Ecological Shifts and Functional Adaptations of Soil Microbial Communities Under Petroleum Hydrocarbon Contamination
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sample Collection
2.2. Soil Properties Analysis
2.3. DNA Extraction and Sequencing
2.4. Data Analyses
3. Results and Discussion
3.1. Geochemical Conditions in Petroleum-Contaminated Soils
3.2. Impact of Petroleum Contamination on Soil Bacterial Phylogenetic Structure
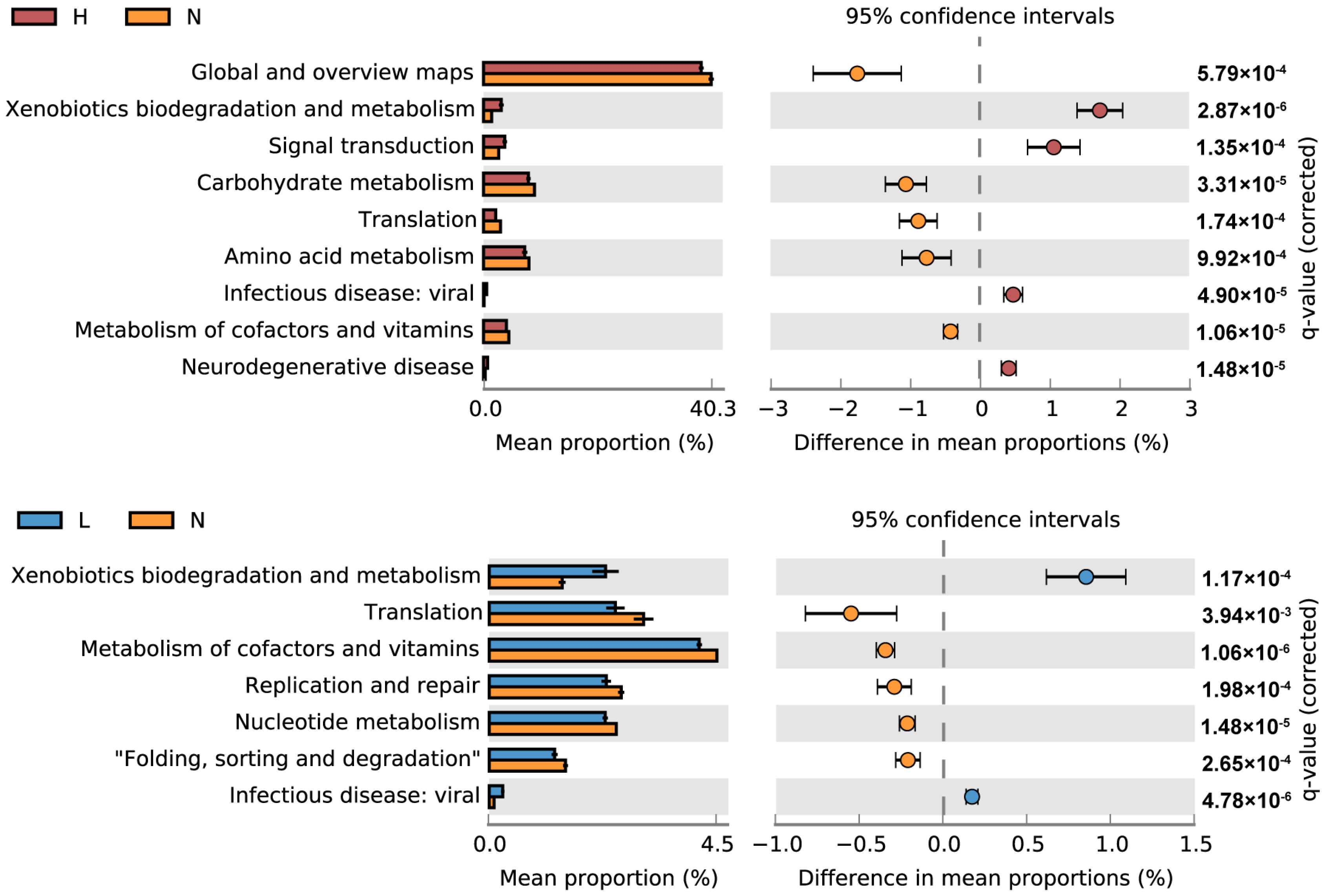
3.3. Overall Microbial Functional Patterns
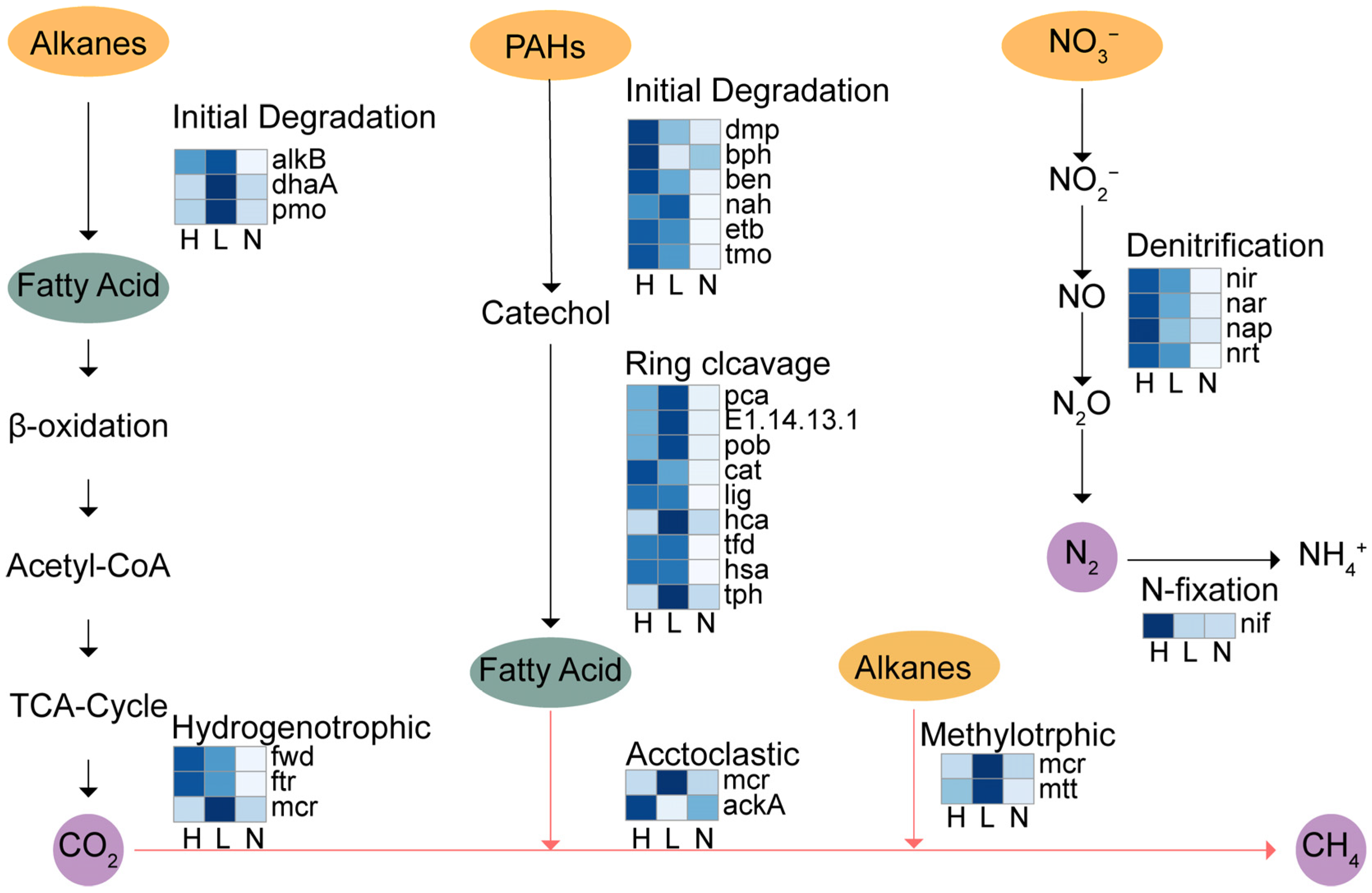
3.4. Key Microbial Functional Genes
3.5. Factors Controlling Soil Bacterial Metabolism Under Petroleum Contamination
3.6. Comparative Ecological Implications and Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basu, S.; Kumar, G.; Chhabra, S.; Prasad, R. Role of soil microbes in biogeochemical cycle for enhancing soil fertility. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 149–157. [Google Scholar]
- Olalekan, R.M.; Ilesanmi, A.; Alima, O.; Omini, D.E.; Raimi, A.G. Exploring how human activities disturb the balance of biogeochemical cycles: Evidence from the carbon, nitrogen and hydrologic cycles. In Science-Based Approaches to Respond to COVID and Other Public Health Threats; IntechOpen: London, UK, 2021. [Google Scholar]
- Raza, T.; Qadir, M.F.; Khan, K.S.; Eash, N.S.; Yousuf, M.; Chatterjee, S.; Manzoor, R.; Ur Rehman, S.; Oetting, J.N. Unraveling the potential of microbes in decomposition of organic matter and release of carbon in the ecosystem. J. Environ. Manag. 2023, 344, 118529. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Griffiths, B.S.; Langenheder, S. Microbial community resilience across ecosystems and multiple disturbances. Microbiol. Mol. Biol. Rev. 2021, 85. [Google Scholar] [CrossRef]
- Nizamani, M.M.; Hughes, A.C.; Qureshi, S.; Zhang, Q.; Tarafder, E.; Das, D.; Acharya, K.; Wang, Y.; Zhang, Z.-G. Microbial biodiversity and plant functional trait interactions in multifunctional ecosystems. Appl. Soil Ecol. 2024, 201, 105515. [Google Scholar] [CrossRef]
- Mohanta, S.; Pradhan, B.; Behera, I.D. Impact and remediation of petroleum hydrocarbon pollutants on agricultural land: A review. Geomicrobiol. J. 2024, 41, 345–359. [Google Scholar] [CrossRef]
- Shah, G.; Soni, V. Comprehensive insights into the impact of oil pollution on the environment. Reg. Stud. Mar. Sci. 2024, 74, 103516. [Google Scholar]
- Pandolfo, E.; Barra Caracciolo, A.; Rolando, L. Recent advances in bacterial degradation of hydrocarbons. Water 2023, 15, 375. [Google Scholar] [CrossRef]
- Zhu, S.; Li, M.; Qian, T.; Chen, J.; Pan, T. Influence of Surfactants on Interfacial Microbial Degradation of Hydrophobic Organic Compounds. Catalysts 2025, 15, 187. [Google Scholar] [CrossRef]
