1. Introduction
Current demographic growth has a direct impact on the generation of domestic wastewater in developing countries, increasing risks to public health and the environment due to elevated nitrogen and phosphorus levels [
1]. Excessive amounts of these contaminants can lead to eutrophication in aquatic ecosystems, causing significant ecological imbalances [
2]. The use of microalgae in wastewater treatment emerges as a highly effective option, as their cultivation in nutrient-rich domestic effluents not only enables water decontamination [
3] but also contributes to reducing CO
2 concentration in the atmosphere, thereby helping mitigate greenhouse gases [
4]. There are many species of microalgae that stand out for their unique qualities, one of which is
Chlorella sp. due to its ubiquitous nature, easy adaptability to adverse conditions, and rapid growth. In this context, open pond systems involving microalgae–bacteria consortia emerge as a viable solution for wastewater treatment, especially in rural areas with technological and economic limitations. These systems offer advantages such as simplicity, low operational costs, and accessibility, making them ideal for these regions [
5].
Strengthening knowledge about consortium-based systems is crucial for the design, optimization, and scaling of these processes, aiming to ensure their accessibility and generate an impact on the development of sustainable systems and circular economies in rural populations. Facilitating access to pollutant removal technologies and waste utilization through the generation of usable biomass (such as biogas for renewable energy or biofertilizers that enhance local agriculture) also promotes efficient resource management and drives sustainable [
6] and decentralized development in these communities. The cultivation of microalgae requires rigorous control of various factors that influence the metabolic and reproductive functions of these microorganisms [
3]. Among the most relevant factors are carbon concentration, temperature, light intensity, available nutrients, and the specific type of microalgae used [
7]. Proper management of these variables is essential to maximize process efficiency and ensure optimal performance in microalgal biomass production [
8]. Unlike pure microalgae cultures, open ponds are characterized by hosting diverse microbial consortia, where consortial relationships, nutrient assimilation dynamics, and biomass generation processes are all complex [
9].
The use of microalgae for wastewater bioremediation has proven to be effective in removing key pollutants such as nitrogen and phosphorus, with removal rates ranging from 24% to 100% for nitrogen and 25% to 100% for phosphorus, depending on the type of wastewater and cultivation conditions. Additionally, microalgae have been shown to reduce COD levels by 23% to 95% when used in raw or primary wastewater treatment [
3]. The co-cultivation of microalgae and bacteria offers a promising alternative to conventional systems by leveraging synergistic interactions between microorganisms, reducing operational costs by utilizing oxygen produced through photosynthesis [
3]. However, challenges such as nutrient competition, mutual inhibition, and system scalability limit its widespread application, highlighting the need for innovative approaches to overcome these barriers.
The study by [
10] developed a kinetic model integrating the Droop function and Logistic equations to predict the growth, nutrient consumption, and CO
2 fixation of
Chlorella vulgaris. This model uniquely incorporated internal nitrogen and phosphorus quotas as key variables, enabling accurate predictions of algal growth behavior under varying CO
2 and NH
4-N concentrations, with correlation coefficients ranging from 0.68 to 0.97. The findings revealed high nutrient removal efficiencies (96.12–99.61% for nitrogen and phosphorus) and significant CO
2 fixation rates (up to 85.72 mg CO
2·L
−1·d
−1) under optimized conditions. Despite its robust theoretical framework and promising results, this study focused solely on monocultures of
C. vulgaris and did not explore algal–bacterial consortia or their potential synergies in wastewater treatment systems.
The use of microalgae–bacteria consortia for wastewater treatment has proven to be an efficient and sustainable strategy, highlighted by their ability to remove nutrients and produce biomass. Studies such as [
11] have explored the dynamics between microalgae and bacteria, analyzing the impact of physicochemical parameters under controlled conditions, including light intensity, nutrient loads, and N:P ratios. Although significant advances have been made, such as the proposal of simple monitoring tools like dissolved oxygen and electrical conductivity for evaluating system performance, challenges remain in hydrodynamic modeling, light attenuation, and gas exchange. Furthermore, experimental validation in real-world conditions is necessary. These gaps present opportunities for improving the design and operation of microalgae–bacteria systems, enhancing their efficiency for industrial applications in diverse environments.
Experimental studies and the use and development of mathematical models play a crucial role in understanding, designing, scaling, and optimizing processes, which are key factors for the implementation and improvement of technologies. In the field of wastewater treatment, these models provide valuable insights into system dynamics, allowing for the prediction of pollutant removal efficiencies and the identification of optimal operating conditions.
Numerous studies and research efforts have been conducted on wastewater treatment, the use of microalgae–microbial consortia, and mathematical models applied to these processes. The integration of these approaches has gained increasing attention due to their potential for efficient nutrient removal and biomass generation.
The study conducted by [
12] presents an innovative approach to simultaneously model the removal of organic matter, ammonium, and phosphate in algal–bacterial consortia, integrating the ASM NO.3 model with a refined algal kinetic model (ASM-A). The findings reveal that phosphate-accumulating organisms (PAOs) and nitrifying bacteria are responsible for over 80% of phosphate removal and 60% of ammonium removal under varying aeration conditions, while algae play a minor role in nutrient uptake. The model illustrates the influence of alkalinity on microbial activity, demonstrating enhanced nutrient removal at an alkalinity level of 1 mol/m
3 (100 mg/L CaCO
3). It also highlights the significance of light intensity and biomass concentration in optimizing reactor performance, recommending low biomass levels to achieve efficient light utilization under artificial illumination. This work provides a robust theoretical framework for optimizing algal–bacterial reactors and addresses challenges associated with microbial interactions and industrial scalability.
