Abstract
Janus nanorods are a special class of nanorods composed of two distinct surface regions, one hydrophilic and one hydrophobic. This amphiphilic characteristic makes them promising candidates for stabilizing water–oil interfaces. Oily wastewater (OWW) contamination, resulting from industrial activities such as petroleum extraction and refining and vegetable oil processing, poses significant risks to ecosystems, water resources, and public health. Traditional surfactants used in enhanced oil recovery (EOR) and wastewater treatment often introduce secondary pollution due to their persistence and toxicity. In this work, we investigate the interfacial behavior of Janus NRs under two different conditions: a thin oil film surrounded by water and a nanoconfined system with purely repulsive walls. Using dissipative particle dynamics (DPD) simulations, we analyze how nanorod length and confinement influence interfacial tension and self-assembly. In bulk systems, shorter NRs (dimers and quadrimers) effectively reduce interfacial tension by adsorbing at the oil–water interface, while longer NRs (hexamers) exhibit bulk aggregation, limiting their surfactant efficiency. In contrast, under nanoconfinement, all NR sizes increase interfacial tension due to steric constraints, with longer NRs preferentially adsorbing onto the solid–liquid interface. These results pave the way for the rational design of nanostructured materials for applications in enhanced oil recovery, wastewater treatment, and membrane filtration.
1. Introduction
Water pollution remains one of the most critical environmental issues today, with impacts ranging from ecosystem degradation to serious public health risks [1,2]. Among the key pollutants, oily hydrocarbons—including vegetable oils, petroleum, and their derivatives—stand out due to their extensive industrial use and high production volumes [3,4,5,6,7,8,9,10,11,12,13]. In 2023 alone, global petroleum production reached nearly 89.8 million barrels per day [14], and the global vegetable oil harvest was estimated at 222 million tons [15]. These large-scale operations inevitably result in environmental contamination across the entire production chain—from extraction to disposal—through spills and the generation of oily wastewater (OWW), with pollutants detected even in remote areas like the Amazon and Antarctica [16,17,18].
In addition to industrial sources, OWW is also generated from domestic and food industry activities, including waste cooking oil (WCO) composed of used vegetable oils and animal fats. Although global data are limited, estimates suggest that WCO production exceeds 18 million tons annually, with roughly 29,000 tons of vegetable oil spilled into marine environments between 1962 and 2017 [19,20,21,22]. This highlights the persistent and widespread nature of oily pollution and the urgent need for new separation and remediation technologies.
The environmental impact of oily waste is both severe and multifaceted. The oil industry alone consumes approximately 16% of the world’s daily freshwater supply, and for every barrel of oil extracted, around three barrels of water are used and potentially contaminated [3]. A significant portion of this water is employed in enhanced oil recovery (EOR), where modified fluids—commonly referred to as smart water (SW)—containing surfactants, salts, and nanoadditives are injected into porous rock formations to improve oil extraction efficiency [23,24,25,26,27]. Although effective, EOR generates complex oily wastewater (OWW) due to the chemical additives involved. Unlike conventional OWW, these processes often occur in nanoconfined environments, where the interactions between water, oil, and additives differ fundamentally from those in bulk systems [28]. Previous studies have shown that confinement significantly alters the behavior of Janus dimers, influencing their self-assembly and interfacial properties [29,30,31]. It is therefore reasonable to expect that larger Janus nanorods (NRs) may exhibit distinct confinement-induced effects on their adsorption and aggregation behavior.
The discharge of OWW and oil spills poses substantial environmental threats, affecting soil, water bodies, and diverse biological communities [16,22,32]. In aquatic systems, oil forms surface films that hinder gas exchange and light penetration, disrupting photosynthesis and depleting oxygen levels. These effects can severely damage aquatic life. In addition, contact with or the ingestion of contaminated water by animals can lead to intoxication, organ damage, neurological disorders, anemia, and, in extreme cases, carcinogenic or reproductive health effects [10,33].
Given the magnitude of these risks, numerous remediation strategies have been developed, including physical, chemical, and biological techniques such as bioremediation, oxidation, electrokinetic remediation, flotation, and solvent extraction [32,34,35]. In recent years, nanostructured materials have emerged as highly promising for water decontamination, enhancing oil–water separation, reducing chemical waste, and improving overall treatment efficiency. Furthermore, molecular modeling has played a pivotal role in guiding the design of such materials, enabling the precise tuning of their interfacial properties for targeted environmental applications [36,37]. This progress has motivated the exploration of amphiphilic and Janus nanoparticles as next-generation alternatives to conventional surfactants in separation technologies [38,39]. More recent simulations also highlight the role of amphiphilic nanomaterials in reducing oil–water interfacial tension and promoting emulsion destabilization, particularly in Janus-type rods where interfacial orientation plays a key role [40].
Nanorods, due to their elongated structure, offer a unique advantage in interfacial stabilization compared to spherical nanoparticles [41,42,43,44]. Currently, single-type nanorods are used to vary the wettability of pre-existing separation mechanisms. The use of nanorods implies an increase in the efficiency and selectivity of the separation of water and oil [45,46,47,48,49,50,51]. Furthermore, in some cases, they act to reduce oil fouling on the surface of membranes [46,48], dye photodegradation activity [47], and photocatalysis capacity [50,51]. Janus nanorods are a special class of nanorods composed of two distinct surface regions—typically one hydrophilic and one hydrophobic—allowing them to preferentially orient at fluid–fluid interfaces and act as solid surfactants. Regarding Janus nanorods, an indication of a tunable surface chemistry and structural anisotropy allows selective wettability, allowing greater separation efficiency while minimizing the need for chemical additives. Due to the possibility of environmentally friendly syntheses producing a reduced environmental impact, the altered wettability of systems and longer residence times at the interface, nanorods of both types are promising candidates for environmental applications such as wastewater treatment, oil spill remediation and technologies such as EOR.
