Abstract
This study conducted a systematic and comparative investigation on how boiling and storage affect the water quality of tap water, spring water, and bottled water, focusing on the molecular cluster size, hardness, nitrite content, and pH value. The findings revealed that boiling reduces water hardness and the size of molecular clusters in both tap and spring water, with these effects lasting for several days. Boiling also decreases the nitrite content, but after one day of storage, the nitrite levels in the boiled water tend to rebound to higher levels than those in un-boiled water. However, boiled spring water stored in a closed bottle maintained lower nitrite levels than un-boiled water for up to seven days. The boiling also slightly increased the pH values of tap and spring water, and its effect could last for several days. There were correlative changes in the water hardness, cluster size, nitrite content, and pH value due to boiling. These results suggest that boiling is beneficial for drinking water because it can improve the water quality for healthy drinking. Additionally, bottled water stored for less than a year remains safe to drink as its quality does not significantly decline in that period.
1. Introduction
Tap and bottled water are the primary drinking water sources for people worldwide. Consequently, water quality, hygiene, and safety are crucial for human health. Drinking water must be free from contaminants and safe for consumption [1]. It should also be of good quality to have suitable hardness and taste for drinking and appropriate molecular structures to play an active role in all biochemical reactions of living organisms to benefit human health [2,3].
Tap waters are usually municipal water supplies, so they would have a strict quality guarantee system to surveil and control the chemical, microbiological, radiological, and heavy metal indices. Bottled waters are also with quality regulations to ensure their quality. Even in those spring water sources open to the public as drinking water sources, there is also surveillance to perform routine water quality tests. Therefore, in the present paper, we are concerned not about whether tap water, bottled water, and open spring waters meet the national or WHO guidelines of drinking water quality but about the effects of boiling and storage time on their water quality.
The parameters we are concerned about as water quality indicators include the water hardness, water cluster, water nitrite content, and pH value. Based on aesthetic acceptability and operational considerations, hardness is significant for drinking water [1]. It would influence the water’s taste. Moreover, the hardness of drinking water has been reported to have a relation with human health problems and even mortality. Several epidemiological investigations have also demonstrated hard water would increase the risk of osteoporosis, nephrolithiasis (kidney stones), colorectal cancer, hypertension and stroke, coronary artery disease, insulin resistance, Alzheimer’s disease, obesity growth retardation, reproductive failure, and eczema [4,5,6]. The hardness of tap and spring water varies widely, depending on the location, treatment, and water source [7], while bottled and packaged waters would be naturally mineralized or soft or demineralized. Therefore, it is necessary to know the hardness of the tap and bottled water you drink daily and whether it is appropriate for your body condition and varied by boiling and storing or other household treatments.
The size of water clusters is also a significant parameter for evaluating water quality. Research has shown that the active component of water is due to the stable water clusters existing in aqueous solutions [3]. Stable water clusters are present in biological systems as active partners in any biochemical reactions occurring inside a living organism. So, they are significant in the functioning of life and related to the pathogenesis of diseases. Several studies have also demonstrated that water with smaller water clusters has better permeability, solubility, metabolic capacity, and immune function and a higher dissolved oxygen concentration [8,9]. In other words, water with smaller water clusters is more favorable to human health and has better water quality [2,10]. Therefore, the size of the water clusters in drinking water has been taken as a vital indicator of its water quality. Recently, several methods, such as 17O NMR, laser-Raman spectra [11,12], infrared spectroscopies [13], atomic force microscopy [3], and IR-UV double-resonance spectroscopy [14], have precisely detected the water clusters in water.
The nitrite content in water was a concern in our study because of its potential risk to human health. Nitrite can induce the oxidation of normal hemoglobin (Hb) to methemoglobin (metHb) in the human body [15]. It also plays a role in forming N-nitrosamines, which are potent carcinogens [16]. Several factors can induce nitrite formation in our drinking water. Without suitable treatment, tap and bottled water may exceed the nitrate values required by legislation. Nitrite can also be formed chemically in the distribution pipes of tap water by the Nitrosomonas bacteria during the stagnation of nitrate-containing and oxygen-poor water in galvanized steel pipes and when employing chlorination for the disinfection of the water [16]. Therefore, due to its harmfulness to human health, nitrite should be used as an indicator of water quality, and one needs to confirm whether boiling and storing would increase or decrease its content in drinking water.
The pH value of water affects the composition of the gut microbiota and hosts glucose regulation to induce health consequences. It would also influence oral health as acidic water would have erosive risks to the enamel and dentine. Therefore, as one of the most important operational water quality parameters, the pH value was also a focus of our study.
As we know, boiling can eliminate or reduce bacteria and parasites or other pathogenic microorganisms in water, so many people usually boil natural and untreated waters, such as spring water, underground water, and tap water, that do not meet the health standard of direct drinking to avoid infection. Boiling drinking water is a part of culture and a habit for the people in Asia, especially in China. Even people in other regions would also like to boil water in winter to have hot water for drinking. Therefore, boiling drinking water is a frequent practice for many people. We should be concerned about whether the water quality would change by boiling. The effect of boiling on drinking water has been studied with so-called repeatedly boiled water (RBW) and prolonged-boil water (PBW) for the nitrite content, pH value, and some other substances (parameters) [17,18]. However, in the cited studies, the waters were boiled many times (18–70 times), much more than what may happen in daily life, and the boiling time was much longer too (from 5 min to 6 h). Since very few people would boil drinking water this way, it was not of significant reference for the present study. Some other studies also reported the effects of boiling on pH values and water clusters [19,20]. However, they only considered a single factor instead of a systematic and simultaneous investigation of various water quality parameters from water’s molecular structure and different physical and chemical properties for the same water sample. They also did not perform a comparative study on the boiling effects for various water samples. Therefore, they were unable to reveal the correlations between the parameters of water quality in the boiling process to obtain a thorough understanding of the mechanism of the boiling effect. Moreover, none of them studied the durations of the boiling effects on various water samples.
