Abstract
Urban lake degradation caused by intensive urbanization necessitates systematic solutions, with water connectivity being a crucial ecological restoration strategy. This study evaluates the two-year effects (2020–2022) of connectivity interventions on seven lakes in Yangshuo, Guilin, classified by connectivity: multi-channel (Mc), single-channel (Sc), and non-connected (Nc). Regular monitoring of the physicochemical parameters and microbial communities revealed significant patterns: multi-channel connected lakes exhibited superior water quality improvement, with trophic state downgrading (weak eutrophic → mesotrophic), but the water quality of Sc-BQ was deteriorating. Seasonal variations showed wet season peaks in pH, DO, CODMn, and Chl-a, versus dry season elevations in NH3-N, NO3-N, TN, and TP. Correlation analysis identified organic matter as the primary driver of eutrophication, with TN strongly linked to NH3-N, indicating persistent domestic sewage contamination. Microbial community restructuring was accompanied by changes in water quality, and the abundance and diversity of OTUs decreased after restoration. Notably, Limnohabitans dominated Mc lakes (31.82–35.1%), while Pleurocapsa prevailed (37.85%) in Nc-LH under weak eutrophic conditions. These findings demonstrate that multi-channel connectivity effectively enhances hydrodynamic conditions and pollutant dispersion, whereas inadequate connectivity exacerbates nutrient accumulation. The study provides critical empirical evidence for optimizing urban lake management, emphasizing the necessity of multi-dimensional connectivity designs and targeted control of untreated sewage inputs in water system rehabilitation projects.
1. Introduction
As an influential body of water in cities, urban lakes not only provide recreational and ecological services for residents, but also play an irreplaceable role in the urban ecosystem, such as rainwater storage and climate regulation [1,2]. The urbanization process has accelerated the unceasing growth of the agglomerated population, and urban construction has crowded out ecological space, leading to the shrinkage of natural lakes and ponds in urban areas and the deterioration of water quality, affecting the biodiversity and ecological balance of water. Strengthening the pollution prevention and control of inner-city lakes, reducing the discharge of pollutants, improving the water quality environment, and protecting lake ecosystems and public health has become one of the critical issues in today’s sustainable urban development.
Currently, measures to restore lake ecosystems include mainly physical, chemical, and biological approaches. Physical methods mainly include dredging, artificial aeration, mechanical algae removal, and water transfer through water system connection, which are primarily applicable to lakes with a small area [3]. Chemical methods, such as flocculation, sedimentation, and oxidation, can quickly remove suspended solids and algae from contaminated water, but their high cost and vulnerability to the risk of secondary contamination preclude their use in the long-term [4,5]. The main physical treatment technologies include microbial remediation, biofilm, ecological floating beds, and artificial wetlands, which are used to improve water quality by degrading organic pollutants and adsorbing inorganic pollutants such as heavy metals. These methods are suitable for lakes with a small degree of pollution, but its remediation process takes a long time, and natural environmental factors such as rainfall and temperature have a greater impact on the effect of water quality improvement, causing many uncertainties [6,7,8].
Among these methods, river and lake water system connectivity not only improves the mutual complementary capacity of river and lake water resources, but also enhances their self-purification capacity, which results in improving water quality [9,10]. Since 1987, when Lake Horowhenua in New Zealand diverted sewage from the town of Levin, external loads of nitrogen and phosphorus decreased by about 20% and 90%, respectively [11]. In order to alleviate the serious eutrophication problem in Lake Tega, Japan constructed the North Chiba Passage in 2000 to transport water from the Tone River to the lake, which has resulted in a reduction of phosphorus concentration in the lake through dilution [12]. In the first phase of a program to improve water quality in Egypt’s Lake Burullus from 2017 to 2019, which included measures to dredge the lake and deepen its connection to the Mediterranean Sea, the water exchange between the lake and the sea water led to a decrease in Chl-a concentration compared to previous studies, thanks to the deepening and widening of the inlet [13]. The water quality of the South-to-North Water Diversion Project in China was “excellent” in terms of seasonal and spatial patterns during the monitoring period from 2016 to 2019, with significant improvements in water quality [14]. In the test of water transfer from 2002 to 2003, the results of a water quality assessment showed that water transfer had a positive effect on the improvement of the water quality of Lake Taihu, in which the key factor of eutrophication, TP, was improved by 31%, and the concentration of CODMn by 19% [15]. The river diversion project carried out in 2007 decreased TP and TN concentrations by 19% and 16%, respectively, and significantly improved the self-purification capacity of Lake Chaohu [16]. From 1985 to 2013, during the implementation of water diversion and other comprehensive remediation of the West Lake in Hangzhou, the TP concentration in the outer lake and Xili Lake of the West Lake decreased by 58% and 78%, respectively, and the TN concentration decreased by 16.7% and 7.7%, respectively, with a significant improvement in transparency [17]. The 2014 Changshu urban water diversion test for water environment assessment showed that structural connectivity of river and lake systems can improve river water quality by more than 30% [18]. Lake Bosten has effectively reduced the lake’s annual endogenous TDS pollutant discharge, and consequently the TDS content of the entire lake, and homogenized it, after three years of engineering implementation since 2018 by taking measures to introduce water from the Kaidu River and the Huangshuigou River [19]. The above engineering measures have had a profound impact on the evolution of urban river and lake systems and the improvement of water quality [20]. The above domestic and international connectivity projects are characterized by a large regional span and a wide area of river and lake basins; however, there are fewer research results on changing the water flow characteristics of miniature lakes in towns and cities for the purpose of improving water quality.
In recent years, with Yangshuo’s rapid social and economic development, the city space continues to extend outward, the population density has increased, and the construction of towns and cities has changed drastically. In particular, the pattern of the water environment in the county has changed significantly compared to the 1980s, with a reduction of about 6.0 hm2 of water surface area in the urban area, the rainwater storage space was reduced and flooding increased during the wet season. At the same time, the poor connectivity of water systems between lakes in urban areas has significantly reduced the capacity of water bodies to accommodate pollution, leading to worsening eutrophication, which had a direct impact on the health of water ecosystems. In order to improve the problem of poor connectivity of the water system in Yangshuo urban area, and enhance the drainage capacity of the urban area as well as improve the water quality of the urban lakes, we built and renovated the urban water system from March 2019 to July 2020, including the construction of a new canal for Shuangyue River–Gongyuan Lake, Gongyuan Lake–Shuangyue River, and Diecui Lake–Pantao Lake, the widening of the Youjiao Lake–Diecui Lake link canal, and connecting Shuangyue River and Guihua River. Due to topographic and elevation constraints and the varying number of connecting channels between the seven inner lakes studied and the surrounding rivers, we classify them into three types with different degrees of connectivity: multi-connected (two channels and more) (multi-connected, Mc), single-channel connected (simply connected, Sc) and non-connected (non-connected, Nc).
In order to explore the changes in water quality of urban lakes after the implementation of the project, we conducted monthly water quality monitoring from July 2020 to July 2022 to evaluate water quality conditions and determined the microbial community structure of the lakes through 16srRNA high-throughput sequencing technology. We analyzed and explored the trend of water quality change in the completed lakes, the influence of seasonal changes in the wet and dry seasons, as well as the changes in microbial community structure in the lakes with different degrees of connectivity in order to provide a theoretical basis for evaluating the impacts of the river–lake system connectivity project on the aquatic ecosystems.
2. Methods and Materials
2.1. Study Area
Yangshuo is situated in the northeastern part of the Guangxi Zhuang Autonomous Region and the southern part of Guilin City, belonging to a mid-subtropical monsoon climate. Yangshuo has an average temperature of 19.2 °C, an average rainfall of 1507 mm, with rainfall concentrated from March to August, and an average evaporation of 382.9 mm. Yangshuo covers an area of 4.60 km2, and the water system mainly consists of Shuangyue River and Guihua River. The length of the main stream of Shuangyue River is 3.19 km, with an average drop of 3%, and a rainfall catchment area of 4.95 km2. Guihua River is a tributary of Shuangyue River, with a total length of 1.18 km. Shuangyue River runs through the northern part of Yangshuo, connected to Baoquan Lake. Lianhua Lake is to the northeast of Baoquan Lake. The water diverted from Shuangyue River to the urban area is channeled into Youjiao Lake through a channel with a width of 0.9 m, a height of 1.1 m, and a length of 21.0 m. When the water surface of Youjiao Lake rises, part of the water flows through the channel on its south side to Diecui Lake, Pantao Lake, and Dujia Lake. The water flows from Dujia Lake into Guihua River, which is located in the southern part of Yangshuo and then passes through Guihua River into Shuangyue River (SY River). The water eventually into Lijiang River (Figure 1). The multi-channel lakes include Youjiao Lake (Mc-YJ), Diecui Lake (Mc-DC), Pantao Lake (Mc-PT), and Dujia Lake (Mc-DJ); the single-connected lake includes Baoquan Lake (Sc-BQ); and the non-connected lakes include Lianhua Lake (Nc-LH) and Gongyuan Lake (Nc-GY). The connectivity project was completed on 10 July 2020. The first year after completion of the water system connection, assessments were conducted from July 2020 to July 2022.
