Metagenomic Analysis of Bacterial Community Structure and Pollutant Removal Process in High-Altitude Municipal Wastewater Treatment Plants of Tibet, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection of WWTPs
2.2. DNA Library Construction and Sequencing
2.3. Bacterial Community Taxonomic
2.4. Metabolic Pathway Analysis
2.5. Data Processing and Statistical Analysis
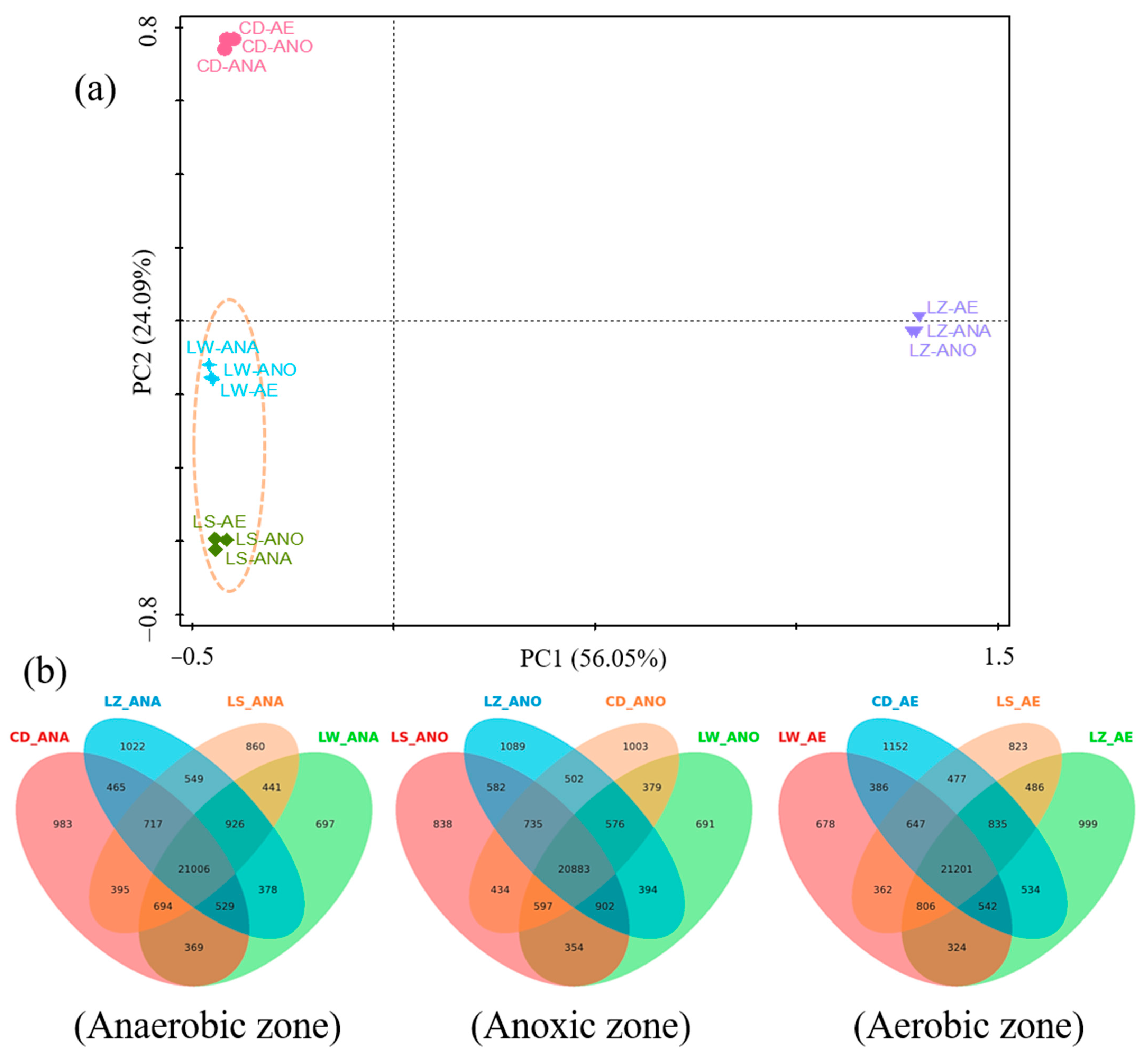
3. Results and Discussion
3.1. Differences in Physicochemical Factors
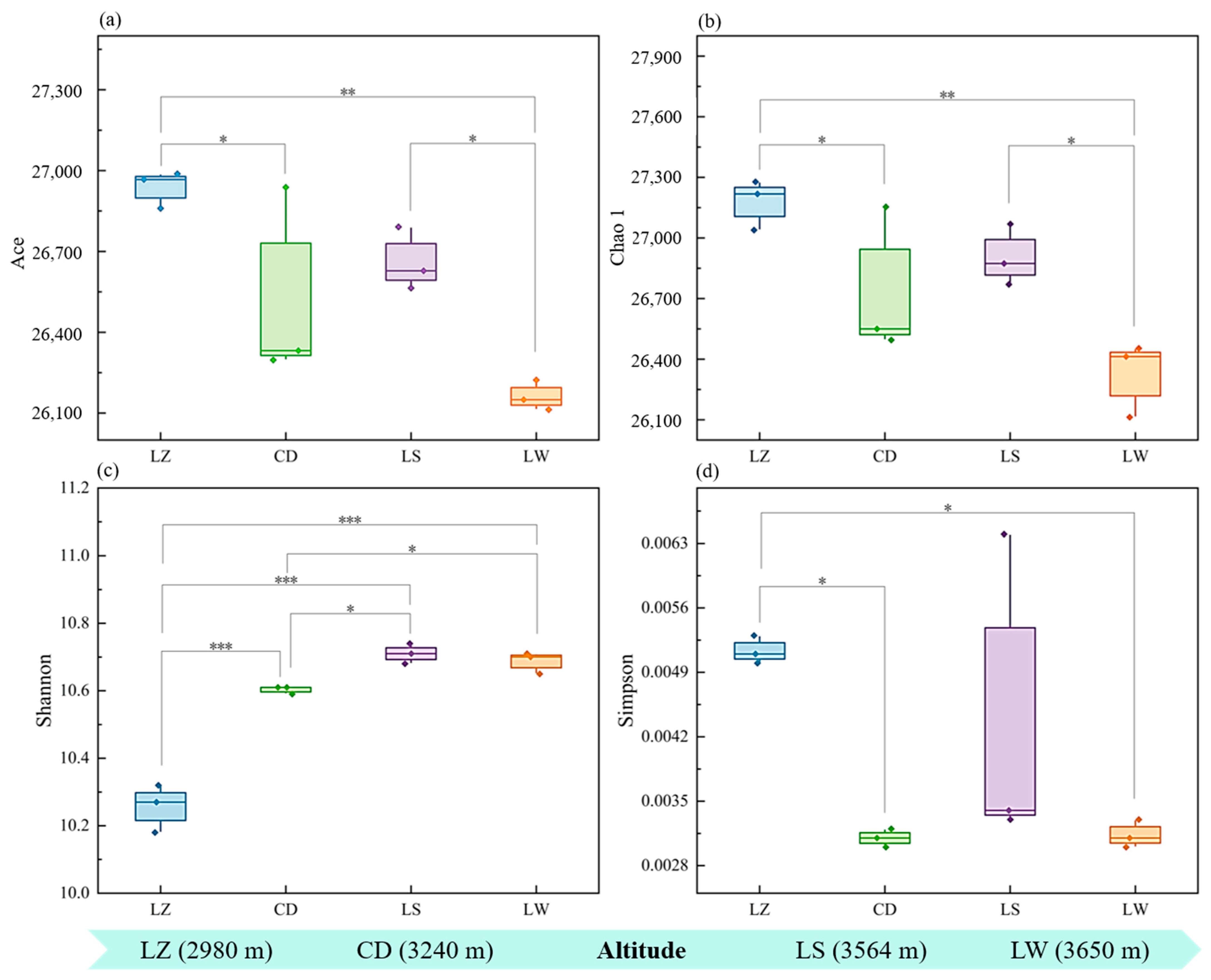
3.2. Bacterial Community Diversity
3.3. Bacterial Community at the Phylum and Genus Levels
3.3.1. The Phylum Level
3.3.2. The Genus Level
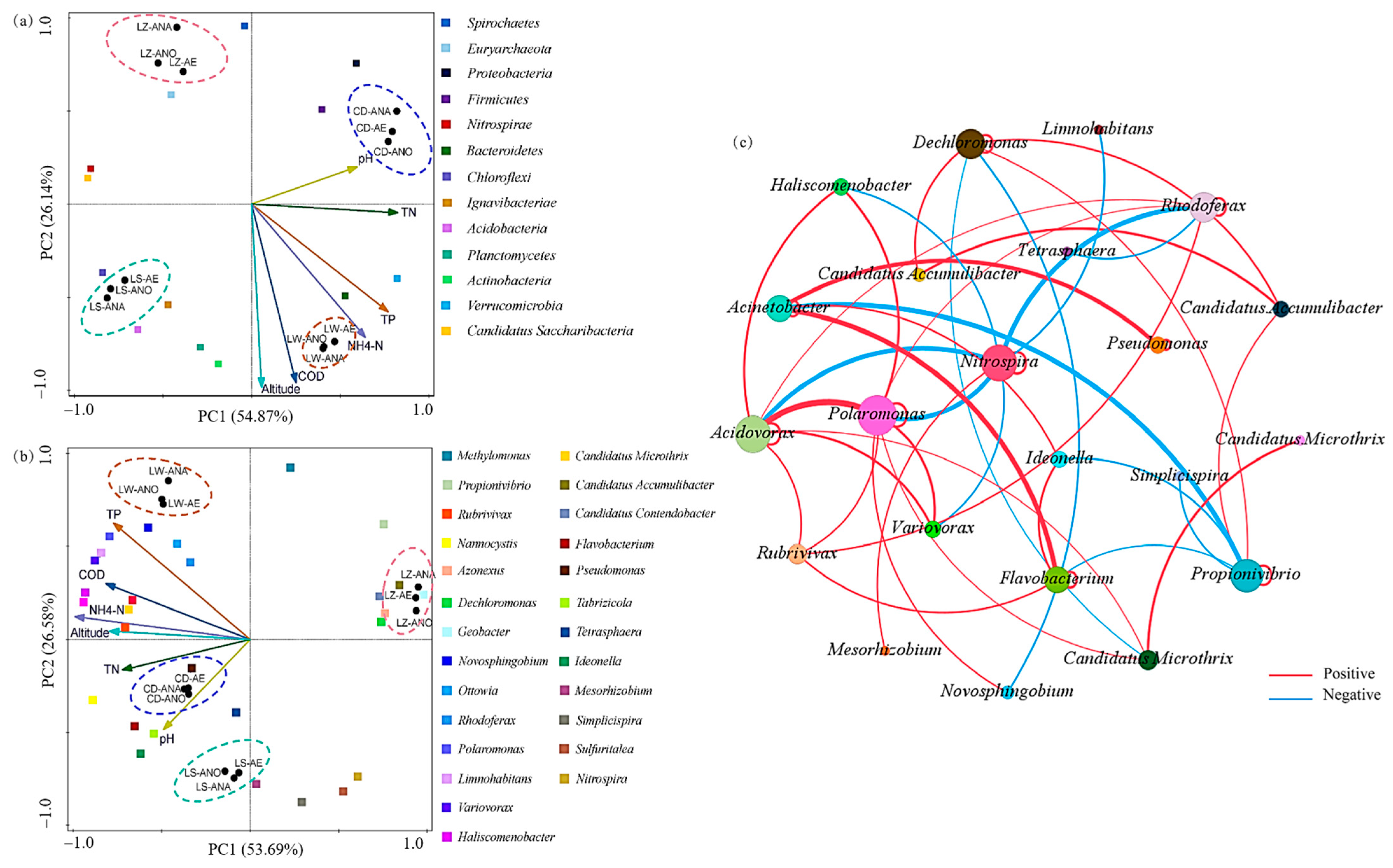
3.4. Correlations Between the Bacterial Community and Environmental Variables
3.4.1. Correlation Analysis at Phylum Level
3.4.2. Correlation Analysis at Genus Level
3.4.3. Analysis of Bacterial Network Diagrams at Genus Level
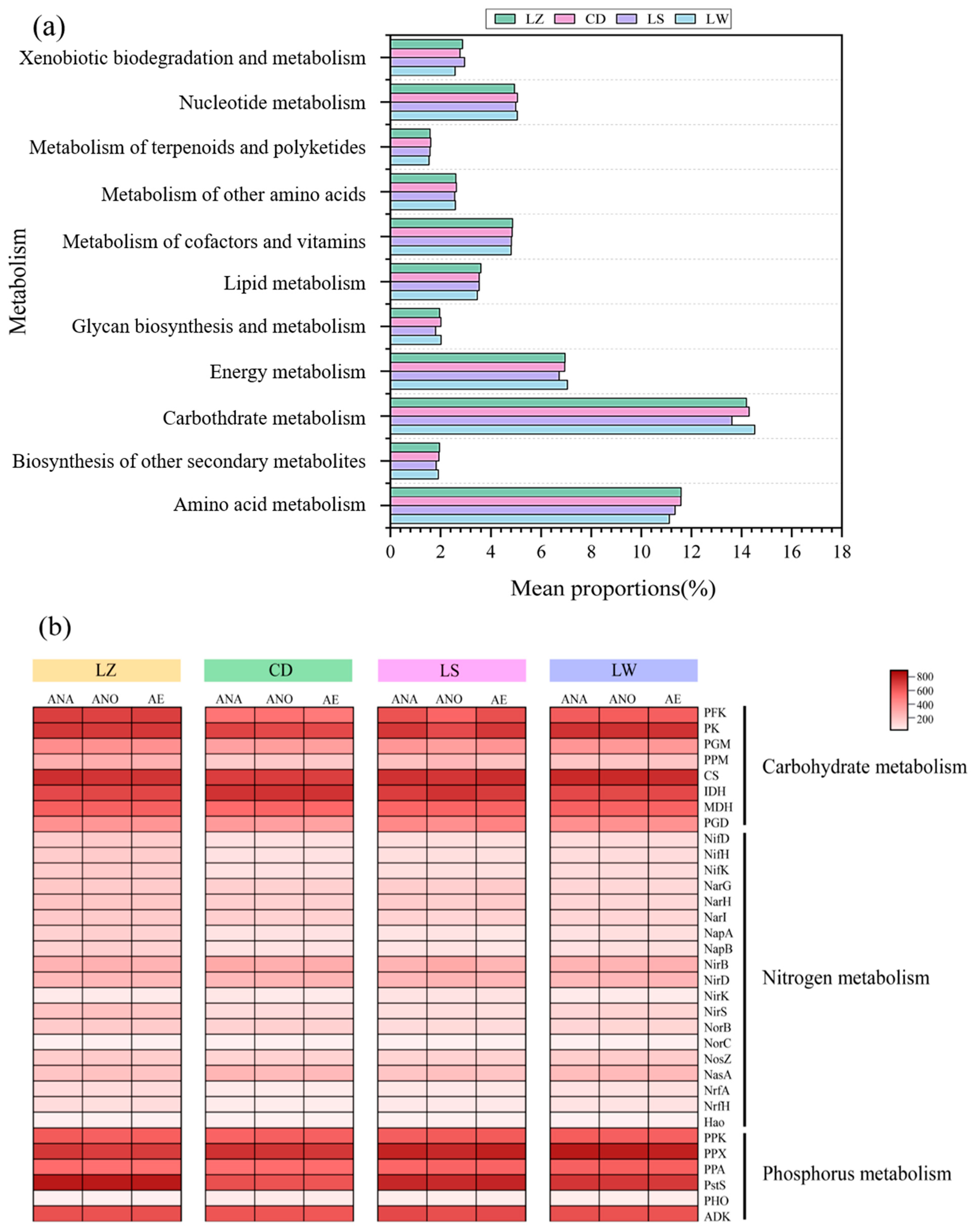
3.5. Functional Metabolism Pathway Analysis
3.5.1. Carbon Metabolism
3.5.2. Nitrogen Metabolism
3.5.3. Phosphorus Metabolism
3.6. Mechanism Analysis of Wastewater Treatment Efficiency by Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, T.; Zhao, Z. Deciphering the Internal Mechanism of Nitrogen Removal from Sludge and Biofilm under Low Temperature from the Perspective of Microbial Function Metabolism. Environ. Res. 2025, 267, 120688. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, D.; Lu, Y.; Li, S.; Zhu, G.; Wang, H. High-performance multi-stage baffled A(2)O treatment process for domestic sewage on plateaus. Water Res. 2025, 268 Pt B, 122695. [Google Scholar] [CrossRef]
- Wang, X.; Peng, S.; Sun, J.; Li, M.; Wang, L.; Li, Y.; Wang, J.; Sun, L.; Zheng, T. Altitude restricts the restoration of community composition and vegetation coverage of quarries on the Qinghai-Tibet Plateau. Ecol. Indic. 2023, 151, 110339. [Google Scholar] [CrossRef]
- Mutegoa, E. Efficient Techniques and Practices for Wastewater Treatment: An Update. Discov. Water 2024, 4, 69. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, Y.; Zhang, G.; Han, G.; Zhao, W. A Novel Sulfidization System for Enhancing Hemimorphite Flotation Through Cu/Pb Binary Metal Ions. Int. J. Min. Sci. Technol. 2024, 34, 1741–1752. [Google Scholar] [CrossRef]
- Wen, S.; Miao, Y.; Tang, Y.; Song, Z.; Feng, Q. Theoretical and Experimental Study on High-Entropy Flotation of Micro-Fine Cassiterite. Int. J. Min. Sci. Technol. 2025, 35, 19–39. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Wastewater Treatment: An Overview. In Green Adsorbents for Pollutant Removal; Environmental Chemistry for a Sustainable World; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Singh, D.; Singh, D.; Mishra, V.; Kushwaha, J.; Sengar, M.; Sinha, S.; Singh, S.; Giri, B.S. Strategies for Biological Treatment of Waste Water: A Critical Review. J. Clean. Prod. 2024, 454, 142266. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, X.; Wang, L.; Wang, Y.; Yang, J.; Wang, Y.; Huan, Y.; Liang, T.; Yu, Q.J. Comprehensive efficiency evaluation of wastewater treatment plants in northeast Qinghai-Tibet Plateau using slack-based data envelopment analysis. Environ. Pollut. 2022, 311, 120008. [Google Scholar] [CrossRef]
- Meng, X.; Huang, Z.; Ge, G. Upgrade and reconstruction of biological processes in municipal wastewater treatment plants. Desalination Water Treat. 2024, 317, 100299. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, X.; Lu, B.; Lin, Y. Real-Time Control of A2O Process in Wastewater Treatment Through Fast Deep Reinforcement Learning Based on Data-Driven Simulation Model. Water 2024, 16, 3710. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, Y.; Wang, H.; Liu, G.; Qi, L.; Xu, X.; Liu, S. Current operation state of wastewater treatment plants in urban China. Environ. Res. 2021, 195, 110843. [Google Scholar] [CrossRef] [PubMed]
- Islam, G.M.; Vi, P.; Gilbride, K.A. Functional relationship between ammonia-oxidizing bacteria and ammonia-oxidizing archaea populations in the secondary treatment system of a full-scale municipal wastewater treatment plant. J. Environ. Sci. 2019, 86, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, L. A meta-analysis of microbial community structures and associated metabolic potential of municipal wastewater treatment plants in global scope. Environ. Pollut. 2020, 263, 114598. [Google Scholar] [CrossRef]
- Begmatov, S.; Dorofeev, A.G.; Kadnikov, V.V.; Beletsky, A.V.; Pimenov, N.V.; Ravin, N.V.; Mardanov, A.V. The structure of microbial communities of activated sludge of large-scale wastewater treatment plants in the city of Moscow. Sci. Rep. 2022, 12, 3458. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Wang, C.; Li, S.; Lau, F.T.K.; Zhou, J.; Zhang, T. Effects of operational parameters on bacterial communities in Hong Kong and global wastewater treatment plants. mSystems 2024, 9, e01333-23. [Google Scholar] [CrossRef]
- Fang, D.; Zhao, G.; Xu, X.; Zhang, Q.; Shen, Q.; Fang, Z.; Huang, L.; Ji, F. Microbial community structures and functions of wastewater treatment systems in plateau and cold regions. Bioresour. Technol. 2018, 249, 684–693. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Wu, D.; Ding, J.; Niu, Y.; Jiang, T.; Yang, X.; Liu, Y. Deciphering the antibiotic resistome and microbial community in municipal wastewater treatment plants at different elevations in eastern and western China. Water Res. 2023, 229, 119461. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Niu, L.; Li, Y.; Zhang, P.; Zhang, W.; Zhang, H. Current state and solution proposal for plateau wastewater treatment plants: A review. Desalination Water Treat. 2019, 155, 120–133. [Google Scholar] [CrossRef]
