Performance of Powdered Activated Coke Produced by One-Step Rapid Process from Lignite: Phenol Adsorption from Synthetic Wastewater and Hydrothermal Regeneration
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Experimental Method
2.2.1. Preparation of PAC
2.2.2. Characterization
2.2.3. Adsorption Experiments
2.2.4. Adsorption Isotherms and Thermodynamics
2.2.5. Adsorption Kinetics
2.2.6. Regeneration Experiments
3. Results and Discussion
3.1. Characterization of PAC
3.2. Effects of Influencing Factors
3.3. Thermodynamic and Kinetic Analysis of Adsorption
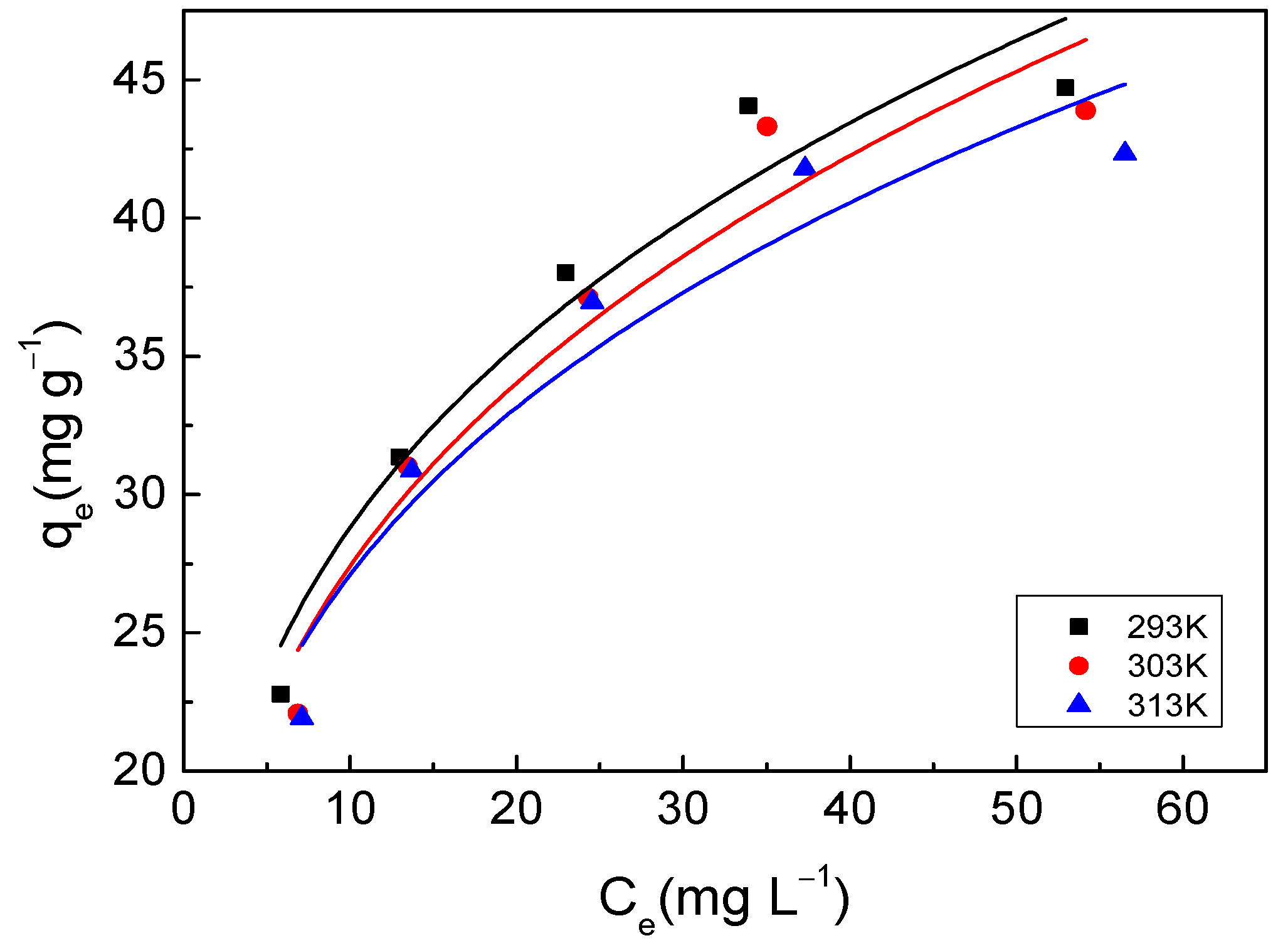
3.3.1. Adsorption Isotherm
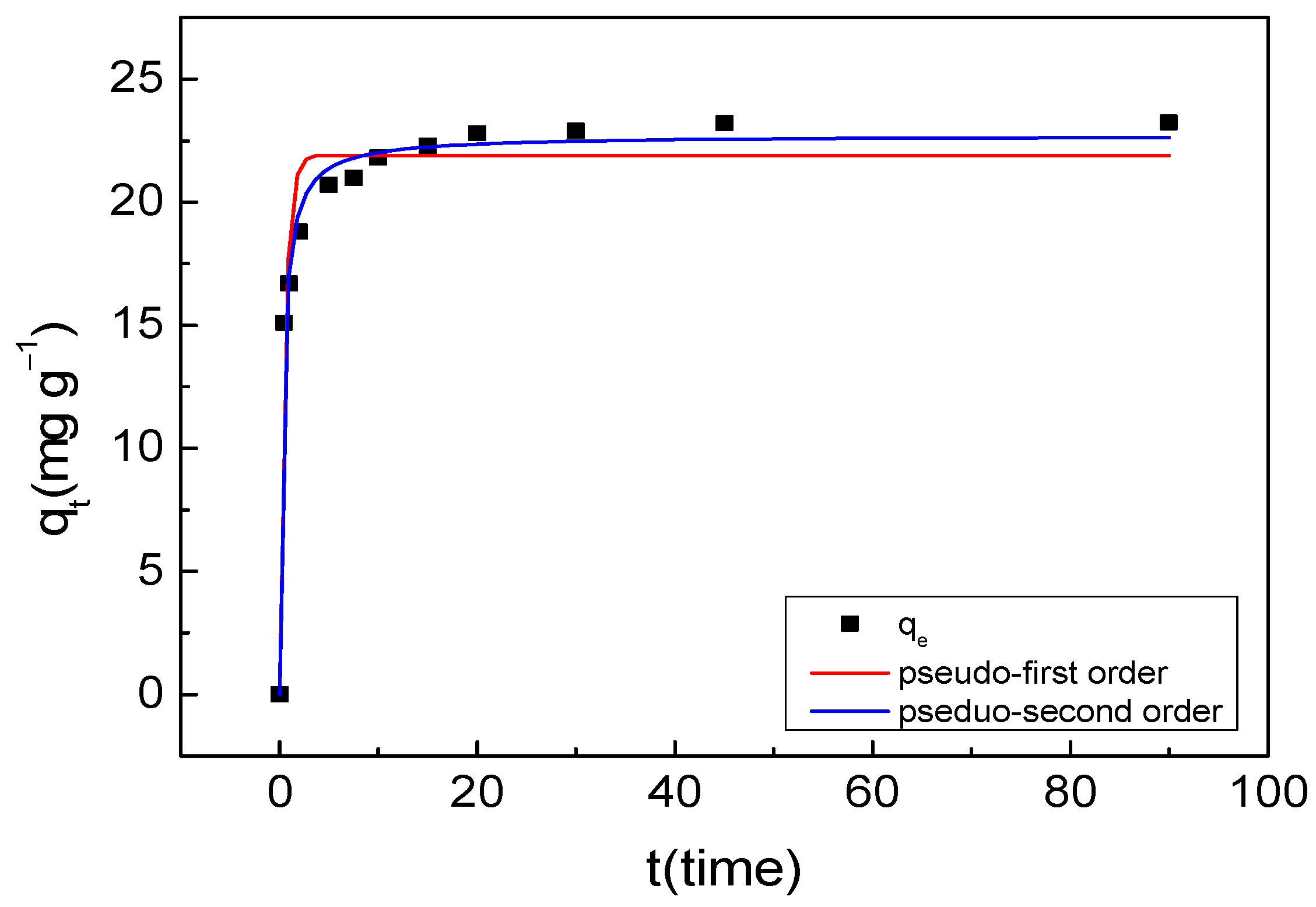
3.3.2. Adsorption Kinetics
3.4. The PAC Regeneration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAC | powdered activated coke. |
| GBAC | granular biological activated carbon. |
| PBAC | powdered biological activated carbon. |
References
- Dobrosz-Gómez, I.; Quintero-Arias, J.D.; Gómez-García, M.Á. Fenton advanced oxidation process for the treatment of industrial textile wastewater highly polluted with acid-black 194 dye. Case Stud. Chem. Environ. Eng. 2024, 9, 100672. [Google Scholar] [CrossRef]
- Fan, X.; Li, S.; Sun, M.; Song, C.; Xiao, J.; Du, J.; Tao, P.; Sun, T.; Shao, M.; Wang, T. Degradation of phenol by coal-based carbon membrane integrating sulfate radicals-based advanced oxidation processes. Ecotoxicol. Environ. Saf. 2019, 185, 109662. [Google Scholar] [CrossRef]
- Smith, S.J.; Lauria, M.; Ahrens, L. Electrochemical Oxidation for Treatment of PFAS in Contaminated Water and Fractionated Foam-A Pilot-Scale Study. ACS EST Water 2023, 3, 1201–1211. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, Y.; Lv, Y. Study on the biological treatment of phenolic-containing wastewater. Mod. Chem. Ind. 2017, 37, 58–62. [Google Scholar]