- Zainab, R.; Hasnain, M.; Ali, F.; Dias, D.A.; El-Keblawy, A.; Abideen, Z. Exploring the bioremediation capability of petroleum-contaminated soils for enhanced environmental sustainability and minimization of ecotoxicological concerns. Environ. Sci. Pollut. Res. 2023, 30, 104933–104957. [Google Scholar] [CrossRef]
- Hoang, S.A.; Sarkar, B.; Seshadri, B.; Lamb, D.; Wijesekara, H.; Vithanage, M.; Liyanage, C.; Kolivabandara, P.A.; Rinklebe, J.; Lam, S.S. Mitigation of petroleum-hydrocarbon-contaminated hazardous soils using organic amendments: A review. J. Hazard. Mater. 2021, 416, 125702. [Google Scholar] [CrossRef]
- Mekonnen, B.A.; Aragaw, T.A.; Genet, M.B. Bioremediation of petroleum hydrocarbon contaminated soil: A review on principles, degradation mechanisms, and advancements. Front. Environ. Sci. 2024, 12, 1354422. [Google Scholar] [CrossRef]
- Khattak, W.A.; Sun, J.; Hameed, R.; Zaman, F.; Abbas, A.; Khan, K.A.; Elboughdiri, N.; Akbar, R.; He, F.; Ullah, M.W. Unveiling the resistance of native weed communities: Insights for managing invasive weed species in disturbed environments. Biol. Rev. 2024, 99, 753–777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Yao, S.; Zhao, X.; Kong, Q.; Cui, L.; Zhang, H. Driving mechanisms for the adaptation and degradation of petroleum hydrocarbons by native microbiota from seas prone to oil spills. J. Hazard. Mater. 2024, 476, 135060. [Google Scholar] [CrossRef]
- Du, S.; Li, X.-Q.; Hao, X.; Hu, H.-W.; Feng, J.; Huang, Q.; Liu, Y.-R. Stronger responses of soil protistan communities to legacy mercury pollution than bacterial and fungal communities in agricultural systems. ISME Commun. 2022, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Zhang, R.; Zeng, Y.; Dai, T.; Ye, Z.; Gao, Q.; Yang, Y.; Guo, X.; Li, G.; Zhou, J. Petroleum pollution changes microbial diversity and network complexity of soil profile in an oil refinery. Front. Microbiol. 2023, 14, 1193189. [Google Scholar] [CrossRef]
- Zhang, R.; Zhuang, J.; Guo, X.; Dai, T.; Ye, Z.; Liu, R.; Li, G.; Yang, Y. Microbial functional heterogeneity induced in a petroleum-polluted soil profile. J. Hazard. Mater. 2024, 465, 133391. [Google Scholar] [CrossRef] [PubMed]
- Amini, F.; Giyahchi, M.; Moghimi, H. Bioremediation of Petroleum Contamination by Microorganisms: Role of Microbial Communities and Applications. Microb. Bioremediation Multiomics Technol. Sustain. Dev. Recent Trends 2024, 13, 136. [Google Scholar]
- Adetitun, D.; Tomilayo, R. Ecological implications of bacterial degradation of alkanes in petroleum-contaminated environments: A review of microbial community dynamics and functional interactions. Glob. J. Pure Appl. Sci. 2023, 29, 133–144. [Google Scholar]