- Lu, Y.-Z.; Han, J.; Zhang, W.-J.; Sun, J.; Li, X.; Yang, Z.-L.; Yang, J.-L.; Li, S.-P.; Zhu, G.-C. Influence of low air pressure on combined nitritation and anaerobic ammonium oxidation process. Sci. Total Environ. 2022, 838, 156556. [Google Scholar] [CrossRef]
- Niu, L.; Li, Y.; Wang, P.; Zhang, W.; Wang, C.; Cai, W.; Wang, L. Altitude-scale variation in nitrogen-removal bacterial communities from municipal wastewater treatment plants distributed along a 3600-m altitudinal gradient in China. Sci. Total Environ. 2016, 559, 38–44. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Huang, S.; Wang, Y.; Liao, S.; Wei, Y. Data on microbial community composition of sludge from high altitude wastewater treatment plants determined by 16S rRNA gene sequencing. Data Brief 2019, 23, 103739. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Z.; Hoshino, A.; Zheng, H.D.; Morley, M.; Arany, Z.; Rabinowitz, J.D. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 2019, 1, 404–415. [Google Scholar] [CrossRef]
- Reisoglu, S.; Aydin, S. Bacteriophage and Their Potential Use in Bioaugmentation of Biological Wastewater Treatment Processes. Sustainability 2023, 15, 12216. [Google Scholar] [CrossRef]
- Wei, C.; Sun, D.; Yuan, W.; Li, L.; Dai, C.; Chen, Z.; Zeng, X.; Wang, S.; Zhang, Y.; Jiang, S.; et al. Metagenomics revealing molecular profiles of microbial community structure and metabolic capacity in Bamucuo lake, Tibet. Environ. Res. 2023, 217, 114847. [Google Scholar] [CrossRef]
- Wiseschart, A.; Mhuantong, W.; Tangphatsornruang, S.; Chantasingh, D.; Pootanakit, K. Shotgun metagenomic sequencing from Manao-Pee cave, Thailand, reveals insight into the microbial community structure and its metabolic potential. BMC Microbiol. 2019, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- GB 18918-2002; Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2002.
- Al Ali, A.A.; Naddeo, V.; Hasan, S.W.; Yousef, A.F. Correlation between bacterial community structure and performance efficiency of a full-scale wastewater treatment plant. J. Water Process. Eng. 2020, 37, 101472. [Google Scholar] [CrossRef]
- Xie, Z.-X.; He, Y.-B.; Wang, M.-H.; Zhang, S.-F.; Kong, L.-F.; Lin, L.; Liu, S.-Q.; Wang, D.-Z. Dissecting microbial community structure and metabolic activities at an oceanic deep chlorophyll maximum layer by size-fractionated metaproteomics. Prog. Oceanogr. 2020, 188, 102439. [Google Scholar] [CrossRef]
