Vertical Binding Characteristics Between Dissolved Organic Matter and Heavy Metals in the Upper Reaches of the Yangtze River Using EEM-PARAFAC and 2D-FTIR-COS
Abstract
1. Introduction
2. Materials and Methods
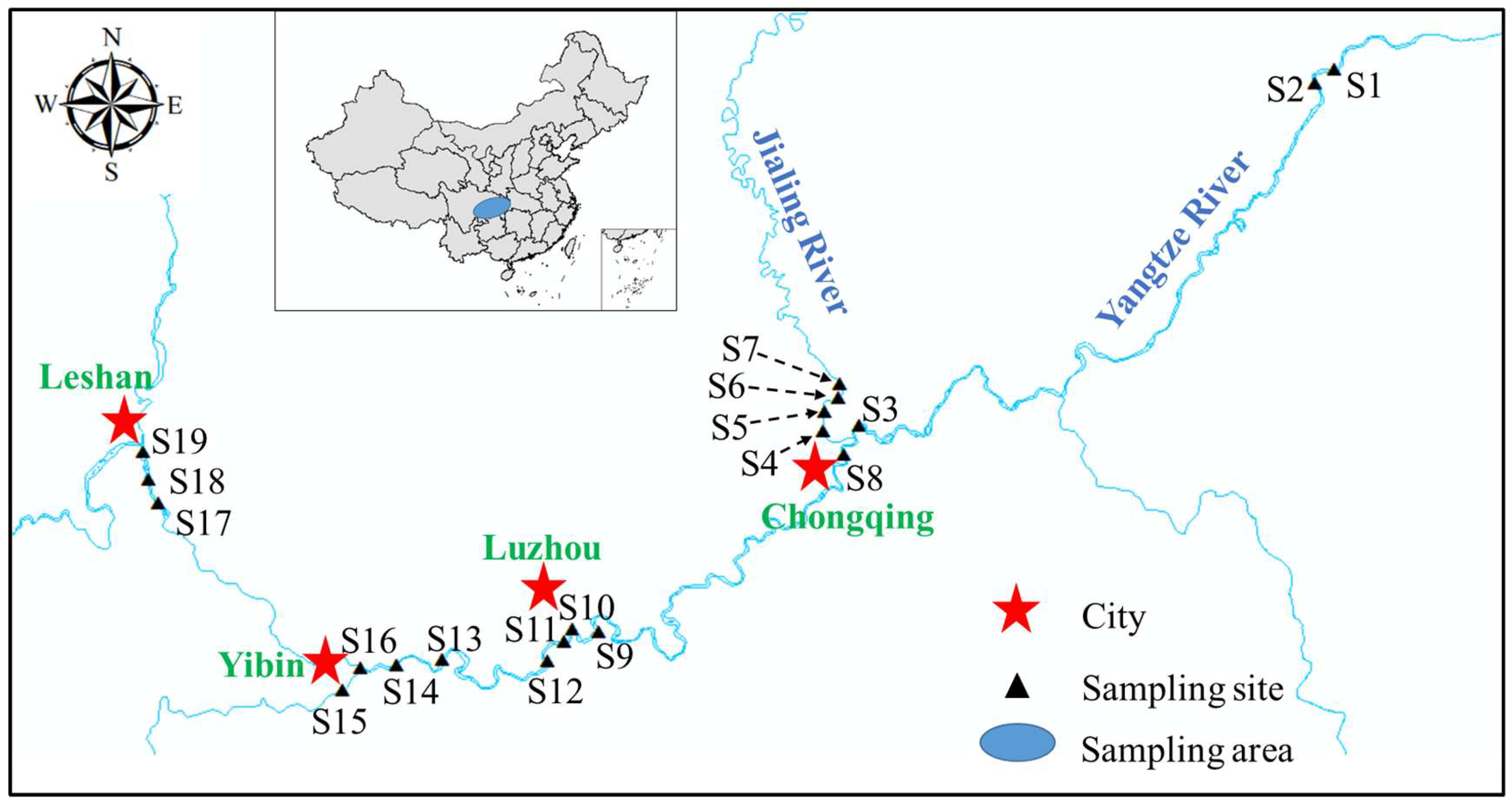
2.1. Study Area and Sample Collection
2.2. Fluorescence Quenching Titration
2.3. Measurements of Optical Properties
2.4. Metal-DOM Binding Modeling
3. Result and Discussion
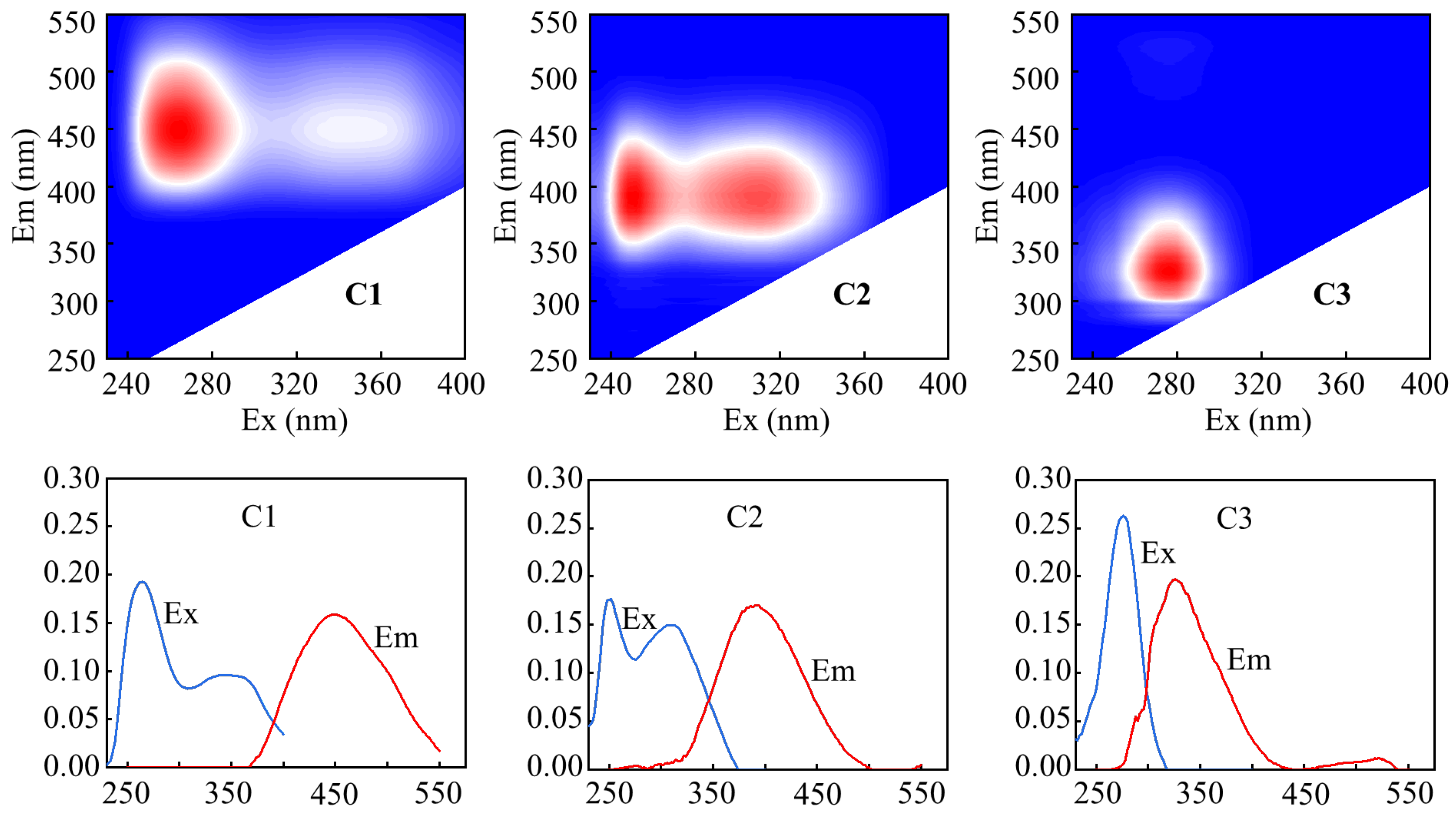
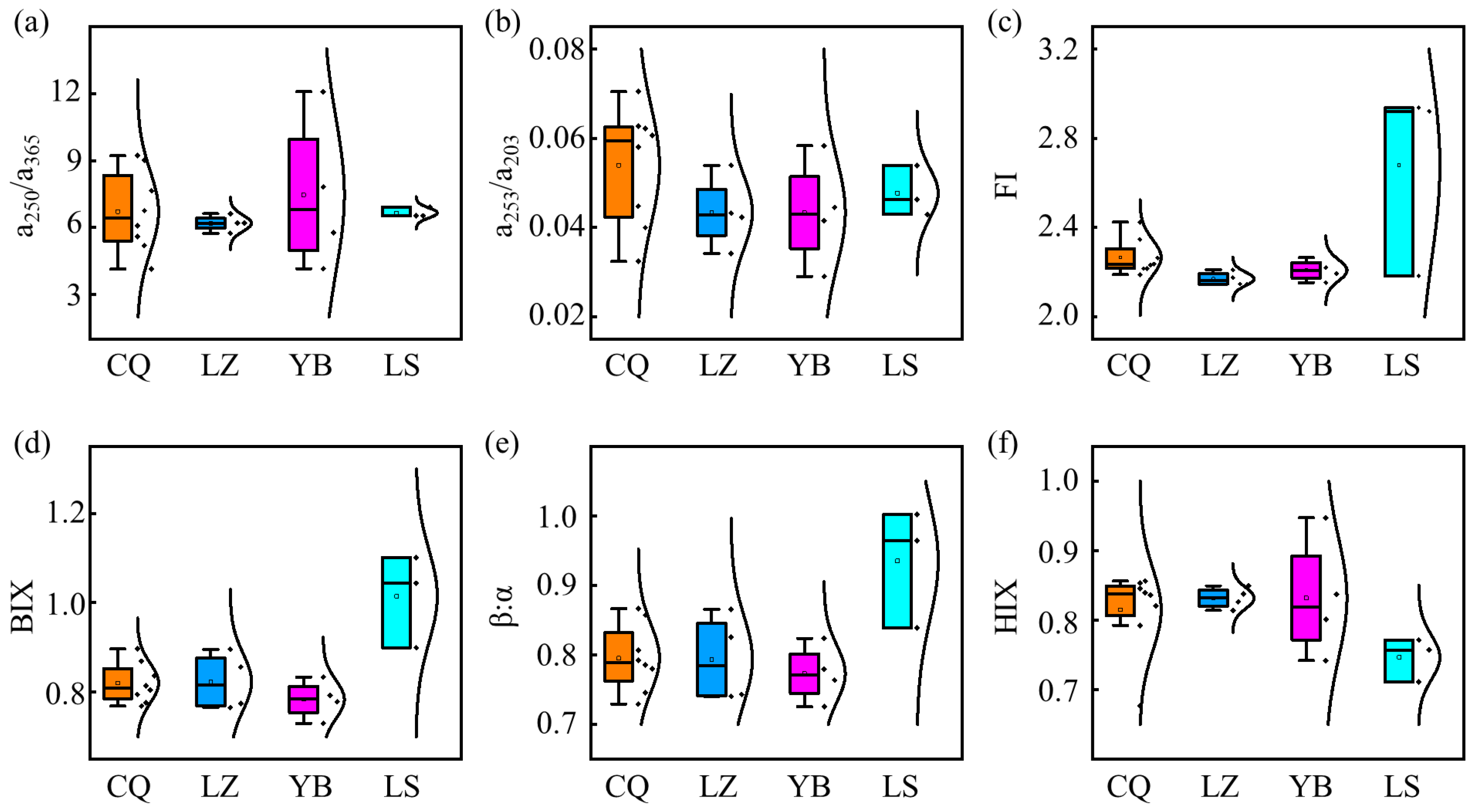
3.1. Characteristics of DOM in the Upstream of the Yangtze River
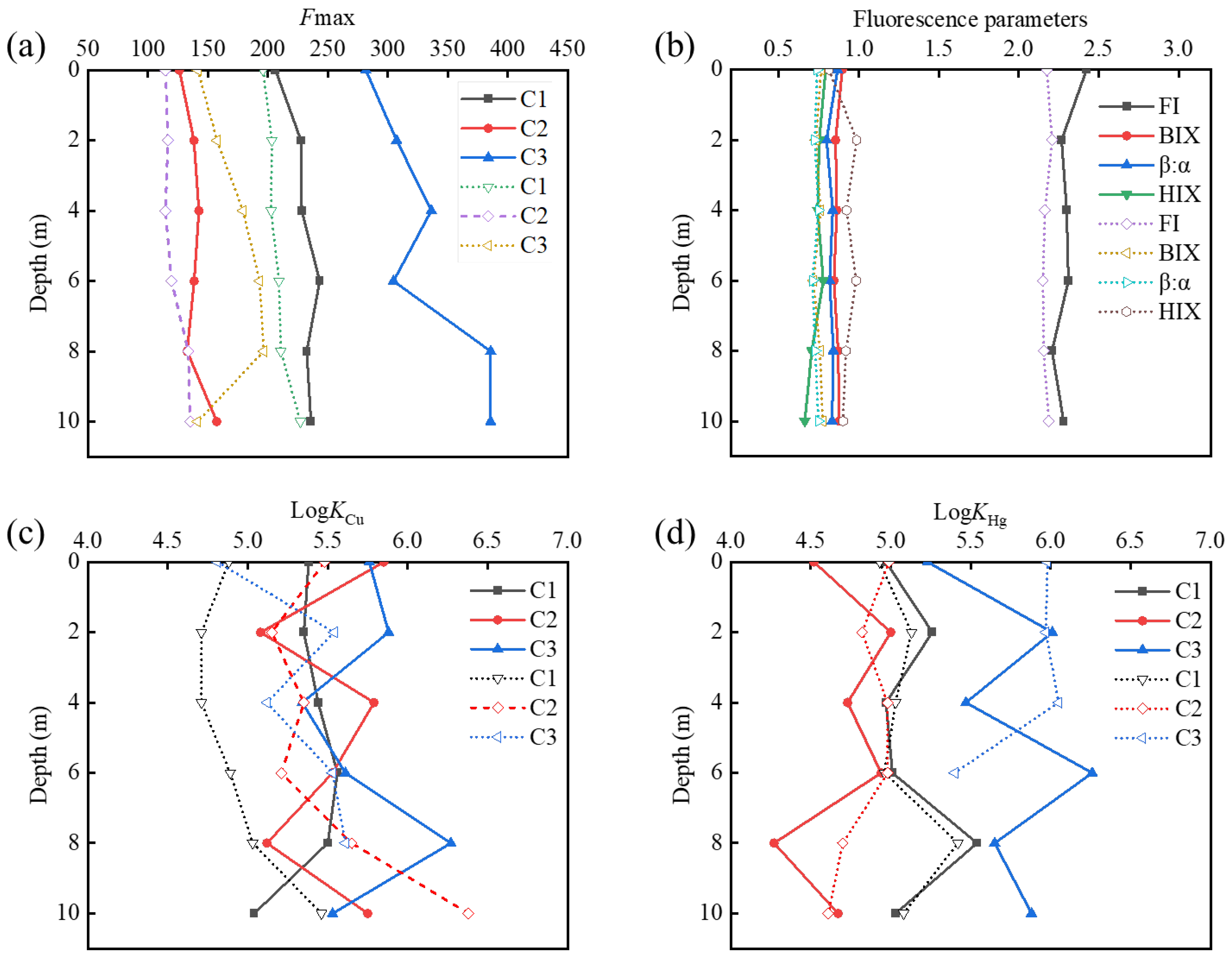
3.2. Evolution of Binding Ability of HMs and DOM
3.3. Analysis of Binding Sequence of HMs and DOM Combined with FTIR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, T.; Chen, X.; Wang, D.; Liang, J.; Bai, W.; Zhang, C.; Wang, Q.; Wei, S. Dynamics of dissolved organic matter (DOM) in a typical inland lake of the Three Gorges Reservoir area: Fluorescent properties and their implications for dissolved mercury species. J. Environ. Manag. 2018, 206, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Tranvik, L.J.; Cole, J.J.; Prairie, Y.T. The study of carbon in inland waters-from isolated ecosystems to players in the global carbon cycle. Limnol. Oceanogr. Lett. 2018, 3, 41–48. [Google Scholar] [CrossRef]
- Ferrer-González, F.X.; Widner, B.; Holderman, N.R.; Glushka, J.; Edison, A.S.; Kujawinski, E.B.; Moran, M.A. Resource partitioning of phytoplankton metabolites that support bacterial heterotrophy. ISME J. 2021, 15, 762–773. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Zhang, Y.; Zhu, G.; Qin, B.; Jang, K.-S.; Spencer, R.G.M.; Kothawala, D.N.; Jeppesen, E.; Brookes, J.D.; et al. Unraveling the Role of Anthropogenic and Natural Drivers in Shaping the Molecular Composition and Biolability of Dissolved Organic Matter in Non-pristine Lakes. Environ. Sci. Technol. 2022, 56, 4655–4664. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Qiu, L.; Zhang, M.; Yang, J.; Ji, R.; Vione, D.; Chen, Z.; Gu, C. Photochemical behavior of dissolved organic matter in environmental surface waters: A review. Eco-Environ. Health 2024, 3, 529–542. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, P.; Liang, Y.; Li, R.; Shi, Z. Modeling the equilibrium and kinetics of heavy metals reactions with dissolved organic matter. Appl. Geochem. 2025, 181, 106300. [Google Scholar] [CrossRef]
- Kikuchi, T.; Fujii, M.; Terao, K.; Jiwei, R.; Lee, Y.P.; Yoshimura, C. Correlations between aromaticity of dissolved organic matter and trace metal concentrations in natural and effluent waters: A case study in the Sagami River Basin, Japan. Sci. Total Environ. 2017, 576, 36–45. [Google Scholar] [CrossRef]
- Fan, T.; Yao, X.; Sun, Z.; Sang, D.; Liu, L.; Deng, H.; Zhang, Y. Properties and metal binding behaviors of sediment dissolved organic matter (SDOM) in lakes with different trophic states along the Yangtze River Basin: A comparison and summary. Water Res. 2023, 231, 119605. [Google Scholar] [CrossRef]