- Othman, A.M.; Mohammed, R.R.; Ismail, M. Adsorption of phenol from aqueous solution using granular activated carbon from walnut shell. AIP Conf. Proc. 2023, 2834, 23. [Google Scholar]
- Al-Ananzeh, N.; Bani-Melhem, K.; Khasawneh, H.E.; Tawalbeh, M.; Al-Qodah, Z.; Al-Bodour, A. Investigating the potential of using solid waste generated from stone cutting factories for phenol removal from wastewater: A study of adsorption kinetics and isotherms. Results Eng. 2023, 20, 10404. [Google Scholar] [CrossRef]
- Piekutin, J.; Kotowska, U.; Puchlik, M. Application of an integrated process for the removal of organic compounds of the phenols group from water. Desalination Water Treat. 2023, 301, 63–70. [Google Scholar] [CrossRef]
- Wen, W.; Wen, C.; Wang, D.; Zhu, G.; Yu, J.; Ling, P.; Xu, M.; Liu, T. A review on activated coke for removing flue gas pollutants (SO2, NOx, Hg0, and VOCs): Preparation, activation, modification, and engineering applications. J. Environ. Chem. Eng. 2024, 12, 11964. [Google Scholar] [CrossRef]
- Mi, Y.; Wang, W.; Zhang, S.; Guo, Y.; Zhao, Y.; Sun, G.; Cao, Z. Ultra-high specific surface area activated carbon from Taihu cyanobacteria via KOH activation for enhanced methylene blue adsorption. Chin. J. Chem. Eng. 2024, 67, 106–116. [Google Scholar] [CrossRef]
- Shahrashoub, M.; Bakhtiari, S. The efficiency of activated carbon/magnetite nanoparticles composites in copper removal: Industrial waste recovery, green synthesis, characterization, and adsorption-desorption studies. Microporous Mesoporous Mater. 2021, 311, 110692. [Google Scholar] [CrossRef]
- Zhou, B.X.; Wang, T.; Xu, T.M.; Li, C.; Li, J.; Fu, J.; Zhang, Z.; Song, Z.; Ma, C. Comparative study on the preparation of powdered activated coke for SO2 adsorption: One-step and two-step rapid activation methods. Fuel 2021, 288, 119570. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, B.X.; Li, C.; Xu, T.; Fu, J.; Ma, C.Y.; Song, Z. Preparation of powdered activated coke for SO2 removal using different coals through a one-step method under high-temperature flue gas atmosphere. J. Anal. Appl. Pyrolysis 2021, 153, 104989. [Google Scholar] [CrossRef]