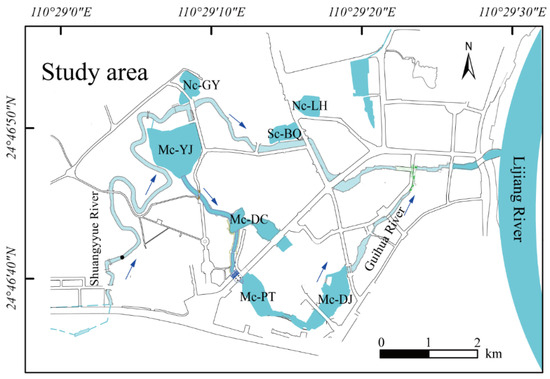
Figure 1.
Connecting water system of Guilin Yangshuo lakes, the blue arrows represents the direction of the water flow.
2.2. Sampling Locations and Analytical Indicators
Samples were collected and monitored once a month from July 2020 to July 2022 at seven lakes with different degrees of connectivity and Shuangyue River in Yangshuo urban area, but water samples were not taken in February 2021. The area is under a wet season from March to August and a dry season from September to February. We operated a multi-parameter water quality analyzer HACH HQ 40d (HACH Corporation, Loveland, CO, USA) in the field to determine the pH and DO of overlying water. The water samples were collected at a depth of 0.5 m into 500 mL polyethylene bottles using a Plexiglas water sampler and stored in the refrigerator at 4 °C for determination as soon as possible. TN was based on the national “Water quality-Determination of total nitrogen-Alkaline potassium persulfate digestion UV spectropho-tometric method” (GB11894-1989) for determination [21]. TP was based on the national “Water quality-Determination of total phosphorus-Ammonium molybdate spectrophotometric method” (GB11893-1989) for determination [22]. NH3-N was based on the national “Water quality-Determination of ammonia nitrogen-Nessler’s reagent spectrophotometry” (HJ535-2009) for determination [23]. NO3-N was based on the national “Water quality-Determination of nitrate-nitrogen-Ultraviolet spectrophotometry” (HJ/T346-2007) for determination [24]. Chl-a was based on the national “Water quality-Determination of chlorophylla-Spectrophotometric method” (HJ897-2017) for determination [25]. CODMn was based on the national “Water quality-Determination of permanganate index” (GB11892-1989) for determination [26].
We collected water samples from Mc-YJ, Mc-DC, Mc-PT, and Nc-LH in December 2021 and December 2022 for an analysis of the microbial community structure in water bodies in different years.
2.3. Statistical Analysis
Microsoft Excel 2019 was utilized to process the relevant raw analytical data. SPSS Statistics 27 was used for principal component analysis and correlation analysis. The correlation between the water quality parameters and the TLIs conformed to the pattern of normal distribution, so Pearson’s coefficient was used for correlation analysis. Mann–Whitney U non-parametric test was used for significance analysis of the differences in the two-year and seasonal variations in the water quality of the lakes. The composition of the microbial community structure of the lakes was analyzed through the 16sRNA high-throughput sequencing technology. The images were analyzed using Origin 2022.
In the alpha diversity index, Chao is an index that estimates the number of OTUs contained in a community using the Chao1 algorithm [27]. The formula is as follows:
where SChao1 denotes the estimated number of OTUs, Sobs denotes the estimated number of OTUs, n1 denotes the number of OTUs containing only one sequence, and n2 denotes the number of OTUs containing only one sequence.
Shannon is one of the indices used to estimate microbial diversity in samples and is often used to reflect alpha diversity [28]. The higher the Shannon value, the higher the diversity of the community. The formula is as follows:
where Sobs denotes the estimated number of OTUs, ni represents the number of sequences contained in the ith OTU, and N represents the number of all individuals, in this case the total number of sequences.
3. Results
3.1. Analysis of Changes in Water Quality Parameters in Lake Water Bodies
The pH of the lakes (Figure 2a) fluctuated within the normal range of 6 to 9 during the two years after completion and was overall higher than that of the upstream SY River. Most lakes had higher mean pH values in the second year compared to the first year. Sc-BQ and Mc-DC increased from 7.81 and 7.66 in the first year and to 8.32 and 7.99 in the second year, or 6.55% and 4.33%, respectively. Mc-PT and Nc-LH increased by 2.26% and 1.34%, respectively, with insignificant changes over the two years compared to Sc-BQ and Mc-DC. Single-channel connected and non-connected lakes had generally higher pH averages than multi-channel connected lakes.
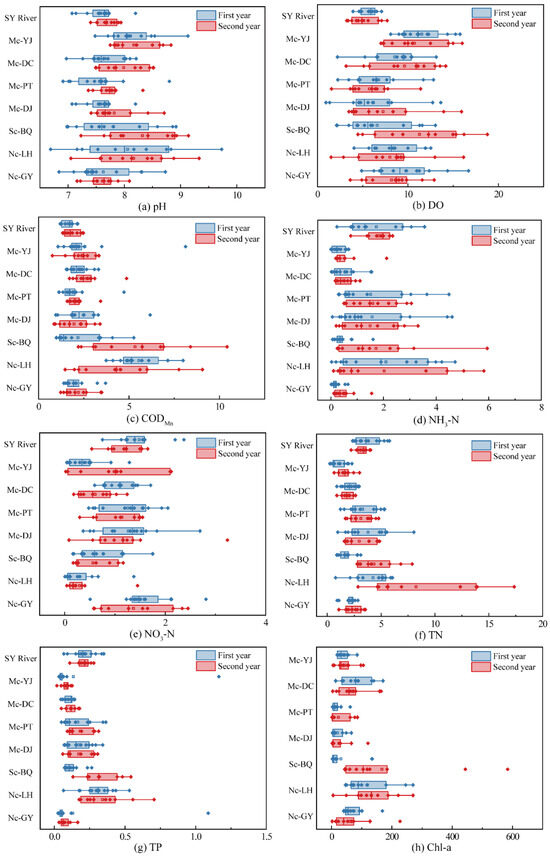
Figure 2.
Boxplots of water quality parameters for seven lakes with different levels of connectivity in two years.
Average DO concentrations (Figure 2b) in the two years after completion of the urban lakes were much greater than the upstream SY River. The average concentration of DO in the second year of Sc-BQ was 11.28 mg/L, which was a significant (p < 0.05) increase of 70.99% compared to the first year, and it is the highest average concentration of all lakes. Mc-DC was raised by 12.76% in the second year. Compared with the first year, the average DO concentrations in the second year of Mc-YJ and Mc-PT were reduced by 7.07% and 14.87%. However, the two-year average DO concentration of Nc-LH did not change significantly, which was from 8.29 mg/L to 8.01 mg/L, but it was generally higher than Mc-PT. The study shows synergy between pH and DO concentration, due to the fact that when aquatic plants release O2, they likewise take up CO2, resulting in a continual accumulation of bicarbonate in the water column and a rise in pH [29]. The pH and DO concentrations of Sc-BQ and Mc-DC showed the same increasing trend, and the increase in DO concentration within a certain range showed an increase in the self-purification ability of the water body; the DO concentration of the Sc-BQ water body reached an oversaturation state, which might be the result of the influence of eutrophication of the water body.
Average CODMn concentrations (Figure 2c) were higher in urban lakes than the SY River in both years, especially Sc-BQ and Nc-LH. The second year of the multi-channel connected lakes was slightly higher than the first year, with Mc-DC increasing from 2.22 mg/L to 2.67 mg/L and Mc-PT increasing from 2.22 mg/L to 2.67 mg/L. The average concentration of Sc-BQ increased from 2.18 mg/L to 5.34 mg/L; the difference between the two years was highly significant (p < 0.01). The average concentration of Nc-LH decreased by 18.37% from 5.54 mg/L to 4.53 mg/L. Sc-BQ was surrounded by approximately two residential buildings and over five hotels and condominiums; therefore, untreated sewage, microplastics, as well as stormwater runoff from roads and rooftops can enter and accumulate in the lake water, thus leading to elevated CODMn concentrations. Compared to Sc-BQ, Nc-LH was not contaminated by organic pollutants entering the water body through the connection with SY River. Although its concentration had decreased, it was surrounded by a vegetable market and numerous roadside vendors. The food scraps, rotting produce, animal waste from the vegetable market, and plastic, oil, and untreated wastewater from roadside vendors may enter the water body. Thus, the active economic activity around the lake is also a contributing factor to water quality [30].