- Xu, H.; Yu, G.; Yang, L.; Jiang, H. Combination of two-dimensional correlation spectroscopy and parallel factor analysis to characterize the binding of heavy metals with DOM in lake sediments. J. Hazard. Mater. 2013, 263, 412–421. [Google Scholar] [CrossRef]
- Konanç, M.U.; Değermenci, G.D.; Kariper, İ.A.; Yavuz, E. After-effects of a closed copper mine: Detailed analysis of environmental impacts in soil and plant samples. Environ. Earth Sci. 2024, 83, 412. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Shan, B. Heavy metal distribution, fractionation, and biotoxicity in sediments around villages in Baiyangdian Lake in North China. Environ. Monit. Assess. 2023, 195, 86. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ren, L.; Hua, C.; Tian, Y.; Yong, X.; Fang, S. Identification of toxic metal contamination in surface sediments of the Xiaoqing River under a long-term perspective (1996–2020): Risks, sources and driving factors. Environ. Res. 2024, 251, 118613. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Liu, G.; Shang, Y.; Wen, Z.; Hou, J.; Song, K. Characterization of dissolved organic matter (DOM) in an urbanized watershed using spectroscopic analysis. Chemosphere 2021, 277, 130210. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.P.; Boyer, E.W.; McKnight, D.M.; Brown, M.G.; Gabor, R.S.; Hunsaker, C.T.; Iavorivska, L.; Inamdar, S.; Johnson, D.W.; Kaplan, L.A.; et al. Variation of organic matter quantity and quality in streams at Critical Zone Observatory watersheds. Water Resour. Res. 2016, 52, 8202–8216. [Google Scholar] [CrossRef]
- Spencer, R.G.M.; Aiken, G.R.; Butler, K.D.; Dornblaser, M.M.; Striegl, R.G.; Hernes, P.J. Utilizing chromophoric dissolved organic matter measurements to derive export and reactivity of dissolved organic carbon exported to the Arctic Ocean: A case study of the Yukon River, Alaska. Geophys. Res. Lett. 2009, 36, 1–4. [Google Scholar] [CrossRef]
- Lu, Q.; He, D.; Pang, Y.; Zhang, Y.; He, C.; Wang, Y.; Zhang, H.; Shi, Q.; Sun, Y. Processing of dissolved organic matter from surface waters to sediment pore waters in a temperate coastal wetland. Sci. Total Environ. 2020, 742, 140491. [Google Scholar] [CrossRef]
- Guo, X.; Peng, Y.; Li, N.; Tian, Y.; Dai, L.; Wu, Y.; Huang, Y. Effect of biochar-derived DOM on the interaction between Cu(II) and biochar prepared at different pyrolysis temperatures. J. Hazard. Mater. 2022, 421, 126739. [Google Scholar] [CrossRef]
- Wu, F.; Evans, D.; Dillon, P.; Schiff, S. Molecular size distribution characteristics of the metal-DOM complexes in stream waters by high-performance size-exclusion chromatography (HPSEC) and high-resolution inductively coupled plasma mass spectrometry (ICP-MS). J. Anal. At. Spectrom. 2004, 19, 979–983. [Google Scholar] [CrossRef]