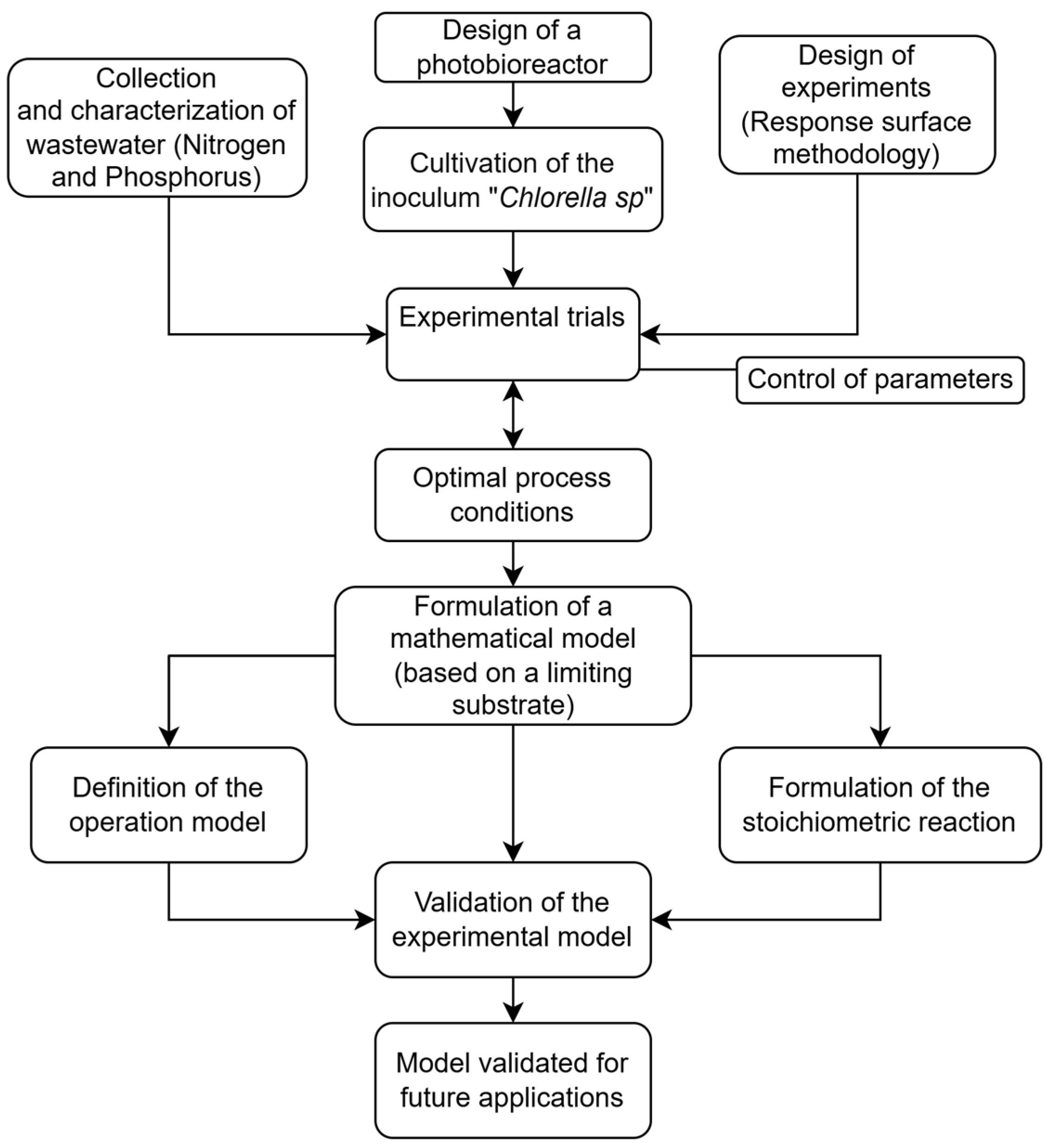
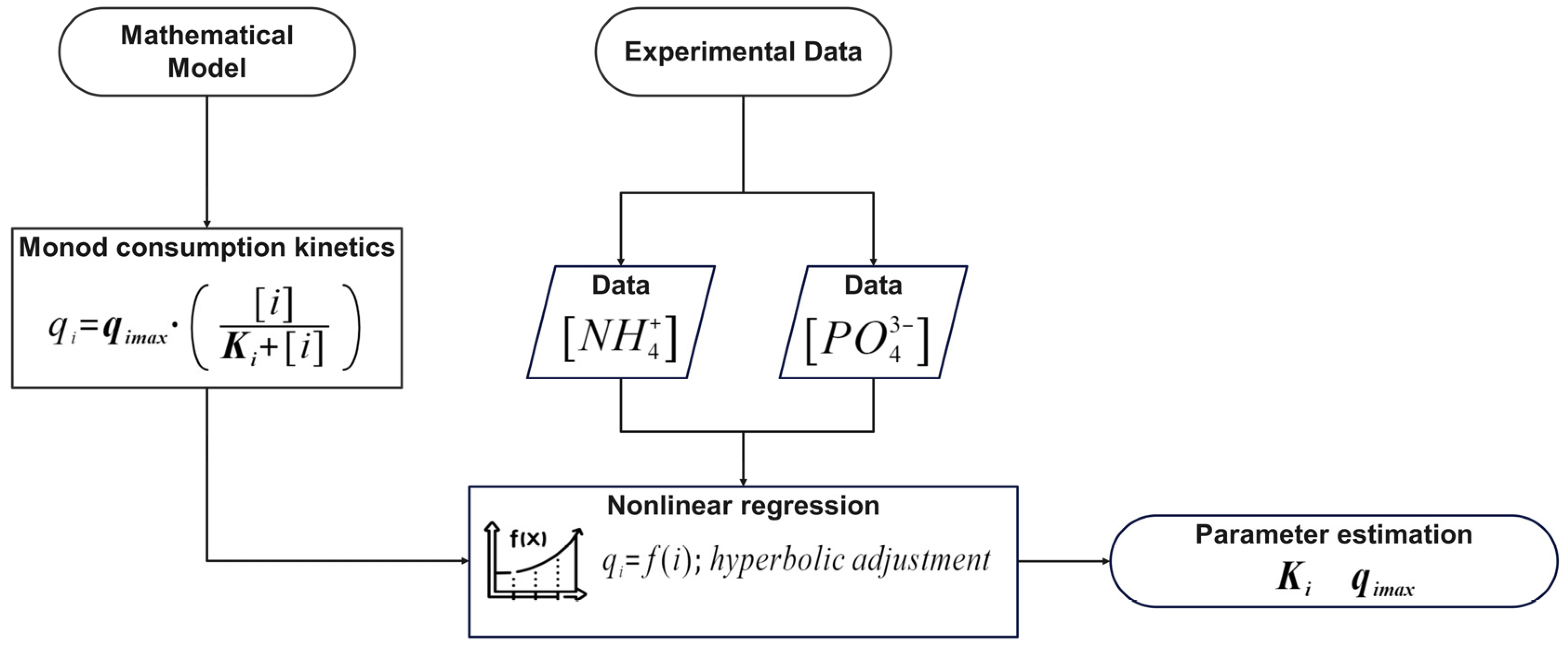
To better understand the dynamics of contaminant assimilation and biomass generation by microalgae–bacteria consortia present in municipal wastewater from open treatment ponds in rural areas of Huanta-Ayacucho, Peru, a study with an experimental design based on the response surface methodology was conducted. This approach allowed the identification of key variables and optimal conditions associated with the cultivation process. Subsequently, a mathematical model was developed to describe the assimilation of nitrogen in the form of ammonium ions (NH
4+), phosphorus in the form of phosphate ions (PO
43−), and microalgal biomass generation through the formulation of independent systems [
12] based on the concept of a limiting substrate.
4. Discussion
In the study on the factors affecting the growth of
Chlorella sp., the graphs and statistical analysis confirmed that cultures exposed to higher light intensity rapidly increased their biomass concentration. This was evidenced by the rise in absorbance levels and coloration, which simultaneously reflected accelerated increases in pH and temperature. The high growth rates of microalgae led to a rapid pH increase, causing additional effects, such as the volatilization of certain nutrients, including nitrogen [
16]. In fact, if the pH increases excessively, the culture becomes inhibited and loses its ability to grow progressively. Therefore, it is necessary to constantly regulate this control parameter by adding carbon dioxide (CO
2), whose concentration is directly proportional to the pH of the medium. According to the findings of [
16], a CO
2 concentration of 10%
v/
v resulted in pH values between 8 and 9, while 17.5%
v/
v CO
2 maintained pH levels between 7 and 8, and 25%
v/
v CO
2 led to pH values between 6 and 7.
Under optimal conditions, similar to those described in study [
16], our experiment maintained a pH range of 6 to 7 with CO
2 injections ranging from 20% to 25%
v/
v. This approach resulted in notable biomass growth and achieved nitrogen and phosphorus removal efficiencies exceeding 85%. These results conclude that the addition of the optimal amount of CO
2 promotes the growth of microalgae by providing favorable pH conditions in the medium, thus improving the biomass production and nitrogen assimilation by the consortium. It is important to mention that the addition of concentrations greater than 30% of CO
2 and continuous flow generates a negative effect on the culture medium since it greatly reduces the pH, destabilizing it immediately and inhibiting the growth of microorganisms.
