1. Introduction
Soil moisture is often limiting for optimum quality and growth of turfgrass during the spring through fall period in southeastern Virginia. Reclaimed water may be a more reliable irrigation source than potable water during times when potable water supplies are limited, such as periods of extreme summer drought.
The Hampton Roads Sanitation District (HRSD) operates the Virginia Initiatives Plant (VIP), a wastewater treatment facility in Norfolk, Virginia that employs biological nutrient removal to generate an effluent containing low concentrations of nitrogen (N) and phosphorus (P). Based on preliminary turfgrass irrigation studies at the VIP, Evanylo
et al. [
1] discovered that the constituents in the recycled water that pose the greatest agronomic and environmental risk are likely to be dissolved solids, nitrogen (N), and phosphorus (P). Potential problems that may arise from the use of such effluent for irrigating turfgrass include: (1) high soluble salt, Na, or Cl concentrations may impair plant growth and quality, (2) high concentrations of Na may disperse soil colloids and degrade soil structure, and (3) excessive concentrations of N and P may contaminate ground and surface water [
2].
Carrow and Duncan [
3] and the USGA [
2] have described the effects of recycled water for turfgrass irrigation on soil, plant and water quality. Irrigation of turfgrass with saline wastewater effluent can cause stress and injury by water deficiency, ion toxicity, and ion imbalances. Salt-induced plant water stress is termed “physiological” or “osmotic” drought. Limited water uptake reduces cell turgor, leaf size, photosynthesis, carbohydrate storage, and rooting. Such effects may result in a lower performing turfgrass that may have poor tolerance to and recovery from wear [
3].
The potential for physiological drought can be determined by measuring the electrical conductivity of the effluent or ECw. The critical EC for creeping bentgrass and hybrid bermudagrass performance have been estimated as 3–6 dS m
−1 and 6–10 dS m
−1 [
3], respectively. The electrical conductivity of the VIP effluent has ranged from 0.9 to 2.8 dS m
−1 [
1]; thus, turfgrass performance would not be expected to be adversely affected by soluble salts from the VIP recycled water.
Continuous use of the saline irrigation source without flushing of salts by rainfall may result in an accumulation of salts detrimental to turfgrass performance. High concentrations of salts in the soil can induce drought symptoms in plants due to reduced osmotic potential [
4]. A 50% inhibition of growth has been reported for bentgrass and bermudagrass grown in soil at electrical conductivity (ECe) values of 8 dS m
−1 and 22 dS m
−1, respectively. Soil ECe values ranged from 0.1 to 0.5 dS m
−1 after a growing season of irrigation with recycled effluent at the HRSD VIP in 2001, but ample rainfall prevented a great reliance on the recycled water to meet soil moisture needs.
Salt-induced physiological drought is caused by high total salinity without regard to the salt type. By contrast, ion toxicity is caused by specific ions, such as Na
+, Cl
−, and H
3BO
3, which are directly toxic to root or shoot tissues [
3]. Injury to foliage occurs at >70 mg Na L
−1 and >100 mg Cl L
−1, and to roots at 70 to 210 mg Na L
−1 and 70 to 355 mg Cl L
−1 [
3].
High levels of Na, Cl, and other ions can induce nutrient imbalances and deficiencies of Ca, K, N, Mg, Mn, and P [
3]. Evanylo
et al. [
1] did not discover any tissue nutrient deficiencies, but the results were obtained from a non-replicated demonstration study; thus, further research is required to assess the potential for plant nutrition effects.
Long standing recommendations for irrigating with water that contains a high soluble salt content includes using a leaching fraction of 10% [
5]. The leaching fraction is the amount of irrigation water above that required by the crop to maintain acceptable root zone salinity. It remains to be seen whether irrigation rates of reclaimed water necessary to prevent excessive salt accumulation in the soil profile will result in acceptable NO
3-N and P leaching amounts.
Bentgrass and bermudagrass are turfgrass species whose extensive root systems are capable of high water use, excellent potential as nitrogen assimilators, and high moisture tolerance [
6]. Such crops can remove a highly mobile ion like nitrate from a volume of water larger than that transpired by the plant through a combination of mass flow and diffusion.
Hayes
et al. [
7] demonstrated that the use of wastewater effluent to supply adequate water for bermudagrass growth in Arizona resulted in increased concentrations of soil nitrate. It is important to determine the optimum combinations of water and nutrient loads to support turfgrass production without impairing groundwater. King and Balogh [
8] were able to significantly decrease offsite nitrate N transport with a resulting improvement in water quality by reducing the application rates of fertilizers as irrigation rates were increased.