- Jiang, T.; Bravo, A.G.; Skyllberg, U.; Bjorn, E.; Wang, D.; Yan, H.; Green, N.W. Influence of dissolved organic matter (DOM) characteristics on dissolved mercury (Hg) species composition in sediment porewater of lakes from southwest China. Water Res. 2018, 146, 146–158. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Tan, W.; Zhang, J.; Gong, X.; Li, Y.; Hui, K.; Chen, H.; Xi, B. New insight into the functional group mechanism and structure-activity relationship of the complexation between DOM and Cr(III) in landfill leachate. J. Hazard. Mater. 2024, 466, 133210. [Google Scholar] [CrossRef]
- He, C.; Liu, N.; Meng, W.; Li, Z. Characterization of metal-binding behavior of DOM component structure in biochar: Analysis of binding sites, binding sequences and impact pathways. J. Environ. Chem. Eng. 2024, 12, 113861. [Google Scholar] [CrossRef]
- Lescord, G.L.; Emilson, E.J.S.; Johnston, T.A.; Branfireun, B.A.; Gunn, J.M. Optical Properties of Dissolved Organic Matter and Their Relation to Mercury Concentrations in Water and Biota Across a Remote Freshwater Drainage Basin. Environ. Sci. Technol. 2018, 52, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Zark, M.; Dittmar, T. Universal molecular structures in natural dissolved organic matter. Nat. Commun. 2018, 9, 3178. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, W.; Chen, H.; Wang, S.; Hao, Z. Molecular properties of dissolved organic matter leached from microplastics during photoaging process. J. Hazard. Mater. 2024, 480, 136154. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, Y.; Zhao, L.; Wei, Z. Characterization of mercury binding to different molecular weight fractions of dissolved organic matter. J. Hazard. Mater. 2022, 431, 128593. [Google Scholar] [CrossRef]
- Ren, H.; Ma, F.; Yao, X.; Shao, K.; Yang, L. Multi-spectroscopic investigation on the spatial distribution and copper binding ability of sediment dissolved organic matter in Nansi Lake, China. J. Hydrol. 2020, 591, 125289. [Google Scholar] [CrossRef]
- Lu, L.; Zou, X.; Yang, J.; Xiao, Y.; Wang, Y.; Guo, J.; Li, Z. Biogeography of eukaryotic plankton communities along the upper Yangtze River: The potential impact of cascade dams and reservoirs. J. Hydrol. 2020, 590, 125495. [Google Scholar] [CrossRef]
- Wang, H.; Lan, J.; Huang, L.; Jiao, X.; Zhao, K.; Guo, W. Longitudinal path analysis of ecosystem water yield effects and its driving forces in the upper Yangtze River basin. Ecol. Indic. 2025, 172, 113273. [Google Scholar] [CrossRef]
- Ning, C.; Sun, S.; Gao, Y.; Xie, H.; Wu, L.; Zhang, H.; Chen, J.; Geng, N. Characterization of natural and anthropogenic dissolved organic matter in the Yangtze river basin using FT-ICR MS. Water Res. 2025, 268, 122636. [Google Scholar] [CrossRef]
- Liang, E.; Li, J.; Li, B.; Liu, S.; Ma, R.; Yang, S.; Cai, H.; Xue, Z.; Wang, T. Roles of dissolved organic matter (DOM) in shaping the distribution pattern of heavy metal in the Yangtze River. J. Hazard. Mater. 2023, 460, 132410. [Google Scholar] [CrossRef]