Besides the boiling effect, whether the sample preservation and storage time would affect the water quality is also worth consideration. As we know, most bottled waters drank by consumers usually should have been stored for some time. Based on our investigation of the Guangzhou bottled water markets, some bottled waters on sale were even manufactured 14 months ago. In addition, the sellers may store bottled water in different conditions with different storage temperatures. Therefore, although the regulatory body would require and supervise the manufactured bottled waters to meet the safe drinking water limitations before sailing, we are not sure whether the water quality parameters would change after such a storage period. Some previous research had studied the variation in nitrite content with storage time in boiled bottled waters [18,21,22]. However, no systematic study had been conducted to investigate the effects of storage time on different water samples, including tap water, spring water, and bottled water, for various water quality parameters, from water’s molecular structure to physical and chemical properties.
Therefore, we performed a systematic study on the effects of boiling and storage time on the water quality of tap water, spring water, and bottled water for the parameters of water’s molecular cluster, hardness, nitrite content, and pH value. This study revealed, for the first time, the correlative change in water’s molecular cluster, hardness, nitrite content, and pH value due to boiling and depicted the mechanisms of the effects of boiling and storage time on water quality. We hope the study will help people understand whether boiling would improve the quality of their drinking water and how the water quality changes with storage time for tap water, spring water, and bottled water. As a result, individuals will recognize that the easy daily practice of boiling their drinking water is beneficial and whether different kinds of drinking water stored for a time in various ways are still safe for drinking.
2. Materials and Methods
2.1. Water Samples
The study area was in Guangzhou, China. Tap water was collected in glass tubes with plastic screw caps from the water pipe of our laboratory. Spring water was sourced from the spring of the Huo Lu mountain in the suburban area of Guangzhou City. The bottled drinking water samples (160 in total) purchased from the local market in Guangzhou were typical 550 mL ones bottled in plastic bottles made from PET (Polyethylene terephthalate). They included three brands: A1 (pure water, C’estbon, Shenzhen, China), A2 (distilled water, Watsons water, Guangzhou, China), and A3 (mineral water, Nongfu Spring water, Hangzhou, China), produced by manufacturers across the country. There were four batches of bottled water with various storage times for A1. These times were 1 month, 3 months, 9 months, and 14 months after delivery. There were two batches for A2, with storage times of two months and 6 months. And there were two batches for A3, with storage times of one month and 9 months. At least 10 bottles were purchased for each batch.
2.2. Methods
2.2.1. Boiling of Water Samples
All water samples were boiled using an electric water heater (Grelide, Huai’an, China). The samples were heated to 100 °C and maintained at that temperature for 25 s. After boiling, the samples were placed in glass bottles closed with caps or open ones without capping and allowed to cool down to room temperature. All experiments were conducted at a temperature of 295 K.
2.2.2. Water Hardness Detection
The water hardness of all the samples was determined using the method of EDTA-2Na titration [23] for the milligrams of calcium carbonate equivalent per liter in the sample. The volume of each sample used for the test was 100 mL.
2.2.3. Size of Water Cluster Determination
The sizes of water clusters were determined using a Bruker NMR spectrometer (Bruker AVANCE III 600M, Bilerica, MA, USA) to obtain the full width at half maximum intensity (FWHM) of 17O-NMR at 14.1 T for the characterization of the water cluster. All experiments were performed at a temperature of 295 K. The volume of each test sample was 10 mL.
2.2.4. Nitrite (NO2) Concentration Detection
The nitrite contents of the water samples were determined using an optical spectrophotometer (Shimadzu UV 1780, Shimadzu, Kyoto, Japan) with the solution of 4 g sulfanilamide, 0.12 g Naphthalene ethylenediamine hydrochloride, and 70 mL hydrochloric acid diluted to 100 mL with pure water as the chromogenic reagent. The detections were conducted on (1) the un-boiled tap water and the boiled tap water immediately after the boiling and stored in different conditions (thermos bottle, closed glass bottle, and open glass bottle) for one day, three days, five days, and seven days and (2) the un-boiled spring water and the boiled spring water immediately after the boiling and stored in different conditions (thermos bottle, closed bottle, and open bottle) for one day, three days, five days, and seven days. (3) The bottled water samples were stored for various periods after delivery and then tested after one, three, and five days, with the bottle cap replaced after each sampling. The volume of each test sample was 50 mL.
2.2.5. pH Value Detection
The pH values of the water samples were measured using a BPHPOCKET pH meter (BELL Analytical Instruments, Dalian Co., Ltd., Dalian, China). All the measurements were conducted on three to five parallel water samples. The resulting data are expressed as means ± standard deviations. Between each measurement, all the water samples were stored in a refrigerator at 8 °C. The volume of each test sample was 200 mL.
2.2.6. Data Processing and Statistical Analysis
In each experiment, samples were routinely analyzed in quintuplicate. The results are presented as the means and standard errors of means. Statistical comparisons were performed using Student’s t test followed by the post hoc Fisher’s least significant difference test. p < 0.05 was considered a significant difference, and p < 0.01 was considered a very significant difference.
3. Results
3.1. Water Hardness
The water hardness of the samples is listed in Table 1. All the bottled waters, including the mineral water (A3), were of low concentrations of calcium carbonate. According to the WHO Guidelines for Drinking-water Quality, they were all soft water (CaCO3 concentration < 60 mg/L). In comparison, the hardness of the tap water was high and in the range of hard water (CaCO3 concentration 120–180 mg/L). After boiling, the hardness of tap water significantly decreased, though it would slightly rebound after several days of storage. Boiling also reduced the hardness of HL spring water, and the effect lasted for days with a slight increase in the index. We can also see that the hardness of the three bottled waters almost remained unchanged after opening their caps for sampling and storing in the bottles with capping for seven more days.

