Multivariate Analysis and Hydrogeochemical Evolution of Groundwater in a Geologically Controlled Aquifer System: A Case Study in North Central Province, Sri Lanka
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.1.1. Geological Setting of the Study Area
2.1.2. Geological and Hydrogeological History of Sri Lanka
2.1.3. Hydrogeology of Fractured Hard-Rock Aquifers
2.1.4. Lithological and Structural Pattern Distribution of the Study Area
2.1.5. Regional Groundwater Salinity Indicators
2.2. Water Sampling and Rock Sampling
2.3. Analytical Procedure
2.4. Hydrogeochemical Analysis
2.5. Software Tools and Platforms
2.5.1. Water Clustering
2.5.2. Geospatial Analysis
2.5.3. Geochemical Processes Analysis
3. Results and Discussion
3.1. Aquifer Characterization and Hydrogeological Setting
3.2. Groundwater Flow Patterns of the Study Area
3.3. Major Ion Chemistry
3.4. Minor and Trace Ion Chemistry
3.5. Piper Classification
3.6. Statistical Classification of Water
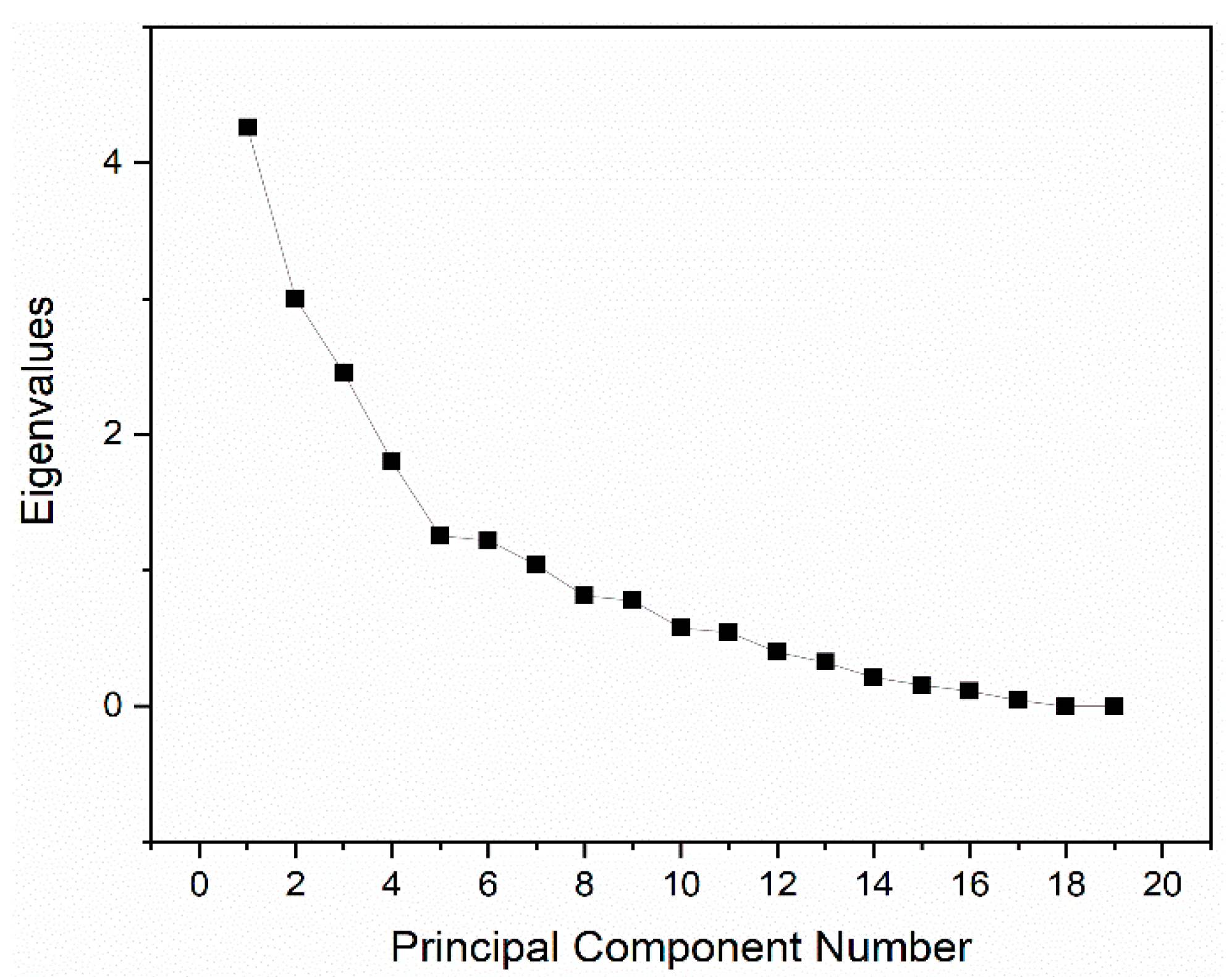
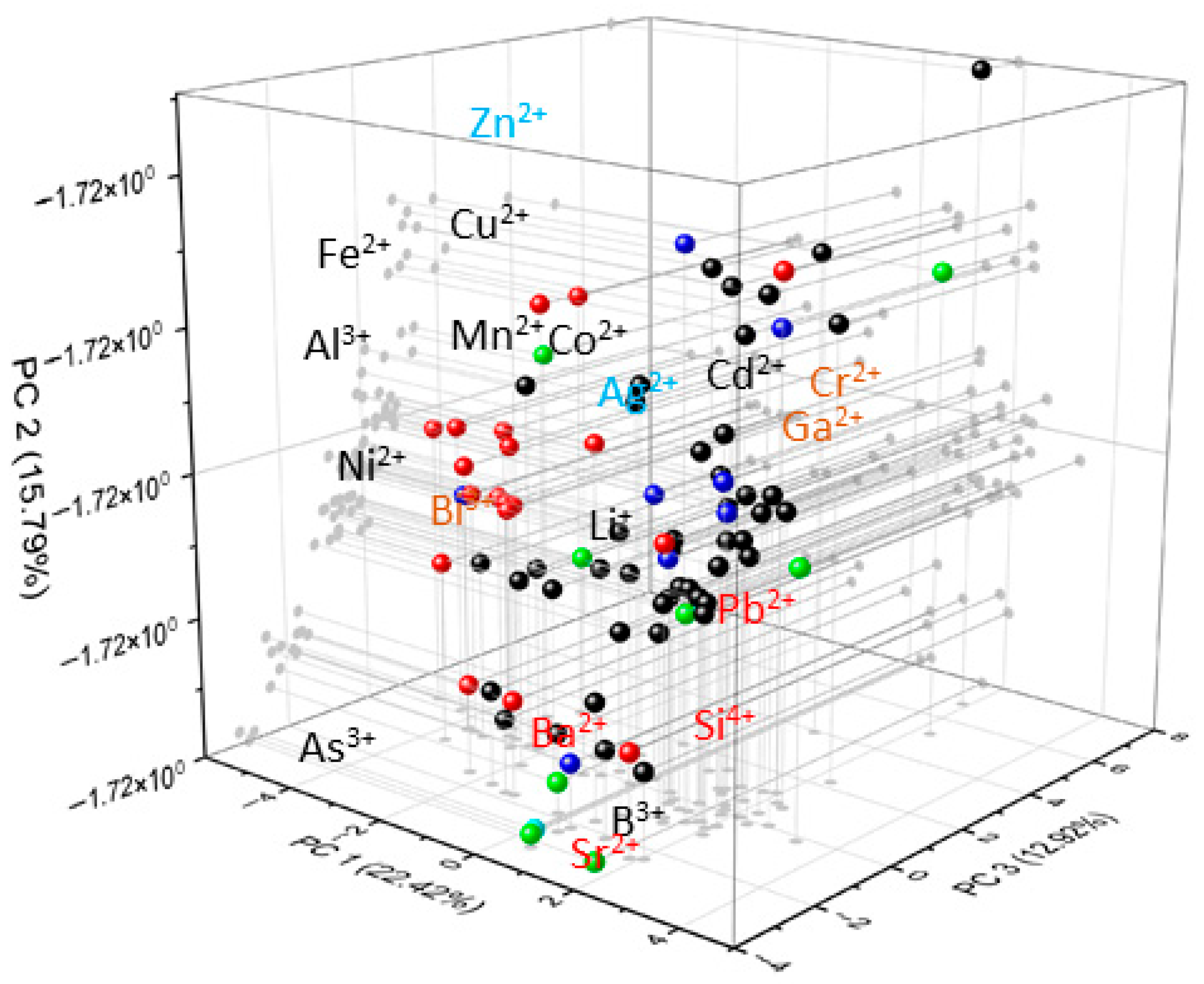
PCA
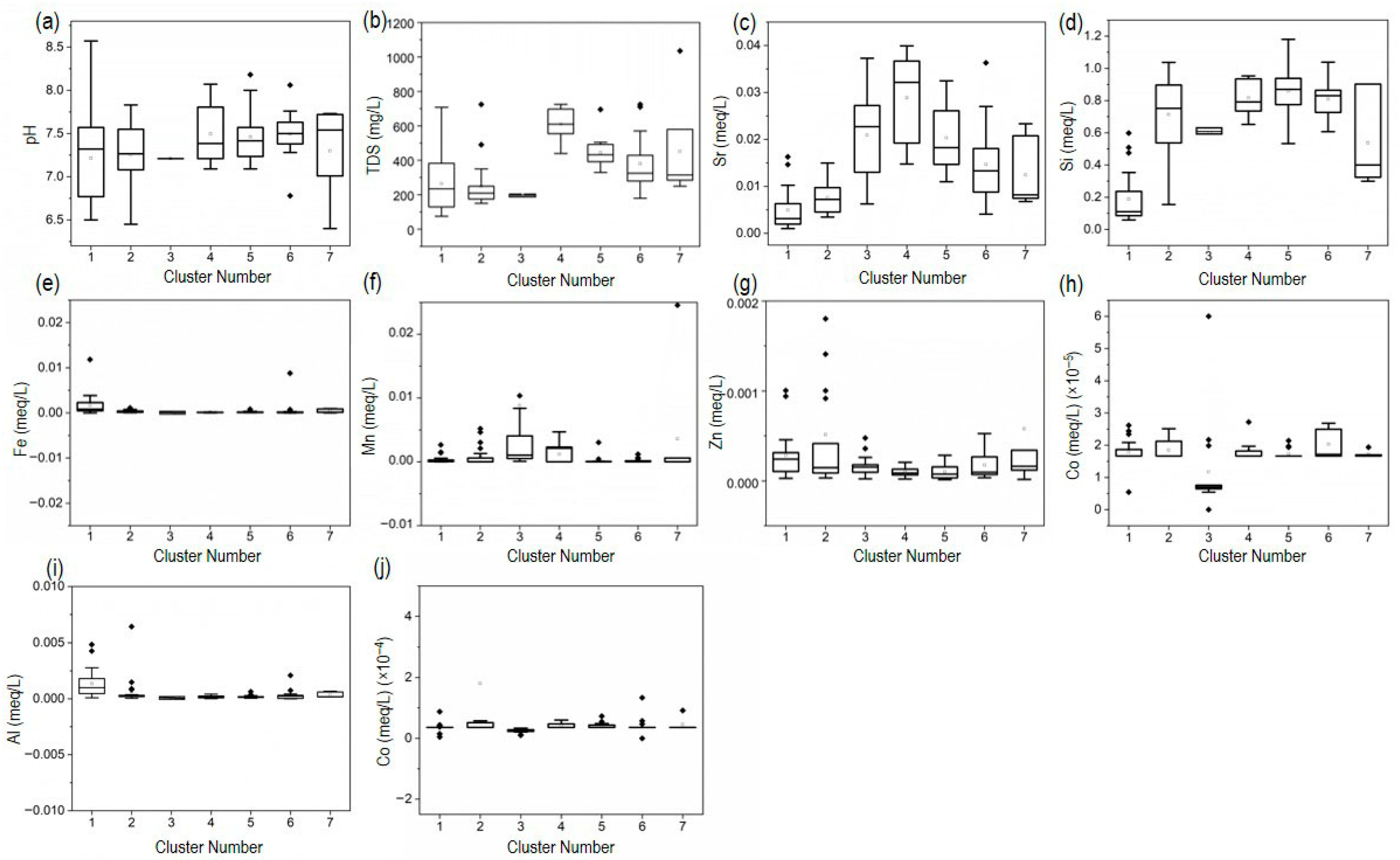
3.7. Hydro-Geochemical Processes and Groundwater Cluster Evolution
3.7.1. Multivariate Analysis Using Litho-Focus Elements
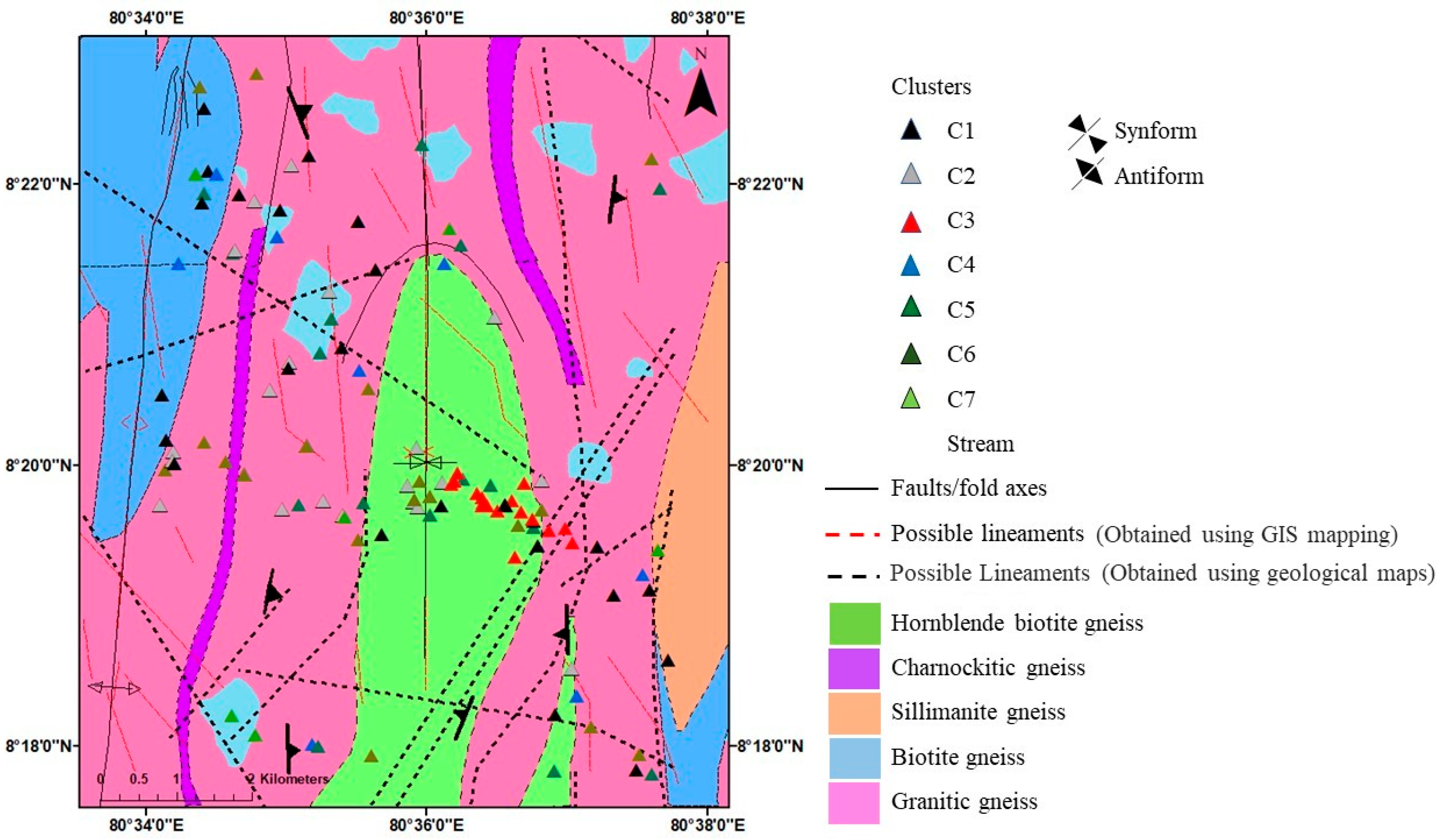
3.7.2. HCA for Litho-Focus Elements and Classified Seven Clusters
3.7.3. Geology and Cluster Association
3.7.4. Combination Diagrams
+ 3 (Mg2+, Fe2+) + H4SiO4 + Cl−
Al2Si2O5(OH)4 +2 Ca2+ + 4 (Mg2+, Fe2+) + HCO3− + H4SiO4 +Cl−
(Anorthite) (Kaolinite)
+ H2O → REE3+ + Fe3+ + Al3+ + SiO2 + Ca2+
(Albite)
(Calcite)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Güler, C.; Thyne, G.D.; McCray, J.E.; Turner, A.K. Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol. J. 2002, 10, 455–474. [Google Scholar] [CrossRef]
