 2005, 60, 245–253. [Google Scholar] [CrossRef]
- Yu, Z.; Xiao, B.; Huang, W.; Peng, P. Sorption of steroid estrogens to soils and sediments. Environ. Toxicol. Chem. 2004, 23, 531–539. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Westall, J. Transport of nonpolar organic compounds from surface water to groundwater. Laboratory sorption studies. Environ. Sci. Technol. 1981, 15, 1360–1367. [Google Scholar] [CrossRef]
- Wilson, J.T.; Enfield, C.G.; Dunlap, W.J.; Cosby, R.L.; Foster, D.A.; Baskin, L.B. Transport and fate of selected organic pollutants in a sandy soil. J. Environ. Qual. 1981, 10, 501–506. [Google Scholar]
- Zwiener, C.; Frimmel, F.H. Short-term tests with a pilot sewage plant and biofilm reactors for the biological degradation of the pharmaceutical compounds clofibric acid, ibuprofen, and diclofenac. Sci. Total Environ. 2003, 309, 201–211. [Google Scholar] [CrossRef]
- Lietz, A.C.; Meyer, M.T. Evaluation of emerging contaminants of concern at the south district wastewater treatment plant based on seasonal sampling events, Miami-Dade County, Florida, 2004. In Scientific Investigations Report 2006–5240; U.S. Geological Survey: Reston, VA, USA, 2006. [Google Scholar]
- Swartz, C.H.; Reddy, S.; Benotti, M.J.; Yin, H.; Barber, L.B.; Brownawell, B.J.; Rudel, R.A. Steroid estrogens, nonylphenol, ethoxylate metabolites, and other wastewater contaminants in groundwater affected by a residential septic system on Cape Cod, MA. Environ. Sci. Technol. 2006, 40, 4892–4900. [Google Scholar]
- Barber, L.B.; Thurman, E.M.; Schroeder, M.P.; LeBlanc, D.R. Long-term fate of organic microbpolutants in sewage-contaminated ground water. Environ. Sci. Technol. 1998, 22, 205–211. [Google Scholar]
- Howdeshell, K.L.; Hotchkiss, A.K.; Thayer, K.A.; Vandenbergh, J.G.; Vom Saal, F.S. Environmental toxins: Exposure to bisphenol A advances puberty. Nature 1999, 401, 763–764. [Google Scholar] [CrossRef]
- Lech, J.J.; Lewis, S.K.; Ren, L. In vivo estrogenic activity of nonylphenol in rainbow trout. Fundam. Appl. Toxicol. 1996, 30, 229–232. [Google Scholar] [CrossRef]
- Orvos, D.R.; Versteeg, D.J.; Inauen, J.; Capdevielle, M.; Rothenstein, A.; Cunningham, V. Aquatic toxicity of triclosan. Environ. Toxicol. Chem. 2002, 21, 1338–1349. [Google Scholar] [CrossRef]
- Roberts, P.; Roberts, J.P.; Jones, D.L. Behavior of the endocrine disrupting chemical nonylphenol in soil: Assessing the risk associated with spreading contaminated waste to land. Soil Biol. Biochem. 2006, 38, 1812–1822. [Google Scholar] [CrossRef]
- Ying, G.G.; Kookana, R.S. Triclosan in wastewaters and biosolids from Australian wastewater treatment plants. Environ. Int. 2007, 33, 199–205. [Google Scholar] [CrossRef]
- Casey, F.M.; Simunek, J.; Lee, J.; Larsen, G.L.; Hakk, H. Sorption, mobility, and transformation of estrogenic hormones in natural soil. J. Environ. Qual. 2005, 34, 1372–1379. [Google Scholar] [CrossRef]
- Fent, G.; Hein, W.J.; Moendel, M.J.; Kubiak, R. Fate of 14C-bisphenol A in soils. Chemosphere 2003, 51, 735–746. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. Environmental Organic Chemistry; Wiley-Interscience: New York, NY, USA, 2003. [Google Scholar]
- Karnjanapiboonwong, A.; Morse, A.N.; Maul, J.D.; Anderson, T.A. Sorption of estrogens, triclosan, and caffeine in a sandy loam and a silt loam soil. J. Soils Sediments 2010, 7, 1300–1307. [Google Scholar] [CrossRef]
- Goloub, T.P.; Koopal, L.K.; Bijsterbosch, B.H.; Sidorova, M.P. Adsorption of cationic surfactants on silica. Surface charge effects. Langmuir 1996, 12, 3188–3194. [Google Scholar] [CrossRef]