- Zhao, W.; Bi, X.; Peng, Y.; Bai, M. Research advances of the phosphorus-accumulating organisms of Candidatus Accumulibacter, Dechloromonas and Tetrasphaera: Metabolic mechanisms, applications and influencing factors. Chemosphere 2022, 307, 135675. [Google Scholar] [CrossRef]
- Chuda, A.; Otlewska, A.; Ziemiński, K. Insights into the Microbial Community Structure in the Biodegradation Process of High-Strength Ammonia Digestate Liquid Fraction in Conventional Activated Sludge System. Bioresources 2023, 18, 3540–3559. [Google Scholar] [CrossRef]
- Chi, T.; Yu, J.; Yin, Y.; Wang, X.; Wang, A.; Wang, X. A Preliminary Study on Core Flora of Activated Sludge during Low-temperature Operation in Wastewater Treatment Plants. Environ. Sci. Technol. 2023, 46, 23–29. [Google Scholar] [CrossRef]
- Yang, K.; Mu, X.; Zhao, Z.; Chen, Y.; Feng, Q.; Wang, S. Efficacy and microbial community analysis of multi-stage AO process for brewing wastewater treatment. Ind. Water Treat. 2021, 41, 112–119. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Mao, Y.-P.; Achenbach, L.A. From canonical nitrite oxidizing bacteria to complete ammonia oxidizer: Discovery and advances. Microbiology 2019, 46, 879–890. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, C.; Lv, W.; Liu, Y.; Sun, J.; Gong, T. Differences of Bacterial Communities in Two Full-Scale A2/O Municipal Wastewater Treatment Plants and Their Effects on Effluent Total Nitrogen Removal. Environ. Technol. Innov. 2021, 21, 101317. [Google Scholar] [CrossRef]
- Tian, W.-D.; Lopez-Vazquez, C.M.; Li, W.-G.; Brdjanovic, D.; van Loosdrecht, M.C.M. Occurrence of PAOI in a low temperature EBPR system. Chemosphere 2013, 92, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Skennerton, C.T.; Barr, J.J.; Slater, F.R.; Bond, P.L.; Tyson, G.W. Expanding our view of genomic diversity in Candidatus Accumulibacter clades. Environ. Microbiol. 2015, 17, 1574–1585. [Google Scholar] [CrossRef]
- Ji, B.; Yang, K.; Zhu, L.; Jiang, Y.; Wang, H.; Zhou, J.; Zhang, H. Aerobic denitrification: A review of important advances of the last 30 years. Biotechnol. Bioprocess Eng. 2015, 20, 643–651. [Google Scholar] [CrossRef]
- Broman, E.; Jawad, A.; Wu, X.; Christel, S.; Ni, G.; Lopez-Fernandez, M.; Sundkvist, J.-E.; Dopson, M. Low temperature, autotrophic microbial denitrification using thiosulfate or thiocyanate as electron donor. Biodegradation 2017, 28, 287–301. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Hu, L.; Li, Y. Phosphorus recovery from municipal wastewater with improvement of denitrifying phosphorus uptake based on a novel AAO-SBSPR process. Chem. Eng. J. 2021, 417, 127907. [Google Scholar] [CrossRef]