- Patekar, M.; Briški, M.; Terzić, J.; Nakić, Z.; Borović, S. Cumulative effects of natural and anthropogenic processes on groundwater chemistry of a small karst island—Case study of Vis (Croatia). Appl. Water Sci. 2024, 14, 214. [Google Scholar] [CrossRef]
- Trabelsi, R.; Zouari, K.; Araguás Araguás, L.J.; Moulla, A.S.; Sidibe, A.M.; Bacar, T. Assessment of geochemical processes in the shared groundwater resources of the Taoudeni aquifer system (Sahel region, Africa). Hydrogeol. J. 2024, 32, 167–188. [Google Scholar] [CrossRef]
- Boumaiza, L.; Walter, J.; Chesnaux, R.; Stotler, R.L.; Wen, T.; Johannesson, K.H.; Brindha, K.; Huneau, F. Chloride-salinity as indicator of the chemical composition of groundwater: Empirical predictive model based on aquifers in Southern Quebec, Canada. Environ. Sci. Pollut. Res. 2022, 29, 59414–59432. [Google Scholar] [CrossRef]
- Abanyie, S.K.; Apea, O.B.; Abagale, S.A.; Amuah, E.E.Y.; Sunkari, E.D. Sources and factors influencing groundwater quality and associated health implications: A review. Emerg. Contam. 2023, 9, 100207. [Google Scholar] [CrossRef]
- Dissanayake, C.B.; Weerasooriya, S.V.R. A geochemical classification of groundwater of Sri Lanka. J. Natl. Sci. Found. Sri Lanka 1985, 13, 147–186. [Google Scholar] [CrossRef]
- Dissanayake, C.B.; Chandrajith, R. The Hydrogeological and Geochemical Characteristics of Groundwater of Sri Lanka. In Groundwater of South Asia; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Stillings, M.; Shipton, Z.K.; Lord, R.A.; Lunn, R.J. Using subtle variations in groundwater geochemistry to identify the proximity of individual geological structures: A case study from the Grimsel Test Site (Switzerland). Geoenergy 2024, 2, geoenergy2024-005. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Carrillo-Rivera, J.J.; Cardona, A. Geochemical evolution of groundwater beneath Mexico City. J. Hydrol. 2002, 258, 1–24. [Google Scholar] [CrossRef]