Average NH3-N concentrations (Figure 2d) in Mc-YJ and Mc-DC were much lower than SY River in both years. The mean concentrations of Mc-PT and Mc-DJ were essentially unchanged from SY River. Overall NH3-N concentrations along the course of the multi-channel connected lakes showed a decreasing and then increasing trend. Most of the multi-channel connection lakes remained largely unchanged for two years. Nc-LH decreased from 2.24 mg/L to 2.03 mg/L in two years; the change was not significant. Increased average NH3-N concentrations in Sc-BQ may also be related to domestic wastewater discharges from neighboring residential areas as well as continued inputs of pollutants from the upstream SY River. The reason for the lower concentration of Mc-YJ in both years may be that nitrogen-containing pollutants entering the lake through connecting channels were diluted by the increased volume of stowed water. At the same time, the sedimentation of suspended particulate matter in the water body that adsorbs ammonia nitrogen in the lake body can also achieve the effect of reducing the concentration. The pattern of decrease in NH3-N concentration of Mc-DC and Mc-PT was also similar to that of Mc-YJ.
Average NO3-N concentrations (Figure 2e) in Mc-PT and Mc-DJ were slightly lower than the SY River in both years, and the concentrations of Sc-BQ and Nc-LH were much lower than the SY River. The average NO3-N concentration in Mc-YJ increased from 0.4 mg/L to 0.99 mg/L, showing a significant increase from the first year (p < 0.01). Although the increase is higher, its content is lower. In addition, there was a decreasing trend in the average concentration of Mc-PT. Sc-BQ and Nc-LH had essentially no significant change in average NO3-N concentrations between the first and second years of the program. As the faster water flow makes more oxygen available to the water body, the aerobic environment promotes the microbial nitrifying flora to oxidize NH4+ to NO3− [31]. This explains the increased NO3-N concentrations in the Mc-YJ water body. The decrease in Sc-BQ concentration may be attributable to the fact that CODMn in the lake water body provides a carbon source to promote denitrification, so nitrate did not accumulate. Nc-LH average concentrations of NO3-N in both years were the lowest of all lakes. Probably due to the lack of connectivity, the water flow in Nc-LH was slower than that in multi-channel lakes and single-channel lakes, and the anaerobic environment at the bottom of the lake provides a good environment for anaerobic microorganisms to survive, so denitrification occurs mainly in the water body. Although Nc-GY is a non-connected lake, NO3-N concentrations were extremely low in both years and did not change significantly. Therefore, the increase in carbon source due to the sink of Nc-LH organic matter and the anaerobic environment at the bottom of the lake were the principal factors that caused more drastic denitrification.
Average TN concentrations (Figure 2f) in urban lakes with different levels of connectivity showed similar patterns to NH3-N in both years. The TN concentrations in the multi-channel connected lakes were less than the SY River. Except for Mc-YJ, which showed an increase in average TN concentrations compared to the first year, all other multi-channel connected lakes showed decreases in average TN concentrations. Mc-DC and Mc-PT decreased by 16.31% and 7.67%, respectively. However, Sc-BQ and Nc-LH showed a significant increase in average TN concentrations. Sc-BQ increased from 1.69 mg/L to 4.33 mg/L by 156.95% (p < 0.01), and Nc-LH increased 88.58% from 4.37 mg/L to 8.25 mg/L. It can be observed that changes in TN concentrations in Sc-BQ and Nc-LH are mainly influenced by NH3-N. The NH3-N concentrations of Mc-YJ increased from 0.29 mg/L to 0.53 mg/L, while NO3-N concentrations increased from 0.4 mg/L to 0.99 mg/L, an increase of 143.3%. It suggests that the TN concentration in Mc-YJ was composed mainly of NO3-N. Similarly, the changes in TN concentration in Mc-DC and Mc-PT were affected by changes in NO3-N concentration; on the other hand, the mean TN concentrations of Sc-BQ and Nc-LH were more significantly elevated, with NH3-N as the major component.
Average TP concentrations (Figure 2g) in the multi-channel connected lakes were less than the SY River in both years. Mc-YJ decreased from 0.14 mg/L to 0.08 mg/L, a 41.2% decrease (p < 0.05), and Mc-DC increased from 0.1 mg/L to 0.12 mg/L. Sc-BQ had lower average concentrations in the first year, while Nc-LH had similar TP concentrations to Mc-YJ and Mc-DC. However, Sc-BQ and Nc-LH remained prominent in the second year at 0.32 mg/L and 0.34 mg/L, respectively, much higher than in the first year. Sc-BQ had the largest increase in average TP concentration of all the lakes at 184.22%. The cause may be that the water body in Sc-BQ and Nc-LH was less mobile, and TP was easily released from the sediment and accumulated, resulting in the increase in TP content in the water body [32]. Secondly, water pH similarly affects the release of sediment phosphorus, as pH affects phosphorus diffusion in sediments mainly through adsorption–dissociation and ion-exchange effects [33]. It has been shown that the release of phosphorus from sediments is promoted when the pH of the water body was elevated, and the release of sediment phosphorus was more significant under alkaline conditions than under acidic conditions [34].
Average Chl-a concentrations (Figure 2h) in Sc-BQ and Nc-LH were overall significantly higher than in the multi-channel connected lakes in both years. In the multi-channel connected lakes, although the average Chl-a concentration in Mc-DC was relatively high, compared to the first year, it decreased by 13.73% in the second year. There is a tendency toward improvement in water quality. In contrast, the Chl-a concentrations of Mc-YJ, Mc-PT, and Mc-DJ did not change significantly between the two years and remained relatively stable. The average Chl-a concentration in Sc-BQ increased from 30.51 μg/L to 167.21 μg/L, which may reflect some changes in the ecological environment or trophic status of the water body in the region. Similarly, although the magnitude of Nc-LH elevation was not as significant as that of Sc-BQ, it also showed some degree of increase and may had been influenced by the external environment or internal ecological processes. Nitrogen and phosphorus concentrations are key factors influencing algal growth in lakes. Concentrations of TP and TN in Mc-YJ, Mc-PT, and Mc-DJ were low in both years, and Chl-a concentrations were also relatively low, fluctuating in the range of 10 to 50 μg/L, but slightly elevated in the second year compared with the first year. Chl-a concentrations were significantly elevated in both Sc-BQ and Nc-LH, which were mainly influenced by TN concentrations.
3.2. Changes in Water Quality Parameters of Lakes During Dry and Wet Seasons
The changes in various water quality parameters of lakes in Yangshuo urban area from July 2020 to July 2022 during the wet and dry seasons are shown in Figure 3. Research showed that urban lake water quality parameters exhibit significant seasonal variations, regulated by hydrological connectivity, temperature, and exogenous pollution. Multi-channel connected lakes (such as Mc-PT and Mc-DJ) had smaller seasonal fluctuations in pH (Figure 3a) and DO (Figure 3b), while single-channel (Sc-BQ) and non-connected lakes (Nc-LH) experienced a significant increase in pH during the wet season (e.g., Nc-LH’s pH increased by 10.8% from the dry to wet season), which may be related to the enhanced photosynthesis of aquatic plants consuming CO2 during the wet season. DO was generally higher in the wet season than in the dry season (e.g., Mc-PT’s DO concentration increased by 24%), but high temperatures in summer reduce oxygen solubility, leading to a decrease in DO from July to August. Elevated surface runoff during the wet season contributed to increased organic pollution, with Mc-PT exhibiting a 21.8% rise in CODMn concentration. In contrast, Sc-BQ reached a peak CODMn concentration of 10.42 mg/L during the dry season, likely due to inflows from surrounding domestic sewage (Figure 3c). There was a significant difference in nitrogen forms: NH3-N (Figure 3d) accumulated significantly in single-channel/non-connected lakes during the dry season (e.g., Sc-BQ’s NH3-N concentration was 1.47 mg/L, 97% higher than the wet season), mainly due to low temperatures inhibiting microbial nitrification; NO3-N (Figure 3e) increased during the wet season due to increased runoff input and aerobic nitrification (Mc-YJ’s NO3-N concentration in the wet season was 0.91 mg/L, 58% higher than the dry season). TN (Figure 3f) and TP (Figure 3g) concentrations were generally higher in the dry season than in the wet season, especially in non-connected lakes like Nc-LH (TN concentration in the dry season was 9.05 mg/L, 123% higher than the wet season; TP concentration in the dry season was 0.41 mg/L, 57.7% higher than the wet season), reflecting hydraulic stagnation promoting nutrient release from sediments. Chl-a (Figure 3h) concentration generally increased during the wet season due to optimized light and nutritional conditions (e.g., Mc-PT’s concentration in the wet season increased by 48%), but Sc-BQ and Nc-LH maintain high values throughout the year (Chl-a > 90 μg/L), indicating their persistent eutrophication risk.