- Yan, M.; Ren, J.; Zhu, Z.; Zhou, B.; Chen, B.; Gao, Y.; Wang, T.; Ma, C. Effect of coal type on the physical properties and CO2 adsorption performance of activated coke prepared by a single-step method. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 5281–5296. [Google Scholar]
- Wang, T.; Liu, K.; Zhang, Z.; Zhou, B.X.; Ma, C.Y. Primary study on the pilot-scale experiment of preparation for powdered activated coke from lignite. Huadian Technol. 2020, 42, 81–87. [Google Scholar]
- Gao, X.; Zhang, Y.Q.; Wang, Z.Q.; Zhao, J.T.; Huang, J.J. Study on perparation and adsorption performance of active coke from lignite. Coal Convers. 2015, 38, 79–83. [Google Scholar]
- Feng, T.; Zhang, S.Z.; Li, J.; Xia, X.; Li, L.; Zhao, X.; Ma, C.Y. Experimental and thermodynamic study on SO2 reduction to elemental sulfur by activated coke and pyrolysis gas: Influence of the reaction atmosphere. Int. J. Hydrogen Energy 2020, 45, 20120–20131. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.Q.; Zhao, X.Q. Insights into the effect of generation temperature on physico chemical properties and SO2 removal over powdered activated coke. Fuel 2021, 288, 119715. [Google Scholar] [CrossRef]
- Shen, L.; Wang, W.; Li, T.; Cui, Y.; Wang, B.; Yu, G.; Wang, X.; Wei, D.; Xiao, J.; Deng, S. Powdered activated coke for COD removal in the advanced treatment of mixed chemical wastewaters and regeneration by Fenton oxidation. Chem. Eng. J. 2019, 371, 631–638. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; Zhang, Y.; Su, Y.; Zhang, H. The deactivation mechanisms, regeneration methods and devices of activated carbon in applications. J. Clean. Prod. 2024, 476, 26. [Google Scholar] [CrossRef]