- de Almeida Salles, L.; Lima, J.E.F.W.; Roig, H.L.; Malaquias, J.V. Environmental factors and groundwater behavior in an agricultural experimental basin of the Brazilian central plateau. Appl. Geogr. 2018, 94, 272–281. [Google Scholar] [CrossRef]
- Udeshani, W.A.C.; Koralegedara, N.H.; Gunatilake, S.K.; Li, S.L.; Zhu, X.; Chandrajith, R. Geochemistry of Groundwater in the Semi-Arid Crystalline Terrain of Sri Lanka and Its Health Implications among Agricultural Communities. Water 2022, 14, 3241. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Dai, S.; French, D. The importance of minerals in coal as the hosts of chemical elements: A review. Int. J. Coal Geol. 2019, 212, 103251. [Google Scholar] [CrossRef]
- Gascoyne, M.; Kamineni, D.C. The Hydrogeochemistry Of Fractured Plutonic Rocks In The Canadian Shield. Appl. Hydrogeol. 1994, 2, 43–49. [Google Scholar] [CrossRef]
- Soysa, R.N.K.; Pallegedara, A.; Kumara, A.S.; Jayasena, D.M.; Samaranayake, M.K.S.M. Adapting Sustainable Development Goals (SDGs) in sustainability reporting (SR) by listed firms in Sri Lanka. J. Trop. Environ. 2015, 11, 1–14. [Google Scholar]
- Kehelpannala, K.V.W. Arc Accretion Around Sri Lanka During the Assembly of Gondwana. Gondwana Res. 2004, 7, 41–46. [Google Scholar]
- Dharmapriya, P.L.; Malaviarachchi, S.P.K.; Kriegsman, L.M.; Galli, A.; Sajeev, K.; Zhang, C. New constraints on the P–T path of HT/UHT metapelites from the Highland Complex of Sri Lanka. Geosci. Front. 2017, 8, 1405–1430. [Google Scholar] [CrossRef]
- Panabokke, C.R.; Perera, A.P.G.R.L. Groundwater Resources of Sri Lanka; Water Resources Board: Colombo, Sri Lanka, 2005. [Google Scholar]
- Panabokke, C.R. Groundwater Conditions in Sri Lanka: A Geomorphic Perspective; National Science Foundation of Sri Lanka: Colombo, Sri Lanka, 2007.