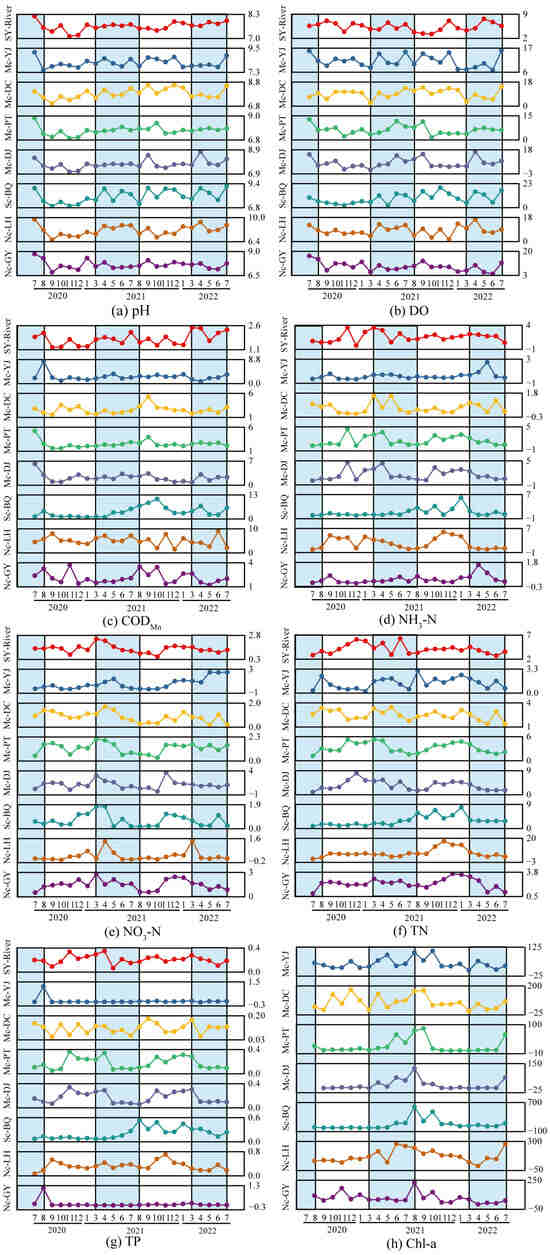
Figure 3.
Seasonal variation in water quality parameters in seven lakes with different degrees of connectivity in two years (blue areas represent wet season, white areas represent dry season).
The seasonal differences in driving mechanisms and their management implications can be attributed to three key factors: (1) temperature regulates microbial activity, with low temperatures during the dry season inhibiting nitrification, leading to NH3-N accumulation [35,36]; (2) hydrological connectivity affects pollutant migration, with multi-channel lakes diluting pollutants through water exchange, while non-connected lakes exacerbate internal source release due to stagnant flow [37]; and (3) spatiotemporal variability of external inputs, with runoff carrying surface source pollutants (CODMn, NO3-N) during the wet season, and point source wastewater input dominating NH3-N increases during the dry season [38]. Non-connected lakes (such as Nc-LH) become “sinks” for nutrients due to the lack of hydraulic disturbance and require priority implementation of ecological water replenishment or sediment dredging. It is recommended to focus on intercepting surface source pollution during the wet season and strengthening internal source control and aeration for oxygen enrichment during the dry season to alleviate the risk of eutrophication. Future management needs to develop dynamic strategies based on seasonal characteristics to optimize the ecological functions of urban water.
4. Discussion
4.1. Evaluation and Comprehensive Analysis of Eutrophication in Lake Water Bodies
4.1.1. Evaluation of Eutrophication of Water Bodies
The degree of eutrophication in seven lakes in Yangshuo urban area was evaluated using the integrated trophic state index (TLI) method [39]. With the exception of Sc-BQ, the TLI of lakes in the second year were all lower than in the first year, indicating that overall eutrophication of urban lake waters improved over time. All of the multi-channel connected lakes were weak eutrophic during the first year, with Mc-DC having the highest TLI at 55.3. Sc-BQ had a TLI of 48.2, which was the lowest of all lakes at the mesotrophic level. Nc-LH was the most eutrophic of all the lakes, with a TLI of 68.7, which was middle eutrophic. Comparing the second year to first year, the TLIs of the multi-channel connected lakes generally reduced, all at mesotrophic levels, with TLIs ranging from 42 to 46. The TLI of Nc-LH decreased to 56.1 in the second year and was at weak eutrophication level. The TLI for the second year of Sc-BQ was 57.1, a shift from mesotrophic to weak eutrophic, with a trend of deteriorating water quality (Figure 4a).
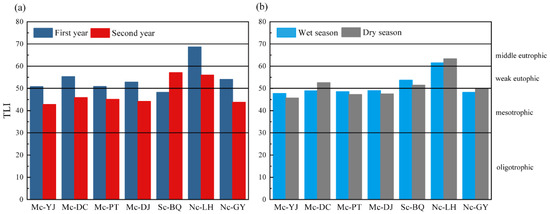
Figure 4.
Comprehensive trophic state indices for seven lakes with different levels of connectivity. (a) The first year and second year; (b) the wet season and dry season.
The dry and wet season TLIs of the multi-channel connected lakes tend to fluctuate between 47 and 49, at mesotrophic levels. Mc-DC had a TLI of 52.6 in the dry season, which was higher than the TLI in the wet season and was in a weak eutrophication level. Sc-BQ had overall higher TLIs than the multi-channel connected lakes in both seasons. The wet and dry season TLIs were 53.7 and 51.5, respectively, and both seasons were at a weak eutrophication level. Nc-LH had a TLI of 61.5 and 63.3 in the wet and dry seasons, respectively, and the TLIs were the highest of all the lakes, which were at the middle eutrophication level. The wet and dry season combined trophic state indices of urban lakes were not significantly different from each other. The wet season index was higher than the dry season for connected lakes, and the dry season was higher than the wet season for non-connected lakes (Figure 4b). The water quality of the connected lakes was poorer during the wet season, and exogenous pollutants were more likely to sink into the lakes with rainwater and spread continuously. The exchange of water between the multi-channel connected lakes dilutes the concentration of pollutants and greatly reduces the accumulation of pollutants within the lakes, leading to lower levels of nutrient fertilization compared to the non-connected lakes. Nc-LH was higher and did not change significantly in TLI during the wet and dry seasons, which indicates that the water body was mainly affected by endogenous pollution and that the water body had insufficient self-purification capacity and poor water quality.
Nitrogen and phosphorus are major factors controlling plant biomass in lakes. It is widely recognized internationally that eutrophication of water bodies is highly likely to occur when the TN concentration in lake water reaches 0.2 mg/L and TP concentration reaches 0.02 mg/L. Redfield has suggested that phosphorus is considered the limiting factor when the nitrogen/phosphorus ratio exceeds 16:1; when the ratio is less than 16:1, nitrogen is usually considered the limiting factor [40]. The average concentration of TP in lakes is 0.03 mg/L, and TN concentration exceeding 1 mg/L can be considered to reach the level of pollution in China [41]. In this study, although most of the lakes in the urban area showed some improvement in water quality, the concentrations of TN and TP were well above the critical values of 0.2 mg/L for TN concentration and 0.02 mg/L for TP concentration for nutrient enrichment of the water body. Therefore, the input of exogenous pollutants should be controlled. The main solution to the wastewater discharged from restaurants and residential areas around Sc-BQ and Nc-LH is to prevent excessive inputs of nitrogen and phosphorus nutrients, which would weaken the inhibition effect on algal growth.
4.1.2. Correlation Analysis
To investigate the relationship between water quality parameters and TLI in urban lakes, Pearson correlation analyses were performed on TLI and eight water quality parameters (Figure 5). The results showed that TLI was significantly and positively correlated with CODMn, Chl-a, TP, and TN with correlation coefficients of 0.7, 0.61, 0.51, and 0.35, respectively. This illustrates that eutrophication is the result of algal blooms caused by organic pollutants and increased nitrogen and phosphorus in water bodies. pH was significantly and positively correlated with DO with a correlation coefficient of 0.74. This is in agreement with the previous results of a similar synergistic trend of elevated pH and DO concentrations for both Sc-BQ and Mc-DC. It was noted that the decrease in pH was due to the decomposition of land plants during flooding, and the decomposition reduced the amount of oxygen while increasing the concentration of CO2 in the environment [42]. This accounts for the positive correlation between pH and DO concentration. The correlation coefficient between NH3-N and TN was 0.74, which was a significant positive correlation. The correlation analysis further illustrated that the NH3-N increased, indicating that Sc-BQ and Nc-LH mainly reflected the trend of TN.
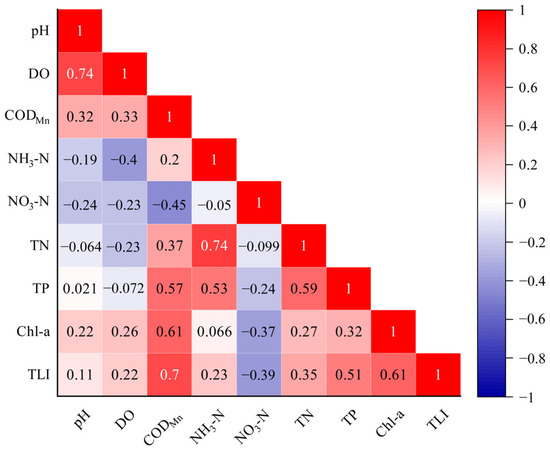
Figure 5.