Table 1.
Water hardness of the water samples.
3.2. FWHM of Water Cluster
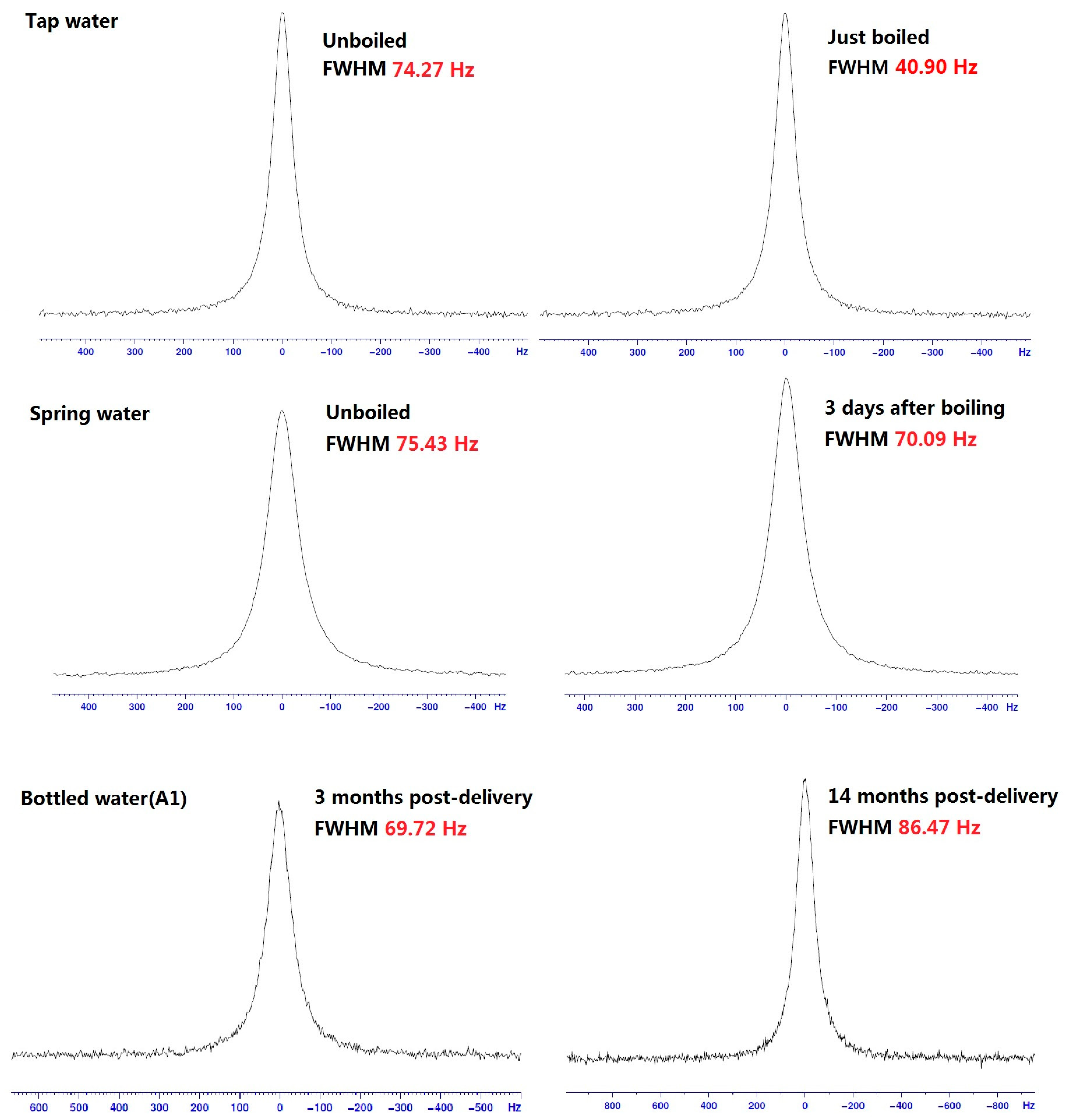
Figure 1 shows some typical spectra from 17O-NMR. Table 2 lists the detailed FWHM results of water clusters in different water samples. As we know, the NMR line broadening of a water sample is inversely proportional to the size of its water cluster. That is, a narrower FWHM indicates smaller water clusters. So after boiling, the water clusters of both the tap water and spring water became smaller but returned to their original sizes five to ten days after the boiling. For the bottled waters, the dimension of water clusters increased with the time after delivery.
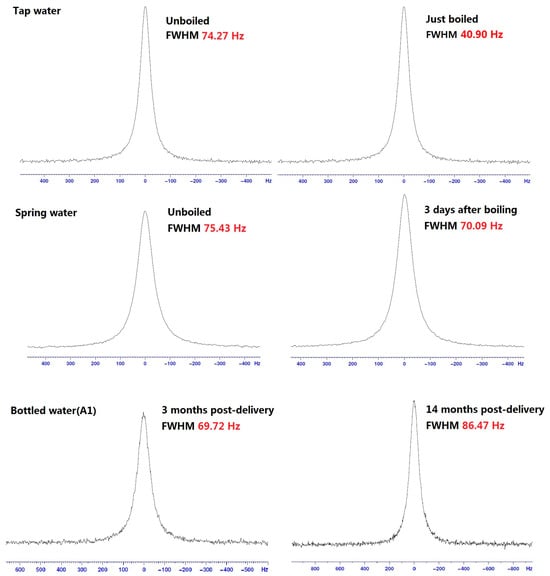
Figure 1.