- Liu, D.; Gao, H.; Yu, H.; Song, Y. Applying EEM-PARAFAC combined with moving-window 2DCOS and structural equation modeling to characterize binding properties of Cu (II) with DOM from different sources in an urbanized river. Water Res. 2022, 227, 119317. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Yao, X.; Ren, H.; Ma, F.; Liu, L.; Huo, X.; Lin, T.; Zhu, H.; Zhang, Y. Multi-spectroscopic investigation of the molecular weight distribution and copper binding ability of dissolved organic matter in Dongping Lake, China. Environ. Pollut. 2022, 300, 118931. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Yao, X.; Ren, H.; Liu, L.; Deng, H.; Shao, K. Regional-scale investigation of the molecular weight distribution and metal-binding behavior of dissolved organic matter from a shallow macrophytic lake using multispectral techniques. J. Hazard. Mater. 2022, 439, 129532. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Song, F.; Wu, F.; Guo, F.; Wang, H.; Feng, W.; Zhou, M.; Deng, Y.; Bai, Y.; Xing, B.; Giesy, J.P. Interactions between stepwise-eluted sub-fractions of fulvic acids and protons revealed by fluorescence titration combined with EEM-PARAFAC. Sci. Total Environ. 2017, 605–606, 58–65. [Google Scholar] [CrossRef]
- Yamashita, Y.; Jaffé, R. Characterizing the Interactions between Trace Metals and Dissolved Organic Matter Using Excitation−Emission Matrix and Parallel Factor Analysis. Environ. Sci. Technol. 2008, 42, 7374–7379. [Google Scholar] [CrossRef]
- Li, S.; Meng, L.; Zhao, C.; Gu, Y.; Spencer, R.G.M.; Álvarez–Salgado, X.A.; Kellerman, A.M.; McKenna, A.M.; Huang, T.; Yang, H.; et al. Spatiotemporal response of dissolved organic matter diversity to natural and anthropogenic forces along the whole mainstream of the Yangtze River. Water Res. 2023, 234, 119812. [Google Scholar] [CrossRef]
- Lin, H.; Guo, L. Variations in Colloidal DOM Composition with Molecular Weight within Individual Water Samples as Characterized by Flow Field-Flow Fractionation and EEM-PARAFAC Analysis. Environ. Sci. Technol. 2020, 54, 1657–1667. [Google Scholar] [CrossRef]
- Graeber, D.; Tenzin, Y.; Stutter, M.; Weigelhofer, G.; Shatwell, T.; von Tuempling, W.; Tittel, J.; Wachholz, A.; Borchardt, D. Bioavailable DOC: Reactive nutrient ratios control heterotrophic nutrient assimilation—An experimental proof of the macronutrient-access hypothesis. Biogeochemistry 2021, 155, 1–20. [Google Scholar] [CrossRef]
- Chen, S.; Lu, Y.; Dash, P.; Das, P.; Li, J.; Capps, K.; Majidzadeh, H.; Elliott, M. Hurricane pulses: Small watershed exports of dissolved nutrients and organic matter during large storms in the Southeastern USA. Sci. Total Environ. 2019, 689, 232–244. [Google Scholar] [CrossRef]
- Ryan, K.A.; Palacios, L.C.; Encina, F.; Graeber, D.; Osorio, S.; Stubbins, A.; Woelfl, S.; Nimptsch, J. Assessing inputs of aquaculture-derived nutrients to streams using dissolved organic matter fluorescence. Sci. Total Environ. 2022, 807, 150785. [Google Scholar] [CrossRef] [PubMed]
- Berggren, M.; Gudasz, C.; Guillemette, F.; Hensgens, G.; Ye, L.; Karlsson, J. Systematic microbial production of optically active dissolved organic matter in subarctic lake water. Limnol. Oceanogr. 2020, 65, 951–961. [Google Scholar] [CrossRef]