- Xiaoping, L.; Laixiang, Z.; Linlin, Y.; Jiale, L.; Liang, X; Mulindankaka, E. Sorption behavior of nonylphenol (NP) on sewage-irrigated soil: Kenetic and thermodynamic studies. Sci. Total Environ. 2012, 473–474, 520–536. [Google Scholar]
- Scheytt, T.J.; Mersmann, P.; Heberer, T. Mobility of pharmaceuticals carbamazepine, diclofenac, ibuprofen, and propyphenazone in miscible-displacement experiments. J. Contam. Hydrol. 2006, 83, 53–69. [Google Scholar] [CrossRef]
- Tsai, W.; Chi-Wei, S.; Ting-Yi, S. Adsorption of bisphenol-A from aqueous solution onto minerals and carbon adsorbents. J. Hazard. Mate. 2006, 134, 169–175. [Google Scholar] [CrossRef]
- Escher, B.I.; Scharzenbach, R.P. Partitioning of substituted phenols in liposome−water, biomembrane−water, and octanol−water systems. Environ. Sci. Technol. 1995, 30, 260–270. [Google Scholar] [CrossRef]
- Tulp, H.C.; Fenner, K.; Schwarzenbach, R.P.; Goss, K.U. pH-dependent sorption of acidic organic chemicals to soil organic matter. Environ. Sci. Technol. 2009, 43, 419–445. [Google Scholar]
- Shareef, A.; Angove, M.J.; Wells, J.D.; Johnson, B.B. Sorption of bisphenol A, 17 -ethynylestradiol and estrone to mineral surfaces. J. Colloid Interface Sci. 2006, 297, 62–69. [Google Scholar] [CrossRef]
- Butler, E.; Whelan, M.J.; Sakrabani, R.; van Egmond, R. Fate of triclosan in field soils receiving sewage sludge. Environ. Pollut. 2012, 167, 101–109. [Google Scholar] [CrossRef]
- Lindstroem, A.; Buerge, I.J.; Poiger, T.; Bergqvist, P.A.; Mueller, M.D.; Buser, H.R. Occurrence and environmental behavior of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ. Sci. Technol. 2002, 36, 2322–2329. [Google Scholar] [CrossRef]
- Staples, C.A.; Dome, P.B.; Klecka, G.M.; Oblock, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Sekela, M.; Brewer, R.; Moyle, G.; Tuominen, T. Occurrence of an environmental estrogen (4-nonylphenol) in sewage treatment plant effluent and the aquatic receiving environment. Water Sci. Technol. 1999, 39, 217–220. [Google Scholar]
- Ferguson, P.L.; Iden, C.R.; Brownawell, B.J. Distribution and fate of neutral alkylphenol ethoxylate metabolites in a sewage-impacted urban estuary. Environ. Sci. Technol. 2001, 35, 2428–2435. [Google Scholar] [CrossRef]
- Isobe, T.; Nishiyama, H.; Nakashima, A.; Takada, H. Distribution and behavior of nonylphenol, octylphenol, and nonylphenol monoethoxylate in Tokyo metropolitan area: Their association with aquatic particles and sedimentary distributions. Environ. Sci. Technol. 2001, 35, 1041–1049. [Google Scholar] [CrossRef]
- Singer, H.; Müller, S.; Tixier, C.; Pillonel, L. Triclosan: Occurrence and fate of a widely used biocide in the aquatic environment: Field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ. Sci. Technol. 2002, 36, 4998–5004. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Ying, G.G.; Kookana, R.S.; Dillon, P. Sorption and degradation of selected five endocrine disrupting chemicals in aquifer material. Water Res. 2003, 37, 3785–3791. [Google Scholar] [CrossRef]
- Li, C.; Li, X.Z.; Graham, N.; Gao, N.Y. The aqueous degradation of bisphenol A and steroid estrogens by ferrate. Water Res. 2008, 42, 109–120. [Google Scholar]
- Müller, S.; Schlatter, C. Estrogenic potency of nonylphenol in vivo—A case study to evaluate the relevance of human non-occupational exposure. Pure Appl. Chem. 1998, 70, 1847–1853. [Google Scholar] [CrossRef]
- National Library of Medicine. Triclosan. Available online: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+7194 (accessed on 12 October 2012).