Typical spectra of 17O-NMR FWHM for different water samples.

Table 2.
The FWHM values of 17O-NMR for different water samples (in Hz).
3.3. The Nitrite (NO2−) Content
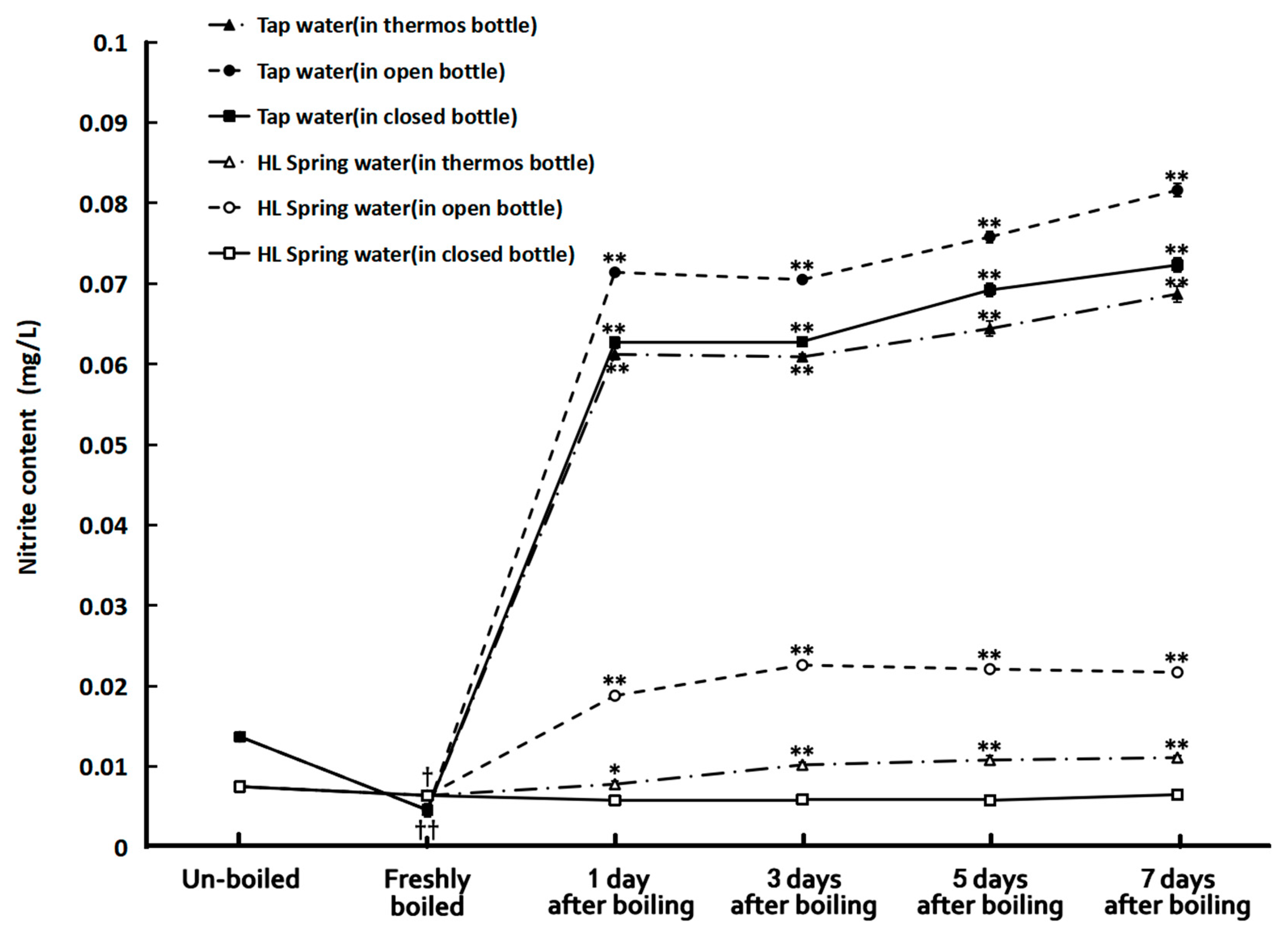
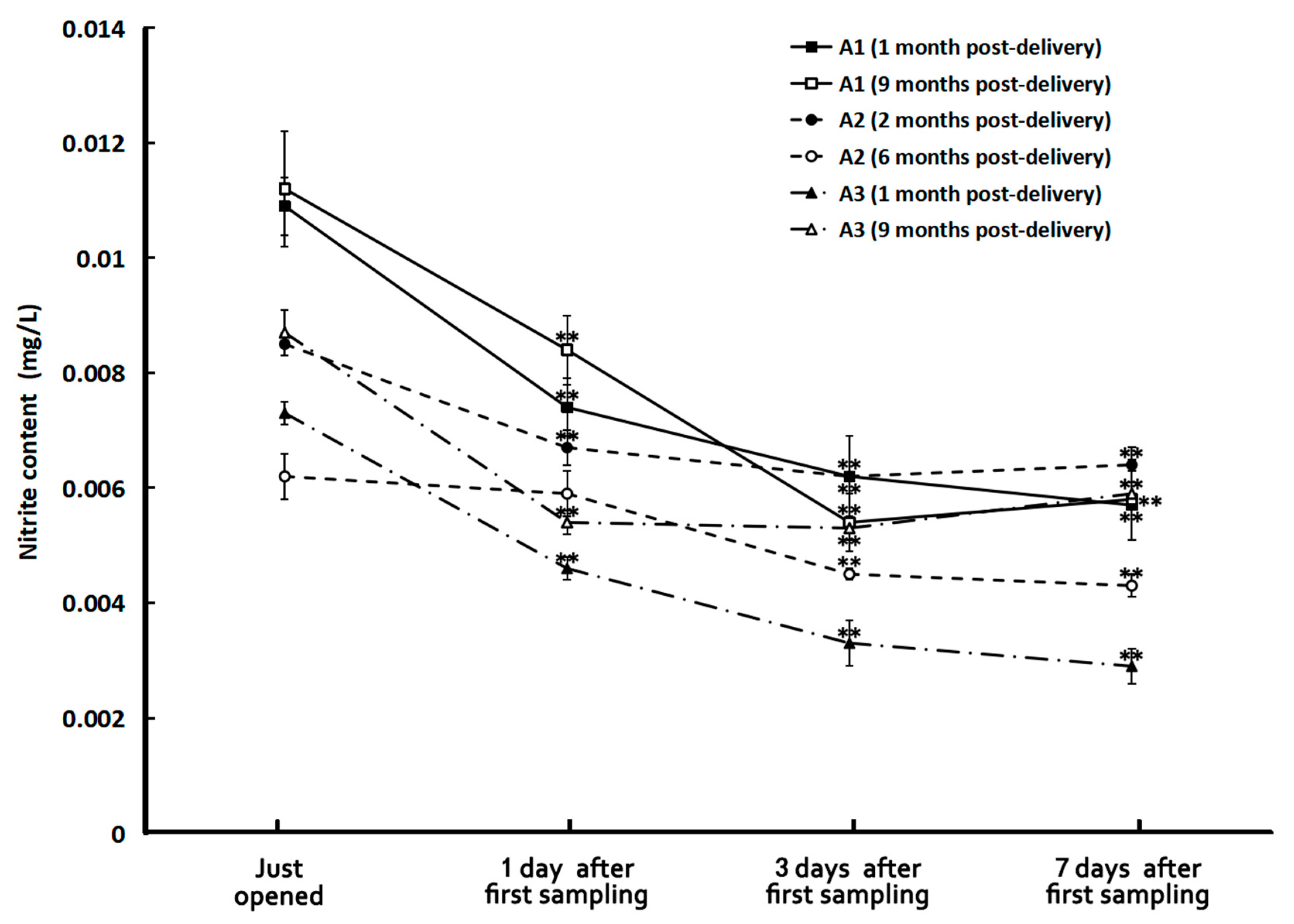
Figure 2 illustrates the nitrite concentrations of the tap and HL spring water samples before and after boiling. It is impressive that boiling could significantly decrease the nitrite content of tap water to about one-third of its original value (0.0137 mg/L). But just storing for one day, the nitrite concentration of boiled tap water increased to more than five folds of the un-boiled ones and then almost did not change anymore with time in seven days. The situation for spring water was quite different. Although boiling also reduced its nitrite content, the reduction was not so much as that in tap water. However, the storage conditions after boiling affected the results quite significantly. The one in a closed bottle almost did not have a nitrite concentration change with time in seven days of storage while the nitrite concentration of boiled spring water in the thermos bottle and open bottle significantly increased. Especially in the latter one, the nitrite concentration increased to more than three folds in three days after boiling. It is also surprising that the nitrite concentration of all the bottled water remained low despite being stored for as long as nine months and decreased with time after opening the bottle for sampling and replacing the cap in seven days (see Figure 3).