A study by [
43] highlighted the impact of carbon sources and nutrient concentrations on the biomass and carbohydrate productivity of
A. platensis. The results demonstrated that supplementing the culture medium with NaHCO
3 concentrations up to 16 g L
−1 significantly enhanced biomass productivity. However, further increasing NaHCO
3 concentration did not enhance biomass growth but instead led to its depletion. It was found that 9.8 g L
−1 of NaHCO
3 was optimal for promoting biomass and carbohydrate accumulation. It was also observed that pH is directly affected by the concentration of bicarbonates supplied in the medium. Indeed, this behavior was also observed by intermittently injecting 25% of gaseous CO2 directly into the culture medium (30 mg/L of aqueous carbonic acid), managing the maintenance of a pH level of 6 to 7 to obtain the best percentages of nutrient removal. Continuing with the report, the mentioned author indicates that two cyanobacteria strains,
Lyngbya limnetica and
Oscillatoria obscura, were cultivated at pH 9.0, producing a maximum biomass of 1196 mg L
−1 and 1226 mg L
−1, with a carbohydrate content of 219 mg g
−1 and 192 mg g
−1, respectively. In addition to bicarbonates, the direct addition of CO
2 and the use of other carbon sources, such as pentose and sucrose, have proven to be beneficial for enhancing carbohydrate accumulation. However, there are also cases where bicarbonate addition improved both protein and carbohydrate production.
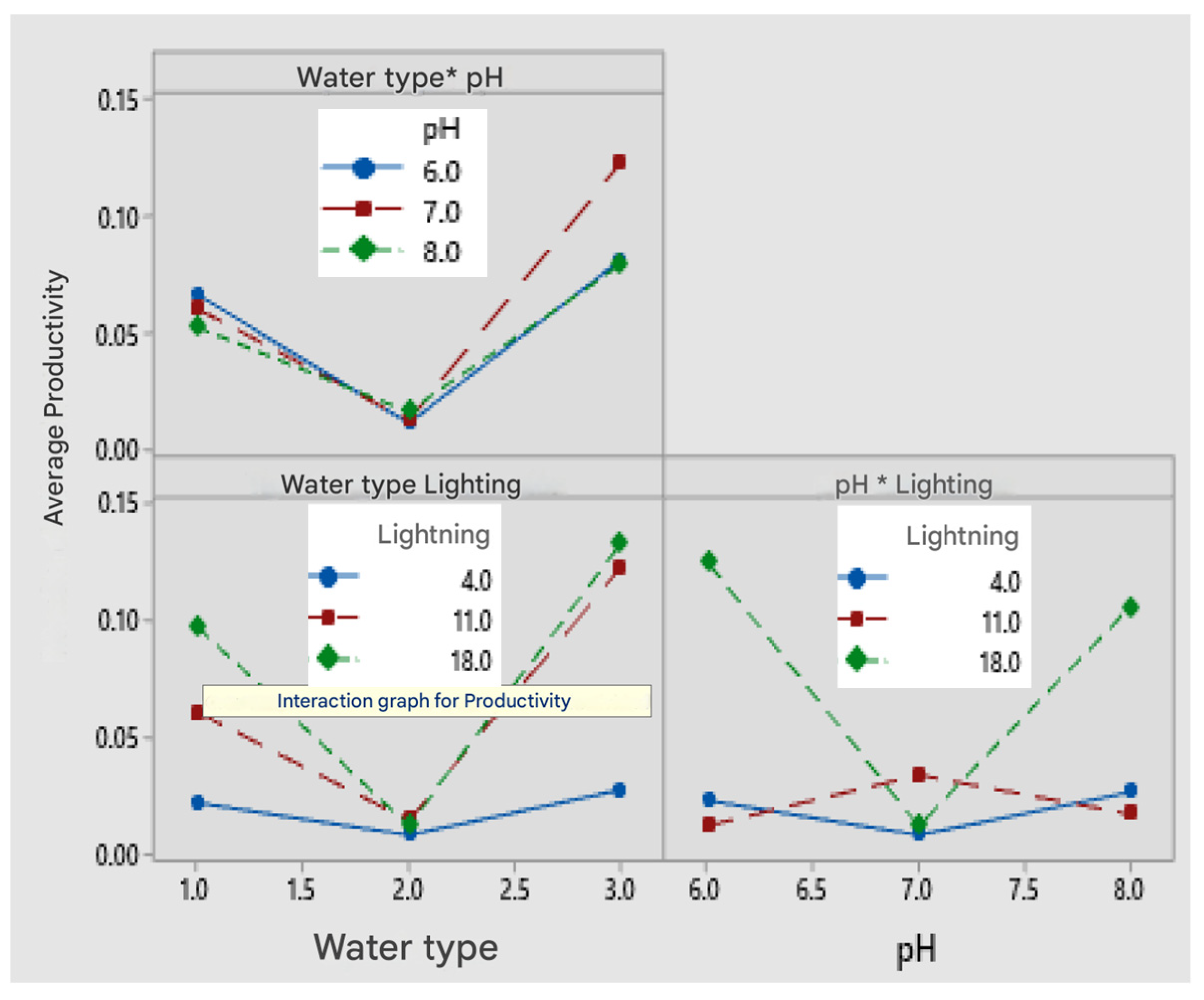
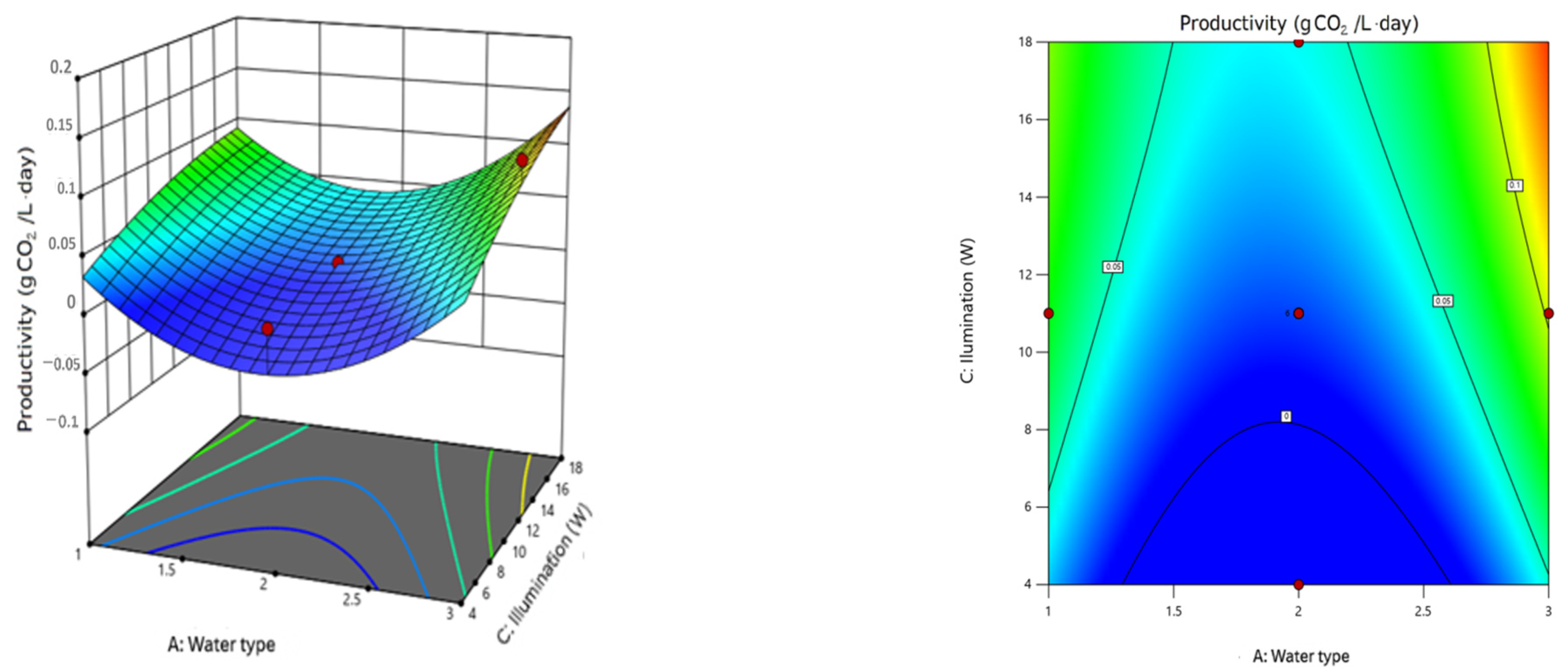
On the other hand, light intensity plays a crucial role in the growth of microalgae; these microorganisms require a sufficient amount of light energy to perform photosynthesis. However, excessive light exposure can also inhibit growth due to overexposure. Our results were consistent with the theory regarding the factors with the greatest influence on microalgae growth. The factors of greatest influence reported in this study are light intensity and culture medium type, while pH has a lesser impact. The graphs and statistical analysis confirmed that cultures exposed to higher light intensity rapidly increased their biomass concentration. En nuetro estudio se probaron 3 intensidades de luz 4μmolm
−2 s
−1, 11μmolm
−2 s
−1y 18μmolm
−2 s
−1, durante la experimentacion se verificó que a medida que las intesidades aumentaban los cultivos tambien incrementaban su población y su porcetaje de remocion de nuetrientes, siendo 18 μmolm
−2 s
−1 la intensidad optima para el ensayo. Similar to this behavior, the author of [
29] reported that the rate of biomass growth and the rate of CO
2 consumption increased with increasing light intensity over the crop. In his study, he found that the optimal intensity for chlorella vulgaris was 150 ± 3 μmolm
−2 s
−1.