- Wayland, E.J.; Davies, A.M. The Miocene of Ceylon. Q. J. Geol. Soc. 1922, 79, 577–602. [Google Scholar] [CrossRef]
- Singhal, B.B.S.; Gupta, R.P. Applied Hydrogeology of Fractured Rocks, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Cooray, P.G. The Precambrian of Sri Lanka: A historical review. Precambrian Res. 1994, 66, 3–18. [Google Scholar] [CrossRef]
- Edmunds, W.M. Geochemistry’s vital contribution to solving water resource problems. Appl. Geochem. 2009, 24, 1058–1073. [Google Scholar] [CrossRef]
- Cooray, P.G. The Tonigala granite, NW Ceylon. Bull. Geol. Soc. Finl. 2017, 43, 19–37. [Google Scholar] [CrossRef]
- Kröner, A. African linkage of Precambrian Sri Lanka. Geol. Rundschau 1991, 80, 429–440. [Google Scholar] [CrossRef]
- Water Resources Board. Distribution of Electrical Conductivity of Groundwater—Water Resources Board. Water Resources Board. Available online: https://wrb.lk/distribution-of-electrical-conductivity-of-groundwater/ (accessed on 13 December 2025).
- Piper, M. 914 Transactions, Americangeophysical Union Timesasgreatas It Should Have Been. and in Thiscas E; American Geophysical Union: Washington, DC, USA, 1944; pp. 914–928. [Google Scholar]
- Jolliffe, I.T. Principal components. Data Handl. Sci. Technol. 2002, 20, 519–556. [Google Scholar] [CrossRef]
- Gibbs, R. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, G.; Ercoli, L.; Rossetto, R. A spatially distributed, physically-based modeling approach for estimating agricultural nitrate leaching to groundwater. Hydrology 2021, 8, 8. [Google Scholar] [CrossRef]
- Kadiasi, N.; Tako, R.; Ibraliu, A.; Stanys, V.; Gruda, N.S. Principal Component and Hierarchical Cluster Analysis of Major Compound Variation in Essential Oil among Some Red Oregano Genotypes in Albania. Agronomy 2024, 14, 1419. [Google Scholar] [CrossRef]