- Sahu, U.K.; Tripathy, S.; Gouda, N.; Mohanty, H.S.; Sahu, M.K.; Panda, S.P.; Krishna, Y.M.; Samantaray, S.; Kumar, V.S.R.; Banu, N.; et al. Activated Carbon-Modified Iron Oxide Nanoparticles for Cr(VI) Removal: Optimization, Kinetics, Isotherms, Thermodynamics, Regeneration, and Mechanism Study. Water Air Soil Pollut. 2023, 234, 561.1–561.20. [Google Scholar] [CrossRef]
- Baruah, M.; Kumar, S.; Ezung, S.L.; Jamir, L.; Sinha, U.B.; Sinha, D. Preparation of Co-doped TiO2 activated carbon nanocomposite and its photocatalytic degradation of phenol wastewater. Inorg. Chem. Commun. 2024, 166, 13. [Google Scholar] [CrossRef]
- Ledesma, B.; Román, S.; Sabio, E.; álvarez-Murillo, A. Improvement of spent activated carbon regeneration by wet oxidation processes. J. Supercrit. Fluids 2015, 104, 94–103. [Google Scholar] [CrossRef]
- Salvador, F.; Martin-Sanchez, N.; Sanchez-Montero, M.J.; Montero, J.; Izquierdo, C. Regeneration of activated carbons contaminated by phenol using supercritical water. J. Supercrit. Fluids 2013, 74, 1–7. [Google Scholar] [CrossRef]
- Kinniburgh, D.G. General purpose adsorption isotherms. Environ. Sci. Technol. 1986, 20, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Li, Z.Q.; Long, X. Adsorption of Diethylenetriamine from Water by Activated Carbon: Kinetics, Isotherms and Thermodynamics. Sep. Sci. Technol. 2023, 58, 21. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids Part I Solids. J. Am. Chem. Soc. 1916, 11, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.Z. Concerning adsorption in solutions. Z. Fur Phys. Chem. 1906, 57, 444–485. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Li, X.; Cui, Y.; Du, W.; Cui, W.; Huo, L.; Liu, H. Adsorption Kinetics and Mechanism of Pb(II) and Cd(II) Adsorption in Water through Oxidized Multiwalled Carbon Nanotubes. Appl. Sci. 2024, 14, 1745. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Nekouei, F.; Nekouei, S.; Tyagi, I.; Gupta, V.K. Kinetic, thermodynamic and isotherm studies for acid blue 129 removal from liquids using copper oxide nanoparticle-modified activated carbon as a novel adsorbent. J. Mol. Liq. 2015, 201, 124–133. [Google Scholar] [CrossRef]
- Li, Z.; Chen, G.; Ma, H.; Huang, F.; Xu, H.; Zhang, L.; Yuan, X.; Zhang, X.; Chen, S.; Zhou, P. Research on the hydrothermal regeneration of powdered activated coke in wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 1. [Google Scholar] [CrossRef]
- Sufnarski, M.D. Regeneration of Granular Activated Carbon Using Hydrothermal Technology; Texas University Department of Engineering: Austin, TX, USA, 1999. [Google Scholar]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined Effect of Nitrogen- and Oxygen-Containing Functional Groups of Microporous Activated Carbon on its Electrochemical Performance in Supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Hao, P.-y.; Meng, Y.-j.; Zeng, F.-g.; Yan, T.-t.; Xu, G.-b. Quantitative study of chemical structures of different rank coals based on infrared spectroscopy. Spectrosc. Spectr. Anal. 2020, 40, 787–792. [Google Scholar]
- Zheng, M.; Han, Y.; Xu, C.; Zhang, Z.; Han, H. Selective adsorption and bioavailability relevance of the cyclic organics in anaerobic pretreated coal pyrolysis wastewater by lignite activated coke. Sci. Total Environ. 2019, 653, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Q.; Li, Z.; Wang, Y. Improved Removal of Quinoline from Wastewater Using Coke Powder with Inorganic Ions. Processes 2020, 8, 156. [Google Scholar] [CrossRef]
- Tamai, H.; Yoshida, T.; Sasaki, M.; Yasuda, H. Dye adsorption on mesoporous activated carbon fiber obtained from pitch containing yttrium complex. Carbon 1999, 37, 983–989. [Google Scholar] [CrossRef]