- Li, R.-H.; Huang, J.; Liu, C.-X.; Yu, K.; Guo, F.; Li, Y.; Chen, Z.-H.; Wang, X.; Zhao, R.-X.; Zhang, J.-Y.; et al. Genome-centric metagenomics provides new insights into metabolic pathways of polyhydroxyalkanoates biosynthesis and functional microorganisms subsisting on municipal organic wastes. Water Res. 2023, 244, 120512. [Google Scholar] [CrossRef]
- Yang, M.; Luo, Q.; Fan, Z.; Zeng, F.; Huang, L.; Ding, S.; Cui, G.; Li, D.; Wei, G.; Liu, C.-Q.; et al. Metagenomics and Stable Isotopes Uncover the Augmented Sulfide-Driven Autotrophic Denitrification in a Seasonally Hypoxic, Sulfate-Abundant Reservoir. Environ. Sci. Technol. 2024, 58, 14225–14236. [Google Scholar] [CrossRef]
- Zhang, F.; Li, P.; Chen, M.; Wu, J.; Zhu, N.; Wu, P.; Chiang, P.; Hu, Z. Effect of operational modes on nitrogen removal and nitrous oxide emission in the process of simultaneous nitrification and denitrification. Chem. Eng. J. 2015, 280, 549–557. [Google Scholar] [CrossRef]
- Wang, K.; Chen, X.; Yan, D.; Xu, Z.; Hu, P.; Li, H. Petrochemical and municipal wastewater treatment plants activated sludge each own distinct core bacteria driven by their specific incoming wastewater. Sci. Total Environ. 2022, 826, 153962. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qaisar, M.; Chen, B.; Xiao, J.; Cai, J. Metagenomic analysis of microbial community and metabolic pathway of simultaneous sulfide and nitrite removal process exposed to divergent hydraulic retention times. Bioresour. Technol. 2022, 354, 127186. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, X.; Luo, J.; Li, H.; How, S.-W.; Wu, D.; He, J.; Cheng, Z.; Gao, Y.; Lu, H. A review of the phosphorus removal of polyphosphate-accumulating organisms in natural and engineered systems. Sci. Total Environ. 2024, 912, 169103. [Google Scholar] [CrossRef]
- Wang, K.; Yan, D.; Chen, X.; Xu, Z.; Cao, W.; Li, H. New Insight to the Enriched Microorganisms Driven by Pollutant Concentrations and Types for Industrial and Domestic Wastewater Via Distinguishing the Municipal Wastewater Treatment Plants. Environ. Pollut. 2024, 361, 124789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yu, Q.; Yan, G.; Zhu, H.; Xu, X.Y.; Zhu, L. Seasonal Bacterial Community Succession in Four Typical Wastewater Treatment Plants: Correlations Between Core Microbes and Process Performance. Sci. Rep. 2018, 8, 4566. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.L.; Yang, R.; Decker, T.; Cavalliere, C.; Andreev, V.; Bircher, N.; Cornell, J.; Dohmen, R.; Pratt, C.J.; Grinnell, A.; et al. Genomes of Novel Myxococcota Reveal Severely Curtailed Machineries for Predation and Cellular Differentiation. Appl. Environ. Microbiol. 2021, 87, e0170621. [Google Scholar] [CrossRef]