Phosphorus transport to surface water is becoming an increasing concern due to the potential for eutrophication. Irrigation with recycled water to supply turfgrass consumptive water use can potentially increase soil P to concentrations greater than those that can be held by coarse-textured soils. Hayes
et al. [
7] measured significant increases in soil P following the application of sewage effluent to irrigated bermudagrass. Tesar
et al. [
9] determined that most of the phosphorus in a wastewater effluent applied over a 5-year experiment was not removed by the plants but remained in the top 15 cm of a fine loamy soil. Little research has been conducted to measure the potential for P in recycled water to be transported through coarse-textured turfgrass soils because the importance of P transport to surface water is such a recent issue.
The characteristics of recycled water and the soil influence the design of a land application system for crop production [
10]. Allhands and Overman [
10] measured decreases in organic matter, cation exchange capacity, exchangeable acidity, and available P with soil depth and increases in bermudagrass dry matter production and N and K uptake. Menzies
et al. [
11] determined that P adsorption capacity of sandy soils was reduced by continual high irrigation rates, which decreased the application site life calculated on the basis of original P sorption capacity.
Further research on soil and plant effects of long term application of recycled water onto sand-based turfgrass systems is required to ensure maintenance of plant quality and to protect water quality. The objectives of this research were to compare the effects of potable and reclaimed (non-potable) water on (1) soil chemical properties that may be affected by irrigation water of varying ionic composition; (2) turfgrass nutrient uptake, growth, and quality and (3) leaching of N and P.
2. Materials and Methods
We coordinated the construction of a turfgrass study site at the HRSD VIP in Norfolk beginning in summer 2003, when a sand-based root zone meeting United States Golf Association [
12] golf green specifications was constructed (
Picture 1). The research plots were plumbed to supply Norfolk city potable water and non-potable water reclaimed at the HRSD VIP to 12 separate plots in which two turfgrass species were to be established. The dimensions of each of the 12 plots were 3 m (width) by 7.5 m (length). A 3 m alley separated each plot.
Each of the 12 plots was instrumented with two lysimeters to collect leachate that percolated through the sand medium. The lysimeters consisted of polyvinyl chloride (PVC) pipe fashioned into a trough by cutting lengthwise pieces 30 cm long and 10 cm in diameter (
Picture 2). The troughs were capped at one end and plumbed at the other end to drain though PVC pipes as conduits to the edges of the plots (
Picture 3). The collection troughs were situated approximately 2 m and 4 m, respectively, from the lower end of each plot, 0.5 m from the outside edges of each plot, and 0.20 m below the sand medium surface. The piping was placed at a 2% slope to enable the leachate collected in the troughs to drain by gravity to the lower end of the plots, where the leachate accumulated in a short, capped PVC pipe prior to sampling. The troughs were filled with acid-washed coarse gravel and sand from the greens’ profile.
Hybrid bermudagrass (
Cynodon dactylon x Cynodon transvaalensis var. Tifsport) and creeping bentgrass (
Agrostis stolonifera var. L-93) were established on six plots each in spring 2004 (
Picture 4). The bentgrass was seeded and the bermudagrass was sprigged in July 2004. Only potable water was used as an irrigation source during establishment. The Tifsport variety of bermudagrass is a warm-season grass that is very deep-rooted and spreads by both stolons and rhizomes. It is commonly used in the Hampton Roads area as the main grass on golf course fairways, tees, and roughs; and is also used on athletic fields and on some home lawns, where it remains green from approximately April 15 to November 30. Tifsport is favored for its excellent wear, heat, drought, disease, and salt tolerance. ‘L-93’ creeping bentgrass is a cool-season turfgrass that spreads by stolons. It is used primarily for putting greens (0.28 to 0.38 cm cutting heights) in Eastern Virginia and less often for golf fairways (1.3 cm cutting height). Creeping bentgrass requires great care to maintain an acceptable performance level during eastern Virginia summers, but it is used because golfers greatly prefer its density, texture, and its 12-month color retention. No other C3 grass can maintain density at these required low mowing heights. Bentgrass is moderately salt tolerant.
The experimental design was a 2 × 2 factorial, completely randomized block consisting of two turfgrass (bermudagrass and bentgrass) and two water (Norfolk city potable and VIP reclaimed) treatments. Each treatment was replicated three times. The reclaimed water was generated at the HRSD VIP from wastewater subjected to secondary treatment and a suspended-growth biological reactor divided into anaerobic, anoxic, and aerobic staged zones for removal of nitrogen and phosphorus (
http://www.hrsd.com/treatmentplants.htm). The effluent used for the source of the reclaimed water in the study was treated by chlorination during 30 minutes of contact.