- Guo, W.; Yang, F.; Li, Y.; Wang, S. New insights into the source of decadal increase in chemical oxygen demand associated with dissolved organic carbon in Dianchi Lake. Sci. Total Environ. 2017, 603–604, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Korshin, G.V.; Li, C.; Benjamin, M.M. Monitoring the properties of natural organic matter through UV spectroscopy: A consistent theory. Water Res. 1997, 31, 1787–1795. [Google Scholar] [CrossRef]
- Song, F.; Wu, F.; Feng, W.; Liu, S.; He, J.; Li, T.; Zhang, J.; Wu, A.; Amarasiriwardena, D.; Xing, B.; et al. Depth-dependent variations of dissolved organic matter composition and humification in a plateau lake using fluorescence spectroscopy. Chemosphere 2019, 225, 507–516. [Google Scholar] [CrossRef]
- Kim, J.; Kim, G. Importance of colored dissolved organic matter (CDOM) inputs from the deep sea to the euphotic zone: Results from the East (Japan) Sea. Mar. Chem. 2015, 169, 33–40. [Google Scholar] [CrossRef]
- Timko, S.A.; Maydanov, A.; Pittelli, S.L.; Conte, M.H.; Cooper, W.J.; Koch, B.P.; Schmitt-Kopplin, P.; Gonsior, M. Depth-dependent photodegradation of marine dissolved organic matter. Front. Mar. Sci. 2015, 2, 66. [Google Scholar] [CrossRef]
- Jørgensen, L.; Stedmon, C.A.; Kragh, T.; Markager, S.; Middelboe, M.; Søndergaard, M. Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter. Mar. Chem. 2011, 126, 139–148. [Google Scholar] [CrossRef]
- Lu, Y.; Edmonds, J.W.; Yamashita, Y.; Zhou, B.; Jaegge, A.; Baxley, M. Spatial variation in the origin and reactivity of dissolved organic matter in Oregon-Washington coastal waters. Ocean. Dyn. 2015, 65, 17–32. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Xu, H.; Zou, L.; Guan, D.; Li, W.; Jiang, H. Molecular weight-dependent spectral and metal binding properties of sediment dissolved organic matter from different origins. Sci. Total Environ. 2019, 665, 828–835. [Google Scholar] [CrossRef] [PubMed]
- D’Andrilli, J.; Silverman, V.; Buckley, S.; Rosario-Ortiz, F.L. Inferring Ecosystem Function from Dissolved Organic Matter Optical Properties: A Critical Review. Environ. Sci. Technol. 2022, 56, 11146–11161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, X.; Ye, Q.; Zhang, Z.; Kong, S.; Cao, C.; Wang, J. Dissolved Metal(loid) Concentrations and Their Relations with Chromophoric and Fluorescent Dissolved Organic Matter in an Urban River in Shenzhen, South China. Water 2020, 12, 281. [Google Scholar] [CrossRef]
- He, X.; Xi, B.; Pan, H.; Li, X.; Li, D.; Cui, D.; Tang, W.; Yuan, Y. Characterizing the heavy metal-complexing potential of fluorescent water-extractable organic matter from composted municipal solid wastes using fluorescence excitation–emission matrix spectra coupled with parallel factor analysis. Environ. Sci. Pollut. Res. 2014, 21, 7973–7984. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.; Huang, B.; Luo, N.; Zhang, Q.; Zhai, X.; Zeng, G. Investigating binding characteristics of cadmium and copper to DOM derived from compost and rice straw using EEM-PARAFAC combined with two-dimensional FTIR correlation analyses. J. Hazard. Mater. 2018, 344, 539–548. [Google Scholar] [CrossRef]