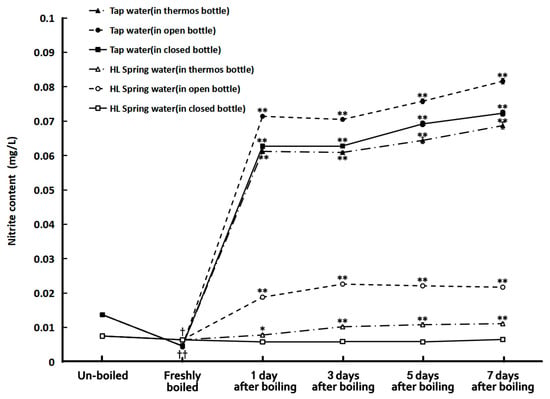
Figure 2.
The nitrite concentration in tap water and HL spring water before and after boiling. †: p < 0.05 and ††: p < 0.01 in comparison with the un-boiled samples. *: p < 0.05 and **: p < 0.01, in comparison with the freshly boiled samples.
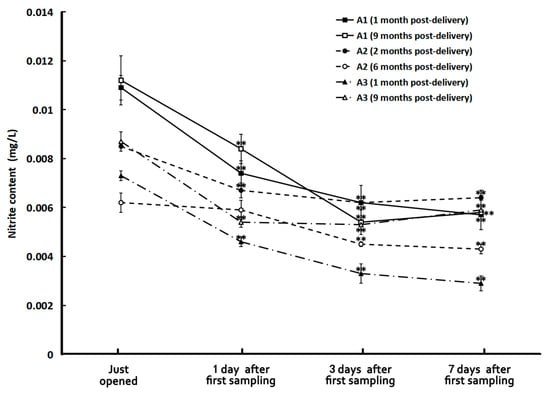
Figure 3.
The nitrite concentrations of different samples of bottled water. **: p < 0.01, in comparison with the ones just opened.
3.4. pH Values
Table 3 lists the pH values of the water samples. All the samples showed a slight increase in the pH value by boiling and a decrease with time during storage after boiling or opening the caps of bottles for sampling.

Table 3.
The pH values of different water samples.
4. Discussion
From the experimental results of our systematic study about the effects of boiling and storage on various water samples, we can see that except for the pH value that only had a slight increase after boiling, boiling significantly affected all the other three studied water quality parameters. It could effectively reduce the sizes of water clusters in both tap and spring water and decrease their hardness. It could also efficiently reduce the concentrations of nitrites to let the nitrite concentration of boiled tap water even down to one-third of that found in un-boiled water.