In this study, we investigate the interfacial behavior of Janus NRs in water–oil separation under two different conditions: (i) a thin oil film surrounded by water under fully periodic boundary conditions, and (ii) a nanoconfined system with the fluid mixture constrained between two purely repulsive plates. These setups allow us to examine how nanorods influence interfacial tension and self-assemble in thin films and confined environments, mimicking conditions relevant to oil recovery, wastewater treatment, and membrane filtration. Through dissipative particle dynamics (DPD) simulations, we aim to identify the influence of the Janus NRs size on the interfacial stability and separation efficiency, providing insights into their potential applications in sustainable environmental technologies. Overall, our results emphasize the potential of Janus NRs as next-generation surfactants and highlight critical design considerations for their application in sustainable technologies.
2. The Method and the Models
2.1. Dissipative Particle Dynamics
To model the water–oil-nanorods system, we employed dissipative particle dynamics (DPD) simulations [52,53,54]. The simulations were performed using the ESPResSo (Extensible Simulation Package for Research on Soft Matter) package [55,56], which allows for an efficient coarse-grained representation of the system. In DPD, the total interaction force between particles consists of three main components:
where is a conservative force; is the dissipative force, related to viscosity; and is the random force, associated with stochastic processes and the temperature of the system. The conservative force is given by
Here, is the maximum repulsion parameter between particles i and j, and is the unit vector of the relative position between i and j. The coefficient is zero if , or if , where is the cutoff radius for particle interactions. The dissipative and random forces are, respectively,
where represents the relative velocity between particles i and j, while and are the dissipation and fluctuation coefficients. The coefficients and refer to the frictional dissipative force and the amplitude of the random force noise, respectively, and the white noise follows a Gaussian distribution centered at zero.
The weight functions and and the coefficients and must obey the following relations [54,57]:
where is the Boltzmann constant and T is the temperature. In order for the nanorods to remain united, fixed and rigid, a harmonic binding force is added to Equation (1), which keeps the monomers fixed [58], and an angular binding force , which keeps the rod inflexible. These forces are, respectively,
where is the spring constant that keeps the rod’s beads bonded together, is the equilibrium position, and is the unit vector position. The angular force is
where is the angular bending constant, is the equilibrium angle between three beads in the same Janus NRs, and the unit position vector is .
2.2. The Model and System Details
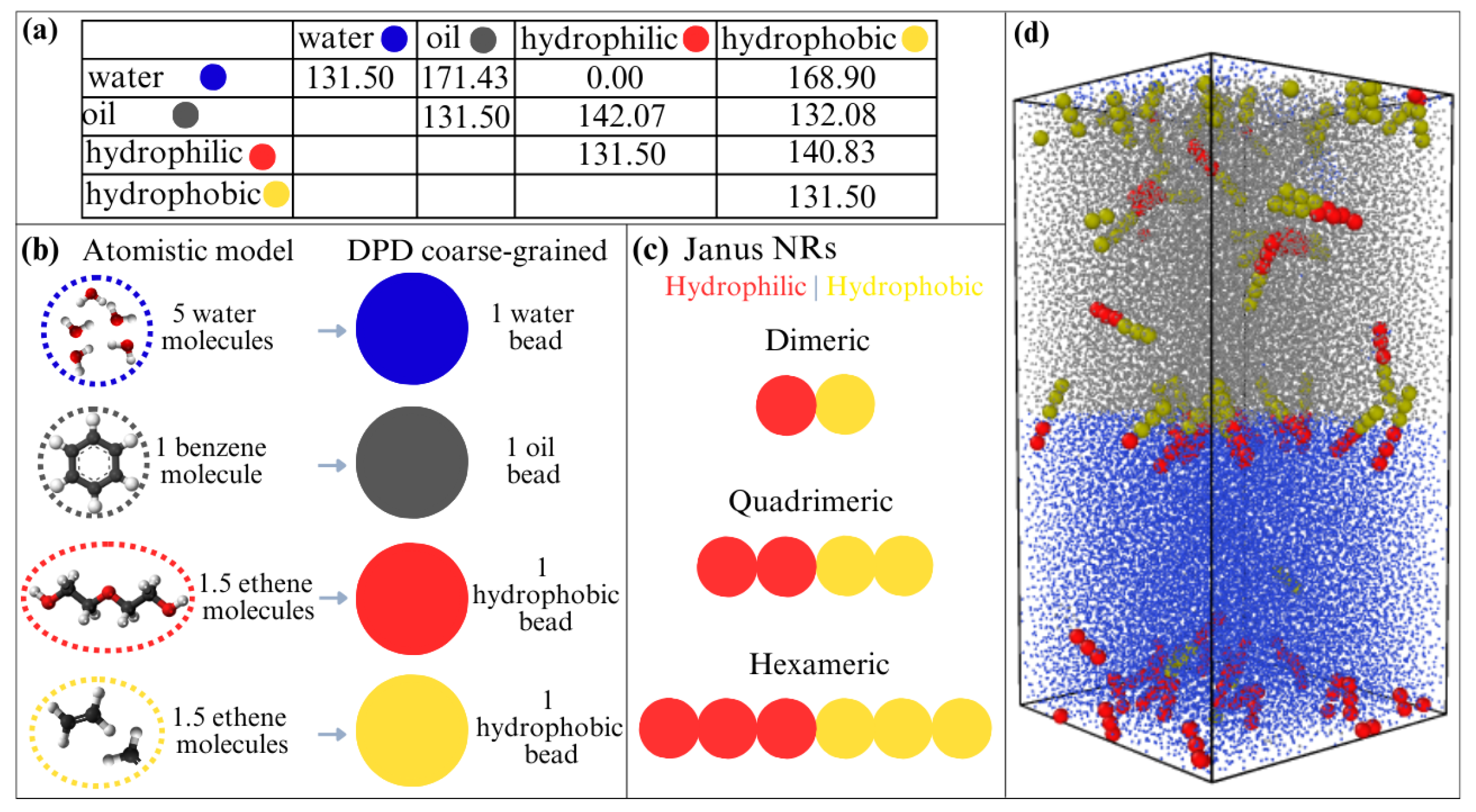
In this work, we use the parametrization proposed by Khedr and Striolo for non-ionic surfactants at a water–oil interface [58]. Their coarse-grained approach defines interaction parameters for water, the organic solvent (which for simplicity we will refer to as oil), and hydrophilic and hydrophobic beads. Here, we retain the same interaction parameters to explore the behavior of nanorods (NRs) at oil–water interfaces under different confinement scenarios. The conservative force parameter is presented in Figure 1a, and the schematic representation of the coarse-grained model is shown in Figure 1b. Each bead has a diameter , with Å. All the Janus NRs are half hydrophilic and half hydrophobic, and 3 sizes of NRs were considered: with 2, 4 or 6 monomers, dimers, quadrimers and hexamers nanorods. A schematic representation can be seen in Figure 1c.

Figure 1.
(a) Interaction parameters for the conservative force in the DPD model, as proposed by reference [58]. (b) Schematic representation of the coarse-grained model for water (blue), oil (gray), and Janus nanorods with hydrophilic (red) and hydrophobic (yellow) beads. (c) Structure of the three types of Janus nanorods—dimeric, quadrimeric and hexameric. (d) Example of the confined simulation box, showing the water and oil regions along with hexameric nanorods.