- Das, N.; Das, A.; Das, S.; Bhatawadekar, V.; Pandey, P.; Choure, K.; Damare, S.; Pandey, P. Petroleum hydrocarbon catabolic pathways as targets for metabolic engineering strategies for enhanced bioremediation of crude-oil-contaminated environments. Fermentation 2023, 9, 196. [Google Scholar] [CrossRef]
- Rezaei, Z.; Moghimi, H. Fungal-bacterial consortia: A promising strategy for the removal of petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 2024, 280, 116543. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Y.; Ju, C.; Zhao, T.; Meng, Q.; Cong, J. Microbial strategies for effective microplastics biodegradation: Insights and innovations in environmental remediation. Environ. Res. 2024, 263, 120046. [Google Scholar] [CrossRef]
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R.; Issac, P.K.; Nasr, M.; Khoo, K.S.; Rajagopal, R.; Greff, B.; Azelee, N.I.W.; Jeon, B.-H. Polyaromatic hydrocarbons (PAHs) in the water environment: A review on toxicity, microbial biodegradation, systematic biological advancements, and environmental fate. Environ. Res. 2023, 227, 115716. [Google Scholar] [CrossRef] [PubMed]
- Tarigholizadeh, S.; Sushkova, S.; Rajput, V.D.; Ranjan, A.; Arora, J.; Dudnikova, T.; Barbashev, A.; Mandzhieva, S.; Minkina, T.; Wong, M.H. Transfer and degradation of PAHs in the soil–plant system: A review. J. Agric. Food Chem. 2023, 72, 46–64. [Google Scholar] [CrossRef]
- McKay, L.J.; Smith, H.J.; Barnhart, E.P.; Schweitzer, H.D.; Malmstrom, R.R.; Goudeau, D.; Fields, M.W. Activity-based, genome-resolved metagenomics uncovers key populations and pathways involved in subsurface conversions of coal to methane. ISME J. 2022, 16, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, T.; Si, B.; Watson, J.; Zhang, Y. Accelerating anaerobic digestion for methane production: Potential role of direct interspecies electron transfer. Renew. Sustain. Energy Rev. 2021, 145, 111069. [Google Scholar] [CrossRef]
- Chamoli, A.; Karn, S.K.; Kumari, M.; Sivaramasamy, E. Biochar mediated fixation of nitrogen compounds (ammonia and nitrite) in soil: A review. Biodegradation 2025, 36, 22. [Google Scholar] [CrossRef]
- Zhang, X.; Sabo, R.; Rosa, L.; Niazi, H.; Kyle, P.; Byun, J.S.; Wang, Y.; Yan, X.; Gu, B.; Davidson, E.A. Nitrogen management during decarbonization. Nat. Rev. Earth Environ. 2024, 5, 717–731. [Google Scholar] [CrossRef]
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, Y.; Cui, W.; Sun, Y.; Zhang, S.; Wang, F. Effects of microplastics and carbon nanotubes on soil geochemical properties and bacterial communities. J. Hazard. Mater. 2022, 433, 128826. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Cheng, L.; Tan, Q.; Liu, Y.; Dou, J.; Yang, K.; Yang, Q.; Wang, S.; Li, J.; Niu, G. Response of the soil microbial community to petroleum hydrocarbon stress shows a threshold effect: Research on aged realistic contaminated fields. Front. Microbiol. 2023, 14, 1188229. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Bakina, L.G.; Mayachkina, N.V.; Chugunova, M.V.; Bityutskii, N.P.; Yakkonen, K.L.; Shavarda, A.L. Long-term effects of oil contamination on soil quality and metabolic function. Environ. Geochem. Health 2024, 46, 13. [Google Scholar] [CrossRef]