These findings indicate that boiling drinking water is not simply to eliminate or reduce bacteria and parasites in water as we usually know or to let us have hot water to drink in winter but also of significant benefits beyond the ordinary imagination for body health. Smaller water clusters can let drinking have better water permeability, solubility, metabolic capacity, and immune function. A lower nitrite concentration in drinking water can reduce the risk of converting normal hemoglobin into methemoglobin, thus lowering the risk of certain cancers. Moreover, reducing the water hardness from 127 mg/L to 76 mg/L transformed tap water from hard to moderately hard, making it have a better taste and be more favorable for human drinking [5]. Boiling could also reduce the presence of some disinfection byproducts in tap water such as halomethanes, haloacetonitriles, and haloacetic acids [24]. Given these advantageous effects of boiling on water quality, we recommend that individuals boil their drinking water as a simple and effective household treatment when possible. It is a step that can significantly improve water quality for healthier drinking, making it a worthwhile practice.
We can also see that the boiling effects on the hardness and water clusters of tap and spring waters could remain for five to seven days, especially for those stored in closed bottles. However, the nitrite contents of the boiled water, either tap water or spring water, except for the spring water deposited in closed bottles, increased immediately on the second day after the boiling. All the boiled tap water samples, whether stored in an opened bottle, closed bottle, or thermos bottle after boiling, had their nitrite concentrations increased to six times as the un-boiled ones after one day of storage. The boiled spring water stored in an open bottle and a thermos bottle also had nitrite concentrations higher than the un-boiled ones. The ones stored in opened bottles had nitrite concentrations of about two to three folds larger than those of the un-boiled ones. Therefore, it is advisable to drink boiled tap water right on the day it is prepared but not overnight, though the nitrite concentrations of the waters in the study were still lower than the intake limit of China (1 mg/L) (1 mg/L) [25] and that of WHO (3 mg/L) [1]. The exception was the boiled spring water stored in closed bottles. Its nitrite concentrations remained lower than those of the un-boiled samples for seven days.
Water hardness primarily arises from dissolved polyvalent metallic ions, mainly calcium and magnesium [26]. The reduction in tap water hardness that occurs when boiling is likely due to the process of decarbonization. Boiling drives off carbon dioxide (CO2) from the water, which promotes the precipitation of calcium carbonate CaCO3 and magnesium carbonate MgCO3, then decreases the water’s hardness. For boiled water stored for several days, not enough CO2 re-enters to dissolve the precipitated CaCO3 and MaCO3 back into the water through a reverse reaction. As a result, the hardness of the boiled tap water does not significantly increase over seven days of storage, especially when kept in a closed bottle.
The formation of water clusters is primarily due to hydrogen bonds. When water is heated to boiling, the increased thermal motion causes water molecules to become more diffusive and spread over a broader range. As these molecules move more rapidly, they collide with each other more frequently. It leads to the dissociation of some hydrogen bonds, resulting in a portion of the water molecules transitioning from bonded states to free ones. Consequently, some large clusters break down into smaller water clusters. After boiling, as the temperature decreases, some of the free water molecules can re-establish hydrogen bonds, causing the sizes of the water clusters to increase again, although this is a gradual thermodynamic process that takes time. Therefore, boiled tap water could retain smaller water clusters for three to five days after heating, gradually returning to the sizes of un-boiled water clusters within seven days of storage. In contrast, the water clusters in HL spring water nearly returned to the size of un-boiled clusters by the second day after boiling, suggesting that the reformation of hydrogen bonds in HL spring water occurred more quickly than in tap water. It might have been due to higher levels of Ca2+, Mg2+, and other cations in spring water, which promoted the association of hydrogen bonds. Similarly, although water cluster sizes in all bottled water samples tended to increase with storage time, the clusters in mineral water returned to larger sizes more rapidly than those in pure or distilled bottled water.
It is important to note that although several studies have demonstrated the beneficial biological effects of smaller water clusters from both experimental and theoretical aspects [8,9,27,28], the mechanisms by which water clusters affect health are not well understood yet and remain a topic of debate [29,30]. Further research is needed to explore the role of water clusters in human cells and their potential impacts on health.
The possible mechanism of decreasing the nitrite content in water by boiling is that heating the water to the boiling point converts some NO2− to NO3−. Compared with nitrite, nitrate is relatively inert and stable and thus is less harmful. However, it can be converted back to nitrite by oxidation. Therefore, after boiling, the nitrite contents in boiled tap water and HL spring water increased during the storage in this study. The exception was the boiled HL spring water samples stored in closed bottles. Their nitrite contents could be retained at levels lower than the un-boiled ones for a week. The boiled HL spring water samples in thermos bottles also had a lower speed of rebounding nitrite contents than those stored in open bottles. The machinery for this phenomenon is unclear yet and awaits further study. Probably, some substances in the spring water, such as metasilicate acid, retarded the nitrate oxidation process in a closed container. As the nitrite content in boiled tap water was even higher than that in the un-boiled samples for one day of storage, it was advisable to drink boiled water within 24 h after boiling it to the boiling point, although it was still much lower than the safety limit (3 mg/L) proposed by the WHO [1].
Nitrite oxidation is a nitrification process that consumes nitrites to produce nitrates. When opening a bottle cap for sampling or drinking, some oxygen molecules would get into the bottle to oxidize some nitrites to become nitrates. Therefore, the nitrite content of the bottled water decreased with time after opening the bottle cap.
We can see from Table 3 that boiling can slightly increase the pH values of both tap water and spring water. The possible machinery is that boiling would decrease the carbon dioxide content in water, while carbon dioxide is the main factor in determining the water pH value, and a lower concentration of carbon dioxide would lead to a higher pH value in water. Therefore, the pH values of the tap water and spring water increased after boiling in our study. The slight increase in pH made the boiled water taste better. Drinking water with weak alkalization could also improve the human body’s acid–base balance and thus be good for health [31]. In the storage of all the water samples, there was no significant change in their carbon dioxide contents, so there were no noteworthy changes in their pH values.