- Latocheski, E.C.; da Rocha, M.C.V.; Braga, M.C.B. Nitrospira in wastewater treatment: Applications, opportunities and research gaps. Rev. Environ. Sci. Bio/Technol. 2022, 21, 905–930. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, J.; Zhang, Y.; Fang, F.; Feng, Q.; Luo, J. Achieving efficient nitrite accumulation in glycerol-driven partial denitrification system: Insights of influencing factors, shift of microbial community and metabolic function. Bioresour. Technol. 2020, 315, 123844. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, X.; Li, Y.; Wang, P.; Zhang, W.; Wang, C.; Wang, Q. Elevational characteristics of the archaeal community in full-scale activated sludge wastewater treatment plants at a 3,660-meter elevational scale. Water Sci. Technol. 2017, 76, 531–541. [Google Scholar] [CrossRef]
- Martínez-Jardines, M.A.; de María Cuervo-López, F.; Martínez-Hernández, S. Physiological and Microbial Community Analysis During Municipal Organic Waste Leachate Treatment by a Sequential Nitrification-Denitrification Process. Water Air Soil Pollut. 2024, 235, 264. [Google Scholar] [CrossRef]
- Ciok, A.; Budzik, K.; Zdanowski, M.K.; Gawor, J.; Grzesiak, J.; Decewicz, P.; Gromadka, R.; Bartosik, D.; Dziewit, L. Plasmids of Psychrotolerant Polaromonas spp. Isolated From Arctic and Antarctic Glaciers—Diversity and Role in Adaptation to Polar Environments. Front. Microbiol. 2018, 9, 1285. [Google Scholar] [CrossRef]
- Kayani, H.; Rasheed, M.A.; Alonazi, W.B.; Jamil, F.; Hussain, A.; Yan, C.; Ahmed, R.; Ibrahim, M. Identification and genome-wide analysis provide insights into the genetic diversity and biotechnological potentials of novel cold-adapted Acinetobacter strain. Extremophiles 2023, 27, 14. [Google Scholar] [CrossRef]
- Yin, J.; Li, J.; Xie, H.; Wang, Y.; Zhao, J.; Wang, L.; Wu, L. Unveiling cold Code: Acinetobacter calcoaceticus TY1′s adaptation strategies and applications in nitrogen treatment. Bioresour. Technol. 2024, 413, 131449. [Google Scholar] [CrossRef]
- Wang, S.; Niu, Q.; Zhu, P.; Huang, Y.; Li, K.; Li, Q. Metagenomics analysis unraveled the influence of sulfate radical-mediated compost nitrogen transformation process. J. Environ. Manag. 2022, 317, 115436. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liang, X.; Li, M.; Ma, M.; Zheng, Q.; Li, D.; An, T.; Wang, G. Advances in the optimization of central carbon metabolism in metabolic engineering. Microb. Cell Factories 2023, 22, 76. [Google Scholar] [CrossRef]
- Huang, X.; Yang, X.; Zhu, J.; Yu, J. Microbial interspecific interaction and nitrogen metabolism pathway for the treatment of municipal wastewater by iron carbon based constructed wetland. Bioresour. Technol. 2020, 315, 123814. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, Y.; Wei, J.; Lyu, C.; Yu, H.; Song, Y. Impacts of dibutyl phthalate on bacterial community composition and carbon and nitrogen metabolic pathways in a municipal wastewater treatment system. Environ. Res. 2023, 223, 115378. [Google Scholar] [CrossRef]