- Xu, X.; Kang, J.; Shen, J.; Zhao, S.; Wang, B.; Zhang, X.; Chen, Z. EEM–PARAFAC characterization of dissolved organic matter and its relationship with disinfection by-products formation potential in drinking water sources of northeastern China. Sci. Total Environ. 2021, 774, 145297. [Google Scholar] [CrossRef]
- Xing, J.; Xu, G.; Li, G. Analysis of the complexation behaviors of Cu(II) with DOM from sludge-based biochars and agricultural soil: Effect of pyrolysis temperature. Chemosphere 2020, 250, 126184. [Google Scholar] [CrossRef]
- Korshin, G.V.; Benjamin, M.M.; Sletten, R.S. Adsorption of natural organic matter (NOM) on iron oxide: Effects on NOM composition and formation of organo-halide compounds during chlorination. Water Res. 1997, 31, 1643–1650. [Google Scholar] [CrossRef]
- Poulin, B.A.; Gerbig, C.A.; Kim, C.S.; Stegemeier, J.P.; Ryan, J.N.; Aiken, G.R. Effects of Sulfide Concentration and Dissolved Organic Matter Characteristics on the Structure of Nanocolloidal Metacinnabar. Environ. Sci. Technol. 2017, 51, 13133–13142. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, Y.; Dong, Y.; Li, D.; Li, W. Metal binding by dissolved organic matter in hypersaline water: A size fractionation study using different isolation methods. Limnologica 2021, 87, 125849. [Google Scholar] [CrossRef]
- Liu, M.; Han, X.; Guo, L.; Ding, H.; Hua, H.; Liu, C.; La, W.; Lang, Y. Role of molecular weight-dependent spectral properties in regulating Cu(II) binding by dissolved organic matter from different sources. Sci. Total Environ. 2023, 873, 162246. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; He, X.; Li, C.; Li, N. The binding properties of copper and lead onto compost-derived DOM using Fourier-transform infrared, UV–vis and fluorescence spectra combined with two-dimensional correlation analysis. J. Hazard. Mater. 2019, 365, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Fan, T.; Yao, X.; Ma, F.; Liu, L.; Ming, J.; Wang, S.; Zhang, Y.; Deng, H. Investigation of the variations in dissolved organic matter properties and complexations with two typical heavy metals under the influence of biodegradation: A survey of an entire lake. Sci. Total Environ. 2022, 806, 150485. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Ye, Q.; Wu, D.; Wang, L.; Yu, Y.; Deng, B.; Du, J. Composition and copper binding properties of aquatic fulvic acids in eutrophic Taihu Lake, China. Chemosphere 2017, 172, 496–504. [Google Scholar] [CrossRef]
- Ding, H.; Su, J.; Sun, Y.; Yu, H.; Zheng, M.; Xi, B. Insight into spatial variations of DOM fractions and its interactions with microbial communities of shallow groundwater in a mesoscale lowland river watershed. Water Res. 2024, 258, 121797. [Google Scholar] [CrossRef]
- Li, W.; Liu, G.; Lei, M.; Zhou, Y.; Cui, H.; Du, H. Spectral fingerprints of DOM-tungsten interactions: Linking molecular binding to conformational changes. J. Hazard. Mater. 2025, 483, 136649. [Google Scholar] [CrossRef]