- Li, L.; Quinlivan, P.A.; Knappe, D.R.U. Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon 2002, 40, 2085–2100. [Google Scholar] [CrossRef]
- Lin, J.Q.; Yang, S.E.; Duan, J.M.; Wu, J.J.; Jin, L.Y.; Deng, Q.L. The Adsorption Mechanism of Modified Activated Carbon on Phenol. MATEC Web Conf. 2016, 67, 03040. [Google Scholar] [CrossRef]
- Feng, Y.; Tian, B.; Xia, B.; Cui, J.; Yang, M. Characterization of phenol ionization state in aqueous solution and transfer feature in electrodialysis. Chin. J. Environ. Eng. 2018, 12, 2466–2474. [Google Scholar]
- Tong, K.; Lin, A.; Ji, G.; Wang, D.; Wang, X. The effects of adsorbing organic pollutants from super heavy oil wastewater by lignite activated coke. J. Hazard. Mater. 2016, 308, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, N.; de Lannoy, C.-F. Upcycling wildfire-impacted boreal peats into porous carbons that efficiently remove phenolic micropollutants. J. Environ. Chem. Eng. 2021, 9, 105305. [Google Scholar] [CrossRef]
- Poddar, A.; Shetty, S.; Nagose, N. Removal of Methylene Blue from Waste Water Using Activated Carbon Prepared by Impregnating it with KOH and Cacl2. Int. J. Adv. Res. Sci. Commun. Technol. 2022, 5, 18. [Google Scholar]
- Arafat Hassan, A.; Franz Marcus Pinto Neville, G. Effect of Salt on the Mechanism of Adsorption of Aromatics on Activated Carbon. Langmuir 1999, 15, 5997–6003. [Google Scholar] [CrossRef]
- Shen, X.; Hussain, T.; Mitchek, M.; Wong, J.; Reible, D. Evaluating the Sorption Kinetics of Polychlorinated Biphenyls in Powdered and Granular Activated Carbon. Water Res. 2023, 236, 10. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, N.S.; Dahalan, F.A.; Zubir, A.A.A.; Hasan, M.; Ibrahim, N.; Lutpi, N.A.; Nazri, R.N.H.R.; Parmin, N.A. Comparative Study of Powdered Activated Carbon and Granular Activated Carbon in Metaldehyde Adsorption: Unraveling Isotherm and Kinetic Insights. Environ. Qual. Manag. 2024, 34, 1. [Google Scholar] [CrossRef]