By employing a DPD approach, we effectively capture the interfacial behavior of Janus nanorods by balancing computational efficiency with accurate mesoscale dynamics. Its coarse-grained approach enables the study of larger systems, making it particularly suited for analyzing processes such as the NRs self-assembly, their adsorption, and the liquid–liquid interfacial tension in oil–water separations. While it simplifies atomic details, this is appropriate for the studied system, where hydrophobic–hydrophilic interactions and steric effects dominate. The chosen time scales and system sizes allow for equilibrated structures and reliable interfacial tension measurements, ensuring that key trends in nanorod behavior are well resolved.
Two distinct scenarios were explored. In the first one, a thin film of oil, with a length of 30.0, is surrounded by a 15.0 film of water on each side. Then, the simulation box in the z-direction is , while in the x and y directions. Standard periodic boundary conditions (PBC) were applied in all directions.
The second scenario consists of water and oil confined inside a parallel pipe with a length of , and . The confinement is achieved by positioning hard spherical walls in the z-direction limits, while standard PBC are applied in the x- and y-directions. The wall beads have the same diameter as the other species in the system but have a repulsion parameter with all particles, acting as a purely repulsive wall with all particles. In the future, the effects of hydrophilic or oleophilic sites in the wall can also be explored. In Figure 1d we can see an example of one simulation box in the confined casew with the water region in blue, oil region in in gray, and nanorods with six monomers—three hydrophilic in red and three hydrophobic in yellow. In all cases, water and oil density were fixed at 3.0 beads/, as proposed in reference [58]. The nanorod density was varied from to 0.040 beads/.
To initiate the simulations, NRs were randomly placed based on the desired density . Half of the nanorods are initially positioned in the water region, and half in the oil region. The system is then equilibrated over time steps, followed by a production phase consisting of time steps to obtain the results. Averages and snapshots were recorded every steps to analyze the system’s behavior and evaluate key properties. Throughout the simulations, various quantities—including energy, interfacial tension, density profiles, instantaneous pressure, and kinetic temperature—were continuously monitored, including during the equilibration phase, to ensure system stability and equilibrium. The random and dissipative parameters are set to = 3 and , and the time step is 0.04 [58]. Here, is the simulation time, which corresponds to 15.2 ps [58]. The temperature of the system was set to 300 K, and the spring constants were [58] and /rad2, with .
The normalized density profile, which we will refer to simply as the density profile for brevity, was evaluated along the z-direction,
In the Results and Discussion section, density and distances will be expressed in units of beads/ and , respectively. For simplicity, these units will be omitted in the figures and discussions, as the relative comparisons remain unaffected by their explicit notation.
The interfacial tension was computed using the components of the pressure tensor (, , and ), following the methodology used in previous studies [58,59]:
where is the number of interfaces in our system. The local stress tensor was computed using the Irving–Kirkwood method, as implemented in ESPResSo [56], by dividing the simulation box into 25 bins along the z-direction; the interfacial tension was then obtained from the average of the normal components and . Since our goal is to understand how the interfacial tension varies in the presence of nanorods, we present our results in terms of relative surface tension, which is defined as the surface tension at a given NR density divided by its value in the absence of nanorods ().