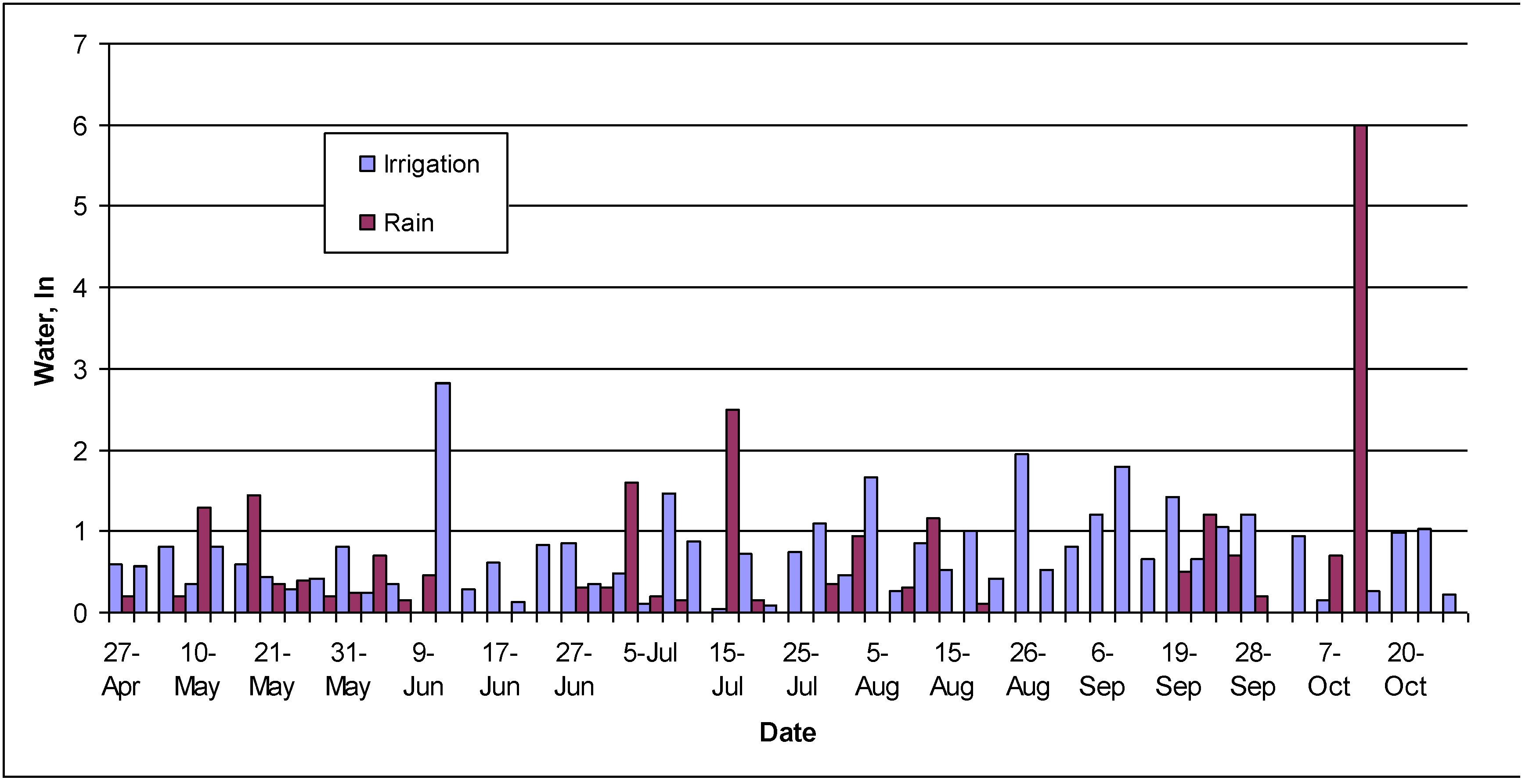
The water treatments were delivered to the appropriate plots via a subsurface irrigation system which was plumbed to provide reclaimed and potable water to the turfgrass plots at known rates (following calibration) by pop-up sprinkler heads arranged around the perimeter of each plot to ensure equal spray coverage. Irrigation was supplied manually by a sports turf professional under contract to the HRSD. Water was applied every 3 or 4 days unless rainfall of at least 6 mm was measured in rain gauges installed on each plot. The amounts of reclaimed and potable water supplied were measured with rain gauges each time the irrigation was applied during the April to October 2005 growth period. On-site natural precipitation was also measured daily during this time.
