Effects of Humus and Solidification Agents on the Solidification/Stabilization Process of Organic-Rich River Sludge: Characteristics of the Stabilized Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. S/S Treatment of Sludge
2.3. Analytical Methods
2.3.1. Physical and Chemical Properties
2.3.2. Mechanical Properties
2.3.3. Extraction of FA and HA
2.3.4. Characterizations of Microscopic Properties
3. Results and Discussion
3.1. Subsection
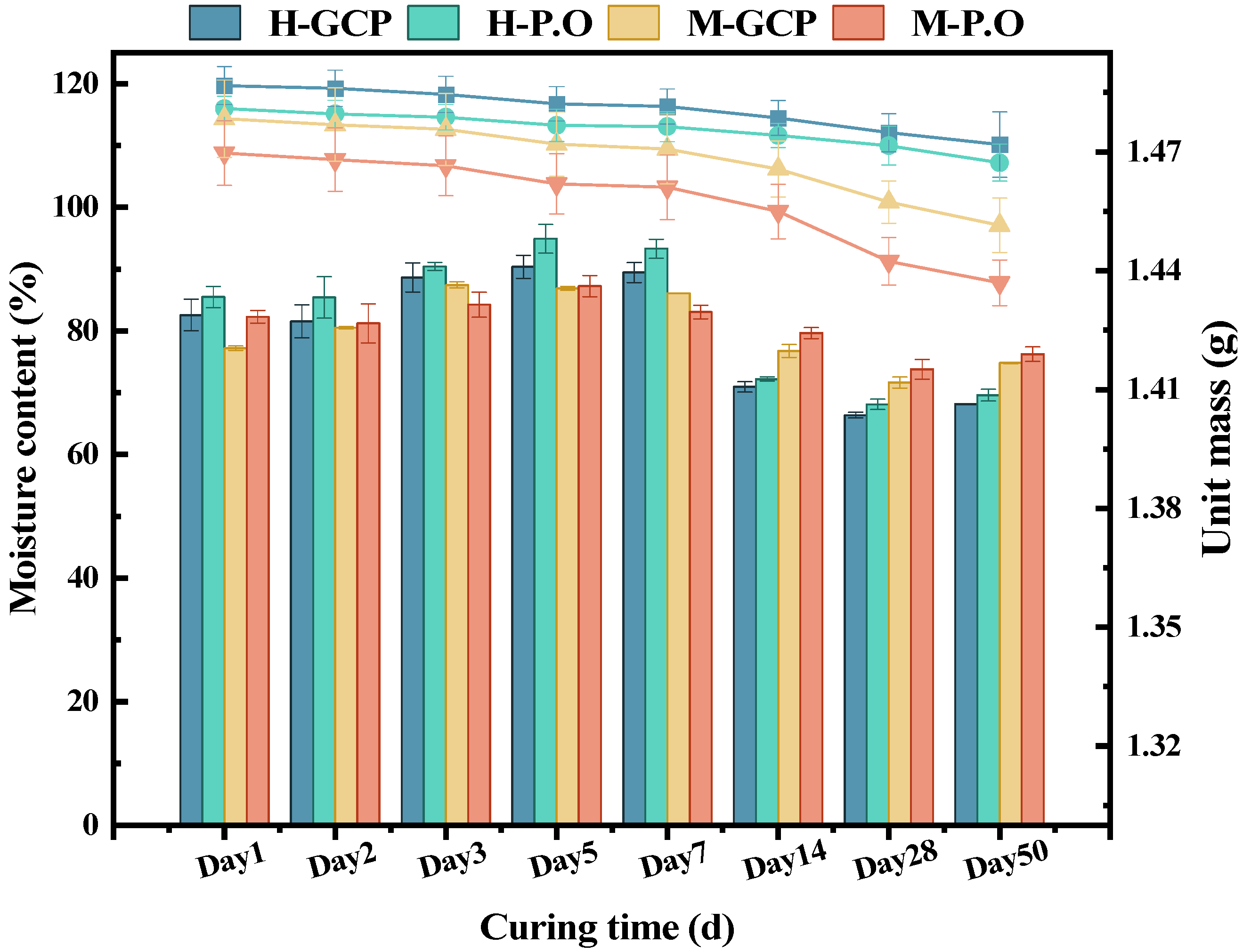
3.2. Water Content and Mass Change of Solidified Sludge
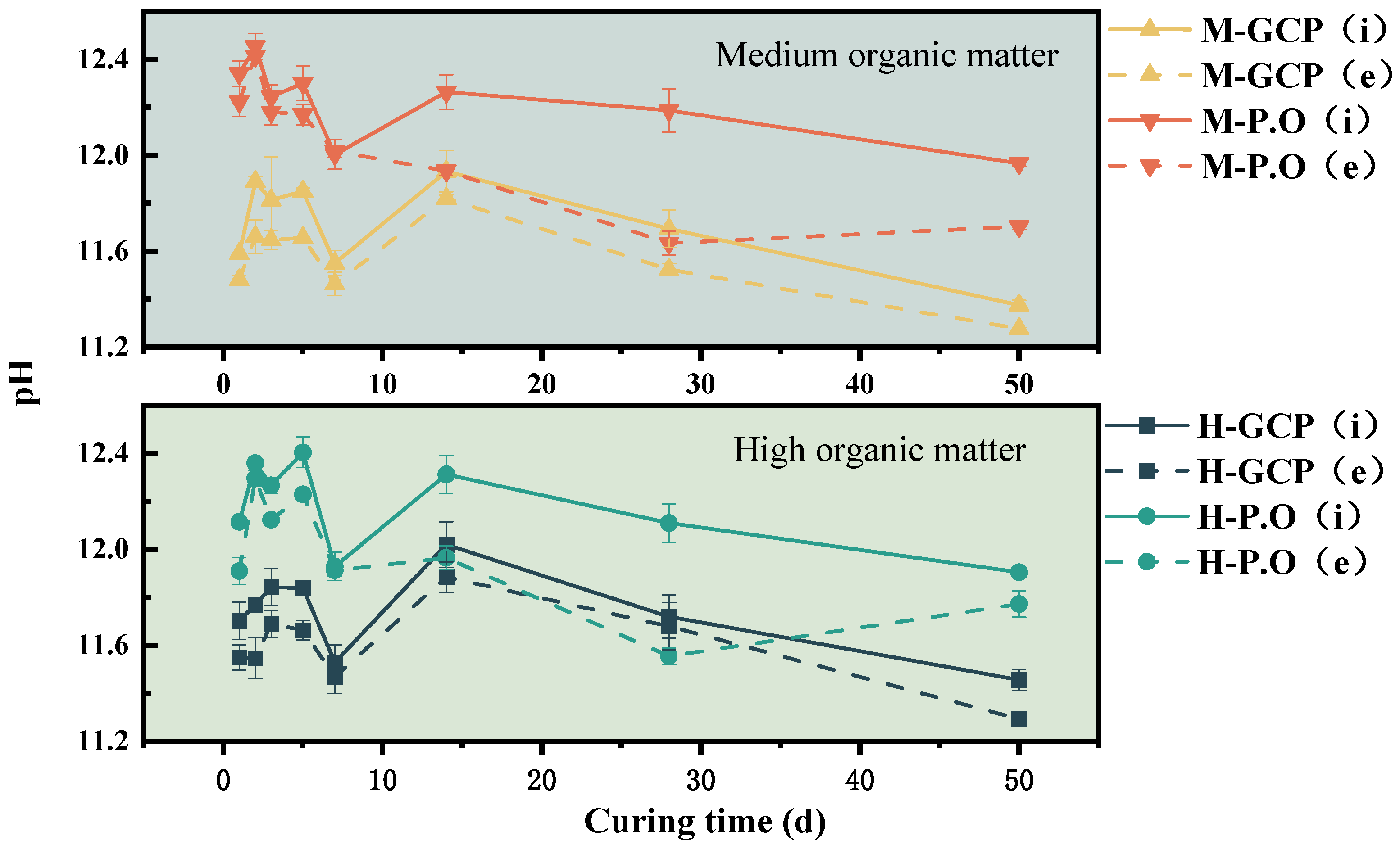
3.3. Internal and External pH of the Solidified Sludge
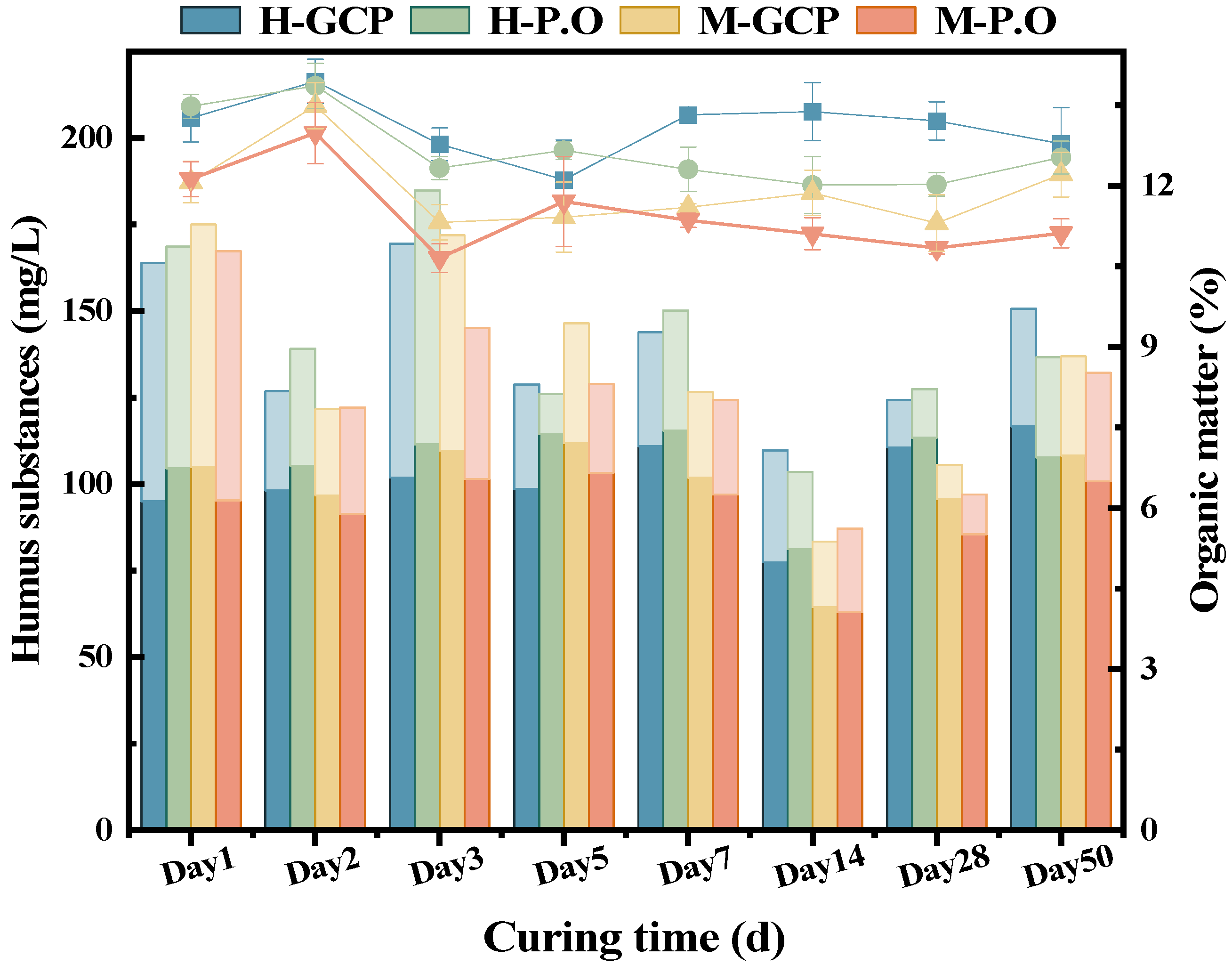
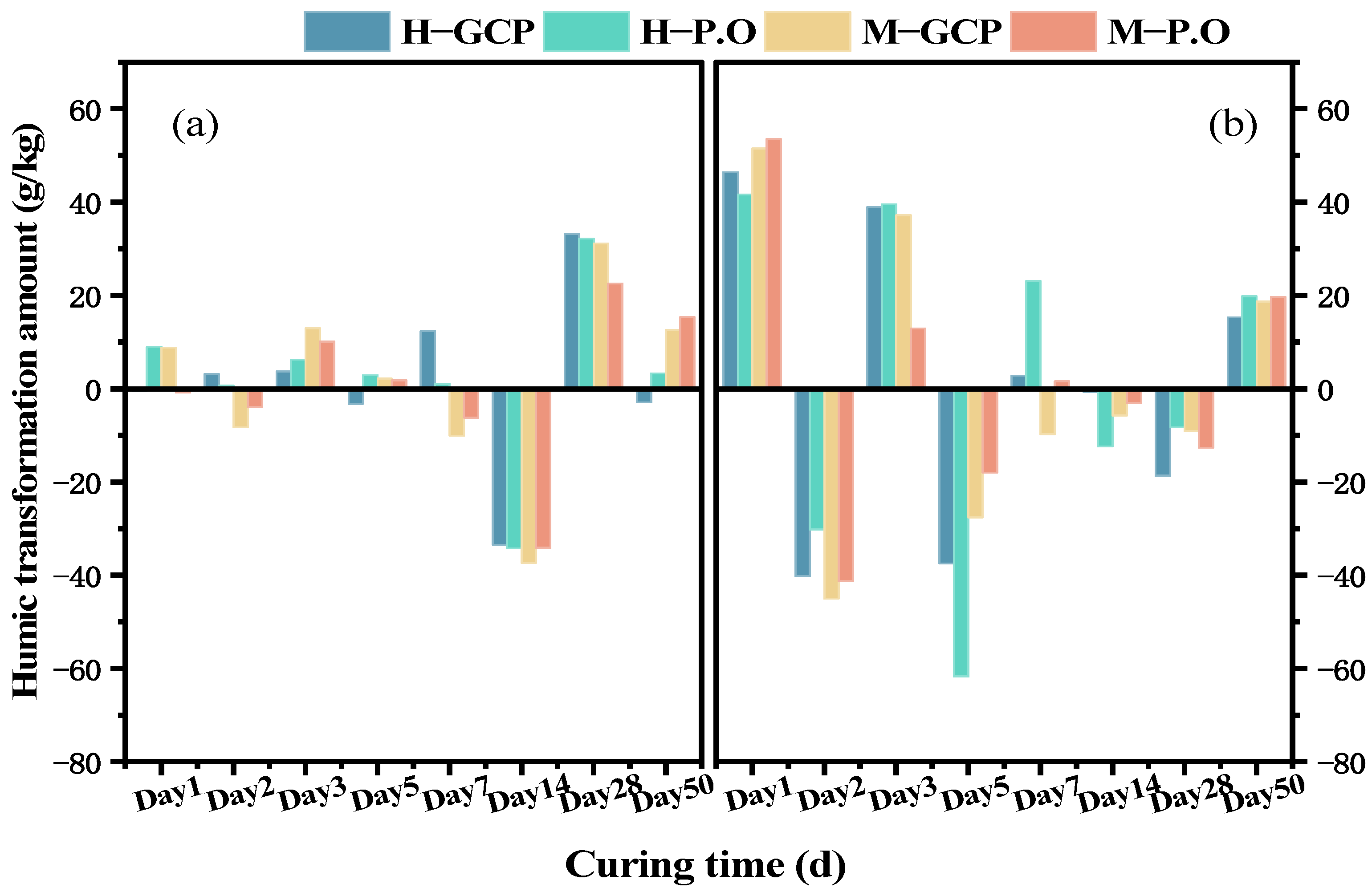
3.4. Changes in Organic Matter and Humus in Solidified Sludge
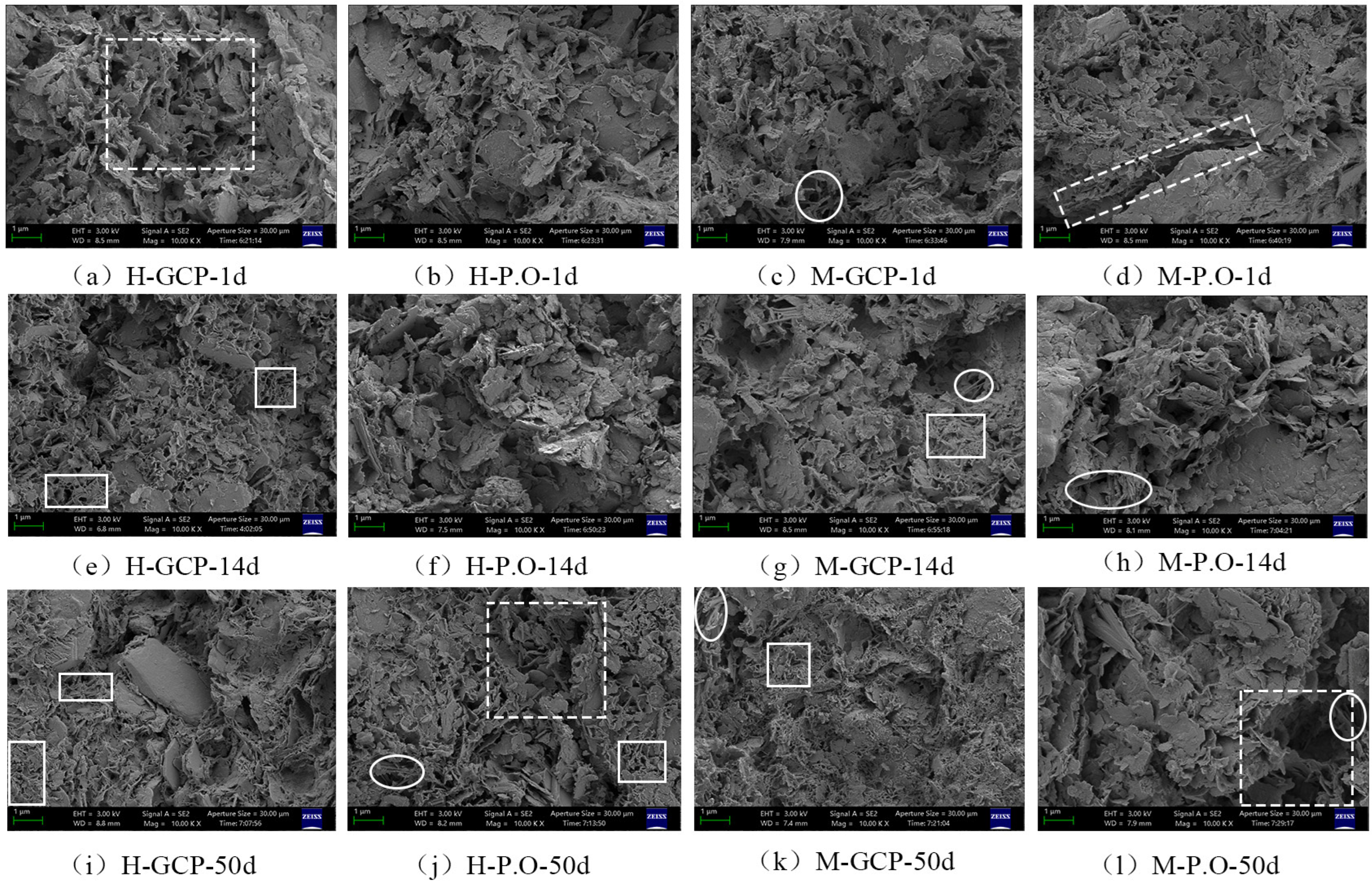