- Dan, Q.; Du, R.; Wang, T.; Sun, T.; Han, H.; Zhu, X.; Li, X.; Zhang, Q.; Peng, Y. Complete nitrogen removal from low-strength wastewater via double nitrite shunt coupling anammox and endogenous nitrate respiration: Functional metabolism and electron transport. Chem. Eng. J. 2023, 465, 143027. [Google Scholar] [CrossRef]
- Ni, Q.; Chen, Y.; Lu, L.; Liu, M. C4-HSL-mediated quorum sensing regulates nitrogen removal in activated sludge process at Low temperatures. Environ. Res. 2024, 244, 117928. [Google Scholar] [CrossRef]
- Long, Y.; Ma, Y.; Wan, J.; Wang, Y.; Tang, M.; Fu, H.; Cao, J. Denitrification efficiency, microbial communities and metabolic mechanisms of corn cob hydrolysate as denitrifying carbon source. Environ. Res. 2023, 221, 115315. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Qin, Y.-L.; Zou, Z.-C.; Appiah, B.; Huang, H.; Yang, Z.-H.; Qun, C. Enhancing intracellular NADPH bioavailability through improving pentose phosphate pathway flux and its application in biocatalysis asymmetric reduction reaction. J. Biosci. Bioeng. 2022, 134, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.-Z.; Ma, Z.; Liu, Y.-J.; Feng, Y.; Sun, Z.; Cheng, Y.; Song, X.; Cui, Q. Overexpression of glucose-6-phosphate dehydrogenase enhanced the polyunsaturated fatty acid composition of Aurantiochytrium sp. SD116. Algal Res. 2016, 19, 138–145. [Google Scholar] [CrossRef]
- Huang, M.-Q.; Cui, Y.-W. Simultaneous aerobic nitrogen and phosphate removal by Pseudomonas aeruginosa SNDPR-01: Comprehensive insights on metabolic potential and mechanisms. Chem. Eng. J. 2024, 481, 148459. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, J.; Zhang, X.; Zhu, Z.; Wu, Z.; Zhang, K.; Wang, Y.; Wu, P.; Zhang, X. Efficient nitrogen removal from municipal wastewater by an autotrophic-heterotrophic coupled anammox system: The up-regulation of key functional genes. Sci. Total Environ. 2023, 904, 166359. [Google Scholar] [CrossRef]
- Wu, L.; Ning, D.; Zhang, B.; Li, Y.; Zhang, P.; Shan, X.; Zhang, Q.; Brown, M.R.; Li, Z.; Van Nostrand, J.D.; et al. Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat. Microbiol. 2019, 4, 1183–1195. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.-X.; Lin, W.; Wang, S.-S.; Zhang, W.-X.; Jiang, Y.-M.; Zhang, Y.; Zhang, H.; Han, Y.-H. Full evaluation of assimilatory and dissimilatory nitrate reduction in a new denitrifying bacterium Leclercia adecarboxylata strain AS3-1: Characterization and functional gene analysis. Environ. Technol. Innov. 2021, 23, 101731. [Google Scholar] [CrossRef]