In a study carried out by [
44] the feasibility of the cultivation of the microalgae
Scenedesmus dimorphus and
Chlorella pyrenoidosa was evaluated, both separately and in combination with the plant growth-promoting bacterium
Azospirillum brasilense, using mixtures of Cheese Acid Whey (SAQ) and Beef Slurry (PV). The results showed that
Scenedesmus dimorphus achieved the highest removal of nitrogenous inorganic compounds, being able to grow in a mixture of 50% of each of these effluents (50% PV-50% SAQ) and removing 90% of P-PO4 and 70% of N-NH4, resulting in a biomass productivity of 0.41 ± 0.03 g L
−1 d
−1 and maintaining its dominance in cultivation. Likewise [
44], our study revealed that
Chlorella sp. exhibited predominance in the culture medium, showing continuous growth and reaching a biomass productivity of 0.22 g L
−1 d
−1 and removal percentages of 94% of P-PO4 and 87% of N-NH4. From this comparison, it can be deduced that the microalgae
Scenedesmus dimorphus and
Chlorella sp. exhibit similar behaviors when cultured in consortium with other bacteria, both showing a notable preference for nitrogen consumption and reaching significant levels of productivity.
The results obtained in this study demonstrated nitrogen and phosphorus removal efficiencies of 87.16% and 94.43%, respectively, which align closely with the values reported by [
11] (92.2% for nitrogen and 71.8–82.4% for phosphorus). Discrepancies between the results can be attributed to differences in the experimental conditions and methodological approaches employed. In terms of biomass production, this study optimized growth conditions through controlled variables such as light intensity and culture medium, resulting in a more comprehensive predictive capability compared to the production rates of 7.1–14.9 mg L
−1 d
−1 reported by [
11]. While the authors of [