- Panda, S.K.; Das, S. Potential of plant growth-promoting microbes for improving plant and soil health for biotic and abiotic stress management in mangrove vegetation. Rev. Environ. Sci. Bio/Technol. 2024, 23, 801–837. [Google Scholar] [CrossRef]
- Yin, Q.; He, K.; Collins, G.; De Vrieze, J.; Wu, G. Microbial strategies driving low concentration substrate degradation for sustainable remediation solutions. npj Clean Water 2024, 7, 52. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Li, Y.; Han, X.; Li, B.; Zhang, X.; Li, Q.; Liang, W. Organic substitutions improve soil quality and maize yield through increasing soil microbial diversity. J. Clean. Prod. 2022, 347, 131323. [Google Scholar] [CrossRef]
- Jemli, M.; Karray, F.; Mansour, L.; Loukil, S.; Bouhdida, R.; Yadav, K.K.; Sayadi, S. Wastewater biotreatment and bioaugmentation for remediation of contaminated sites at an oil recycling plant. Water Sci. Technol. 2025, 91, 139–159. [Google Scholar] [CrossRef]
- Rawat, C.D.; Phian, S.; Gupta, R.; Verma, H.; Kumar, M.; Kaur, J.; Rawat, V.S. Microbial bioprocesses in remediation of contaminated environments and resource recovery. In Microbial Bioprocesses; Elsevier: Amsterdam, The Netherlands, 2023; pp. 225–274. [Google Scholar]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Fu, Y.; Lin, D.; Hou, M.; Li, X.; Hu, D.; Wang, Z. Transformation of soil organic matter subjected to environmental disturbance and preservation of organic matter bound to soil minerals: A review. J. Soils Sediments 2023, 23, 1485–1500. [Google Scholar] [CrossRef]
- Xiang, L.; Harindintwali, J.D.; Wang, F.; Redmile-Gordon, M.; Chang, S.X.; Fu, Y.; He, C.; Muhoza, B.; Brahushi, F.; Bolan, N. Integrating biochar, bacteria, and plants for sustainable remediation of soils contaminated with organic pollutants. Environ. Sci. Technol. 2022, 56, 16546–16566. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Liao, J.; Balcazar, J.L.; Ye, M.; Wu, R.; Wang, D.; Alvarez, P.J.; Yu, P. Adaptive modification of antiviral defense systems in microbial community under Cr-induced stress. Microbiome 2025, 13, 34. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Q.; Su, J.; Li, M.; Lin, B.; Wu, N.; Shen, H.; Chen, J. Unraveling the mechanisms and responses of aniline-degrading biosystem to salinity stress in high temperature condition: Pollutants removal performance and microbial community. Chemosphere 2024, 362, 142688. [Google Scholar] [CrossRef]