Picture 1.
Construction of the sand-based study site in summer 2003.
Picture 1.
Construction of the sand-based study site in summer 2003.
Picture 2.
Lysimeter collection trough embedded in sand medium.
Picture 2.
Lysimeter collection trough embedded in sand medium.
Picture 3.
Lysimeter collection containers extending beyond the lower end of the instrumented turfgrass plot (summer 2004).
Picture 3.
Lysimeter collection containers extending beyond the lower end of the instrumented turfgrass plot (summer 2004).
Picture 4.
Establishment of turfgrass showing 5-day old bentgrass seedlings (foreground) and bermudagrass sprigged plots (background) on July 29, 2004.
Picture 4.
Establishment of turfgrass showing 5-day old bentgrass seedlings (foreground) and bermudagrass sprigged plots (background) on July 29, 2004.
Assessment of quality of the irrigation water was based on monthly mean analyses of wastewater effluent and City of Norfolk potable water analyzed by HRSD analytical technicians (
Table 1). Analyses were conducted using Standard Methods for the Examination of Water and Wastewater [
13]. Loading rates of water constituents were calculated as the products of the constituents’ concentrations and irrigation volumes.
Table 1.
Chemical and physical analysis of potable (2005) and reclaimed, non-potable (2005 and 2006) water used to irrigate the turfgrass, and groundwater standards for southeastern Virginia. Values are means of analyses conducted several times per week throughout the application period.
Table 1.
Chemical and physical analysis of potable (2005) and reclaimed, non-potable (2005 and 2006) water used to irrigate the turfgrass, and groundwater standards for southeastern Virginia. Values are means of analyses conducted several times per week throughout the application period.
| Parameter | Potable–2005 | Reclaimed–2005 | Reclaimed–2006 | Groundwater standards |
| pH | 7.53 | 7.00 | 7.02 | 6.5–9.0 |
| Electrical conductivity, dS m−1 | 0.27 | 1.5 | | |
| BOD5, mg L−1 | <2 | 8.2 | 4.0 | |
| Total suspended solids, mg L−1 | 1.0 | 4.5 | 4.8 | 1000 |
| Total Kjeldahl nitrogen, mg L−1 | 0.8 | 2.1 | 2.0 | |
| NO3-N, mg L−1 | 0.6 | 5.2 | 6.5 | 5.0 |
| Total phosphorus, mg L−1 | 0.25 | 0.42 | 0.34 | |
| Sodium, mg L−1 | 36 | 238 | | 100 |
| Chloride, mg L−1 | 16 | 317 | 420 | 50 |
We estimated that the amounts of N and P supplied by irrigating with the reclaimed and potable water would necessitate supplemental fertilizer N and P based on previous analyses [
1]. The entire turfgrass nutrient requirements were, therefore, provided by commercial fertilizer as supplied according to Virginia Cooperative Extension Soil Test recommendations for turfgrass [
14].
Summer to fall 2004 was used as the establishment period for the turfgrasses. Turfgrass was maintained according to typical golf course maintenance standards for mowing heights, mowing frequency, and pesticide use [
15]. Diseases, weeds, and insects were scouted and controlled on an early curative basis. Our goal was to maintain the plots to a quality level required at a mid to high expectation golf course fairway. A 19–2–19 (N–P
2O
5–K
2O) fertilizer was used each year to supply 202 kg N ha
−1 to the bentgrass and 253 kg N ha
−1 to the bermudagrass, regardless of irrigation source.