11] relied on physicochemical parameters such as dissolved oxygen and electrical conductivity to monitor system performance in real time, the current study expanded the methodological approach by incorporating a mathematical model to predict nutrient removal dynamics and evaluate system behavior under optimized conditions. These methodological distinctions underscore the complementary approaches used to advance the understanding of microalgae–bacteria consortia for sustainable wastewater treatment.
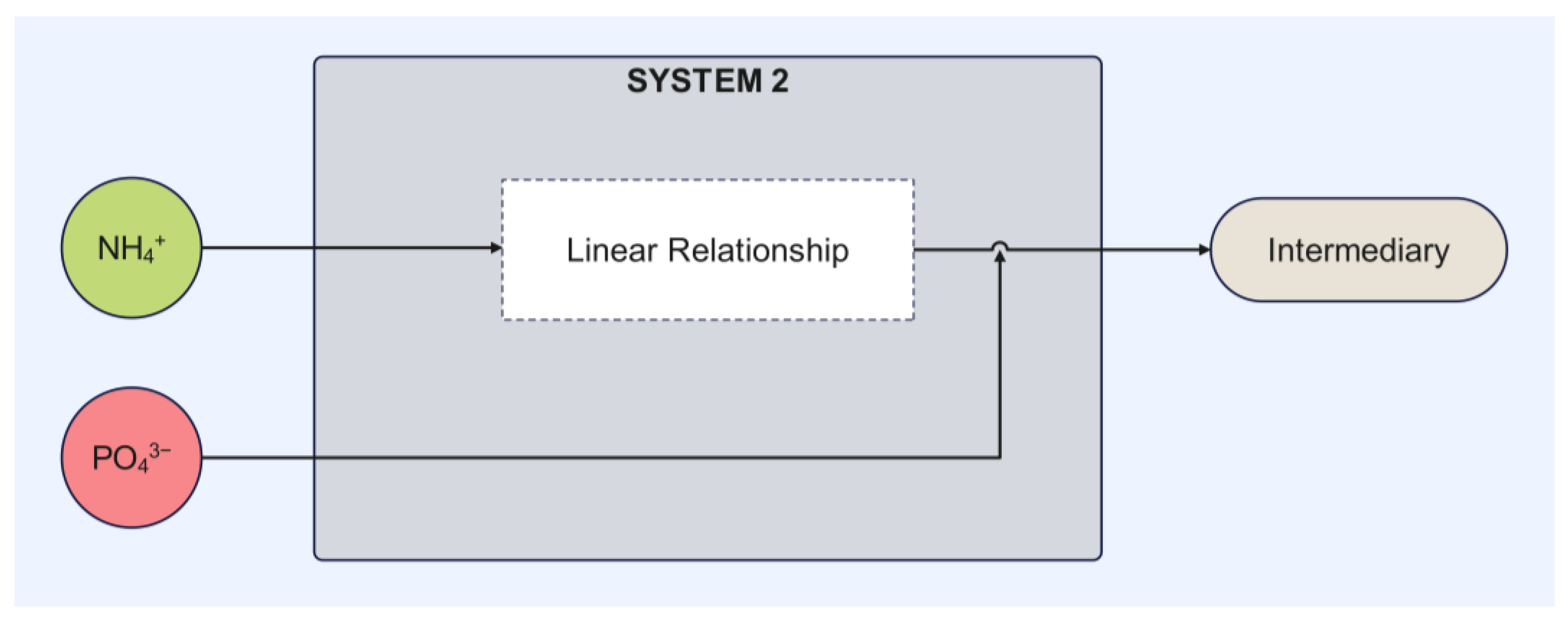
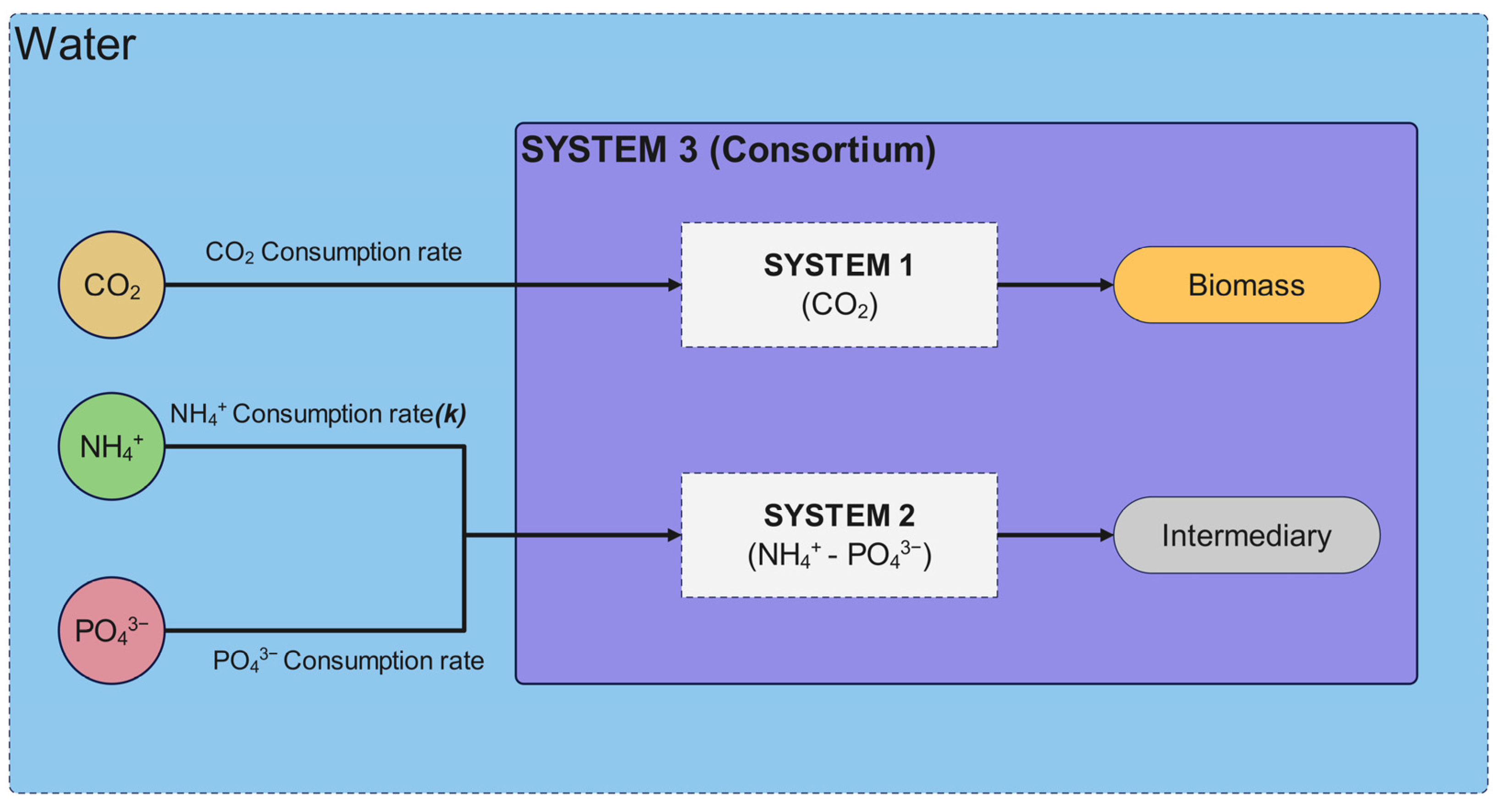
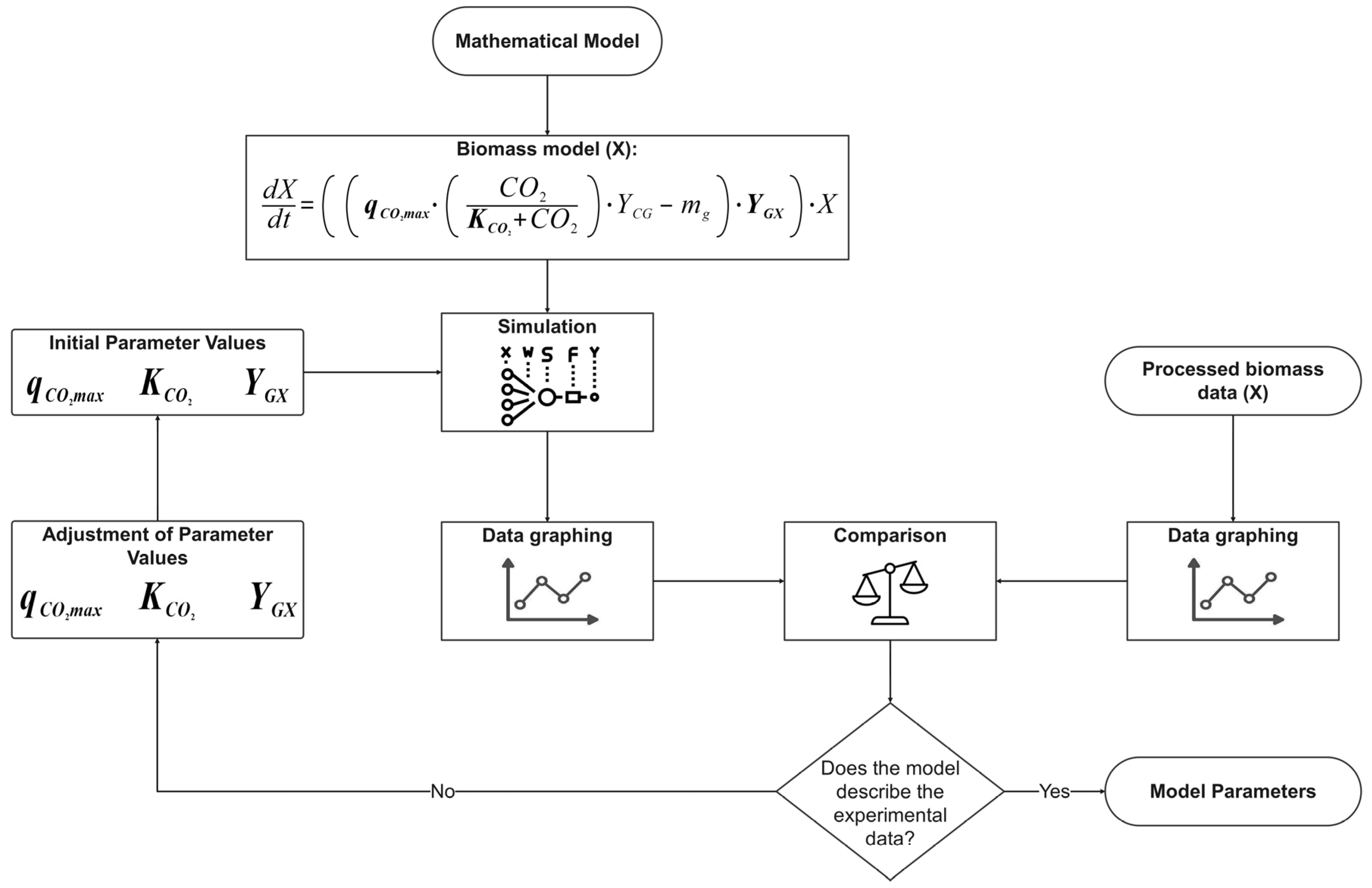
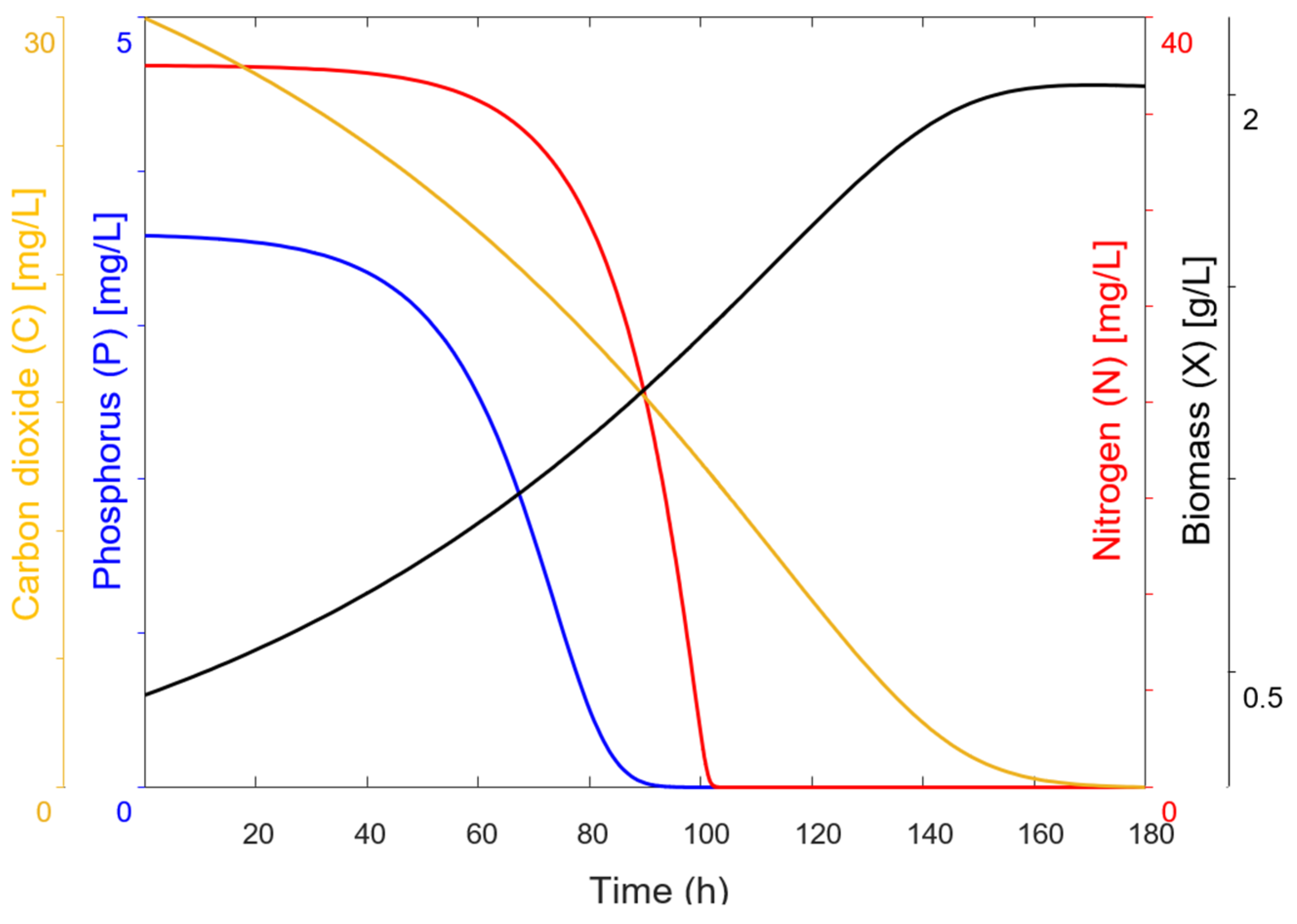
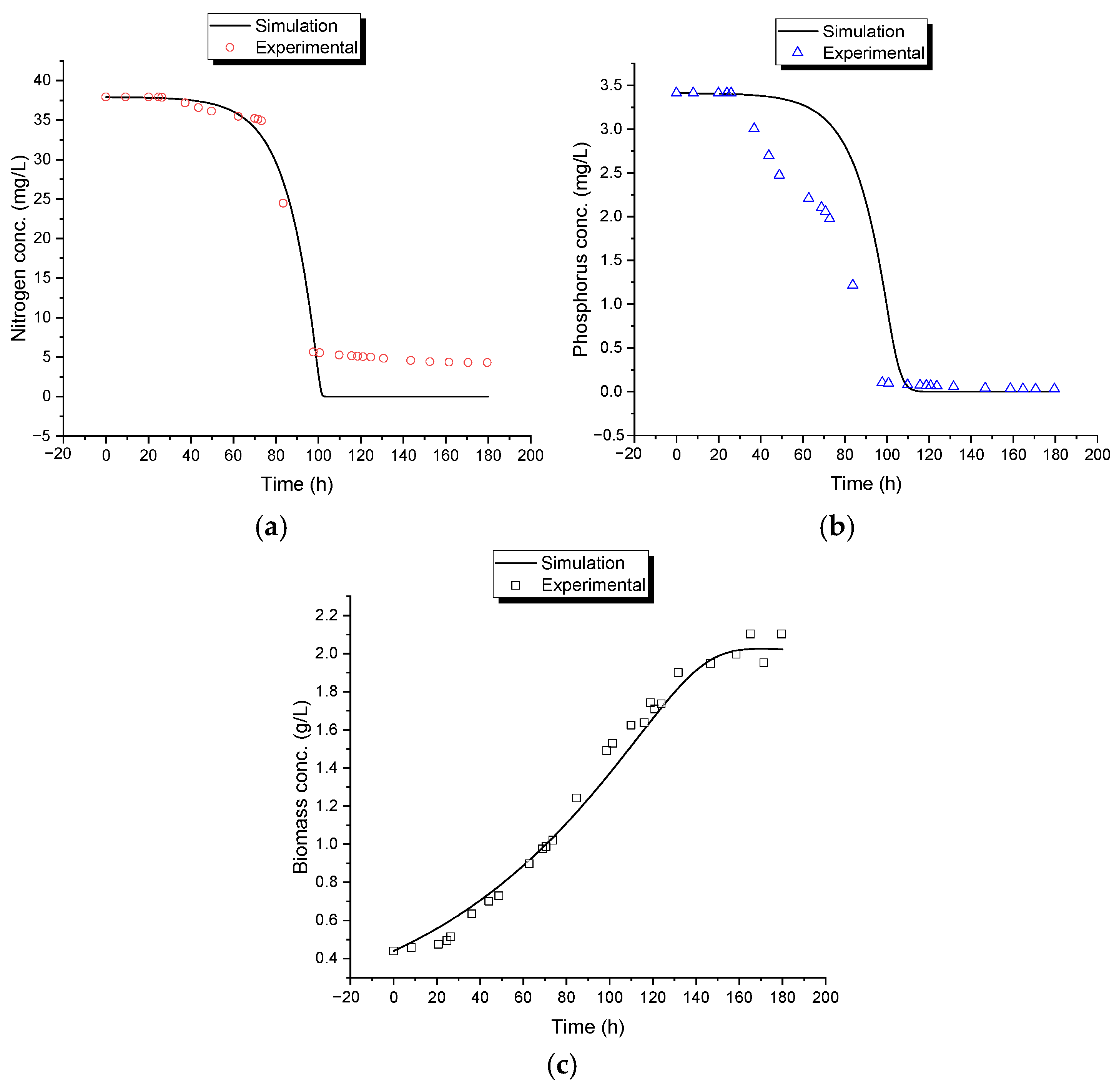
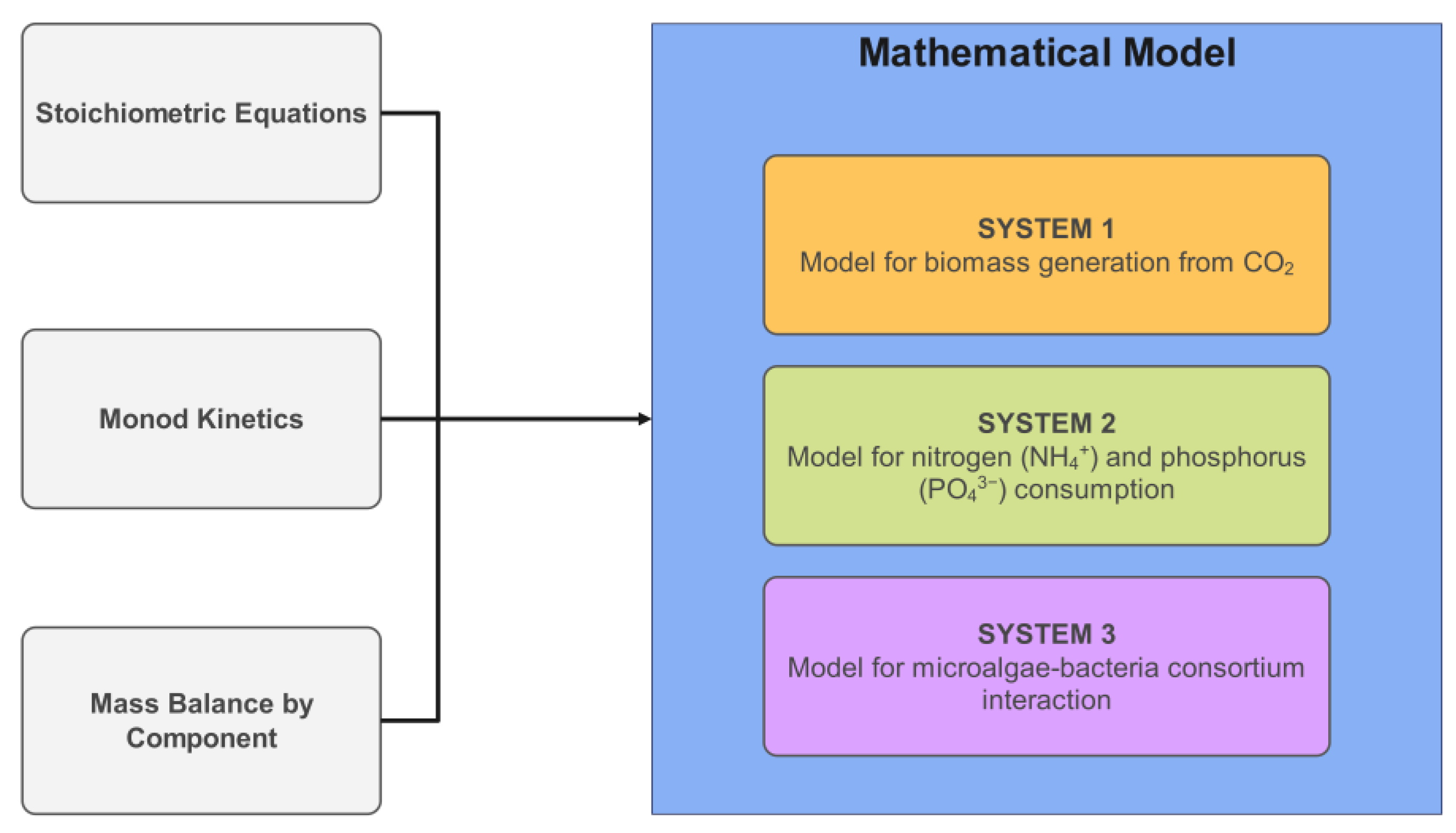
The mathematical model proposed in this study was structured into two independent systems to analyze biomass generation and contaminant removal. System 1, which describes the conversion of CO
2 into biomass, exhibited high precision in its experimental behavior, with a standard error of 0.07186. The model predicted a biomass concentration of 2.02 g/L, closely matching the 1.95 g/L obtained experimentally. System 2, which describes the assimilation or consumption of nitrogen and phosphorus based on the assumption of an intermediate, exhibited a lower degree of correlation compared to System 1. The phosphorus model was the second most accurate, with a standard error of estimation of 0.63065, while the nitrogen model was the least accurate, with a standard error of estimation of 3.40285. Graphically, the discrepancies between the mathematical model and the experimental data were confirmed in the behavior of these variables, particularly in the final phase of the assimilation process, with greater differences observed for nitrogen. The final values obtained for these variables also support these discrepancies, as seen in the differences in removal percentages between the simulated and experimental data (
Table 9). The experimental results showed that both contaminants were not fully consumed, leaving residual traces of 4.88 mg/L of nitrogen and 0.19 mg/L of phosphorus, whereas the model predicted complete removal (0.00 mg/L for both variables), achieving 100% theoretical removal efficiency.