- Rellegadla, S.; Prajapat, G.; Jain, S.; Agrawal, A. Microbial communities succession post to polymer flood demonstrate a role in enhanced oil recovery. Appl. Microbiol. Biotechnol. 2023, 107, 5531–5544. [Google Scholar] [CrossRef]
- Medić, A.B.; Karadžić, I.M. Pseudomonas in environmental bioremediation of hydrocarbons and phenolic compounds-key catabolic degradation enzymes and new analytical platforms for comprehensive investigation. World J. Microbiol. Biotechnol. 2022, 38, 165. [Google Scholar] [CrossRef] [PubMed]
- Konya, A.; Fiddler, B.A.; Bunch, O.; Hess, K.Z.; Ferguson, C.; Krzmarzick, M.J. Lead or cadmium co-contamination alters benzene and toluene degrading bacterial communities. Biodegradation 2023, 34, 357–369. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Wu, B.; Ma, T.; Jiang, H.; Mi, Y.; Jiang, C.; Zang, H.; Zhao, X.; Li, C. Potential and mechanism for bioremediation of papermaking black liquor by a psychrotrophic lignin-degrading bacterium, Arthrobacter sp. C2. J. Hazard. Mater. 2022, 439, 129534. [Google Scholar] [CrossRef] [PubMed]
- Fitriyanto, N.; Natalia, D.; Prasetyo, R.; Erwanto, Y.; Ngadiono, N. Properties of rabbit feces composting using indigenous Alcaligenes sp. LS2T and Arthrobacter sp. LM1KK. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; p. 012014. [Google Scholar]
- Yamini, V.; Rajeswari, V.D. Metabolic capacity to alter polycyclic aromatic hydrocarbons and its microbe-mediated remediation. Chemosphere 2023, 329, 138707. [Google Scholar] [CrossRef] [PubMed]
- Ehis-Eriakha, C.B.; Ajuzieogu, C.A.; Orogu, J.O.; Akemu, S.E. Overview of petroleum hydrocarbon pollution and bioremediation technologies. Bioremediation J. 2024, 1–23. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Sher, F.; Navarrete, A.A.; Américo-Pinheiro, J.H.P. Microbial adaptation to different environmental conditions: Molecular perspective of evolved genetic and cellular systems. Arch. Microbiol. 2022, 204, 144. [Google Scholar] [CrossRef]
- Maqsood, Q.; Sumrin, A.; Waseem, R.; Hussain, M.; Imtiaz, M.; Hussain, N. Bioengineered microbial strains for detoxification of toxic environmental pollutants. Environ. Res. 2023, 227, 115665. [Google Scholar] [CrossRef]
- Huang, L.; Ye, J.; Jiang, K.; Wang, Y.; Li, Y. Oil contamination drives the transformation of soil microbial communities: Co-occurrence pattern, metabolic enzymes and culturable hydrocarbon-degrading bacteria. Ecotoxicol. Environ. Saf. 2021, 225, 112740. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P. A review on catalytic-enzyme degradation of toxic environmental pollutants: Microbial enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef]