- Ilayaraja, K.; Ambica, A. Spatial distribution of groundwater quality between injambakkam-thiruvanmyiur areas, south east coast of India. Nat. Environ. Pollut. Technol. 2015, 14, 771–776. [Google Scholar]
- Freeze, R.A.; Cherry, J.A. Groundwater; Prentice-Hall: Hoboken, NJ, USA, 1979; Volume 16. [Google Scholar]
- Hem. Groundwater Hydrogeology, 3rd ed.; U.S. Geological Survey: Reston, VA, USA, 1985. [CrossRef]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.N.; Bemment, C.D. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef] [PubMed]
- Helsel, D.R.; Hirsch, R.M.; Ryberg, K.R.; Archfield, S.A.; Gilroy, E.J. Statistical Methods in Water Resources; USGS Publications Warehouse: Reston, VA, USA, 2020; Volume 2020, pp. 1–484. [CrossRef]
- Drever, J.I.; Stillings, L.L. The role of organic acids in mineral weathering. Colloids Surfaces A Physicochem. Eng. Asp. 1997, 120, 181. [Google Scholar] [CrossRef]
- Ueno, Y.; Kitajima, Y. Suppression of Methane Gas Emissions and Analysis of the Electrode Microbial Community in a Sediment-Based Bio-Electrochemical System. Adv. Microbiol. 2014, 04, 252–266. [Google Scholar] [CrossRef]
- White, A.F.; Brantley, S.L. The effect of time on the weathering of silicate minerals: Why do weathering rates differ in the laboratory and field? Chem. Geol. 2003, 202, 479–506. [Google Scholar] [CrossRef]
- Lasaga, A.C.; Soler, J.M.; Ganor, J.; Burch, T.E.; Nagy, K.L. Chemical weathering rate laws and global geochemical cycles. Geochim. Cosmochim. Acta 1994, 58, 2361–2386. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M.; Rao, C.N.R.; Raveau, B.; Jolivet, J.-P.; Henry, M.; Livage, J. The Iron Oxides. 2003. Available online: https://content.e-bookshelf.de/media/reading/L-602886-6f6c768889.pdf (accessed on 13 December 2025).