- Oesterle, P.; Gallampois, C.; Jansson, S. Fate of trimethoprim, sulfamethoxazole and caffeine after hydrothermal regeneration of activated carbon. J. Clean. Prod. 2023, 429, 139477. [Google Scholar] [CrossRef]


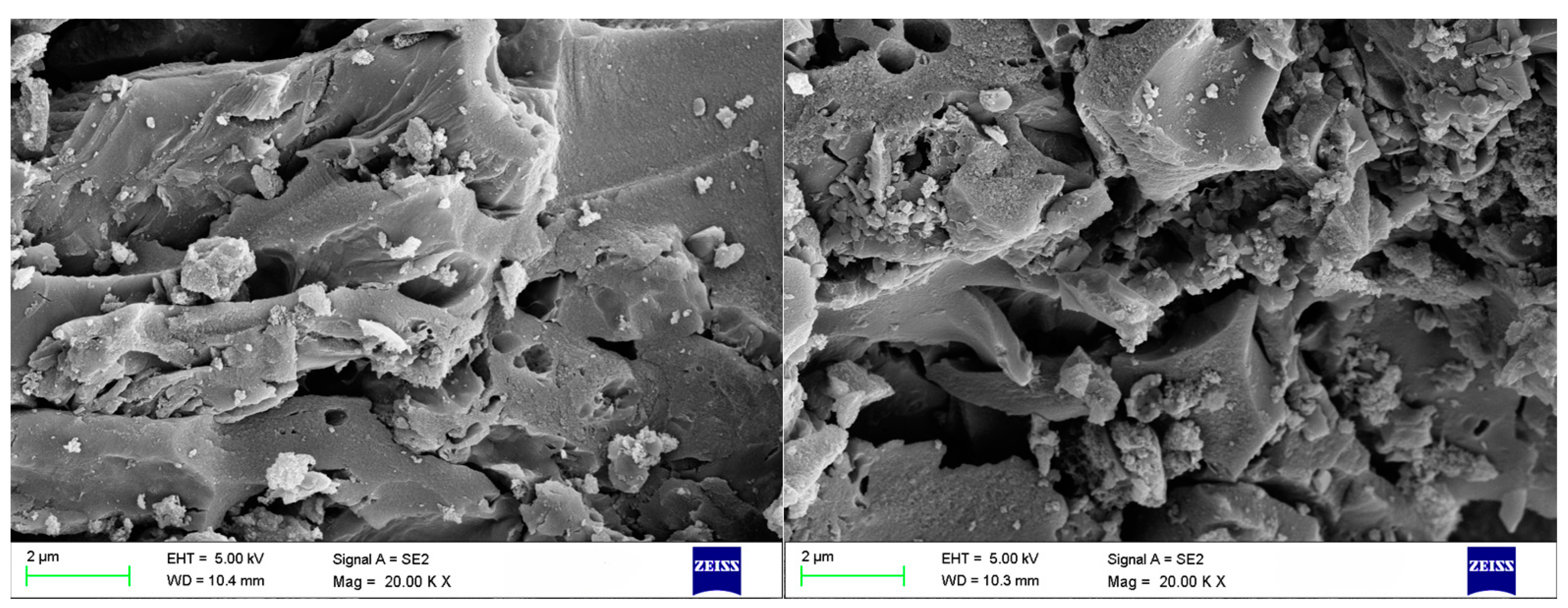
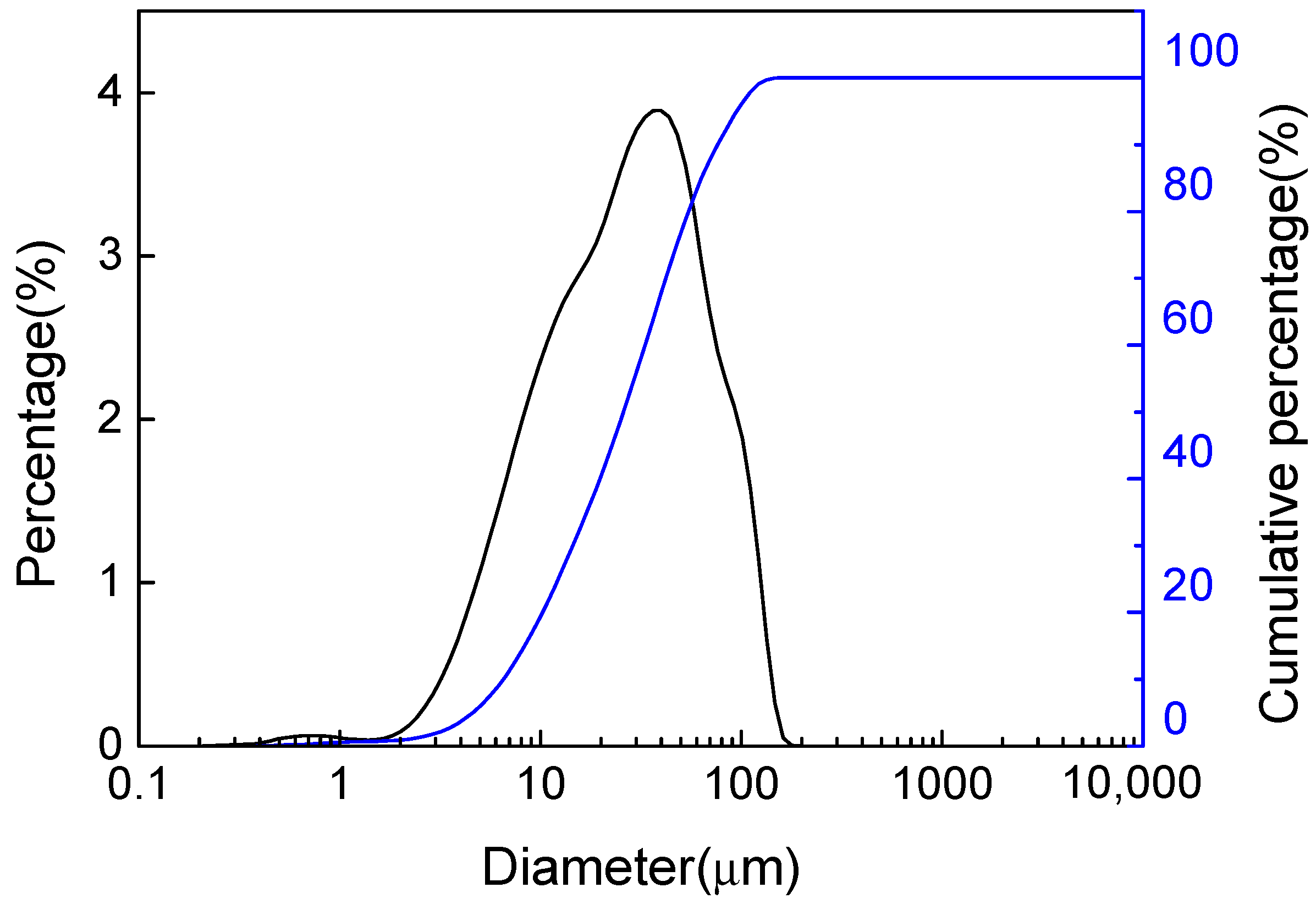

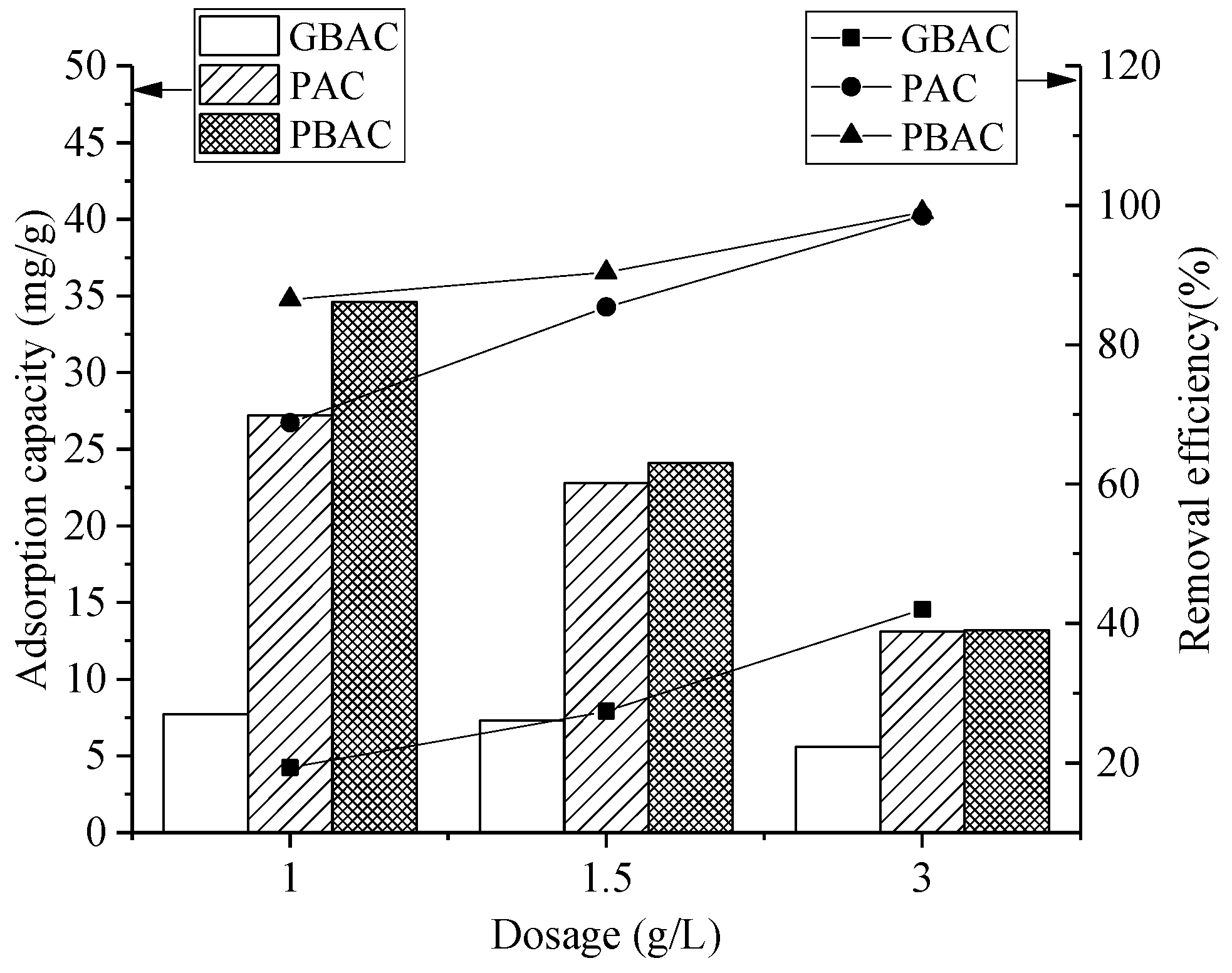
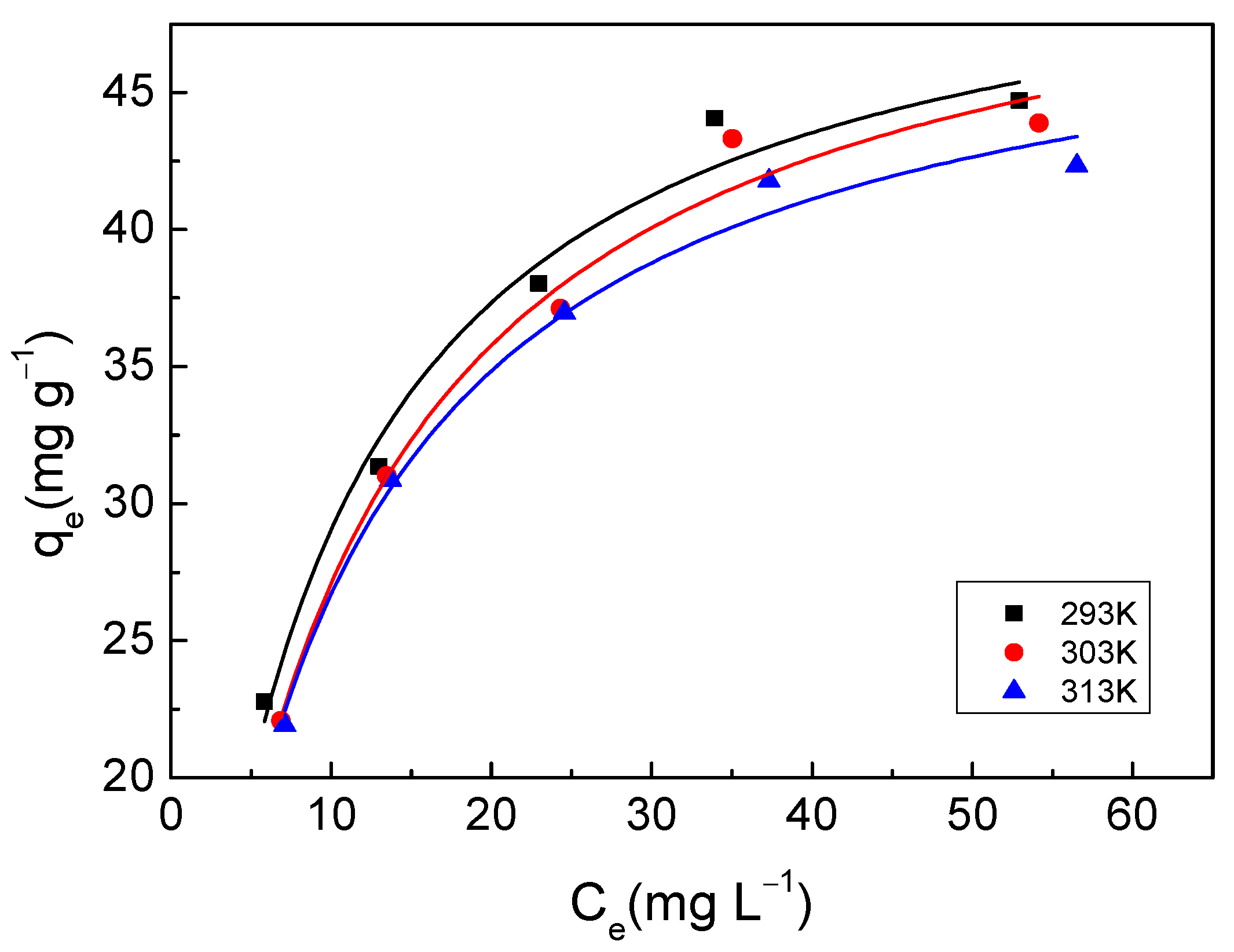

| Machine Name | Model | Manufacturer |
|---|---|---|
| Double beam UV/VIS spectrophotometer | TU1901 | Beijing General Instruments Co., Ltd. (Beijing, China) |
| Electronic balance | SQP | Sartorius Mechatronics T&H GmbH (Beijing, China) |
| Constant temperature magnetic stirring water bath | HH-2 | Changzhou Langyue Instrument Manufacturing Co., Ltd. (Changzhou, China) |
| Scanning electron microscope | SUPRATM55 | Carl Zeiss AG (Oberkochen, Germany) |
| pH meter | PHS-3 E | Shanghai INESA Analytical Instrument Co., Ltd. (Shanghai, China) |
| Laser diffraction analyzer | LS 13 320 XR | Beckman Instruments Inc. (Brea, CA, USA) |
| Fourier Transform Infrared Spectrometer | Nicolet 6700 | Thermo Fisher Scientific(Waltham, MA, USA) |
| Automatic Surface Area and Pore Size Analyzer | Autosorb-iQ | Quantachrome Instruments(Boynton Beach, FL, USA) |
| Absorbent | Raw | Particle Size (µm) | Manufacturer | Prices (USD/t) |
|---|---|---|---|---|
| PAC (powdered activated coke) | lignite coal | <61 μm | Laboratory of one-step preparation of activated coke at Shandong University (Jinan, China) | 487 |
| GBAC (granular biological activated carbon) | coconut shell | 2000~4000 | Shanghai Activated Carbon Factory Co. | 278 |
| PBAC (powdered biological activated carbon) | coconut shell | <61 μm | Shanghai Activated Carbon Factory Co. (Shanghai, China) | 1391 |
| Absorbent | BET Surface Area (m2/g) | Micropore Specific Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Average Pore (nm) |