- Arora, J.; Kumari, A.; Ranjan, A.; Rajput, V.D.; Shende, S.; Prazdnova, E.V.e.; Mandzhieva, S.S.; Sushkova, S.; Minkina, T.; Chauhan, A. Microbial Adaptation in Different Extreme Environmental Conditions and Its Usefulness in Differently Polluted Soil. In Extremophiles for Sustainable Agriculture and Soil Health Improvement; Springer: Berlin/Heidelberg, Germany, 2024; pp. 47–62. [Google Scholar]
- Kour, D.; Kaur, T.; Devi, R.; Yadav, A.; Singh, M.; Joshi, D.; Singh, J.; Suyal, D.C.; Kumar, A.; Rajput, V.D. Beneficial microbiomes for bioremediation of diverse contaminated environments for environmental sustainability: Present status and future challenges. Environ. Sci. Pollut. Res. 2021, 28, 24917–24939. [Google Scholar] [CrossRef]
- Peng, C.; Wan, X.; Zhang, J.; Zhang, B.; Wang, S.; Ma, T.; Bian, Y.; Wang, W. Bacterial diversity and competitors for degradation of hazardous oil refining waste under selective pressures of temperature and oxygen. J. Hazard. Mater. 2022, 427, 128201. [Google Scholar] [CrossRef]
- Reineke, W.; Schlömann, M. Microorganisms at different sites: Living conditions and adaptation strategies. In Environmental Microbiology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 349–396. [Google Scholar]
- Saranya, S.; Thamanna, L.; Sreekutty, V.; Dhayanithi, S.; Chellapandi, P. Bioremediation of oil and natural gas industry waste using methanogens: Current status and future perspective to biohythane production. Arab. J. Sci. Eng. 2024, 50, 4457–4475. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Chen, Y. Biochar mitigates N2O emission of microbial denitrification through modulating carbon metabolism and allocation of reducing power. Environ. Sci. Technol. 2021, 55, 8068–8078. [Google Scholar] [CrossRef] [PubMed]
- Chamoli, A.; Bhambri, A.; Karn, S.K.; Raj, V. Ammonia, nitrite transformations and their fixation by different biological and chemical agents. Chem. Ecol. 2024, 40, 166–199. [Google Scholar] [CrossRef]
- Yin, Q.; Feng, Z.; Hu, Y.; Zhan, X.; Wu, G. Microbial interactions in pollution control ecosystems. Curr. Pollut. Rep. 2021, 7, 104–114. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, X.; Li, Y.; Feng, Q.; Wu, Y.; Luo, J. Signaling molecule alleviates inhibitory impacts of surfactant on methane production during sludge and food waste co-digestion: Insights of electron bifurcation and quorum sensing. J. Hazard. Mater. 2025, 484, 136810. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gu, H.; Liu, J.; Wei, D.; Zhu, P.; Cui, X.a.; Zhou, B.; Chen, X.; Jin, J.; Liu, X.; et al. Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 2022, 418, 115846. [Google Scholar] [CrossRef]