The fact that phosphorus was almost completely consumed, while nitrogen was not, suggests two possible hypotheses: (1) nitrogen may not be acting as a limiting substrate, or (2) other unidentified factors and variables may be influencing the assimilation of both contaminants.
The second hypothesis is supported by studies from various authors who reported removal percentages similar to those in this study. These studies indicate that the removal of these contaminants is influenced by multiple factors, such as ammonium availability in the medium [
10,
12]; CO
2 availability, which stimulates microalgal metabolism [
10]; dissolved oxygen levels and the presence of other microorganisms, such as PAOs (polyphosphate-accumulating organisms), which play a role in phosphorus removal [
12]; and alkalinity levels, which favor phosphorus removal but also enhance ammonium [
12].
It is important to note that the conditions for comparing the results of this study with those obtained by [
10] are limited due to several factors, including the different microalgae species used in both studies, the differences in water type and conditions, and most importantly, the microalgae–bacteria consortial relationships, which [
10] did not include in their study.
Analyzing Hypothesis (2), it is known that when ammonium concentration is reduced too much, both microalgae and bacteria struggle to efficiently sustain phosphorus removal, preventing its complete [
12]. In this study, despite achieving 94.43% phosphorus removal, the presence of residual nitrogen (87.16% ammonium removal) suggests that ammonium availability was sufficient to allow phosphorus uptake. This finding reinforces Hypothesis (2)—although phosphorus is removed in higher proportions, its elimination is not entirely autonomous but rather depends on the sufficient presence of ammonium in the system and other factors that require further investigation.
We would not rule out Hypothesis (1), which considers the possibility that phosphorus could also act as a limiting substrate under certain conditions, especially given that this study was conducted in a consortium where other microorganisms, such as PAOs [