3.5. Microstructure of Solidified Sludge
3.6. Mineral Composition of Solidified Sludge
4. Conclusions
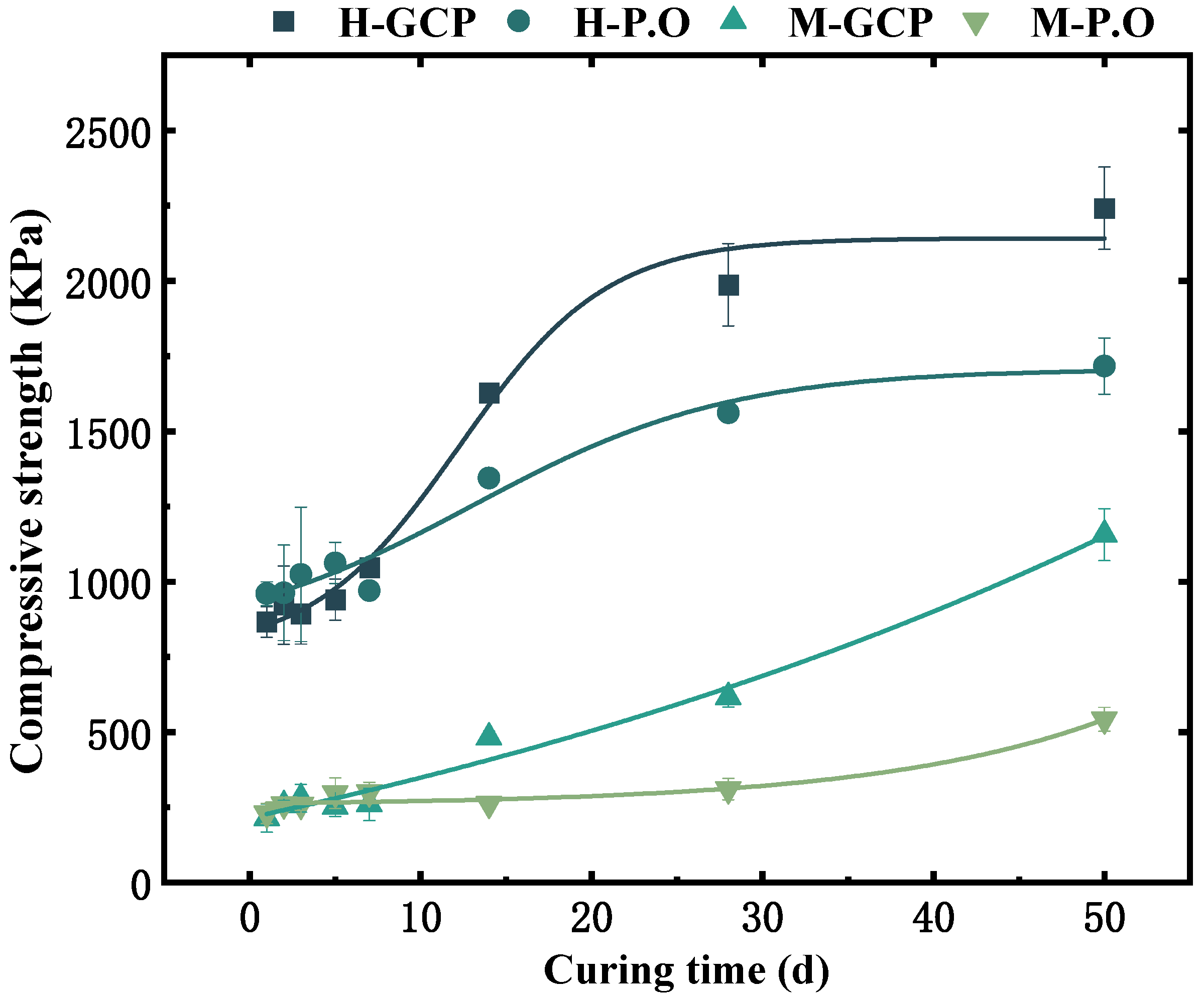
- The presence of humus in organic matter of sludge could affect the compressive strength of the solidified sludge, resulting in a slow increasing rate of the strength of the solidified sludge in the initial stage. The content of organic matter was not a major factor for the large difference in compressive strength between SHOC and SMOC. Curing agents exhibited a stronger influence than organic matter. GCP exhibited a stronger solidification effect on organic-rich sludge due to the blast furnace slag and phosphogypsum, achieving a higher compressive strength of its solidified sludge (2242.24 Kpa) at 50 d than that of P.O-solidified sludge, which was also supported by microstructure analysis.
- The water content of the solidified sludge was affected by the hydration reaction. It stabilized in a certain range after 14 d, which reflected the solidification level of the solidified sludge. The mass of the solidified sludge gradually decreased with the increase in S/S treatment time, and a higher mass loss corresponded to a lower solidification level.