- Hu, B.; Wang, P.; Wang, C.; Qian, J.; Bao, T.; Shi, Y. Investigating spectroscopic and copper-binding characteristics of organic matter derived from sediments and suspended particles using EEM-PARAFAC combined with two-dimensional fluorescence/FTIR correlation analyses. Chemosphere 2019, 219, 45–53. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, Y.; Liu, X.; Miao, T.; Guan, Q.; Yang, R.; Qu, J. Insight into interactions of heavy metals with livestock manure compost-derived dissolved organic matter using EEM-PARAFAC and 2D-FTIR-COS analyses. J. Hazard. Mater. 2021, 420, 126532. [Google Scholar] [CrossRef]
- Sun, Z.; Yao, X.; Sang, D.; Wang, S.; Lü, W.; Sun, X.; Zhang, Y.; Deng, H.; Li, T. Effects of photodegradation on the composition characteristics and metal binding behavior of sediment-derived dissolved organic matter (SDOM) in Nansi Lake, China. Environ. Res. 2024, 261, 119682. [Google Scholar] [CrossRef]
- Ifon, B.E.; Kiki, C.; Lasisi, K.H.; Suanon, F.; Adyari, B.; Wotto, V.; Yu, C.-P.; Hu, A. Effects of bisphenols and perfluoroalkylated substances on fluorescence properties of humic and amino acids substances of dissolved organic matter: EEM-PARAFAC and ATR-FTIR analysis. J. Environ. Chem. Eng. 2022, 10, 108186. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, X.; Wang, Z.; Yang, X.; Li, S.; Liang, G.; Xie, X. Insight into metal binding properties of biochar-derived DOM using EEM-PARAFAC and differential absorption spectra combined with two-dimensional correlation spectroscopy. Environ. Sci. Pollut. Res. 2021, 28, 13375–13393. [Google Scholar] [CrossRef] [PubMed]
- Noda, I. Techniques useful in two-dimensional correlation and codistribution spectroscopy (2DCOS and 2DCDS) analyses. J. Mol. Struct. 2016, 1124, 29–41. [Google Scholar] [CrossRef]
- Peng, S.; Wang, F.; Wei, D.; Wang, C.; Ma, H.; Du, Y. Application of FTIR two-dimensional correlation spectroscopy (2D-COS) analysis in characterizing environmental behaviors of microplastics: A systematic review. J. Environ. Sci 2025, 147, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tian, Y.; Xie, L.; Liu, Y.; Dai, B.; Guo, X.; Yang, Y. The application of two-dimensional correlation spectroscopy for the binding properties of heavy metals onto digestate-derived DOM from anaerobic digestion of chicken manure. Ecotoxicol. Environ. Saf. 2020, 204, 111129. [Google Scholar] [CrossRef]
- Birdwell, J.E.; Engel, A.S. Characterization of dissolved organic matter in cave and spring waters using UV-Vis absorbance and fluorescence spectroscopy. Org. Geochem. 2010, 41, 270–280. [Google Scholar] [CrossRef]

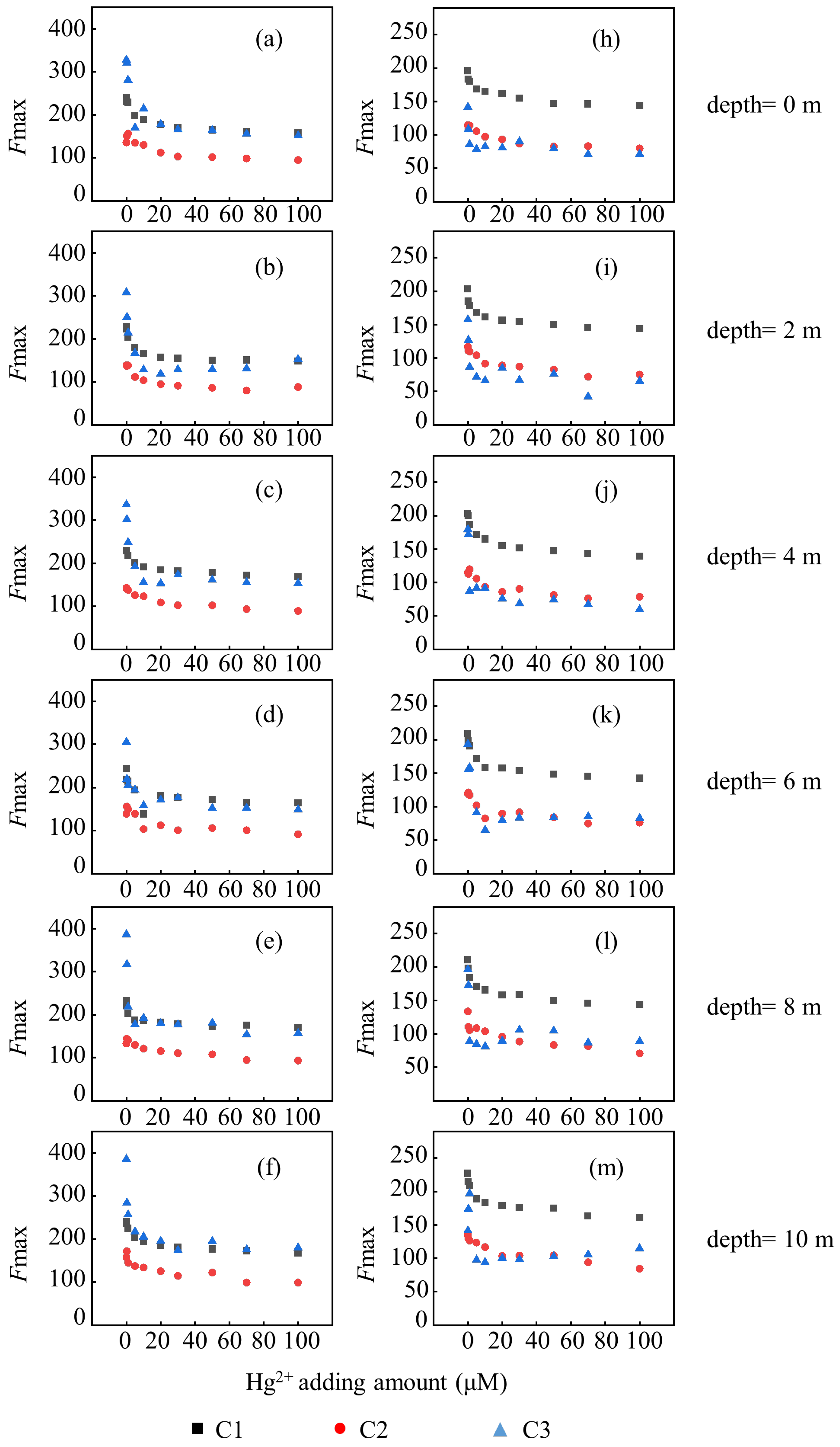
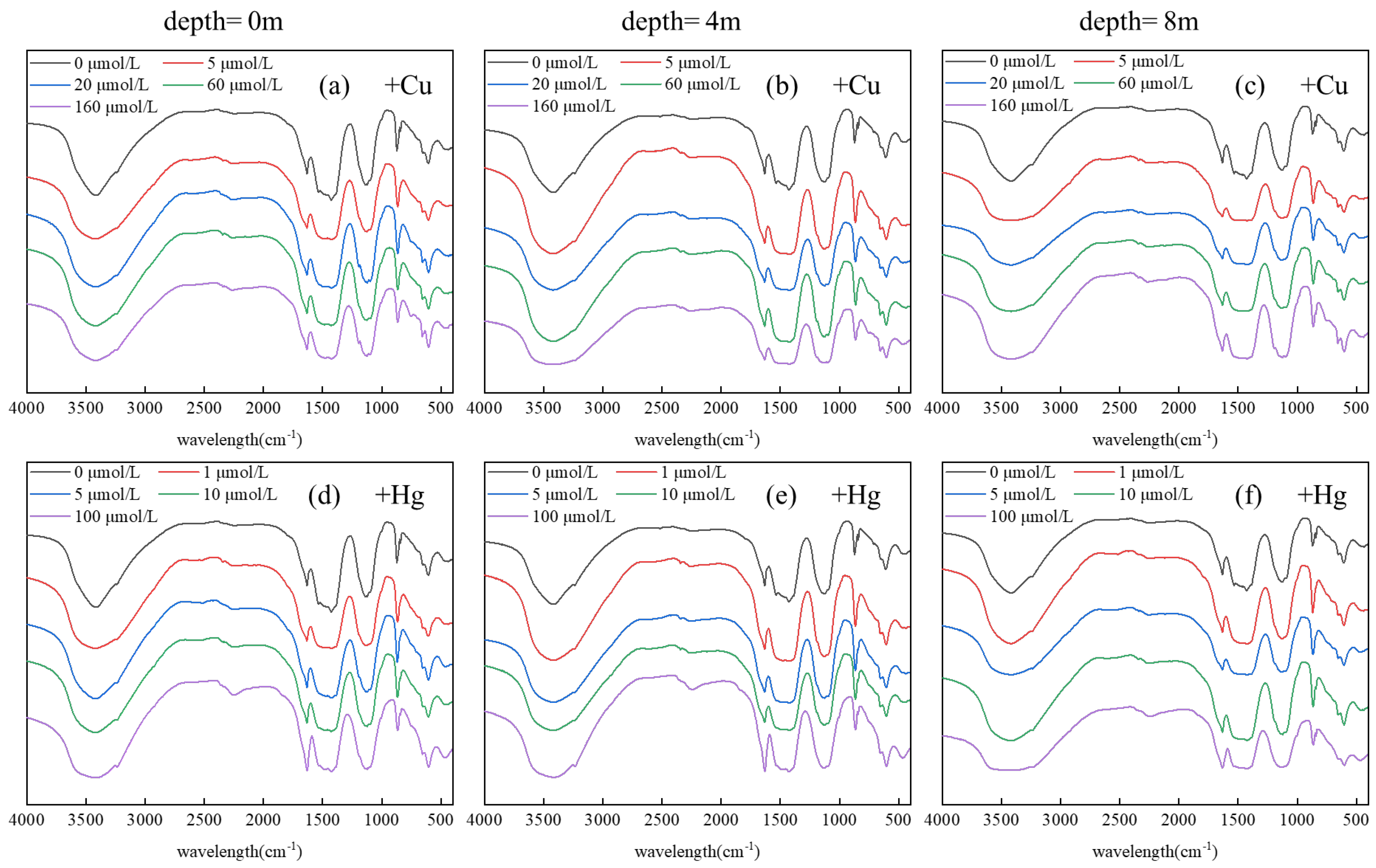
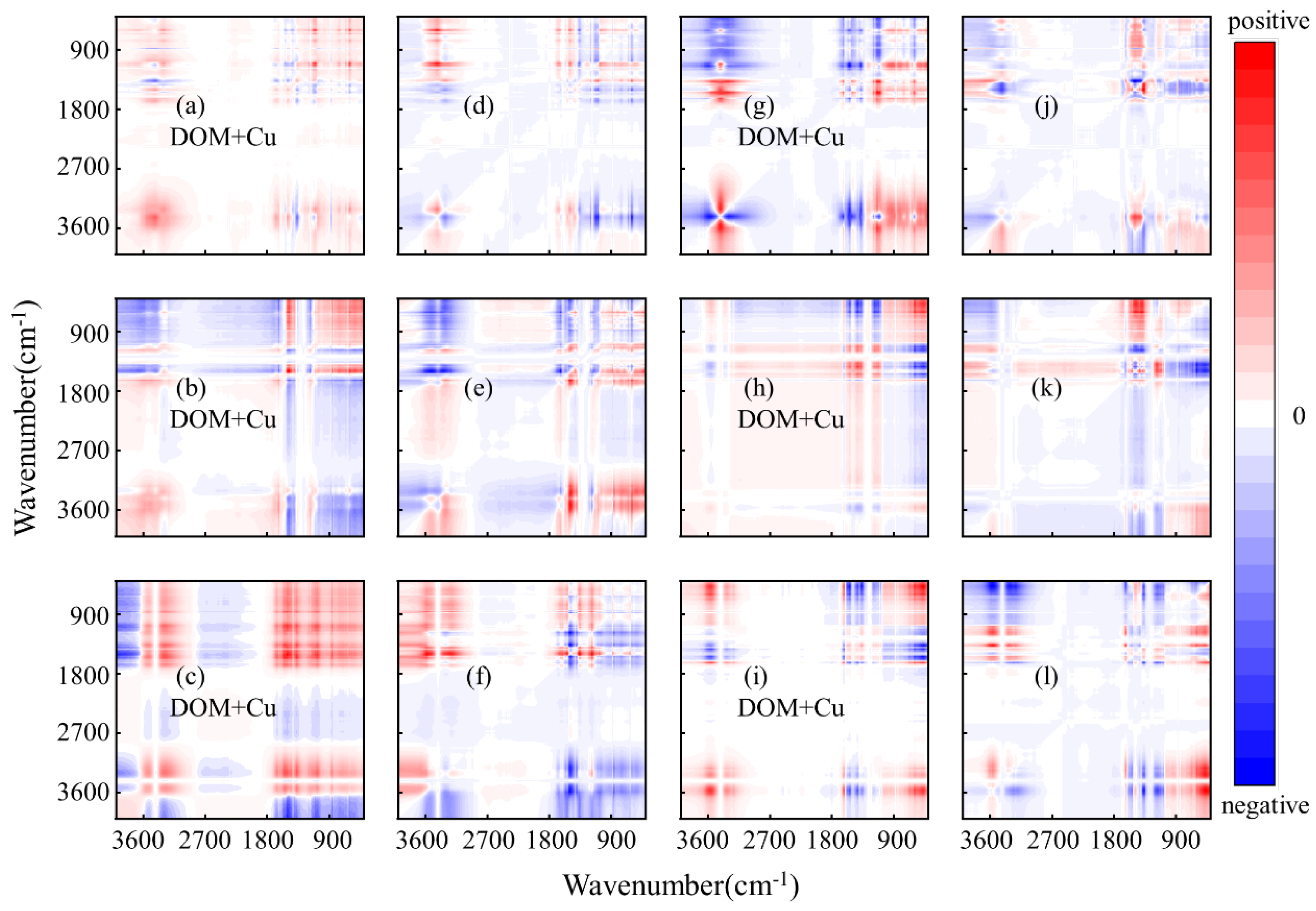
| Site | Components | Deepth (m) | Cu2+ | Hg2+ | ||||
|---|---|---|---|---|---|---|---|---|
| logK | Fend (%) | R2 | logK | Fend (%) | R2 | |||
| S2 | C1 | 0 | 5.38 | 51.41 | 0.92 | 4.97 | 64.03 | 0.94 |