Finally, to characterize the NRs alignment at the interfaces [60], the global nematic order parameter was calculated from the alignment tensor , defined as
where is the unit vector along the axis of nanorod i, obtained from the normalized displacement between its first and last monomers, and is the Kronecker delta. The nematic director is identified as the eigenvector associated with the largest eigenvalue of .
The nematic order parameter S is then given by
where the average is taken over all nanorods that satisfy the positional criteria.
3. Results and Discussion
3.1. Water–Oil Thin Films
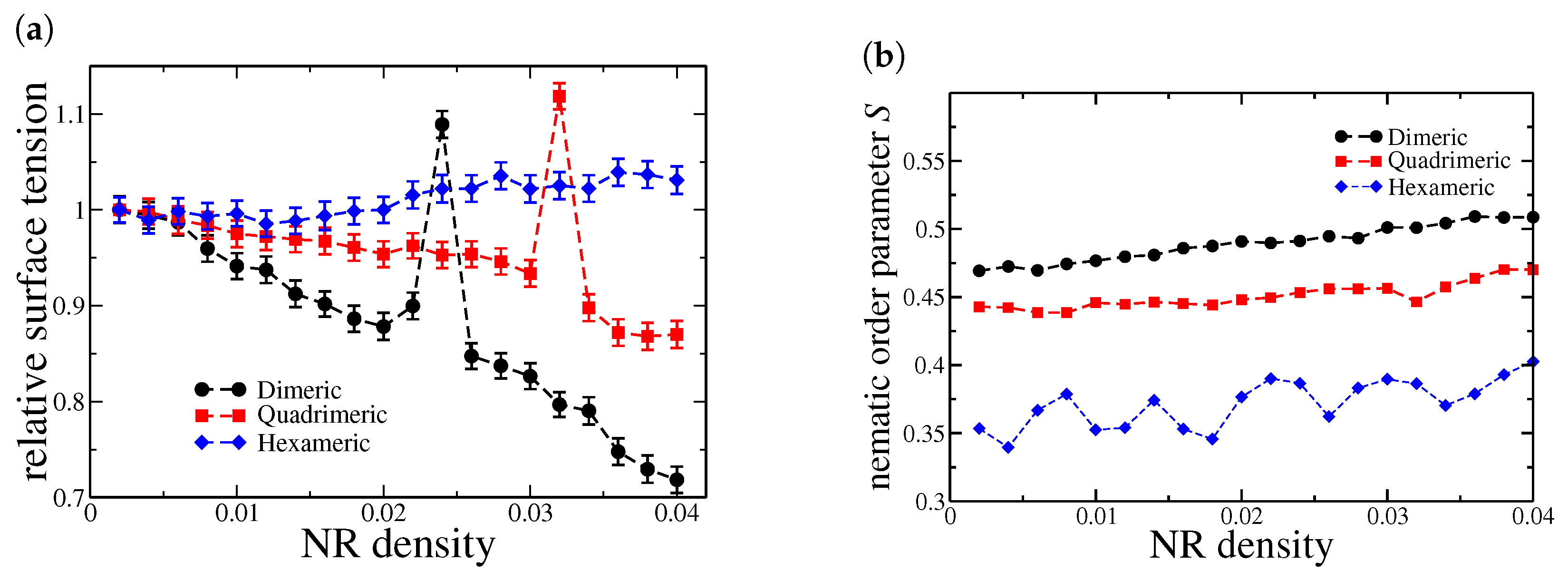
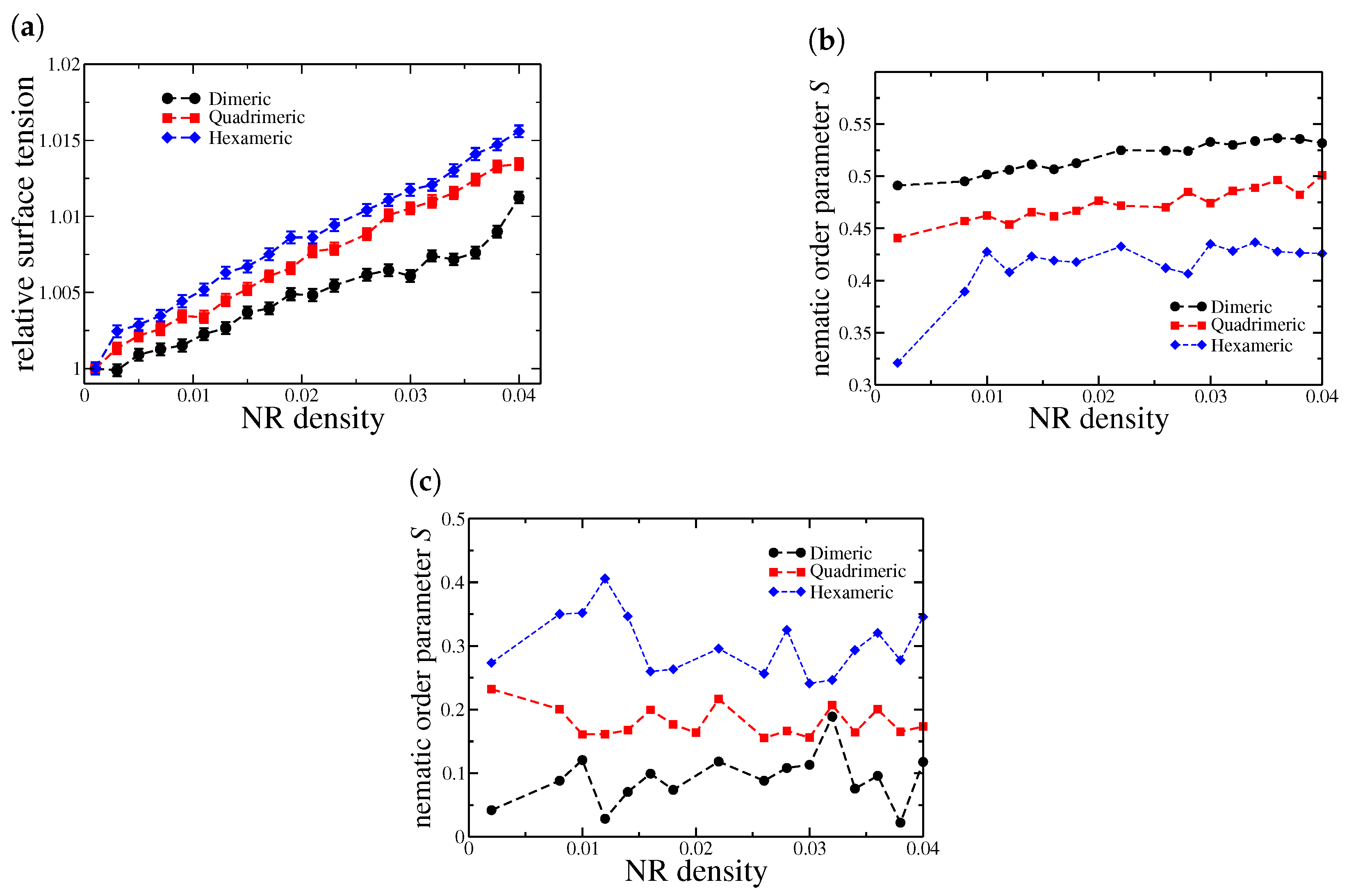
We begin our discussion by analyzing the behavior of interfacial tension at the water–oil interface in the case of a thin oil film, as shown in Figure 2a. We will call this case bulk since PBCs are applied in all directions. Here, the tension is normalized by its value in the absence of nanorods, i.e., when . We should expect that Janus NRs act as surfactants, reducing the free energy of the interface by preferentially adsorbing at the oil–water boundary. However, the efficiency of this process depends on the nanorod length and the balance between their hydrophobic and hydrophilic regions.

Figure 2.
(a) Relative interfacial tension as a function of nanorod density for the bulk system. The values are normalized by the surface tension of the system in the absence of nanorods (). (b) Nematic order parameter S for NRs at the oil–water interfaces.
As observed, the behavior varies depending on the number of beads in each nanorod present. Dimeric nanorods reduce the interfacial tension and exhibit a discontinuity at a density of . A similar trend is observed for quadrimeric nanorods, with the discontinuity occurring at . This decreasing behavior is characteristic of surfactants, which typically lower interfacial tension. However, hexameric nanorods exhibit a different behavior. Instead of decreasing interfacial tension, they slightly increase it compared to the nanorod-free case, and no discontinuity is observed.