12], may be present. The interactions of these organisms within the consortium were not considered in this study. In wastewater treatment systems, phosphorus is typically present in lower concentrations than nitrogen, which could influence its availability for biomass assimilation. Mathematically, under the experimental conditions, using phosphorus as a limiting factor for intermediate formation would, in turn, condition nitrogen assimilation. This hypothesis could be explored by incorporating a stoichiometric relationship that considers phosphorus, nitrogen, and bacterial biomass formation. Evaluating this scenario would allow a comparison between both conditions and help determine under what circumstances phosphorus might restrict nitrogen removal.
Despite the discussed discrepancies, the consortial model proposed in this study, structured as two independent systems, aligns with the independent models proposed by [
12] and accurately describes the experimental data with a high degree of precision.
It can be interpreted that the simulated model predicts optimal removal under ideal conditions; however, in reality, limitations imposed by alkalinity, oxygen availability, CO2 availability, and interactions with other microorganisms may affect nutrient removal efficiency.
Given the observed interactions in the system, there is a possibility of reformulating the model into three independent systems, allowing for a more precise representation of nutrient removal dynamics: System 1 would describe microalgae growth (CO
2 → Biomass), System 2 would represent the nitrification process by bacteria (NH
4+ → NO
3−), and System 3 would account for phosphorus removal mediated by PAOs (PO
43− → Polyphosphates) [
12]. The model reformulation should also include a stoichiometric equation that links nitrogen and phosphorus removal (considering PAO metabolism and nitrifying bacteria activity) and account for the dynamics of an open aqueous system, including gas exchange, the evaporation rate, and the equilibrium of key species such as carbon dioxide and carbonic acid, which influence alkalinity and pH regulation.