Correlation analysis of physical and chemical properties of urban lakes.
4.1.3. Principal Component Analysis
The first principal component (PC1) has a high variance contribution of 35.2%, which is higher than the 31.0% of the second principal component (PC2). The first principal component has large loadings on Chl-a, TP, TN, NH3-N, and CODMn of 0.33, 0.487, 0.454, 0.397, and 0.447, respectively. The second principal component has large loadings on pH and DO, 0.507 and 0.564, respectively (Figure 6). This explains the fact that the first principal component mainly reflects the pollution level of organic and inorganic oxidizable substances and the concentration of nitrogen and phosphorus nutrients in Nc-LH and Sc-BQ. Combined with the previous correlation analysis, it can be shown that eutrophication is more severe in Sc-BQ. The second principal component characterizes the concentration of hydrogen ions in the water body of the lake as well as oxygen in the water body. This result revealed differences in environmental factors among connectivity lakes. Non-connected and single-channel connected lakes had more significant changes in environmental factors compared to the multi-channel connected lakes. This result further illustrates that multi-channel connected lakes made it easier for pollutants to be diluted and dispersed due to their better hydrodynamic conditions, thus reducing the impact of pollutant loads on water quality and maintaining its relative stability. Hydrologic connectivity can improve water quality in multi-channel connected lakes, while negatively affecting water quality in single-channel connected lakes. However, the influence of the water system connection project on the water quality of lakes was a long-term process with a certain lag [43,44,45], and the water quality condition of lakes will fluctuate. Therefore, long-term and continuous monitoring of the water quality of lakes is necessary to evaluate the effectiveness of the project.
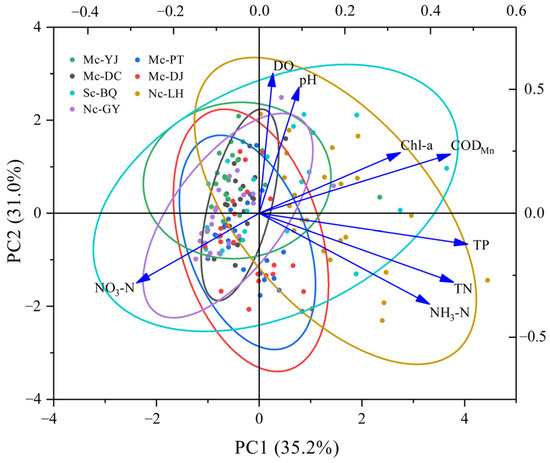
Figure 6.
Principal component analysis of physical and chemical properties of urban lakes.
4.2. Characterization of Microbial Community Structure in Lake Water Bodies
4.2.1. Alpha Diversity of Microbial Communities
Four lakes with different degrees of connectivity, Mc-YJ, Mc-DC, Mc-PT, and Nc-LH, were chosen for this study. We collected three parallel samples from each lake for 16sRNA high-throughput sequencing in December 2021 and 2022, and calculated the Shannon, Chao1 index, which reflects the alpha diversity of the microbial community from the OTUs.
Compared to 2021, the number of OTUs for Mc-YJ, Mc-DC, Mc-PT, and Nc-LH in 2022 declined from 986, 1016, 1824, and 1369 to 718, 420, 1249, and 826, respectively, indicating a decrease in the number of bacterial species in the water of the lakes. The largest number of bacterial species was observed in Mc-PT in both years, followed by Nc-LH, Mc-YJ, and Mc-DC (Figure 7a).
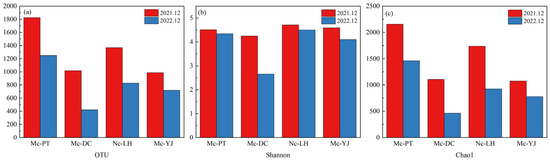
Figure 7.
Alpha diversity of microorganisms in lakes with different levels of connectivity. (a) OTU; (b) Shannon; (c) Chao1.
The Shannon index reflects biodiversity; the larger the Shannon, the higher the biodiversity. The Shannon indices for Mc-YJ, Mc-DC, Mc-PT, and Nc-LH showed a decreasing trend from 4.60, 4.25, 4.51, and 4.71 in 2021 to 4.09, 2.65, 4.34, and 4.50 in 2022, respectively, with the most significant decrease for Mc-DC (p < 0.05). Shannon’s index was largest for Nc-LH and smallest for Mc-DC in both years. The decreasing trend in Shannon’s index indicates a decrease in biodiversity (Figure 7b).
The Chao1 index reflects the richness of the community; the greater the Chao1, the richer the community. The Chao1 index was consistent with the pattern of change in the Shannon index, with a decreasing trend in the second year compared to the first year. Mc-YJ, Mc-DC, Mc-PT, and Nc-LH decreased from 1074.9, 1105.2, 2155.5, and 1736.9 to 777.9, 426.0, 1459.5, and 923.9, respectively. The Chao1 in Mc-PT was the highest and lowest in Mc-DC (Figure 7c).
The Chao1 index and Shannon index of Nc-LH in 2021 were 1736.9 and 4.71, respectively, and the nutrient level of the water body in that year was at the level of middle eutrophic; the eutrophication level of the water body declined to the level of weak eutrophication in 2022. However, the Chao1 index of Mc-PT was greater than that of Nc-LH for two years, and that of Mc-DC and Mc-YJ for two years smaller than Nc-LH. The abundance of microorganisms may not be a valid reflection of the water quality of the lake. On the contrary, the two-year Shannon indices of Nc-LH were higher than those of the other multi-channel connected lakes, indicating that the water bodies had more microbial species and were more seriously polluted. The results showed that the water bodies with Shannon indices of 4.7 or more were middle eutrophic, and the overall water quality was weak eutrophic or mesotrophic in the range of 4–4.7.
4.2.2. Composition of Microbial Phylum Level Communities in Lake Water Bodies
Changes in the relative abundance of microorganisms in lake waters are important indicators of the health of lake ecosystems, environmental changes, and the impact of human activities [46,47]. The composition and dynamics of microbial communities are closely related to the physicochemical properties, nutrient cycling, pollutant degradation, and ecological functions of water bodies, and changes in their relative abundance have an important impact on the carbon and nitrogen cycles [48]. For example, an increase in the relative abundance of methanogenic bacteria in the carbon cycle reflects enhanced anaerobic decomposition of organic matter, and a decrease in the relative abundance of denitrifying bacteria in eutrophic lakes may lead to the accumulation of nitrate and increased water pollution. In addition, changes in the relative abundance of microbial communities for different pollution indicators allow for monitoring the degree of pollution in lakes.
Both temporal and spatial variations affect microbial community structure in water bodies [49]. There were high relatively abundances of Pseudomonadota, Bacteroidota, Actinomycetota, and Cyanobacteriota in four lakes. The highest relative abundance in 2021 was Pseudomonadota, which accounted for 24.8% to 54.6% of the total bacterial abundance; Mc-PT had the highest relative abundance of Pseudomonadota among all lakes. Bacteroidota accounted for 10.4% to 26.9% of the total abundance, with Pseudomonadota and Bacteroidota in Mc-PT being the highest among all lakes. Actinomycetota accounted for 9.4% to 26.9% of the total abundance, while Nc-LH was the highest. Cyanobacteriota accounted for 1.6–30.2% of the total abundance, with Mc-DC, Nc-LH, and Mc-YJ were higher at 30.2%, 26.8%, and 24.6%, respectively (Figure 8a).
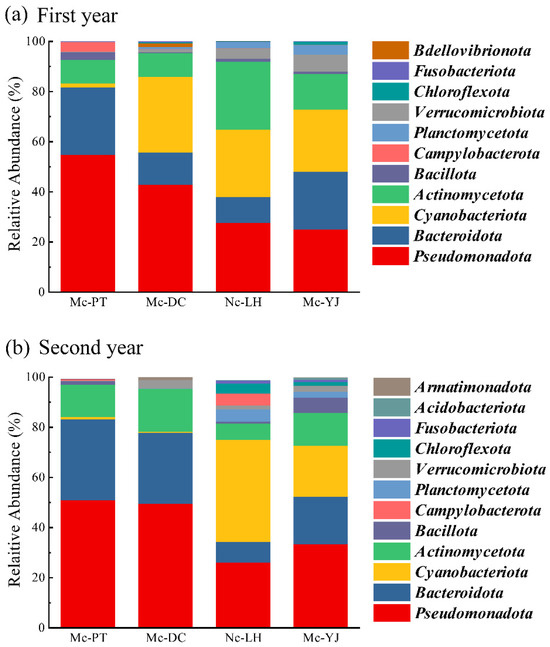
Figure 8.
Community structure of microorganisms in water bodies of urban lakes at phylum level in (a) 2021 and (b) December 2022.