- The pH of the solidified sludge exhibited a fluctuation trend, followed by a continual decrease with the continual increase in the humus content. The external pH was always lower than the internal pH due to the influence of the carbonation reaction. The P.O-solidified sludge displayed more alkalinity and lower stability.
- The changing trend of humus content in the solidified sludge was opposite to the pH, indicating that the hydration reaction was antagonistic to the decomposition of humus. The main difference in organic matter between the solidified SHOC and SMOC was the content of HA. Neutralization of humus with the hydration products resulted in the fluctuation of pH. The humus content in the solidified sludge was negatively correlated with the compressive strength.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lirer, S.; Liguori, B.; Capasso, I.; Flora, A.; Caputo, D. Mechanical and chemical properties of composite materials made of dredged sediments in a fly-ash based geopolymer. J. Environ. Manag. 2017, 191, 1–7. [Google Scholar] [CrossRef]
- Todaro, F.; Gisi, S.D.; Notarnicola, M. Contaminated marine sediment stabilization/solidification treatment with cement/lime: Leaching behaviour investigation. Environ. Sci. Pollut. Res. 2020, 27, 21407–21415. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zuo, D.; Wang, F.; Liang, C. Investigation on stabilization/solidification characteristics of lead-contaminated soil using innovative composite model of cement and soda residue. Environ. Earth Sci. 2022, 81, 2–19. [Google Scholar] [CrossRef]
- Couvidat, J.; Chatain, V.; Bouzahzah, H.; Benzaazoua, M. Characterization of how contaminants arise in a dredged marine sediment and analysis of the effect of natural weathering. Sci. Total Environ. 2018, 624, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Wu, S.; Jing, X.; Liu, K.; Ren, L. Long-term internal erosion mechanism of organic matter in sediments solidified by cement, lime, and metakaolin. Front. Earth Sci. 2023, 10, 1047079. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, D.; Feng, P.; Hao, B.; Guo, Y.; Wang, S. Municipal sewage sludge incineration and its air pollution control. J. Clean. Prod. 2021, 295, 126456. [Google Scholar] [CrossRef]
- Raheem, A.; Sikarwar, V.S.; He, J.; Dastyar, W.; Dionysiou, D.D.; Wang, W.; Zhao, M. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: A review. Chem. Eng. J. 2018, 337, 616–641. [Google Scholar] [CrossRef]
- Ding, A.; Zhang, R.; Ngo, H.H.; He, X.; Ma, J.; Nan, J.; Li, G. Life cycle assessment of sewage sludge treatment and disposal based on nutrient and energy recovery: A review. Sci. Total Environ. 2021, 769, 144451. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Cho, D.W.; Tsang, D.C.W.; Yang, J.; Hou, D.; Baek, K.; Kua, H.W.; Poon, C.S. Novel synergy of Si-rich minerals and reactive MgO for stabilisation/solidification of contaminated sediment. J. Hazard. Mater. 2019, 365, 695–706. [Google Scholar] [CrossRef]
- Božena, D.; Rostislav, D.; Jakub, H. New possibilities of neutralisation sludge solidification technology. J. Clean. Prod. 2018, 204, 1097–1107. [Google Scholar] [CrossRef]
- Liang, D.; Yan, J.; Wang, F.; Lv, G. The long-term leaching behavior of heavy metals in geopolymers and Portland cement bricks containing MSWI fly ash: A comparative study. J. Environ. Chem. Eng. 2024, 12, 112894. [Google Scholar] [CrossRef]
- Chen, Y.; He, X.; Zhang, S.; Tan, X.; Wan, Y. Strength and microstructure properties of solidified sewage sludge with two types of cement-based binders. Sci. Rep. 2020, 10, 20769. [Google Scholar] [CrossRef]
- Pacewska, B.; Wilinska, I. Usage of supplementary cementitious materials: Advantages and limitations. J. Therm. Anal. Calorim. 2020, 142, 371–393. [Google Scholar] [CrossRef]
- Matsimbe, J.; Dinka, M.; Olukanni, D.; Musonda, I. Geopolymer: A Systematic Review of Methodologies. Materials 2022, 15, 6852. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, C.; Qu, G.; Liu, L.; Chen, B.; Liu, S.; Li, J.; Ren, Y.; Yang, Y. Study of Semi-Dry High Target Solidification/Stabilization of Harmful Impurities in Phosphogypsum by Modification. Molecules 2022, 27, 462. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Oliveira, M.L.S.; Crissien, T.J.; Santosh, M.; Schindler, M. A review on the environmental impact of phosphogypsum and potential health impacts through the release of nanoparticles. Chemosphere 2021, 286, 131513. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Y.G.; He, J.H.; Zhou, H. Solidification/stabilization of composite heavy metals using red mud-blast furnace slag based geopolymer. Environ. Earth Sci. 2024, 83, 533. [Google Scholar] [CrossRef]