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Brantley, C.G.; Day, J.W.; Lane, R.R.; Hyfield, E.; Day, J.N.; Ko, J.Y. Primary production, nutrient dynamics, and accretion of a coastal freshwater forested wetland assimilation system in Louisiana. Ecol. Eng. 2008, 34, 7–22. [Google Scholar] [CrossRef]
- Riebe, C.S.; Kirchner, J.W.; Finkel, R.C. Sharp decrease in long-term chemical weathering rates along an altitudinal transect. Earth Planet. Sci. Lett. 2004, 218, 421–434. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Hussien, B.M.; Fayyadh, A.S. Preferable Districts for Groundwater Exploitation Based on Hydrogeologic Data of Aquifers-West Iraq. J. Water Resour. Prot. 2014, 06, 1173–1197. [Google Scholar] [CrossRef]
- Selvam, R.A.; Ravindran, A.; Jebamalai, A.; Ravindran, G.; Viswasam, S.P. Hydrochemical evaluation of the strip aquifer in Srivaikundam region, Southern India: Implications for drinking and irrigation. Discov. Geosci. 2025, 3, 27. [Google Scholar] [CrossRef]
- Chandrajith, R.; Bandara, U.G.C.; Diyabalanage, S.; Senaratne, S.; Barth, J.A.C. Groundwater for Sustainable Development Application of Water Quality Index as a vulnerability indicator to determine seawater intrusion in unconsolidated sedimentary aquifers in a tropical coastal region of Sri Lanka. Groundw. Sustain. Dev. 2022, 19, 100831. [Google Scholar] [CrossRef]
- Kullerud, K. Occurrence and origin of Cl-rich amphibole and biotite in the Earth’s crust—Implications for fluid composition and evolution. In Hydrogeology of Crystalline Rocks; Springer: Dordrecht, The Netherlands, 2000; pp. 205–225. [Google Scholar]
- Marandi, A.; Shand, P. Applied Geochemistry Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Feth, J.H. Chloride in Natural Continental Water A Review; United States Government Printing Office: Washington, DC, USA, 1981. [CrossRef]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Stallard, R.F.; Edmond, J.M. Geochemistry of the Amazon 2. The influence of geology and weathering environment on the dissolved load. J. Geophys. Res. 1983, 88, 9671–9688. [Google Scholar] [CrossRef]
- Meybeck, M. Global chemical weathering of surficial rocks estimated from river dissolved loads. Am. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- Vandenbohede, A.; Lebbe, L. Groundwater chemistry patterns in the phreatic aquifer of the central Belgian coastal plain. Appl. Geochem. 2012, 27, 22–36. [Google Scholar] [CrossRef]









| Parameter | Shallow | Deep | Surface | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Median | Max | CV | Mean | Min | Median | Max | CV | Mean | Min | Median | Max | CV | |
| pH | 7.38 | 6.45 | 7.40 | 8.18 | 0.066 | 7.17 | 6.40 | 7.25 | 8.06 | 0.064 | 7.37 | 6.56 | 7.40 | 8.57 | 0.064 |
| EC (μS/cm) | 686.70 | 150.00 | 634.50 | 1450.00 | 0.473 | 754.38 | 300.00 | 729.00 | 1850.00 | 0.635 | 419.43 | 124.40 | 460.00 | 650.00 | 0.437 |
| TDS (mg/L) | 344.02 | 75.00 | 325.00 | 725.00 | 0.044 | 320.80 | 369.00 | 390.00 | 1035.00 | 0.701 | 280.49 | 83.30 | 308.00 | 435.00 | 0.474 |