Pseudomonadota remains the more abundant phylum in the four lakes in 2022. Mc-PT decreased from 54.6% to 50.8%; Mc-DC and Mc-YJ increased from 42.7% and 24.8% to 49.5% and 33.4%, respectively. Pseudomonadota remained the dominant phylum in Mc-PT and Mc-DC. Nc-LH decreased significantly from 27.4% to 0.3%, and its relative abundance changed more significantly compared to other lakes. The most pronounced change in relative abundance of Bacteroidota was Mc-DC, which increased from 12.7% to 28.3%. The changes in the relative abundance of Actinomycetota were more pronounced in Mc-DC and Nc-LH. Mc-DC increased from 9.4% to 17.2%, while Nc-LH decreased significantly from 26.9% to 6.5%. The most significant changes in the relative abundance of Cyanobacteriota were Mc-DC and Nc-LH. Mc-DC decreased significantly from 30.2% to 0.3% (p < 0.01), and Nc-LH increased from 26.8% to 40.5% (p < 0.05) (Figure 8b).
Microbial community composition at the gate level in the four lakes in this study was similar to that of most lakes. The abundance of Pseudomonadota in the Fu River in winter was 42.59%, which was the highest abundance among all the groups of bacteria [50]. The relative abundance of Pseudomonadota ranged from 27.80% to 81.04% in the river and lake mouths of the Poyang Lake basin, and similarly, the relative abundance of Bacteroidota and Actinomycetota was also high [51]. Pseudomonadota has been shown to remove nitrogen and phosphorus pollutants from water bodies and may play a key role in the degradation of pollutants [52,53,54]. In particular, NO3-N is utilized to make it proliferate, and Pseudomonadota is strongly correlated with NO3-N concentration. This also accounts for the lower average NO3-N concentrations in Mc-DC and Mc-PT compared to the previous year, which was possibly due to the consumption of NO3-N concentrations by the breeding of Pseudomonadota. Actinomycetes are usually microorganisms that represent better water quality, whereas an increase in Cyanobacteriota is a sign of severe eutrophication of the water body [55]. The elevated relative abundance of Cyanobacteriota in Nc-LH also explains the higher average Chl-a concentration in both years. The same is consistent with the previous results that the TLI of Nc-LH was 68.7 and 56.1 in the first and second years, respectively, at the level of middle and weak eutrophication degrees. Secondly, the decrease in the abundance of Actinomycetota was also associated with the growth of cyanobacterial blooms, which were negatively correlated with each other [56]. Cyanobacteriota abundance in Nc-LH was significantly higher than in the multi-channel connected lakes in the second year, and the abundance of Actinomycetota was lower than that of the multi-channel connected lakes. This may be due to the lack of exchange of Nc-LH with other lake water bodies, and the easy accumulation of nutrients such as nitrogen and phosphorus. The frequent exchange of water between lakes in multiple channels dilutes the concentration of nutrients to a certain extent, making it difficult for Cyanobacteriota to reproduce and grow.
4.2.3. Genus Level Community Composition of Microorganisms in Lake Water Body
The top 20 populations in microbial abundance levels in four lakes were chosen for the analysis of genus level structure. The dominant genera in Mc-PT in 2021 were Limnohabitans (29.33%), Flavobacterium (19.21%), and Tabrizicola (12.80%). Neochroococcus was the dominant genus in Mc-DC (35.09%) and Mc-YJ (30.14%), which belongs to Cyanobacteria and it is the dominant genus in the occurrence of blooms. Trichocoleus (16.23%) and Tetrasphaera (14.20%) were the dominant genera in Nc-LH. Limnohabitans is a genus of bacterioplankton with diverse metabolism, rapid growth, and diverse morphology, occurring in high abundance in almost all lake systems worldwide [57,58] (Figure 9a). Limnohabitans typically have higher abundance in alkaline environments. This strain transfers carbon from primary producers to more advanced producers [59,60], and has a strong denitrification function, can remove nitrogen in the water body, improve water quality, and remove organic matter in the water body, playing an important role in water quality [61]. Flavobacterium is a denitrifying microorganism of high abundance [62] and can survive cold environments [63,64]. In full sunlight, it is able to utilize the degradation of cyanobacteria and other algal organic matter to obtain the energy needed for growth [65], which is important for the improvement of water quality in lakes.
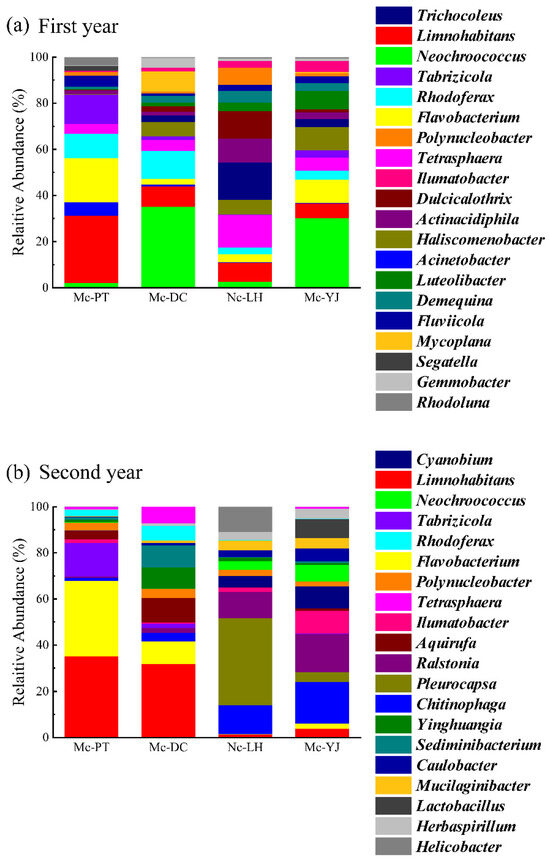
Figure 9.
Community structure of microorganisms in water bodies of urban lakes at genus level in (a) 2021 and (b) December 2022.
The abundance of Limnohabitans and Flavobacterium in Mc-PT in 2022 increased to 35.1% and 32.8%, respectively, while there was not any significant change in the abundance of Tabrizicola. The shift in Mc-PT from middle eutrophic to mesotrophic levels may be a consequence of Flavobacterium inhibiting algal growth by ingesting input organic matter. Nitrogenous nutrients were also consumed by denitrification to remove nitrates from the water body. In addition, Flavobacterium immobilized in alginate was able to degrade PCP efficiently in scale-up batch reactor experiments, with a gradual decrease in degradation efficiency as the rate of PCP loading in influent water increased or the hydraulic retention time decreased. This finding revealed the potential of Flavobacterium in PCP degradation and its characterization by exterior factors [66]. Therefore, the ability of Flavobacterium to become a dominant genus in Mc-PT connectivity to improve water quality was due to the appropriate rate of water movement and hydraulic retention time. The abundance of Neochroococcus in Mc-DC decreased significantly from 35.09% to 0.15%, at which point the dominant genus was Limnohabitans, with a relative abundance of 31.82%. The relative abundance of Neochroococcus in Mc-YJ decreased from 30.14% to 3.68%, and at this time, Chitinophaga (18.13%) and Ralstonia (16.52%) became the dominant genera. Microorganisms such as Chitinophaga and Ralstonia are capable of degrading organic pollutants in water bodies, which serve to maintain the health and stability of water bodies. Pleurocapsa (37.85%) was the dominant genus in Nc-LH in 2022, and the increase in these microorganisms can likely lead to turbidity, odor, and other undesirable effects in water bodies (Figure 9b).
5. Conclusions
The two-year investigation demonstrated that hydrological connectivity critically regulates water quality evolution in urban lacustrine systems. Interannual monitoring revealed divergent trajectories: multi-channel connected lakes exhibited overall improvement, particularly Mc-YJ with elevated parameters contrasting with declines in Mc-DJ and Mc-PT. Conversely, single-channel and non-connected lakes displayed marked deterioration. Seasonal patterns were characterized by wet-phase maxima in pH, DO, CODMn, and chlorophyll-a, opposing dry-phase peaks in TN, TP, NH3-N, and NO3-N. Trophic state transitions corroborated these dynamics, with Mc systems progressing to mesotrophic status, whereas Sc-BQ and Nc-LH reverted to weak eutrophic conditions. Critically, microbial signatures substantiated connectivity-mediated mechanisms—Cyanobacteriota suppression coupled with Bacteroidota enrichment in Mc-DC indicated remediation efficacy, contrasting with Cyanobacteriota dominance in Nc-LH signaling degradation. Taxon-specific shifts (e.g., Flavobacterium prevalence in Mc-PT) further delineated hydrological connectivity as a pivotal driver of microbial succession and nutrient cycling processes.