|---|---|---|---|---|---|
| PAC | 514.413 | 41.292 | 0.600 | 0.015 | 4.822 |
| GBAC | 786.363 | 705.564 | 0.514 | 0.368 | 3.046 |
| PBAC | 987.264 | 910.281 | 0.702 | 0.466 | 2.332 |
| Temperature | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | Ka (L mg−1) | R2 | Kf ((mg g−1) (L mg−1)1/n) | n | R2 | |
| 293 K | 52.21 | 0.1254 | 0.9779 | 14.56 | 0.2963 | 0.9297 |
| 303 K | 52.66 | 0.1060 | 0.9803 | 13.36 | 0.3121 | 0.9148 |
| 313 K | 50.13 | 0.1139 | 0.9864 | 13.87 | 0.2908 | 0.8997 |
| Temperature | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (1 min−1) | R2 | qe (mg g−1) | K2 (g.(mg min)−1) | R2 | |
| 293 K | 21.91 | 1.821 | 0.9514 | 22.71 | 0.1409 | 0.9892 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Xu, H.; Chen, S.; Zhao, D. Performance of Powdered Activated Coke Produced by One-Step Rapid Process from Lignite: Phenol Adsorption from Synthetic Wastewater and Hydrothermal Regeneration. Water 2025, 17, 1161. https://doi.org/10.3390/w17081161
Chen G, Xu H, Chen S, Zhao D. Performance of Powdered Activated Coke Produced by One-Step Rapid Process from Lignite: Phenol Adsorption from Synthetic Wastewater and Hydrothermal Regeneration. Water. 2025; 17(8):1161. https://doi.org/10.3390/w17081161
Chicago/Turabian StyleChen, Guifang, Hao Xu, Shouyan Chen, and Dachuan Zhao. 2025. "Performance of Powdered Activated Coke Produced by One-Step Rapid Process from Lignite: Phenol Adsorption from Synthetic Wastewater and Hydrothermal Regeneration" Water 17, no. 8: 1161. https://doi.org/10.3390/w17081161
APA StyleChen, G., Xu, H., Chen, S., & Zhao, D. (2025). Performance of Powdered Activated Coke Produced by One-Step Rapid Process from Lignite: Phenol Adsorption from Synthetic Wastewater and Hydrothermal Regeneration. Water, 17(8), 1161. https://doi.org/10.3390/w17081161