This phenomenon can be attributed to two main factors. First, as the nanorod length increases, geometric constraints may hinder their effective incorporation at the interface. Longer nanorods may struggle to align optimally, thereby reducing their surfactant-like effect. Second, interparticle interactions become more pronounced with increasing nanorod length, potentially leading to aggregation or misalignment. Our findings indicate that dimeric (two-monomer) nanorods effectively reduce interfacial tension by aligning efficiently at the oil–water boundary. Their small size minimizes steric hindrance, allowing for a homogeneous distribution at the interface. Similarly, quadrimeric (four-monomer) nanorods also promote a reduction in interfacial tension, suggesting they retain efficient adsorption and contribute to interface stabilization. To support this analysis, Figure 2b shows the behavior of the nematic order parameter S for nanorods adsorbed at the oil–water interfaces located at and , as further confirmed by the density profiles. Both dimeric and quadrimeric nanorods exhibit higher values of S compared to hexameric ones, indicating a greater degree of orientational order. This enhanced alignment at the interface is directly associated with their more effective interfacial activity and stabilization capabilities.
In contrast, hexameric nanorods exhibit a markedly different behavior. Rather than distributing uniformly at the interface, they tend to form lateral aggregates, leading to a saturation effect where additional nanorods do not further reduce interfacial tension. Furthermore, a fraction of the hexameric nanorods appear to remain dispersed within the bulk aqueous or oil region rather than residing at the interface, further diminishing their surfactant efficiency.