There are correlations between the four parameters on the water quality in the boiling process. For example, when the pH values of the tap and spring waters rose to higher than 7.0, it would induce more OH− in the water. The OH− ions would bind with the Ca2+ ions and then precipitate, thus reducing the dissolution of CaCO3 and the hardness of the water. On the other hand, the rise of the pH would increase the NO2− oxidation rate to oxidize more nitrites, thus decreasing the nitrite content in water [32]. The pH variation would also destabilize the hydrogen bonding to influence the water cluster [33].
The effect of the storage duration on the quality of bottled water revealed that, aside from the water cluster size, which changed by 30% after 14 months, there were no significant variations in any other parameters across all samples, including in the nitrite content in the water stored for nine months. The fact suggests that bottled water can maintain quality for up to a year, even under varying storage conditions and having been manufactured by different manufacturers. Therefore, drinking bottled water delivered within one year is safe.
5. Conclusions
We performed systematical and comparative studies on the effects of boiling and storage on the water quality of tap water, spring water, and bottled water for the parameters of water’s molecular cluster, hardness, nitrite content, and pH value. It was the first time someone had conducted such systematical studies about the effects of boiling and storage on the correlative changes in various physical and chemical properties of the same water samples. It was also the first comparative study of the effects on different types of water samples and multiple storage methods and durations. We found that boiling could induce a decrease in water hardness and cluster sizes in tap and spring waters and the effects could last for days. It would also decrease the nitrite contents in both kinds of water, but after one day of storage, the nitrite content in the boiled waters would rebound to even higher than in the un-boiled samples. The exception was the boiled spring water stored in a closed bottle whose nitrite content could keep a value lower than the un-boiled samples for seven days. The boiling also slightly increased the pH values of tap and spring water, and its effect could last several days. There were correlative changes in the water hardness, cluster size, nitrite content, and pH value due to boiling.
These findings lead to the following conclusions:
- Boiling is very beneficial for healthy water drinking because it could improve the water quality in all the water samples and parameters we studied, from the molecular structure to physical and chemical properties. The boiling-induced reduction in the sizes of water clusters, hardness, and nitrite content of drinking water can help improve the metabolic capacity and immune function of our body and reduce the risk of developing cancers. Meanwhile, the slight increase in pH and the reduction in hardness make boiled water taste better and more favorable for drinking. As we drink water daily, the cumulative action of these beneficial effects would be significantly conducive to our body’s health. So, it is advisable to drink boiled water as a regular practice.
- Storing boiled water for more than one day would deteriorate almost all the water samples stored in various ways. Their nitrite contents would be even higher than those of the un-boiled samples for one day of storage, though they were still much lower than the safety limit proposed by WHO. There was an exception for the boiled spring water stored in closed bottles, which could retain lower nitrite contents than the un-boiled samples for a week. For this reason, it is better to consume boiled water on the same day it is prepared.
- The storage within one year of bottled water samples only slightly affects the sizes of their water clusters but not the other water parameters, meaning that bottled water stored for a year remains safe for drinking.
The limitation of this study was that it only tested three types of bottled water: A1 (pure water), A2 (distilled water), and A3 (mineral water), with just one brand representing each type. Moreover, they were purchased from local markets at different times, from one month to 14 months after their deliveries. The storage conditions, including temperature and lighting, were unclear and might have been quite different. Therefore, in future studies, it would be better to include a broader range of water samples immediately after delivery from the manufacturer so that these samples can be stored under the same controlled conditions to ensure that the obtained results would be more accurate and comparable.
We hope our study will help people fully understand the health benefits of drinking freshly boiled water so that they would like to consume the water boiled on the same day as their daily practice to let the water they drink each time be helpful for their health.
Author Contributions
Y.H. conceived and designed the experiments. Y.Z., B.C. and L.N. performed the experiments; Y.H., Y.Z. and B.C. analyzed the data. Y.H. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported partly by the National Natural Science Foundation of China (No. 30940019), Guangdong Provincial Applied Science and Technology Research and Development Program (Nos. 2015B010105006 and 2013B060100011), and Guangzhou Science Technology and Innovation Commission (Nos. 2014Y2-00508 and 201604020146).
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017.