This revised approach would allow for a more accurate representation of the interdependence between nitrogen and phosphorus removal, considering the differentiated influence of each process and the role of alkalinity in their regulation.
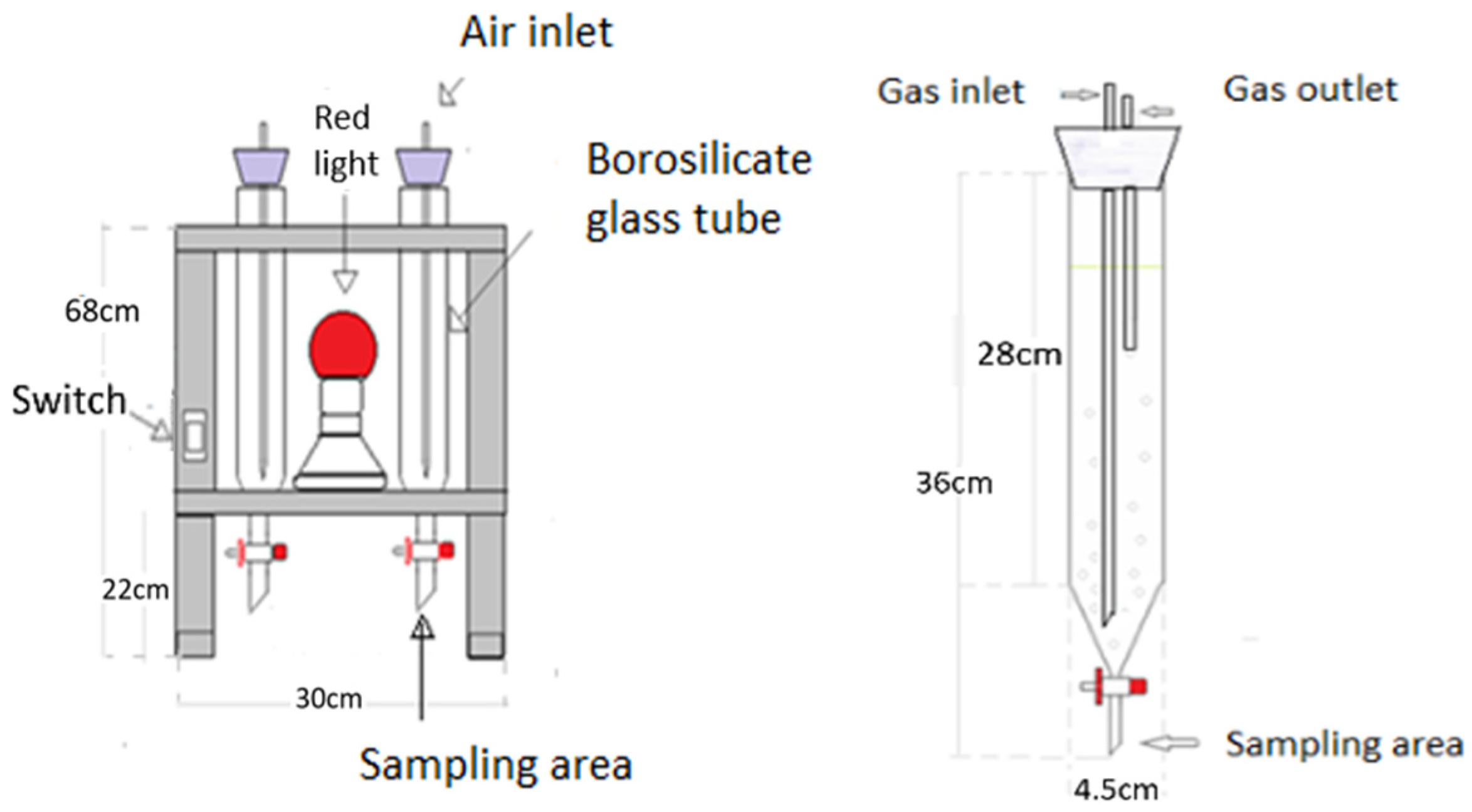
From an economic and practical point of view, the proposed approach is based on open pond technology, which is already implemented and operational in various regions. Therefore, its practical viability is supported by existing infrastructure. The main additional requirements for its application include a process for the inoculation of Chlorella sp. and the establishment of appropriate monitoring and control methods to ensure process stability and efficiency. While a detailed economic assessment was not conducted, the use of an already established system suggests that implementation costs could be minimized by leveraging existing facilities. However, further evaluation is needed to optimize scalability and operational feasibility, particularly in terms of biomass productivity, nutrient removal efficiency, and long-term system performance under real-world conditions.
5. Conclusions
The key influencing factors identified through the response surface analysis were light intensity, with an optimal value of 18 μmolm−2 s−1, and the culture medium type, which was municipal wastewater. Both factors had p-values below 0.05, indicating a significant influence on biomass growth, with light intensity having a stronger effect than the culture medium type. Additionally, the controlled complementary parameters in this study included 20–25% v/v intermittent CO2, a pH range of 6–7, and an aeration rate of 1 L/min.
The experiments in the photobioreactor showed a removal of 94.43% of phosphorus and 87.16% of nitrogen, while the model simulations predicted a 100% removal for both nutrients. This indicates that the model can adequately describe the system’s dynamics, although under real conditions, differences in removal efficiency may exist.
The differentiation of the process into two independent systems—where biomass generation from CO
2 occurs separately from the assimilation of nitrogen and phosphorus through the formation of a theoretical intermediate —represents a key contribution of this study. This separation allows for a more precise description of the consortium’s dynamics, as supported by [
12], which notes that CO
2 uptake by microalgae and nutrient removal are not always coupled.
In this context, the nitrogen consumption rate by bacteria (kqN), representing 90% of the total consortium consumption (qN), directly influences the formation of the intermediate through the introduced factor k. This intermediate, in turn, affects phosphorus removal according to the proposed model. The model established a coupling relationship between nitrogen and phosphorus removal, where phosphorus assimilation depended on the availability of the intermediate generated from nitrogen. Phosphorus variation over time was not only directly linked to its concentration but also to the prior transformation of nitrogen to intermediate within the system.
The model’s fit to experimental data showed some deviations, particularly in nitrogen prediction. These differences may be attributed to the consideration of nitrogen as the limiting reactant for intermediate formation in System 2. The initial concentration of nitrogen was 11 times greater than the initial concentration of phosphorous; therefore, considering that it was also the limiting substrate, mathematically, the remotion of phosphorus was expected to be 100%
From an operational perspective, these findings suggest that optimizing the nitrogen-to-phosphorus ratio in the system could enhance process efficiency. However, due to the complexity of microalgae–bacteria interactions, a more detailed assessment is needed to optimize the model’s scalability and operational feasibility for real-world applications.
There are two hypotheses that should be tested in future studies: one is that nitrogen acted as a limiting substrate, but its removal—and that of phosphorus—was influenced by other factors; the other is that, under certain conditions, phosphorus could also act as a limiting substrate.
Future research should focus on the following:
Considering the hypothesis of using phosphorus as a limiting substrate for modeling.
Taking into account other factors, possibly environmental, that have affected the contaminant assimilation process.
Expanding the model to incorporate phenomena, such as gas exchange with the environment, energy balances that include irradiation as an important environmental factor in open systems, and the interaction of carbonic acid and CO2 as aqueous-phase species and their effect on the system.
Coupling the stoichiometry of nitrifying bacteria and PAOs with the CO2 generation of these species and their impact on microalgal biomass.