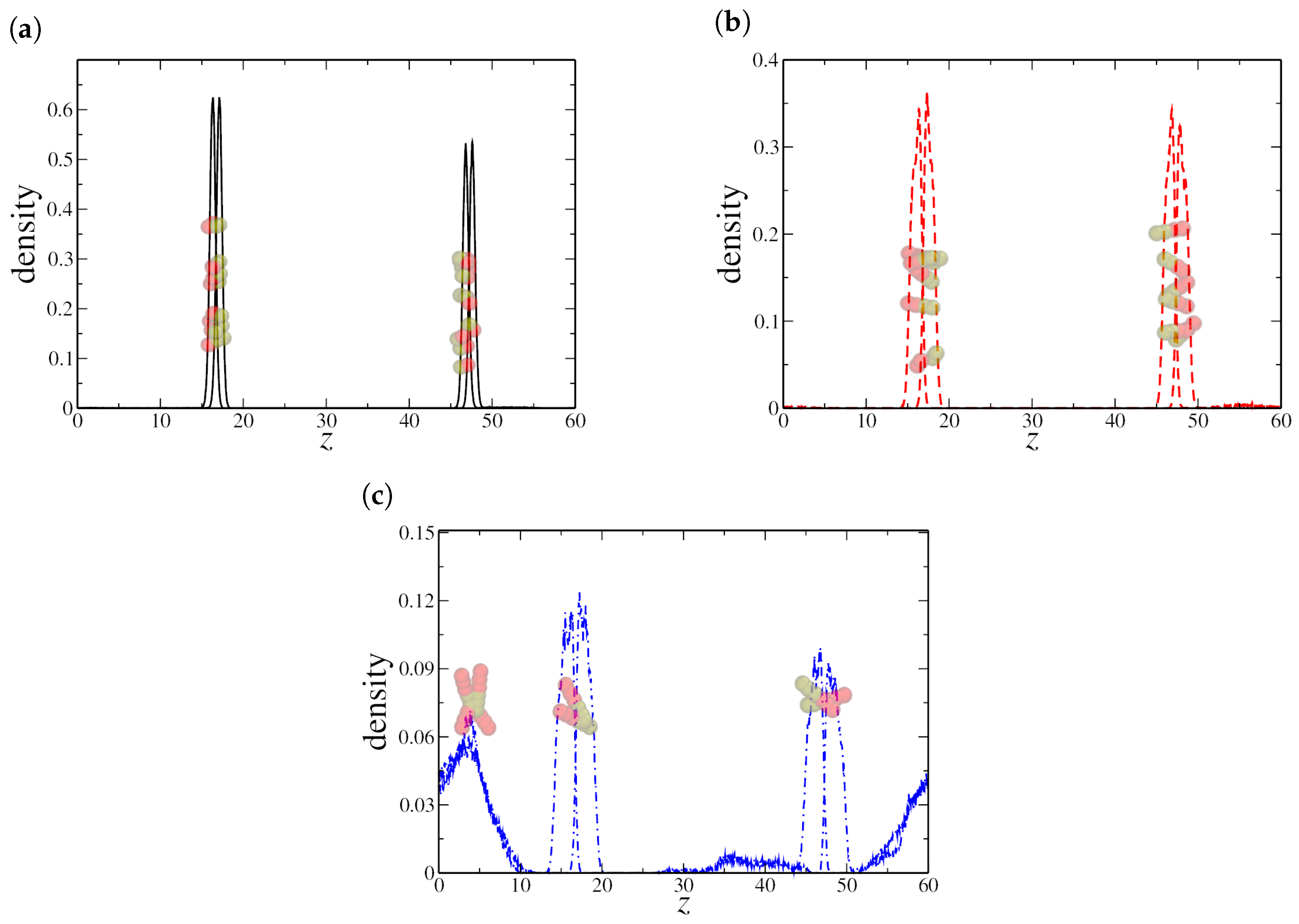
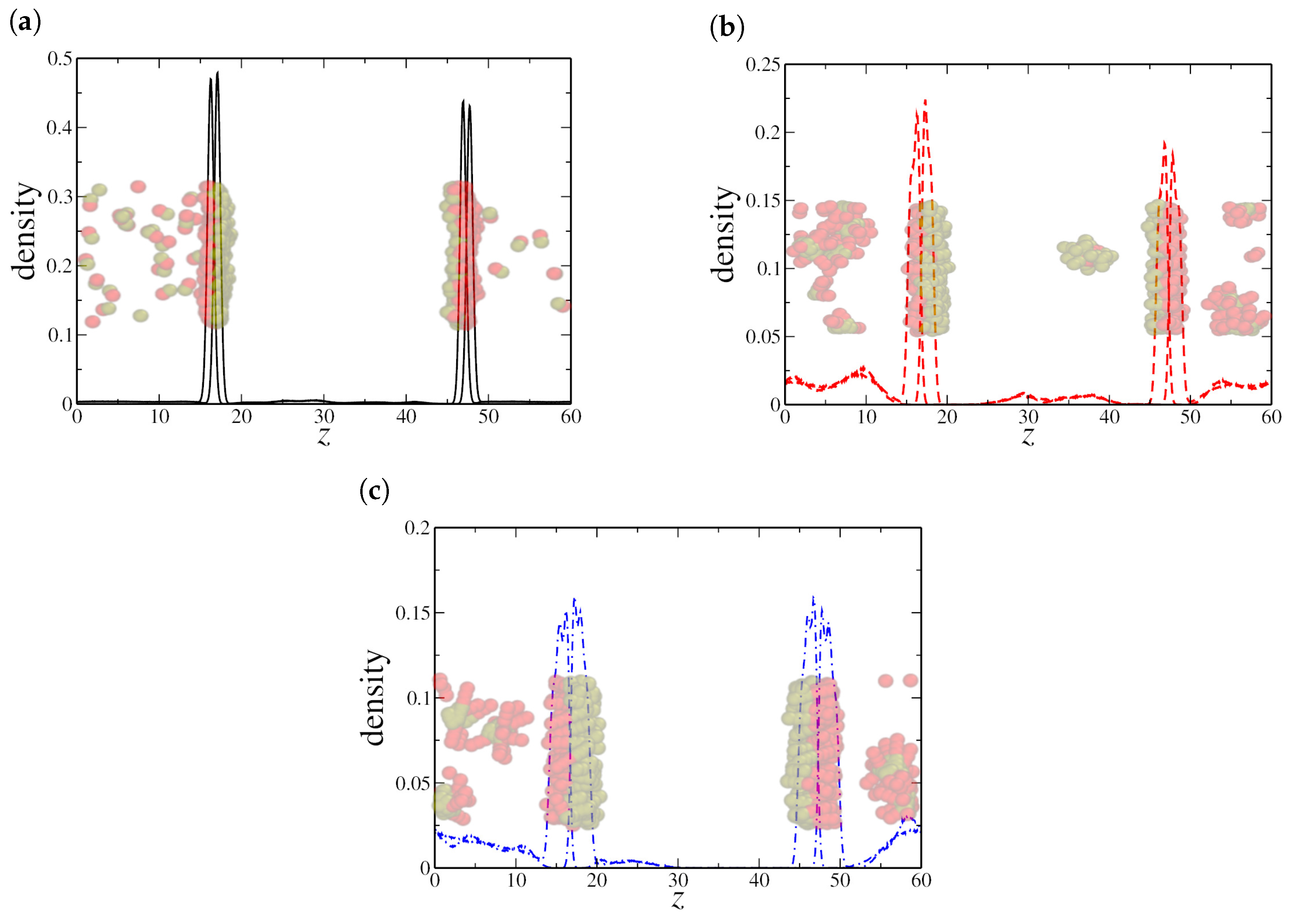
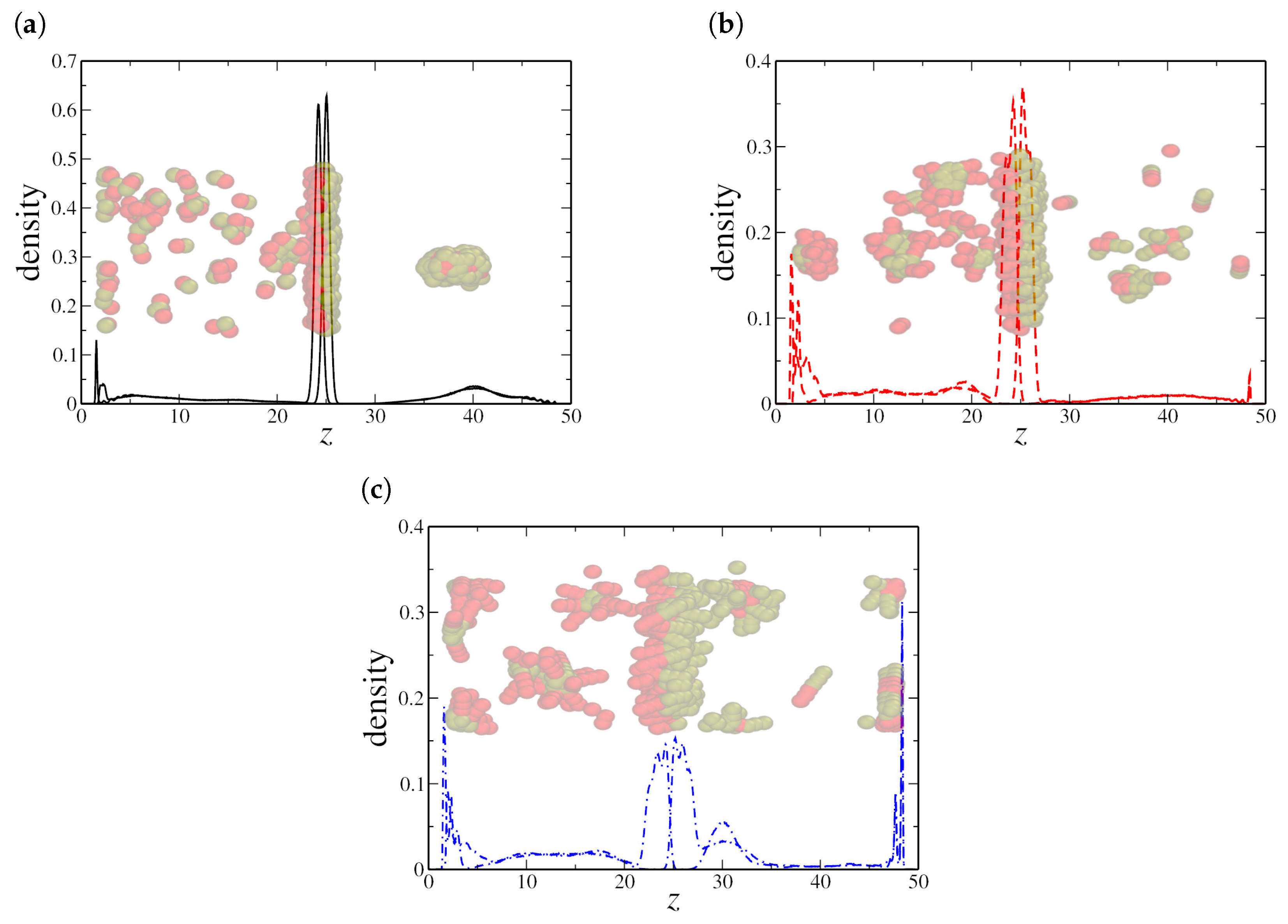
To validate these hypotheses, we analyzed the density distribution of hydrophilic and hydrophobic monomers along the z-direction. We first examine the low-density regime, with , as shown in Figure 3. For both dimeric and quadrimeric nanorods, the density profiles and the corresponding snapshots confirm that they are predominantly aligned at the water–oil interface (see Figure 3a,b). However, even at this low concentration, hexameric nanorods exhibit a tendency to aggregate within the aqueous phase, as evident in Figure 3c. This aggregation further supports the hypothesis that steric and interparticle effects become dominant for longer nanorods, limiting their efficiency as interfacial agents.