| Na+ (mg/L) | 76.74 | 15.46 | 60.88 | 317.22 | 0.785 | 74.17 | 22.06 | 52.47 | 159.46 | 0.610 | 46.94 | 14.09 | 56.59 | 78.95 | 0.480 |
| K+ (mg/L) | 5.29 | 0.52 | 2.28 | 26.14 | 1.181 | 4.59 | 2.07 | 3.99 | 9.72 | 0.515 | 5.64 | 3.19 | 4.73 | 7.25 | 0.215 |
| Ca2+(mg/L) | 74.79 | 14.51 | 74.23 | 230.05 | 0.541 | 63.73 | 18.08 | 54.72 | 169.32 | 0.707 | 21.40 | 6.67 | 18.13 | 90.64 | 0.857 |
| Mg2+ (mg/L) | 7.36 | 0.04 | 0.04 | 31.88 | 0.622 | 6.37 | 0.01 | 0.04 | 56.71 | 0.511 | 0.04 | 2.89 | 0.01 | 5.93 | 1.081 |
| Cl− (mg/L) | 125.19 | 0.00 | 78.52 | 668.23 | 0.081 | 124.72 | 21.94 | 78.35 | 308.40 | 0.701 | 83.81 | 10.86 | 81.34 | 152.18 | 0.552 |
| NO3− (mg/L) | 8.38 | 0.01 | 0.94 | 86.08 | 2.020 | 37.46 | 0.04 | 4.39 | 243.21 | 1.965 | 3.08 | 0.06 | 1.43 | 26.69 | 2.001 |
| SO42− (mg/L) | 45.75 | 8.21 | 37.19 | 193.58 | 0.699 | 48.78 | 18.38 | 45.82 | 78.74 | 0.418 | 18.81 | 0.05 | 19.37 | 39.44 | 0.434 |
| HCO3− (mg/L) | 268.17 | 10.01 | 229.56 | 734.10 | 0.450 | 250.80 | 22.41 | 263.63 | 564.32 | 0.664 | 112.07 | 37.06 | 118.36 | 200.86 | 0.408 |
| CO32− (mg/L) | 24.40 | 0.49 | 22.32 | 73.74 | 0.867 | 25.60 | 1.10 | 26.52 | 56.70 | 0.699 | 11.40 | 3.72 | 11.94 | 20.22 | 0.388 |
| Si (mg/L) | 43.27 | 14.19 | 46.36 | 62.82 | 0.303 | 52.41 | 34.43 | 54.22 | 70.90 | 0.203 | 6.17 | 3.53 | 5.87 | 11.19 | 0.314 |
| Element | Shallow | Deep | Surface | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N Total | Mean (meq/L) | CV | N Total | Mean (meq/L) | CV | N Total | Mean (meq/L) | CV | |
| Mn | 81 | 0.00208 | 5.918 | 15 | 0.00289 | 2.221 | 17 | 3.81 × 10−4 | 1.808 |
| Co | 71 | 1.68 × 10−5 | 0.458 | 12 | 1.81 × 10−5 | 0.126 | 16 | 1.86 × 10−5 | 0.142 |
| Cu | 75 | 6.13 × 10−5 | 3.564 | 13 | 1.21 × 10−4 | 2.090 | 16 | 3.62 × 10−5 | 0.000 |
| Zn | 75 | 2.18 × 10−4 | 1.889 | 12 | 6.11 × 10−4 | 1.417 | 16 | 2.74 × 10−4 | 0.855 |
| Cd | 70 | 1.56 × 10−5 | 0.344 | 12 | 1.58 × 10−5 | 0.358 | 16 | 1.13 × 10−5 | 0.111 |
| Al | 64 | 4.51 × 10−4 | 2.188 | 13 | 1.93 × 10−4 | 1.121 | 17 | 0.00149 | 0.701 |
| B | 64 | 0.02033 | 0.549 | 13 | 0.01721 | 0.383 | 17 | 0.02158 | 0.752 |
| Pb | 81 | 0.7408 | 4.414 | 15 | 7.41 × 10−5 | 0.272 | 17 | 0.88238 | 4.123 |
| As | 73 | 5.16 × 10−4 | 0.522 | 12 | 4.99 × 10−4 | 0.523 | 16 | 5.19 × 10−4 | 0.208 |
| Ni | 57 | 7.41 × 10−6 | 0.250 | 10 | 7.17 × 10−6 | 0.000 | 17 | 3.52 × 10−5 | 0.692 |
| Sr | 81 | 0.01532 | 0.656 | 15 | 0.01558 | 0.576 | 17 | 0.00471 | 0.584 |
| Cs | 36 | 0.00165 | 0.699 | 7 | 0.00202 | 0.603 | 16 | 0.00255 | 0.442 |
| Ba | 64 | 0.0038 | 0.515 | 13 | 0.00336 | 0.714 | 17 | 0.00109 | 0.574 |
| Bi | 57 | 1.50 × 10−4 | 0.000 | 10 | 1.50 × 10−4 | 0.000 | 16 | 1.50 × 10−4 | 0.000 |
| Ag | 57 | 3.98 × 10−5 | 0.388 | 10 | 3.92 × 10−5 | 0.367 | 16 | 4.27 × 10−5 | 0.075 |
| Ga | 57 | 8.46 × 10−4 | 0.000 | 10 | 8.46 × 10−4 | 0.000 | 16 | 8.46 × 10−4 | 0.000 |
| Li | 64 | 4.78 × 10−4 | 0.879 | 13 | 0.00138 | 1.128 | 17 | 2.65 × 10−5 | 0.593 |
| Cr | 57 | 4.50 × 10−5 | 0.000 | 10 | 4.50 × 10−5 | 0.000 | 16 | 4.50 × 10−5 | 0.000 |
| Fe | 64 | 2.57 × 10−4 | 1.348 | 13 | 2.17 × 10−4 | 1.341 | 17 | 0.00256 | 1.213 |