- Shen, H.; Huang, Y.; Lin, X.; Dai, Z.; Zhao, H.; Su, W.-Q.; Dahlgren, R.A.; Xu, J. Recoupling of Soil Carbon, Nitrogen, and Phosphorus Cycles along a 30 Year Fire Chronosequence in Boreal Forests of China. Environ. Sci. Technol. 2025, 59, 4432–4443. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Wu, F.; Zhang, J.; Ai, S.; Liu, Z. Microbial community composition and degradation potential of petroleum-contaminated sites under heavy metal stress. J. Hazard. Mater. 2023, 457, 131814. [Google Scholar] [CrossRef]
- Kong, L.; Xu, T.; Wang, Z.; Wen, X.; Jiao, Z.; Liu, J. Metagenomic analysis of petroleum biodegradation coupled to specific N-cycling process in oil-contaminated soil. Appl. Soil Ecol. 2024, 193, 105144. [Google Scholar] [CrossRef]
- Karishma, S.; Saravanan, A.; Deivayanai, V.; Ajithkumar, U.; Yaashikaa, P.; Vickram, A. Emerging strategies for enhancing microbial degradation of petroleum hydrocarbons: Prospects and challenges. Bioresour. Technol. Rep. 2024, 26, 101866. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Zhou, X.; Waigi, M.G.; Gudda, F.O.; Odinga, E.S.; Mosa, A.; Ling, W. Nitrogen addition enhanced the polycyclic aromatic hydrocarbons dissipation through increasing the abundance of related degrading genes in the soils. J. Hazard. Mater. 2022, 435, 129034. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Chen, J.; Castellano, M.J.; Ye, C.; Zhang, N.; Miao, Y.; Zheng, H.; Li, J.; Ding, W. Oxygen availability regulates the quality of soil dissolved organic matter by mediating microbial metabolism and iron oxidation. Glob. Change Biol. 2022, 28, 7410–7427. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, L.; Yang, S.; Xun, Y.; Zhang, T.; Wei, W. Thermally enhanced anoxic biodegradation of polycyclic aromatic hydrocarbons (PAHs) in a highly contaminated aged soil. J. Environ. Chem. Eng. 2022, 10, 107236. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Zhang, J.; Geng, B.; Zhao, J.; Jia, W.; Cheng, L. Ecological Shifts and Functional Adaptations of Soil Microbial Communities Under Petroleum Hydrocarbon Contamination. Water 2025, 17, 1216. https://doi.org/10.3390/w17081216
Ren L, Zhang J, Geng B, Zhao J, Jia W, Cheng L. Ecological Shifts and Functional Adaptations of Soil Microbial Communities Under Petroleum Hydrocarbon Contamination. Water. 2025; 17(8):1216. https://doi.org/10.3390/w17081216
Chicago/Turabian StyleRen, Lei, Jie Zhang, Bao Geng, Jie Zhao, Wenjuan Jia, and Lirong Cheng. 2025. "Ecological Shifts and Functional Adaptations of Soil Microbial Communities Under Petroleum Hydrocarbon Contamination" Water 17, no. 8: 1216. https://doi.org/10.3390/w17081216
APA StyleRen, L., Zhang, J., Geng, B., Zhao, J., Jia, W., & Cheng, L. (2025). Ecological Shifts and Functional Adaptations of Soil Microbial Communities Under Petroleum Hydrocarbon Contamination. Water, 17(8), 1216. https://doi.org/10.3390/w17081216