- Silva, L.H.P.; Nehring, V.; de Paiva, F.F.G.; Tamashiro, J.R.; Galvín, A.P.; López-Uceda, A.; Kinoshita, A. Use of blast furnace slag in cementitious materials for pavements—Systematic literature review and eco-efficiency. Sustain. Chem. Pharm. 2023, 33, 101030. [Google Scholar] [CrossRef]
- Shao, L.; Ding, Z.; Wang, S.; Pan, K.; Hu, C. Effect of Organic Matter Components on the Mechanical Properties of Cemented Soil. Materials 2023, 16, 5889. [Google Scholar] [CrossRef]
- Zander, F.; Shakeel, A.; Kirichek, A.; Chassagne, C.; Gebert, J. Effects of organic matter degradation in cohesive sediment: Linking sediment rheology to spatio-temporal patterns of organic matter degradability. J. Soils Sediments 2022, 22, 2873–2882. [Google Scholar] [CrossRef]
- Cui, P.; Li, H.; Cui, L.; Su, F. Sediment particle size distribution, source of organic matter and environmental implications in the Liao River, northeast China. Catena 2025, 249, 108696. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere Environ. Toxicol. Risk Assess. 2018, 202, 420–437. [Google Scholar] [CrossRef]
- Ge, S.; Pan, Y.; Zheng, L.; Xie, X. Effects of organic matter components and incubation on the cement-based stabilization/solidification characteristics of lead-contaminated soil. Chemosphere 2020, 260, 127646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, J.; Li, Z. Strength and leaching behavior of heavy metal contaminated sludge solidified/stabilized by compound binders. Environ. Res. 2021, 197, 111053. [Google Scholar] [CrossRef] [PubMed]
- Okuyucu, O.; Jayawickrama, P.; Senadheera, S. The relationship between curing regime and mechanical properties of controlled low-strength material. Constr. Build. Mater. 2022, 315, 125460. [Google Scholar] [CrossRef]
- Gao, H.; Tao, H.; Yang, Y.; Che, Q.; Tang, Q.; Gu, Y. Effect of Humus on the Solidification and Stabilization of Heavy Metal Contaminated River Sediment. Int. J. Environ. Res. Public Health 2023, 20, 4882. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, J.; Zhao, Z.; Liu, J.; Lin, H. Strength Properties of Cement-Solidified Dredged Sludge Affected by Curing Temperature. Buildings 2022, 12, 1889. [Google Scholar] [CrossRef]
- Maierdan, Y.; Gu, K.; Chen, B.; Haque, M.A.; Zhang, Y.; Zhao, L. Recycling of heavy metal contaminated river sludge into unfired green bricks: Strength, water resistance, and heavy metals leaching behavior—A laboratory simulation study. J. Clean. Prod. 2022, 342, 130882. [Google Scholar] [CrossRef]
- Wang, T.; Medepalli, S.; Zheng, Y.; Zhang, W.; Ishida, T.; Bishnoi, S.; Hou, D.; Shi, Z. Retardation effect of the pozzolanic reaction of low-calcium supplementary cementitious materials on clinker hydration at later age: Effects of pore solution, foreign ions, and pH. Cem. Concr. Res. 2024, 177, 107416. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, X.; Chen, X.; Huo, X.; Yu, Z. Microbial-Induced Carbonate Precipitation: A Review on Influencing Factors and Applications. Adv. Civ. Eng. 2021, 2021, 9974027. [Google Scholar] [CrossRef]
- Ma, C.; Chen, B.; Chen, L. Effect of organic matter on strength development of self-compacting earth-based construction stabilized with cement-based composites. Constr. Build. Mater. 2016, 123, 414–423. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Wang, H.; Li, M.; Liu, S.; Wan, L. Carbonation behavior of solidified/stabilized cadmium in phosphogypsum slag-based cementitious materials. Constr. Build. Mater. 2024, 437, 136848. [Google Scholar] [CrossRef]
- Davis, W.M.; Erickson, C.L.; Johnston, C.T.; Delfino, J.J.; Porter, J.E. Quantitative Fourier Transform Infrared spectroscopic investigation humic substance functional group composition. Chemosphere 1999, 38, 2913–2928. [Google Scholar] [CrossRef]