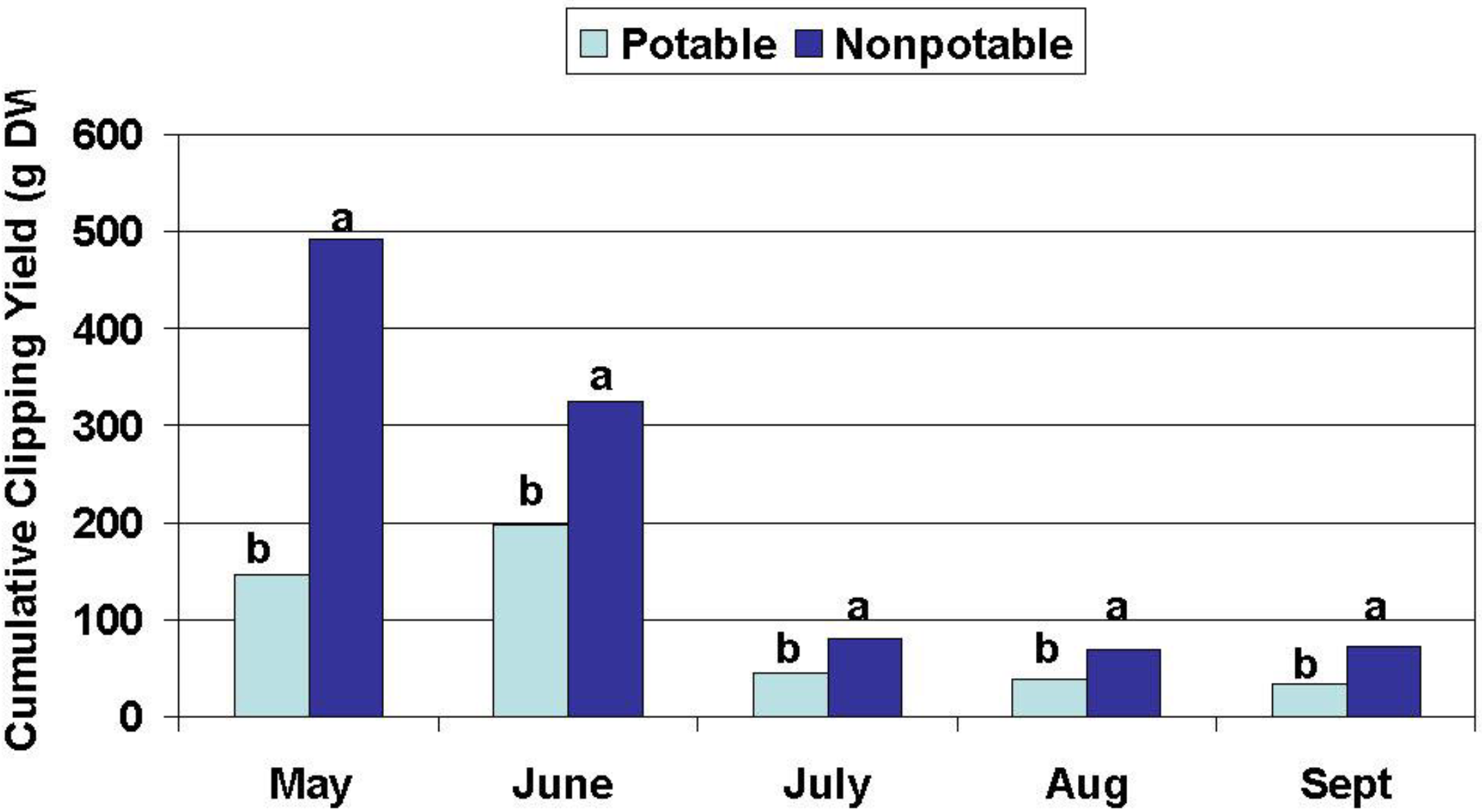
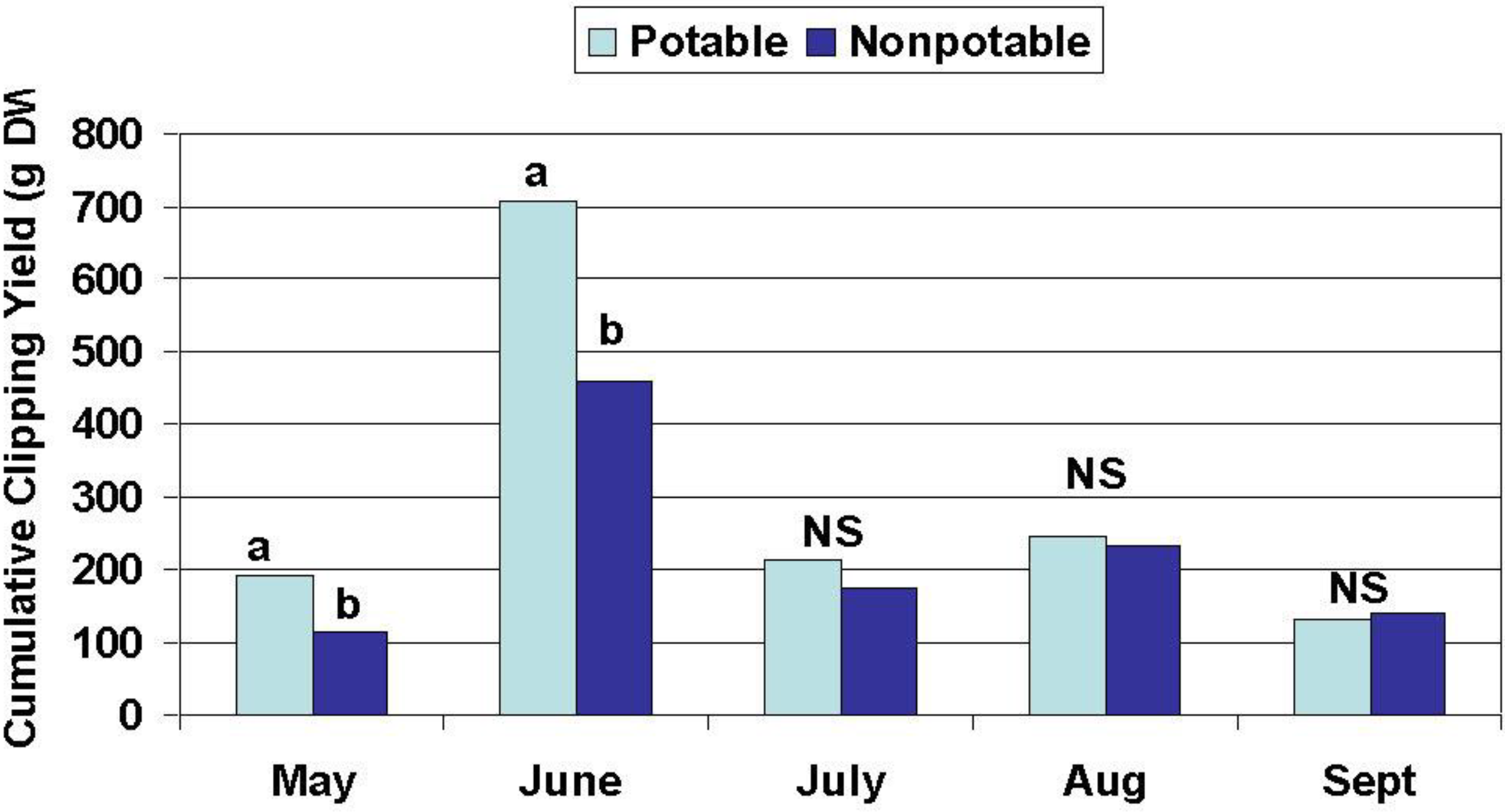
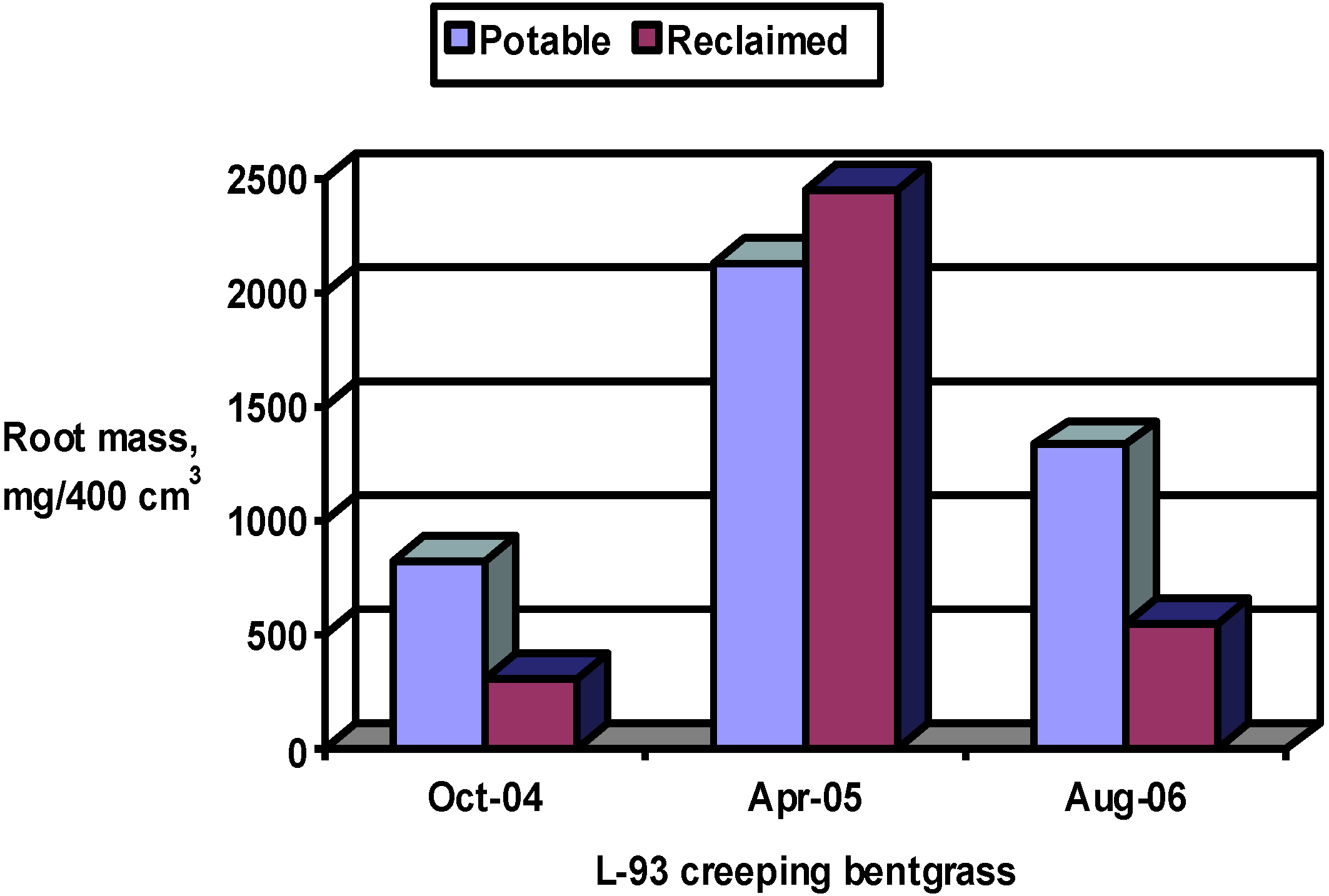
The effects of water source on turfgrass visual quality, wear tolerance and recovery, root growth, and tiller density were assessed periodically beginning after establishment. Root mass was measured in October 2004 and in April 2005 and tiller density was measured in October 2004 and August 2005. Turfgrass growth and quality monitoring were conducted from July to December 2004 and from May to October 2005 as described below.
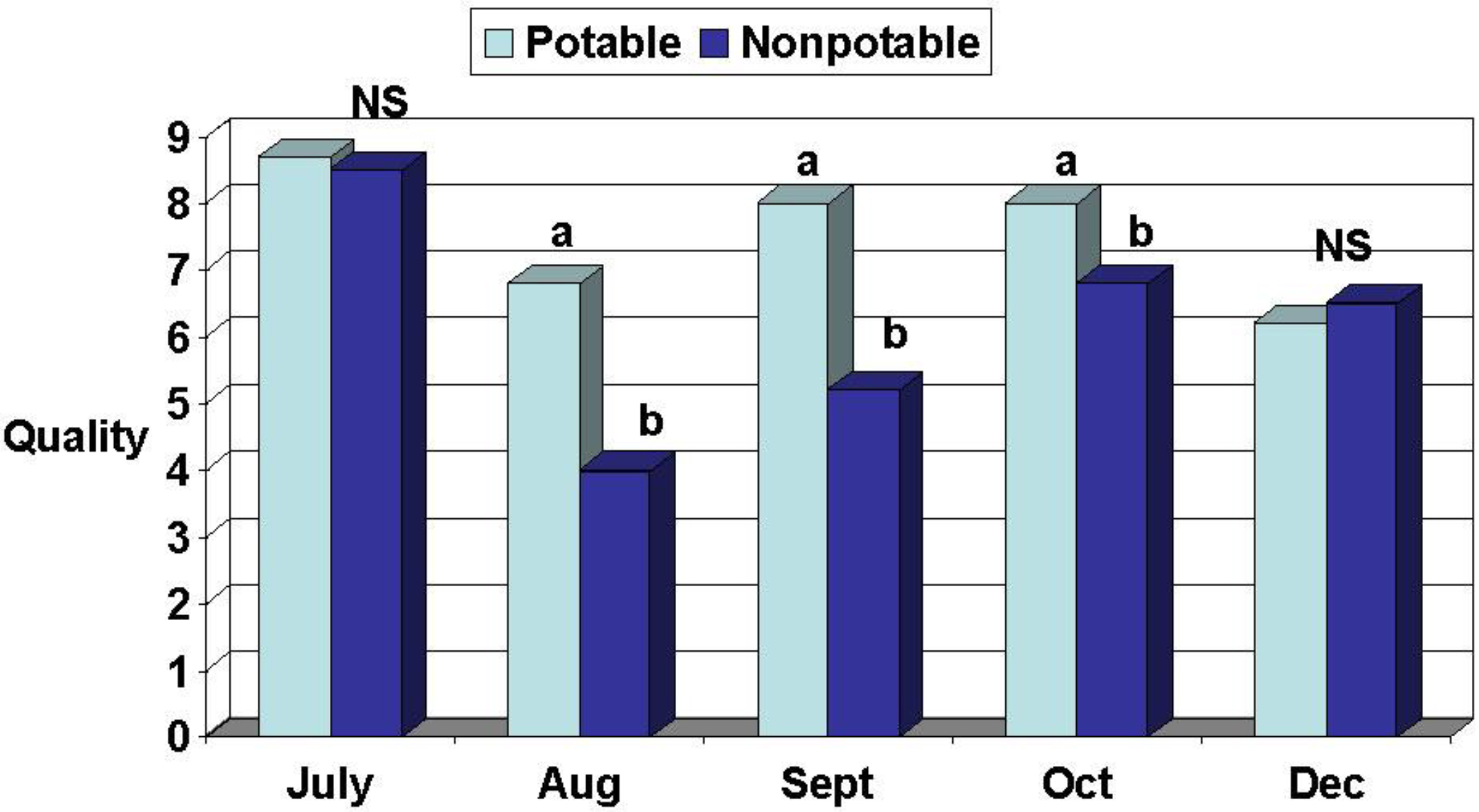
Wear tolerance and recovery: A studded roller was used to simulate spring (May, 2005), summer (July, 2004 and 2005), and fall (September 15 to October 15, 2004 and 2005) soccer seasons consisting of 20 “matches” each. Five matches per week were simulated for one month. Four days clippings were collected each week from May through October 2005 for determination of biomass production and N and P