| 2 | 5.35 | 51.85 | 0.94 | 5.26 | 61.41 | 0.97 | ||
| 4 | 5.44 | 46.17 | 0.95 | 4.97 | 71.02 | 0.97 | ||
| 6 | 5.56 | 47.29 | 0.89 | 5.01 | 62.77 | 0.84 | ||
| 8 | 5.50 | 51.10 | 0.92 | 5.54 | 73.00 | 0.90 | ||
| 10 | 5.04 | 48.38 | 0.95 | 5.03 | 68.47 | 0.98 | ||
| C2 | 0 | 5.85 | 66.78 | 0.91 | 4.52 | 48.21 | 0.95 | |
| 2 | 5.08 | 64.57 | 0.94 | 5.00 | 54.31 | 0.97 | ||
| 4 | 5.79 | 55.28 | 0.94 | 4.73 | 56.39 | 0.98 | ||
| 6 | 5.53 | 64.50 | 0.85 | 4.94 | 65.57 | 0.84 | ||
| 8 | 5.12 | 77.12 | 0.86 | 4.27 | 53.46 | 0.97 | ||
| 10 | 5.75 | 72.14 | 0.97 | 4.67 | 56.17 | 0.90 | ||
| C3 | 0 | 5.76 | 41.27 | 0.99 | 5.23 | 39.99 | 0.88 | |
| 2 | 5.88 | 44.71 | 0.97 | 6.01 | 40.77 | 0.90 | ||
| 4 | 5.34 | 30.52 | 0.88 | 5.47 | 39.87 | 0.87 | ||
| 6 | 5.61 | 41.36 | 0.77 | 6.26 | 30.96 | 0.90 | ||
| 8 | 6.27 | 37.38 | 0.97 | 5.65 | 37.52 | 0.85 | ||
| 10 | 5.53 | 39.54 | 0.97 | 5.88 | 45.03 | 0.84 | ||
| S9 | C1 | 0 | 4.88 | 41.79 | 0.94 | 4.93 | 69.49 | 0.86 |
| 2 | 4.71 | 34.19 | 0.96 | 5.13 | 68.15 | 0.83 | ||
| 4 | 4.71 | 34.19 | 0.96 | 5.03 | 65.84 | 0.97 | ||
| 6 | 4.89 | 38.85 | 0.95 | 4.96 | 62.13 | 0.87 | ||
| 8 | 5.03 | 42.35 | 0.95 | 5.42 | 68.96 | 0.91 | ||
| 10 | 5.46 | 42.68 | 0.84 | 5.08 | 69.18 | 0.88 | ||
| C2 | 0 | 5.48 | 67.59 | 0.94 | 4.99 | 66.82 | 0.98 | |
| 2 | 5.15 | 67.70 | 0.96 | 4.82 | 58.04 | 0.95 | ||
| 4 | 5.35 | 68.00 | 0.88 | 4.98 | 64.33 | 0.96 | ||
| 6 | 5.21 | 62.48 | 0.96 | 4.98 | 59.90 | 0.87 | ||
| 8 | 5.65 | 56.15 | 0.93 | 4.70 | 44.94 | 0.83 | ||
| 10 | 6.38 | 66.92 | 0.77 | 4.61 | 56.98 | 0.92 | ||
| C3 | 0 | 4.81 | 26.97 | 0.86 | 5.98 | 42.37 | 0.91 | |
| 2 | 5.54 | 33.71 | 0.98 | 5.97 | 26.80 | 0.92 | ||
| 4 | 5.12 | 23.58 | 0.94 | 6.05 | 37.49 | 0.91 | ||
| 6 | 5.53 | 36.23 | 0.86 | 5.40 | 35.17 | 0.82 | ||
| 8 | 5.61 | 33.35 | 0.90 | - | ||||
| 10 | - | - | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zou, T.; Zhang, W.; Fan, Y.; Bai, Y. Vertical Binding Characteristics Between Dissolved Organic Matter and Heavy Metals in the Upper Reaches of the Yangtze River Using EEM-PARAFAC and 2D-FTIR-COS. Water 2025, 17, 1359. https://doi.org/10.3390/w17091359
Wang X, Zou T, Zhang W, Fan Y, Bai Y. Vertical Binding Characteristics Between Dissolved Organic Matter and Heavy Metals in the Upper Reaches of the Yangtze River Using EEM-PARAFAC and 2D-FTIR-COS. Water. 2025; 17(9):1359. https://doi.org/10.3390/w17091359
Chicago/Turabian StyleWang, Xihuan, Tiansen Zou, Weibo Zhang, Yili Fan, and Yingchen Bai. 2025. "Vertical Binding Characteristics Between Dissolved Organic Matter and Heavy Metals in the Upper Reaches of the Yangtze River Using EEM-PARAFAC and 2D-FTIR-COS" Water 17, no. 9: 1359. https://doi.org/10.3390/w17091359
APA StyleWang, X., Zou, T., Zhang, W., Fan, Y., & Bai, Y. (2025). Vertical Binding Characteristics Between Dissolved Organic Matter and Heavy Metals in the Upper Reaches of the Yangtze River Using EEM-PARAFAC and 2D-FTIR-COS. Water, 17(9), 1359. https://doi.org/10.3390/w17091359