- Saride, S.; Puppala, A.J.; Chikyala, S.R. Swell-shrink and strength behaviors of lime and cement stabilized expansive organic clays. Appl. Clay Sci. 2013, 85, 39–45. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Bejger, R.; Debaene, G.; Smreczak, B. Characterization of Soil Organic Matter Individual Fractions (Fulvic Acids, Humic Acids, and Humins) by Spectroscopic and Electrochemical Techniques in Agricultural Soils. Agronomy 2021, 11, 1067. [Google Scholar] [CrossRef]
- Pardal, X.; Pochard, I.; Nonat, A. Experimental study of Si–Al substitution in calcium-silicate-hydrate (C-S-H) prepared under equilibrium conditions. Cem. Concr. Res. 2009, 39, 637–643. [Google Scholar] [CrossRef]
- Andrade Neto, J.d.S.; De la Torre, A.G.; Kirchheim, A.P. Effects of sulfates on the hydration of Portland cement—A review. Constr. Build. Mater. 2021, 279, 122428. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Yan, Y.; Lothenbach, B.; Skibsted, J. Incorporation of Sodium and Aluminum in Cementitious Calcium-Alumino-Silicate-Hydrate C-(A)-S-H Phases Studied by 23Na, 27Al, and 29Si MAS NMR Spectroscopy. J. Phys. Chem. C 2021, 125, 27975–27995. [Google Scholar] [CrossRef]
- Feng, D.; Ye, F.; Chen, D.; Liang, S. Research progress on solidification/stabilization of sludge with Alkali–Activated cementitious materials: A review. Sustain. Chem. Pharm. 2025, 43, 101904. [Google Scholar] [CrossRef]
- Liu, J.; Xie, G.; Wang, Z.; Li, Z.; Fan, X.; Jin, H.; Zhang, W.; Xing, F.; Tang, L. Synthesis of geopolymer using municipal solid waste incineration fly ash and steel slag: Hydration properties and immobilization of heavy metals. J. Environ. Manag. 2023, 341, 118053. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, W.; Xing, F.; Wang, S.; Tang, L.; Lin, S.; Dong, Z. Carbonation of the synthetic calcium silicate hydrate (C-S-H) under different concentrations of CO2: Chemical phases analysis and kinetics. J. CO2 Util. 2020, 35, 303–313. [Google Scholar] [CrossRef]
- Hay, R.; Li, J.; Celik, K. Phase evolution, micromechanical properties, and morphology of calcium (alumino)silicate hydrates C-(A-)S-H under carbonation. Cem. Concr. Res. 2022, 152, 106683. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, H.; El-Korchi, T.; Zhang, G.; Tao, M. Experimental feasibility study of geopolymer as the next-generation soil stabilizer. Constr. Build. Mater. 2013, 47, 1468–1478. [Google Scholar] [CrossRef]


| Sludge | Moisture Content (%) | pH | Organic Matter Content (%) | Zeta Potential (mv) |
|---|---|---|---|---|
| SHOC | 228.5 | 7.8 | 12.9 | −40.5 |
| SMOC | 235.8 | 7.2 | 8.3 | −25.7 |
| Sludge | Cr | As | Ni | Cu | Zn | Pb |
|---|---|---|---|---|---|---|
| SHOC | 393.8 | 807.7 | 190.9 | 297.7 | 1545.3 | 172.7 |
| SMOC | 670.0 | 751.5 | 357.5 | 361.5 | 2065.8 | 175.6 |
| Curing Agent | CaO | SiO2 | SO3 | Al2O3 | MgO | Fe2O3 | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|
| GCP | 51.3 | 18.1 | 13.0 | 7.9 | 4.8 | 3.0 | 0.6 | 0.3 |
| Cement | 66.2 | 17.5 | 4.1 | 4.7 | 2.1 | 3.8 | 0.8 | 0.6 |
| Sample Name | Organic Matter Content (%) | Curing Agent | S/S Time (d) |
|---|---|---|---|
| H-GCP | 12.9 | GCP | 1, 2, 3, 5, 7, 14, 28, 50 |
| H-P.O | 12.9 | P.O | 1, 2, 3, 5, 7, 14, 28, 50 |
| M-GCP | 8.3 | GCP | 1, 2, 3, 5, 7, 14, 28, 50 |
| M-P.O | 8.3 | P.O | 1, 2, 3, 5, 7, 14, 28, 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Ran, F.; Liu, S.; Wang, L.; Fan, C. Effects of Humus and Solidification Agents on the Solidification/Stabilization Process of Organic-Rich River Sludge: Characteristics of the Stabilized Sludge. Water 2025, 17, 1153. https://doi.org/10.3390/w17081153
Zhu Y, Ran F, Liu S, Wang L, Fan C. Effects of Humus and Solidification Agents on the Solidification/Stabilization Process of Organic-Rich River Sludge: Characteristics of the Stabilized Sludge. Water. 2025; 17(8):1153. https://doi.org/10.3390/w17081153
Chicago/Turabian StyleZhu, Yuqi, Fuyuan Ran, Sihong Liu, Liujiang Wang, and Chunzhen Fan. 2025. "Effects of Humus and Solidification Agents on the Solidification/Stabilization Process of Organic-Rich River Sludge: Characteristics of the Stabilized Sludge" Water 17, no. 8: 1153. https://doi.org/10.3390/w17081153
APA StyleZhu, Y., Ran, F., Liu, S., Wang, L., & Fan, C. (2025). Effects of Humus and Solidification Agents on the Solidification/Stabilization Process of Organic-Rich River Sludge: Characteristics of the Stabilized Sludge. Water, 17(8), 1153. https://doi.org/10.3390/w17081153