assimilation. Visual turf quality (density, uniformity, and color) ratings were determined monthly from July through December 2004 and from May through October 2005. Ratings of visual quality are a standard accepted practice amongst turf scientists for making treatment comparisons in terms of perceived aesthetic value. Turf quality was rated on a 1–9 scale with 1 = dead/dormant/completely brown turf, 6 = a minimally acceptable rating, and 9 = highest possible quality. Quality was based on color, leaf texture (width), uniformity of coverage, and tiller density.
Two 10-cm diameter, 10 cm deep plugs per plot were sampled in October 2004 and August 2004 and 2005 to measure tiller density. Roots were washed from the plugs and dried at 70 °C for measurement of total root biomass.
The bermudagrass and bentgrass were mowed every two to three days or at least three times per week to a 12 mm height. Collection of plant growth and N and P plant uptake data were initiated in spring 2005 after establishment periods for the bermudagrass (July to October in 2003 and May to October 2004) and bentgrass (March to December 2004). Clippings of each species were collected each week throughout the 2005 growing season and during May and July only in 2006 for calculation of biomass production, N and P leaf clipping concentrations and aboveground N and P assimilation. All samples were dried in a forced air oven at 70 °C for 72 h or until constant mass was achieved, weighed for dry matter, and ground in a stainless steel Wiley mill to pass a 0.5-mm sieve in preparation for chemical analysis. Plant tissue was digested and analyzed for total Kjeldahl N and P using block digestion and QuickChem autoanalyzer industrial methods [
16] to determine monthly N and P concentrations and (in conjunction with dry matter production) assimilation.
Ten soil cores were sampled to a 10-cm depth from each plot at the beginning (July 2004 and April 2005 and 2006) and end (October 2005 and August 2006) of each growing season for analysis of routine soil test parameters. The samples were air-dried and passed through a 2-mm sieve in preparation for chemical extraction. Soil samples were analyzed for pH in soil-water mix; Bray 1-P; ammonium acetate exchangeable K, Ca, Mg, Na, and H for calculation of base saturation and exchangeable sodium percentage (ESP); and cation exchange capacity using standard soil test procedures [
17] and organic matter by Walkley-Black wet oxidation [
18].
Leachate was collected monthly from each lysimeter, volumes were determined, and subsamples were analyzed colorimetrically for NH
4-N and NO
3-N with a QuickChem autoanalyzer (Methods No. 12-107-04-1-B and No. 12-107-06-2-A [
19]) and total P by EPA 365.4 [
20] in order to calculate leachate N and P masses.
All data were statistically analyzed by analysis of variance, and mean separations were calculated using least significant differences at a 0.05 probability level employing SAS [
21].