Author Contributions
Conceptualization, H.L. and S.B.; Methodology, G.H.; Validation, M.W., Q.Z., D.X. and Y.D.; Formal analysis, H.C., M.Z., M.W., Q.Z., D.X. and Y.D.; Data curation, S.H.; Writing—original draft, S.H.; Writing—review & editing, H.L., S.B. and Y.Z.; Supervision, H.L., S.B. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (52360024) and the Guangxi Science and Technology Program (Guike AB22080067, Guike AA20161001, Guike AD25069074). We also thank the Collaborative Innovation Center for Water Pollution Control and Water Safety in Karst Area, Areas and Key Laboratory of Carbon Emission and Pollutant Collaborative Control (Guilin University of Technology) for equipment support.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Mei Wang was employed by the company Hengsheng Water Environment Treatment Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Lu, S.; Si, J.; Hou, C.; Li, Y.; Wang, M.; Yan, X.; Xie, M.; Sun, J.; Chen, B.; Li, S. Spatiotemporal distribution of nitrogen and phosphorus in alpine lakes in the Sanjiangyuan Region of the Tibetan Plateau. Water Sci. Technol. 2017, 76, 396–412. [Google Scholar] [CrossRef]
- Wang, W.-H.; Wang, Y.; Sun, L.-Q.; Zheng, Y.-C.; Zhao, J.-C. Research and application status of ecological floating bed in eutrophic landscape water restoration. Sci. Total Environ. 2020, 704, 135434. [Google Scholar] [CrossRef]
- Anawar, H.M.; Chowdhury, R. Remediation of Polluted River Water by Biological, Chemical, Ecological and Engineering Processes. Sustainability 2020, 12, 7017. [Google Scholar] [CrossRef]
- Peilin, G.; Meng, C.; Lichao, Z.; Yuejun, S.; Minghao, M.; Lingyun, W. Study on Water Ecological Restoration Technology of River. IOP Conf. Ser. Earth Environ. Sci. 2019, 371, 032025. [Google Scholar] [CrossRef]
- Lu, Y.; Li, M.-C.; Lee, J.; Liu, C.; Mei, C. Microplastic remediation technologies in water and wastewater treatment processes: Current status and future perspectives. Sci. Total Environ. 2023, 868, 161618. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Chen, G.; Liu, Y.; Zhou, L.; Cai, M.; Song, X.; Zou, G. Comparison of two different ecological floating bio-reactors for pollution control in hyper-eutrophic freshwater. Sci. Rep. 2018, 8, 14306. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hu, Y.; Li, S.; Peng, S.; Zhao, H. Microbial mechanisms of using enhanced ecological floating beds for eutrophic water improvement. Bioresour. Technol. 2016, 211, 451–456. [Google Scholar] [CrossRef]
- Reddy, S.; Osborne, J. An insight on the advancements of biological technologies in the bioremediation of textile effluents. Urban Water J. 2022, 19, 468–480. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, W.; Lu, Y.; Sun, S.; Yin, Q. Construction and application of urban water system connectivity evaluation index system based on PSR-AHP-Fuzzy evaluation method coupling. Ecol. Indic. 2023, 153, 110421. [Google Scholar] [CrossRef]
- Yang, S.; Yang, G.; Li, B.; Wan, R. Water quality improves with increased spatially surface hydrological connectivity in plain river network areas. J. Environ. Manag. 2025, 377, 124703. [Google Scholar] [CrossRef]
- Vant, W.N.; Gilliland, B.W. Changes in water quality in Lake Horowhenua following sewage diversion. N. Z. J. Mar. Freshw. Res. 1991, 25, 57–61. [Google Scholar] [CrossRef]
- Amano, Y.; Sakai, Y.; Sekiya, T.; Takeya, K.; Taki, K.; Machida, M. Effect of phosphorus fluctuation caused by river water dilution in eutrophic lake on competition between blue-green alga Microcystis aeruginosa and diatom Cyclotella sp. J. Environ. Sci. 2010, 22, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, F.A.; Hosny, S.; Faragallah, H.M.; Mohamed, E.; Shabaka, S. Preliminary assessment of water quality post-the first phase of the development plans in Lake Burullus, Deltaic coast of the Mediterranean Sea, Egypt. Sci. Afr. 2022, 16, e01193. [Google Scholar] [CrossRef]
- Nong, X.; Shao, D.; Zhong, H.; Liang, J. Evaluation of water quality in the South-to-North Water Diversion Project of China using the water quality index (WQI) method. Water Res. 2020, 178, 115781. [Google Scholar] [CrossRef]
- Zhai, S.; Zhang, H.; Hu, W.; Yu, X. Evaluation on result of Yangtze-Taihu Water Diversion. China Water Resour. 2008, 1, 21–23. [Google Scholar] [CrossRef]
- Xie, X.; Qian, X.; Zhang, Y.; Qian, Y.; Tian, F. Effect on Chaohu Lake Water Environment of Water Transfer from Yangtze River to Chaohu Lake. Res. Environ. Sci. 2009, 22, 897–901. [Google Scholar] [CrossRef]
- You, A.; Wu, Z.; Han, Z.; Yang, J.; Hua, L. Spatial and temporal distributions and variations of nutrients in the West Lake, Hangzhou, after the implementation of integrated water management program (1985–2013). J. Lake Sci. 2015, 27, 371–377. [Google Scholar] [CrossRef]
- Cui, G.; Chen, X.; Xiang, L.; Zhang, Q.; Xu, Q. Evaluation of water environment improvement by interconnected river network in plain area. J. Hydraul. Eng. 2017, 48, 1429–1437. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Y.; Ye, Z.; Li, Y.; Zhu, C. River-Lake System Connectivity Effectively Reduced the Salinity of Lake Water in Bosten Lake, Northwest China. Water 2022, 14, 4002. [Google Scholar] [CrossRef]
- Gurnell, A.M.; Shuker, L.; Lee, M.; Boitsidis, A.J. Gradients in the Biophysical Structure of Urban Rivers and Their Association with River Channel Engineering. River Res. Appl. 2012, 28, 908–925. [Google Scholar] [CrossRef]
- GB11894-1989; Water Quality-Determination of Total Nitrogen-Alkaline Potassium Persulfate Digestion UV Spectrophotometric Method. State Environmental Protection Administration: Beijing, China, 1989.
- GB11893-1989; Water Quality-Determination of Total Phosphorus-Ammonium Molybdate Spectrophotometric Method. State Environmental Protection Administration: Beijing, China, 1989.
- HJ535-2009; Water Quality-Determination of Ammonia Nitrogen-Nessler’s Reagent Spectrophotometry. State Environmental Protection Administration: Beijing, China, 2009.
- HJ/T346-2007; Water Quality-Determination of Nitrate-Nitrogen-Ultraviolet Spectrophotometry. State Environmental Protection Administration: Beijing, China, 2007.
- HJ897-2017; Water Quality-Determination of Chlorophyll a-Spectrophotometric method. State Environmental Protection Administration: Beijing, China, 2017.
- GB11892-1989; Water Quality-Determination of Permanganate Index. State Environmental Protection Administration: Beijing, China, 1989.