| Eigenvalue | Percentage of Variance | Cumulative |
|---|---|---|
| 4.25952 | 22.42% | 22.42% |
| 3 | 15.79% | 38.21% |
| 2.45525 | 12.92% | 51.13% |
| 1.80367 | 9.49% | 60.62% |
| 1.25429 | 6.60% | 67.22% |
| 1.22096 | 6.43% | 73.65% |
| 1.04111 | 5.48% | 79.13% |
| 0.81315 | 4.28% | 83.41% |
| 0.77978 | 4.10% | 87.51% |
| 0.57597 | 3.03% | 90.55% |
| 0.54575 | 2.87% | 93.42% |
| 0.39839 | 2.10% | 95.51% |
| 0.32505 | 1.71% | 97.23% |
| 0.21386 | 1.13% | 98.35% |
| 0.15598 | 0.82% | 99.17% |
| 0.11264 | 0.59% | 99.77% |
| 0.04465 | 0.23% | 100.00% |
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| Mn | 0.06368 | 9.90 × 10−16 | 0.23758 | −0.29224 |
| Co | 0.04069 | 9.17 × 10−15 | 0.29662 | 0.28234 |
| Cu | 0.05266 | 2.12 × 10−15 | 0.3468 | −0.31749 |
| Zn | 0.03487 | 2.82 × 10−15 | 0.45643 | −0.4005 |
| Cd | 0.36437 | 1.94 × 10−15 | 0.27127 | 0.19941 |
| Al | −0.26404 | 1.19 × 10−16 | 0.05608 | −0.03213 |
| B | 0.08257 | 3.58 × 10−16 | −0.15654 | 0.35152 |
| Pb | 0.41939 | 7.44 × 10−16 | 0.05024 | 0.0376 |
| As | 0.02485 | −5.48 × 10−15 | −0.4458 | −0.36854 |
| Ni | −0.29864 | 1.42 × 10−16 | 0.03855 | 0.23955 |
| Sr | 0.2949 | −1.69 × 10−15 | −0.25595 | 0.10965 |
| Ba | 0.31156 | −1.37 × 10−15 | −0.18881 | 0.04238 |
| Bi | −4.60 × 10−16 | 0.57735 | −5.78 × 10−15 | −2.61 × 10−15 |
| Ag | 0.09318 | 3.91 × 10−15 | 0.32305 | 0.41696 |
| Ga | −4.62 × 10−16 | 0.57735 | −5.78 × 10−15 | −2.60 × 10−15 |
| Li | 0.24577 | 7.00 × 10−16 | 0.08903 | −0.07738 |
| Cr | −4.62 × 10−16 | 0.57735 | −5.78 × 10−15 | −2.60 × 10−15 |
| Si | 0.4396 | −4.32 × 10−16 | −0.06993 | −0.10741 |
| Fe | −0.26258 | 4.02 × 10−16 | 0.06819 | 0.09747 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Hansani, U.; Witharana, S.A.; Dharmapriya, P.L.; Wijekoon, P.; Wu, Z.; Chen, X.; Jinadasa, S.; Weerasooriya, R. Multivariate Analysis and Hydrogeochemical Evolution of Groundwater in a Geologically Controlled Aquifer System: A Case Study in North Central Province, Sri Lanka. Water 2026, 18, 89. https://doi.org/10.3390/w18010089
Hansani U, Witharana SA, Dharmapriya PL, Wijekoon P, Wu Z, Chen X, Jinadasa S, Weerasooriya R. Multivariate Analysis and Hydrogeochemical Evolution of Groundwater in a Geologically Controlled Aquifer System: A Case Study in North Central Province, Sri Lanka. Water. 2026; 18(1):89. https://doi.org/10.3390/w18010089
Chicago/Turabian StyleHansani, Uthpala, Sapumal Asiri Witharana, Prasanna Lakshitha Dharmapriya, Pushpakanthi Wijekoon, Zhiguo Wu, Xing Chen, Shameen Jinadasa, and Rohan Weerasooriya. 2026. "Multivariate Analysis and Hydrogeochemical Evolution of Groundwater in a Geologically Controlled Aquifer System: A Case Study in North Central Province, Sri Lanka" Water 18, no. 1: 89. https://doi.org/10.3390/w18010089
APA StyleHansani, U., Witharana, S. A., Dharmapriya, P. L., Wijekoon, P., Wu, Z., Chen, X., Jinadasa, S., & Weerasooriya, R. (2026). Multivariate Analysis and Hydrogeochemical Evolution of Groundwater in a Geologically Controlled Aquifer System: A Case Study in North Central Province, Sri Lanka. Water, 18(1), 89. https://doi.org/10.3390/w18010089