- Laurson, P.; Mäeorg, U. Water and water clusters in biological systems. Agron. Res. 2015, 13, 1253–1259. [Google Scholar]
- Lo, S.Y.; Li, W.C.; Huang, S.H. Water clusters in life. Med. Hypotheses 2000, 54, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Ternan, J.L. Comments on the use of a calcium hardness variability index in the study of carbonate aquifers: With reference to the central pennines, England. J. Hydrol. 1972, 16, 317–321. [Google Scholar] [CrossRef]
- Akram, S.; Rehman, F. Hardness in Drinking-Water, its Sources, its Effects on Humans and its Household Treatment. J. Chem. Appl. 2018, 4, 4. [Google Scholar]
- Bao, Y.; Li, Y.; Zhou, Y.; Zhou, J.; Mu, W.; Deng, X.; Shen, C.; Han, L.; Ran, J. Water quality and neurodegenerative disease risk in the middle-aged and elderly population. Ecotoxicol. Environ. Saf. 2025, 289, 117647. [Google Scholar] [CrossRef] [PubMed]
- Ingin, Y.P.; Mahringer, D.; El-Athman, F. Hardness properties of calcium and magnesium ions in drinking water. Appl. Food Res. 2024, 5, 100600. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, Y.; Huang, X.; Zhang, X.; Li, J.; Huang, Y.; Li, K.; Weng, H.; Xu, Y.; Zhang, Y. Exploration of the Existence Forms and Patterns of Dissolved Oxygen Molecules in Water. Nano-Micro Lett. 2024, 16, 208. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.P. The shape of water—How cluster formation explains the hydrophobic effect. J. Mol. Liq. 2024, 400, 124491. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, F.; Chen, B.; Li, Y.; Na, P.; Zhuo, J. Small water clusters stimulate microcystin biosynthesis in cyanobacterial Microcystis aeruginosa. J. Appl. Phycol. 2013, 25, 329–336. [Google Scholar] [CrossRef]
- Kusanagi, H. 17O-NMR spectra of the isolated water molecules in hydrophobic poly (ε-caprolactone). Polym. J. 1996, 28, 825–826. [Google Scholar] [CrossRef][Green Version]
- Starzaka, M.; Mathlouthi, M. Cluster composition of liquid water derived from laser-Raman spectra and molecular simulation data. Food Chem. 2003, 82, 3–22. [Google Scholar] [CrossRef]
- Woutersen, S.; Emmerichs, U.; Bakker, H. Femtosecond mid-IR pump-probe spectroscopy of liquid water: Evidence for a twocomponent structure. Science 1997, 278, 658–660. [Google Scholar] [CrossRef]
- Pribble, R.N.; Zwier, T.S. Size-specific infrared spectra of benzene-(H2O)n clusters (n = 1 through 7): Evidence for noncyclic (H2O)n structures. Science 1994, 265, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Jaffé, E. Methaemoglobinaemia. Clin. Haematol. 1981, 10, 99–122. [Google Scholar] [CrossRef] [PubMed]
- WHO. Toxicological Evaluation of Certain Food Additives and Contaminants. Prepared by the Forty-Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 1996.
- Zhai, Y.; Yang, J.; Zhu, Y.; Du, Q.; Yuan, W.; Lu, H. Quality change mechanism and drinking safety of repeatedly-boiled water and prolonged-boil water: A comparative study. J. Water Health 2020, 18, 631–653. [Google Scholar] [CrossRef]
- Hu, W.; Cai, Z.; Liang, S. Nitrite Content in “Thousand Boiling Water” and “Overnight Water”. Modern Food 2021, 12, 140–144+149. [Google Scholar] [CrossRef]
- Li, L.; Wei, M.; Ling, D. To investigate the change of pH in drinking water by different ways. J. Med. Pest Control. 2017, 33, 851–853. [Google Scholar]
- Li, F.-Z.; Zhang, X.-J.; Wang, Z.-S.; Lv, M.-J. Study on structures of boiling water and chilled water with NMR. Water Wastewater Eng. 2003, 29, 27–30. [Google Scholar]
- Chen, Y.; Xiao, Y.; Liu, X. Changes of Nitrite and Nitrate Content at Different Standing Time in Boiled Drinking Water. J. Jinzhou Med. Univ. 2018, 39, 70–72. [Google Scholar]
- Sun, D.-H.; Gou, X.-D. Changes of Nitrite Content with Time in Drinking Water. Shandong Environ. 2000, 71. [Google Scholar]
- Ferreira, D.; Barros, M.; Oliveira, C.M.; Silva, R.J.N.B.d. Quantification of the uncertainty of the visual detection of the end-point of a titration: Determination of total hardness in water. Microchem. J. 2019, 146, 856–863. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, J.; Zhang, P.; Wu, Y.; Deng, J.; Dong, F.; Li, X.; Dietrich, A.M. Impact of boiling on chemical and physical processes for reduction of halomethanes, haloacetonitriles, and haloacetic acids in drinking water. Sci. Total Environ. 2024, 906, 167657. [Google Scholar] [CrossRef] [PubMed]
- Standardization Administration of China. Drinking Water Hygiene Standard; Standardization Administration of China: Beijing, China, 2022.
- WHO. Hardness in Drinking-Water; WHO: Geneva, Switzerland, 2010.
- Sin, J.-S.; Jang, Y.-M.; Kim, C.-H.; Kim, H.-C. Steric effect of water molecule clusters on electrostatic interaction and electroosmotic transport in aqueous electrolytes: A mean-field approach. AIP Adv. 2018, 8, 105222. [Google Scholar] [CrossRef]
- Dement’ev, V.B.; Haghi, A.K.; Kodolov, V.I. Nanoscience and Nanoengineering; Apple Academic Press: New York, NY, USA, 2019. [Google Scholar]
- Kontogeorgis, G.M.; Holster, A.; Kottaki, N.; Tsochantaris, E.; Topsøe, F.; Poulsen, J.; Bache, M.; Liang, X.; Blom, N.S.; Kronholm, J. Water structure, properties and some applications—A review. Chem. Thermodyn. Therm. Anal. 2022, 6, 100053. [Google Scholar] [CrossRef]
- Hiraoka, A.; Shinohara, A.; Yoshimura, Y. Studies on the Physicochemical Properties and Existence of Water Products (as Drinks) Advertised as Having Smaller Cluster Sizes of H2O Molecules than Those of Regular Water. J. Health Sci. 2010, 56, 717–720. [Google Scholar] [CrossRef][Green Version]
- Chycki, J.; Zając, T.; Maszczyk, A.; Kurylas, A. The effect of mineral-based alkaline water on hydration status and the metabolic response to short-term anaerobic exercise. Biol. Sport 2017, 34, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Shammas, N.K. Interactions of Temperature, pH, and Biomass on the Nitrification Process. Water Environ. Fed. 1986, 58, 52–59. [Google Scholar]
- Khorolskyi, O.; Malomuzh, N.P. pH and H-bonding energy for pure water. Chem. Phys. Lett. 2023, 828, 140713. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).