- Cousins, I.T.; Staples, C.A.; Kleĉka, G.M.; Mackay, D. A multimedia assessment of the environmental fate of bisphenol A. Hum. Ecol. Risk Assess. 2002, 8, 1107–1135. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I. Adsorption and transport of trace contaminant estrone in NF/RO membranes. Environ. Eng. Sci. 2002, 19, 441–451. [Google Scholar] [CrossRef]
- Hurwitz, A.R.; Liu, S.T. Determination of aqueous solubility and pKa values of estrogens. J. Pharm. Sci. 1977, 66, 624–627. [Google Scholar] [CrossRef]
- Ahel, M.; Giger, W. Partitioning of alkylphenols and alkylphenol polyethoxylates between water and organic solvents. Chemosphere 1993, 26, 1471–1478. [Google Scholar] [CrossRef]
- Machatha, S.G.; Yalkowsky, S.H. Comparison of the octanol/water partition coefficients calculated to experimentally determined values. Int. J. Pharm. 2005, 294, 185–192. [Google Scholar] [CrossRef]
- Gerstl, Z. Estimation of organic chemical sorption by soils. J. Contam. Hydrol. 1990, 6, 357–375. [Google Scholar] [CrossRef]
- Patrolecco, L.; Capri, S.; Angelis, S.D.; Pagnotta, R.; Polesello, S.; Valsecchi, S. Partition of nonylphenol and related compounds among different aquatic compartments in Tiber River (Central Italy). Water Air Soil Pollut. 2006, 172, 151–166. [Google Scholar] [CrossRef]
- Porter, A.J.; Hayden, N.J. Nonylphenol in the Environment: A Critical Review. Available online: http://www.emba.uvm.edu/~nhayden/npreview.Pdf (accessed on 12 August 2002).
- Huang, W.; Weber, W.J. A distributed reactivity model for sorption by soils and sediments. Relationships between desorption, hysteresis, and the chemical characteristics of organic domains. Environ. Sci. Technol. 1997, 31, 2562–2569. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Roberts, S.; Higgins, C.; McCray, J. Sorption of Emerging Organic Wastewater Contaminants to Four Soils. Water 2014, 6, 1028-1042. https://doi.org/10.3390/w6041028
Roberts S, Higgins C, McCray J. Sorption of Emerging Organic Wastewater Contaminants to Four Soils. Water. 2014; 6(4):1028-1042. https://doi.org/10.3390/w6041028
Chicago/Turabian StyleRoberts, Sarah, Christopher Higgins, and John McCray. 2014. "Sorption of Emerging Organic Wastewater Contaminants to Four Soils" Water 6, no. 4: 1028-1042. https://doi.org/10.3390/w6041028
APA StyleRoberts, S., Higgins, C., & McCray, J. (2014). Sorption of Emerging Organic Wastewater Contaminants to Four Soils. Water, 6(4), 1028-1042. https://doi.org/10.3390/w6041028