- Huang, M.-Q.; Cui, Y.-W.; Yang, H.-J.; Xu, M.-J.; Cui, Y.; Chen, Z. A halophilic aerobic-heterotrophic strain Halomonas venusta SND-01: Nitrogen removal by ammonium assimilation and heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2023, 374, 128758. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, Z.; Mo, S.; Li, J.; He, S.; Kashif, M.; Liang, Z.; Shen, P.; Yan, B.; Jiang, C. Desulfobacterales stimulates nitrate reduction in the mangrove ecosystem of a subtropical gulf. Sci. Total Environ. 2021, 769, 144562. [Google Scholar] [CrossRef]
- Ni, M.; Pan, Y.; Li, D.; Huang, Y.; Chen, Z.; Li, L.; Bi, Z.; Wu, R.; Song, Z. Metagenomics, metatranscriptomics, and proteomics reveal the metabolic mechanism of biofilm sequencing batch reactor with higher phosphate enrichment capacity under low phosphorus load. Environ. Res. 2023, 238, 117237. [Google Scholar] [CrossRef]



| Sample | LZ | CD | LS | LW | ||||
|---|---|---|---|---|---|---|---|---|
| Influent | Effluent | Influent | Effluent | Influent | Effluent | Influent | Effluent | |
| COD (mg/L) | 45.80 | 7.20 | 147.90 | 17.15 | 223.64 | 24.44 | 340.19 | 24.37 |
| NH4+-N (mg/L) | 5.70 | 2.02 | 20.343 | 0.007 | 16.31 | 0.42 | 23.09 | 2.46 |
| TN (mg/L) | 6.70 | 6.47 | 49.19 | 3.98 | 18.23 | 2.15 | 25.05 | 5.73 |
| TP (mg/L) | 1.10 | 0.26 | 4.291 | 0.1716 | 1.70 | 0.19 | 6.99 | 0.27 |
| pH | 7.15 | 7.07 | 7.70 | 7.36 | 7.32 | 7.48 | 7.2 | 7.06 |
| Temperature (°C) | 16.28 | 15.56 | 14.9 | 16.55 | 15.6 | 16.4 | 15.5 | 16.2 |
| Altitude (m) | 2980 | 3240 | 3564 | 3650 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Liu, Y.; Li, H.; Xiong, J.; Zhang, Q.; Li, P.; Liu, L.; Lu, X. Metagenomic Analysis of Bacterial Community Structure and Pollutant Removal Process in High-Altitude Municipal Wastewater Treatment Plants of Tibet, China. Water 2025, 17, 1284. https://doi.org/10.3390/w17091284
Zhang R, Liu Y, Li H, Xiong J, Zhang Q, Li P, Liu L, Lu X. Metagenomic Analysis of Bacterial Community Structure and Pollutant Removal Process in High-Altitude Municipal Wastewater Treatment Plants of Tibet, China. Water. 2025; 17(9):1284. https://doi.org/10.3390/w17091284
Chicago/Turabian StyleZhang, Rui, Yiwen Liu, Haibo Li, Jian Xiong, Qiangying Zhang, Pengtao Li, Lingjie Liu, and Xuebin Lu. 2025. "Metagenomic Analysis of Bacterial Community Structure and Pollutant Removal Process in High-Altitude Municipal Wastewater Treatment Plants of Tibet, China" Water 17, no. 9: 1284. https://doi.org/10.3390/w17091284
APA StyleZhang, R., Liu, Y., Li, H., Xiong, J., Zhang, Q., Li, P., Liu, L., & Lu, X. (2025). Metagenomic Analysis of Bacterial Community Structure and Pollutant Removal Process in High-Altitude Municipal Wastewater Treatment Plants of Tibet, China. Water, 17(9), 1284. https://doi.org/10.3390/w17091284