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Guo, Q.; Lu, B.; Qin, Y.; Wang, C. Effect of Cage Aquaculture on Aquatic Bacteria Community Structure in a Deep Karst Lake: A Case Study of Wanfeng Lake. J. Hydroecology 2024, 45, 175–183. [Google Scholar]
- Xia, W.; Zhang, M.; Zhou, M.; Wu, J.; Yao, N.; Feng, B.; Ouyang, T.; Liu, Z.; Zhang, Q. Spatio-temporal dynamics of dissolved oxygen and its influencing factors in Lake Xiannv Jiangxi, China. J. Lake Sci. 2023, 35, 334–340. [Google Scholar] [CrossRef]
- Peng, S.; Wu, P.; Lu, Y.; Chen, L.; Wang, Z.; Lu, Y. Influence of river structure and hydrodynamics on water quality in the upper Taihu Basin, China. J. Clean. Prod. 2024, 453, 142262. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, R.P.; Geng, C.; Fu, D. Carbon-to-nitrogen ratio influence on the performance of bioretention for wastewater treatment. Environ. Sci. Pollut. Res. 2020, 27, 17652–17660. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, S.; Ni, Z.; Jiao, L. The response of water quality variation in Poyang Lake (Jiangxi, People’s Republic of China) to hydrological changes using historical data and DOM fluorescence. Environ. Sci. Pollut. Res. 2015, 22, 3032–3042. [Google Scholar] [CrossRef]
- Zhao, K.; Fu, H.; Zhu, Y.; Wang, Y.; Wang, S.; Li, F. Environmental impacts of nitrogen and phosphorus nutrient diffusion fluxes at a sediment-water interface: The case of the Yitong River, China. Sustainability 2023, 15, 1210. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, Y.; Zhou, J.; Wu, Y. Phosphorus release from lake sediments: Effects of pH, temperature and dissolved oxygen. KSCE J. Civ. Eng. 2014, 18, 323–329. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Li, X.; Liu, J.; Ao, H.; Li, Q. Seasonal variation of water quality of Xinyunliang River, Dianchi Basin and the impact of the riparian ecological restoration. J. Lake Sci. 2012, 24, 334–340. [Google Scholar]
- Liu, H.; Qi, Z.; Pan, H.; Tang, X.; Shao, K.; Gao, G. Research on effect of ecological restoration of low flow urban rivers: Taking Qinshui River for ecample. Environ. Eng. 2015, 33, 5–10. [Google Scholar]
- Wang, C.; Wang, X.; Xu, Y.J.; Lv, Q.; Ji, X.; Jia, S.; Liu, Z.; Mao, B. Nitrogen and phosphorus evolution process and driving mechanisms of three major freshwater lakes with different river-lake connectivity (DRLC) in the lower reaches of the Yangtze river, the largest river in Asia. J. Clean. Prod. 2025, 486, 144471. [Google Scholar] [CrossRef]
- Hu, B.; Liu, Y.; Chen, Y.; Yao, Y.; Liu, H.; Wang, Z. Water quality and pollution source apportionment responses to rainfall in steppe lake estuaries: A case study of Hulun Lake in northern China. Ecol. Indic. 2024, 168, 112791. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Zhang, J. Evaluate method and classification standard on lake eutrophication. Environ. Monit. China 2002, 18, 47–49. [Google Scholar] [CrossRef]
- Domagalski, J.L.; Morway, E.; Alvarez, N.L.; Hutchins, J.; Rosen, M.R.; Coats, R. Trends in nitrogen, phosphorus, and sediment concentrations and loads in streams draining to Lake Tahoe, California, Nevada, USA. Sci. Total Environ. 2021, 752, 141815. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, W.; Wang, X.; Couture, R.-M.; Larssen, T.; Zhao, Y.; Li, J.; Liang, H.; Liu, X.; Bu, X.; et al. Decline in Chinese lake phosphorus concentration accompanied by shift in sources since 2006. Nat. Geosci. 2017, 10, 507–511. [Google Scholar] [CrossRef]
- Araoye, P.A. The seasonal variation of pH and dissolved oxygen (DO2) concentration in Asa lake Ilorin, Nigeria. Int. J. Phys. Sci. 2009, 4, 271–274. [Google Scholar]
- Duan, T.; Feng, J.; Chang, X.; Li, Y. Evaluation of the effectiveness and effects of long-term ecological restoration on watershed water quality dynamics in two eutrophic river catchments in Lake Chaohu Basin, China. Ecol. Indic. 2022, 145, 109592. [Google Scholar] [CrossRef]
- Luo, Z.; Zhao, S.; Wu, J.; Zhang, Y.; Liu, P.; Jia, R. The influence of ecological restoration projects on groundwater in Yongding River Basin in Beijing, China. Water Supply 2019, 19, 2391–2399. [Google Scholar] [CrossRef]
- Gao, W.; Lu, J.-J. A restoration trial of bird habitat on the intertidal flats in the Yangtze Estuary and its short-term effects. Acta Ecol. Sin. 2008, 28, 2080–2089. [Google Scholar] [CrossRef]
- Shen, Z.; Xie, G.; Gong, Y.; Shao, K.; Gao, G.; Tang, X. Seasonal dynamics of environmental heterogeneity augment microbial interactions by regulating community structure in different trophic lakes. Environ. Res. 2024, 263, 120031. [Google Scholar] [CrossRef]
- Xiao, P.; Wu, Y.; Zuo, J.; Grossart, H.P.; Sun, R.; Li, G.; Jiang, H.; Cheng, Y.; Wang, Z.; Geng, R.; et al. Differential microbiome features in lake–river systems of Taihu basin in response to water flow disturbance. Front. Microbiol. 2024, 15, 1479158. [Google Scholar] [CrossRef]
- Zhao, A.; Lu, Y.; Li, Q.; Li, T.; Zhao, J. Metagenomics reveals the diversity and role of surface-water microbes in biogeochemical cycles in lakes at different terrain ladders. Front. Environ. Sci. 2023, 11, 1121775. [Google Scholar] [CrossRef]
- Bashenkhaeva, M.V.; Zakharova, Y.R.; Petrova, D.P.; Khanaev, I.V.; Galachyants, Y.P.; Likhoshway, Y.V. Sub-Ice Microalgal and Bacterial Communities in Freshwater Lake Baikal, Russia. Microb. Ecol. 2015, 70, 751–765. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Wang, Y.; Zhang, S.; Zhang, G.; Han, Y.; Li, M.; Liu, L. Spatial and temporal dynamics of microbial community composition and factors influencing the surface water and sediments of urban rivers. J. Environ. Sci. 2023, 124, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Xu, G.; Huang, J.; Jian, Z.; Qin, T.; Ouyang, L.; Huang, X. Study on Microbial Diversity in Estuary of Poyang Lake Basin. Acta Agric. Jiangxi 2022, 34, 138–142+148. [Google Scholar] [CrossRef]
- Yan, Q.; Min, J.; Yu, Y.; Zhu, Z.; Feng, G. Microbial community response during the treatment of pharmaceutically active compounds (PhACs) in constructed wetland mesocosms. Chemosphere 2017, 186, 823–831. [Google Scholar] [CrossRef]
- Hou, L.; Zhou, Q.; Wu, Q.; Gu, Q.; Sun, M.; Zhang, J. Spatiotemporal changes in bacterial community and microbial activity in a full-scale drinking water treatment plant. Sci. Total Environ. 2018, 625, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Droppo, I.G.; Krishnappan, B.G.; Lawrence, J.R. Microbial interactions with naturally occurring hydrophobic sediments: Influence on sediment and associated contaminant mobility. Water Res. 2016, 92, 121–130. [Google Scholar] [CrossRef]
- Niu, Y.; Shen, H.; Chen, J.; Xie, P.; Yang, X.; Tao, M.; Ma, Z.; Qi, M. Phytoplankton community succession shaping bacterioplankton community composition in Lake Taihu, China. Water Res. 2011, 45, 4169–4182. [Google Scholar] [CrossRef]
- Ghai, R.; Megumi Mizuno, C.; Picazo, A.; Camacho, A.; Rodriguez-Valera, F. Key roles for freshwater Actinobacteria revealed by deep metagenomic sequencing. Mol. Ecol. 2014, 23, 6073–6090. [Google Scholar] [CrossRef]
- Props, R.; Denef, V.J. Temperature and Nutrient Levels Correspond with Lineage-Specific Microdiversification in the Ubiquitous and Abundant Freshwater Genus Limnohabitans. Appl. Environ. Microbiol. 2020, 86, e00140-20. [Google Scholar] [CrossRef] [PubMed]
- Simek, K.; Kasalicky, V.; Jezbera, J.; Jezberova, J.; Hahn, M.W. Broad Habitat Range of the Phylogenetically Narrow R-BT065 Cluster, Representing a Core Group of the Betaproteobacterial Genus Limnohabitans. Appl. Environ. Microbiol. 2010, 76, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Hornak, K.; Kasalicky, V.; Simek, K.; Grossart, H.-P. Strain-specific consumption and transformation of alga-derived dissolved organic matter by members of the Limnohabitans-C and Polynucleobacter-B clusters of Betaproteobacteria. Environ. Microbiol. 2017, 19, 4519–4535. [Google Scholar] [CrossRef]
- Shabarova, T.; Kasalicky, V.; Simek, K.; Nedoma, J.; Znachor, P.; Posch, T.; Pernthaler, J.; Salcher, M.M. Distribution and ecological preferences of the freshwater lineage LimA (genus Limnohabitans) revealed by a new double hybridization approach. Environ. Microbiol. 2017, 19, 1296–1309. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, L.; Cai, M.; Cui, N.; Pang, S.; Zou, G.; Zhao, Z.; Yuan, Q.; Huang, W.; Zhang, Y. Metagenomics-based analysis of microbial community structure and function composition in aquaculture pond for Chinese mitten crab. Prog. Fish. Sci. 2024, 45, 112–124. [Google Scholar] [CrossRef]
- Wang, H.; Chen, N.; Feng, C.; Deng, Y.; Lu, W. Differences in microbial diversity, composition and function during V(v) release and reduction in nitrate-V(v) co-contaminated water from liquid carbon sources. Environ. Sci.-Water Res. Technol. 2023, 9, 1890–1902. [Google Scholar] [CrossRef]
- Sun, M.-L.; Zhao, F.; Shi, M.; Zhang, X.-Y.; Zhou, B.-C.; Zhang, Y.-Z.; Chen, X.-L. Characterization and Biotechnological Potential Analysis of a New Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef]
- Gu, Z.; Liu, K.; Pedersen, M.W.; Wang, F.; Chen, Y.; Zeng, C.; Liu, Y. Community assembly processes underlying the temporal dynamics of glacial stream and lake bacterial communities. Sci. Total Environ. 2021, 761, 143178. [Google Scholar] [CrossRef]
- Eiler, A.; Bertilsson, S. Flavobacteria Blooms in Four Eutrophic Lakes: Linking Population Dynamics of Freshwater Bacterioplankton to Resource Availability. Appl. Environ. Microbiol. 2007, 73, 3511–3518. [Google Scholar] [CrossRef]
- Lo, K.V.; Zhu, C.M.; Cheuk, W. Biodegradation of pentachlorophenol by Flavobacterium species in batch and immobilized continuous reactors. Environ. Technol. 1998, 19, 91–96. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).