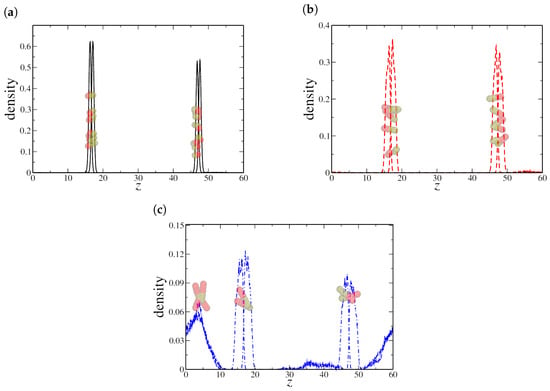
Figure 3.
Density profile along the z-axis for (a) dimeric, (b) quadrimeric, and (c) hexameric nanorods at a low density (). Snapshots from the final simulation frame illustrate nanorod distribution, showing interfacial localization for (a,b), while (c) displays both interfacial adsorption and aggregation in the bulk. Here, the oil–water interfaces are located at the peaks in the NRs density profile, near and , respectively.
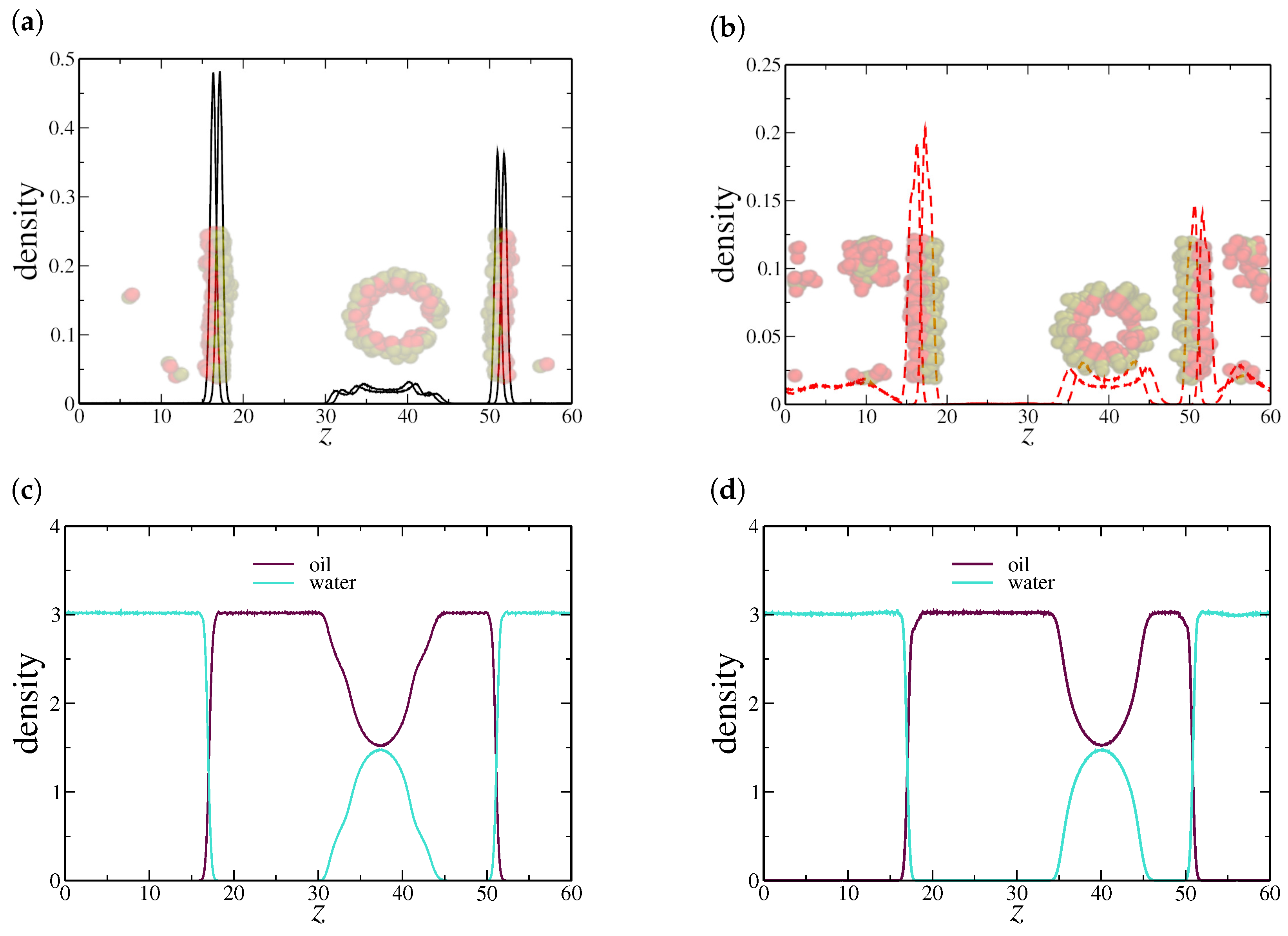
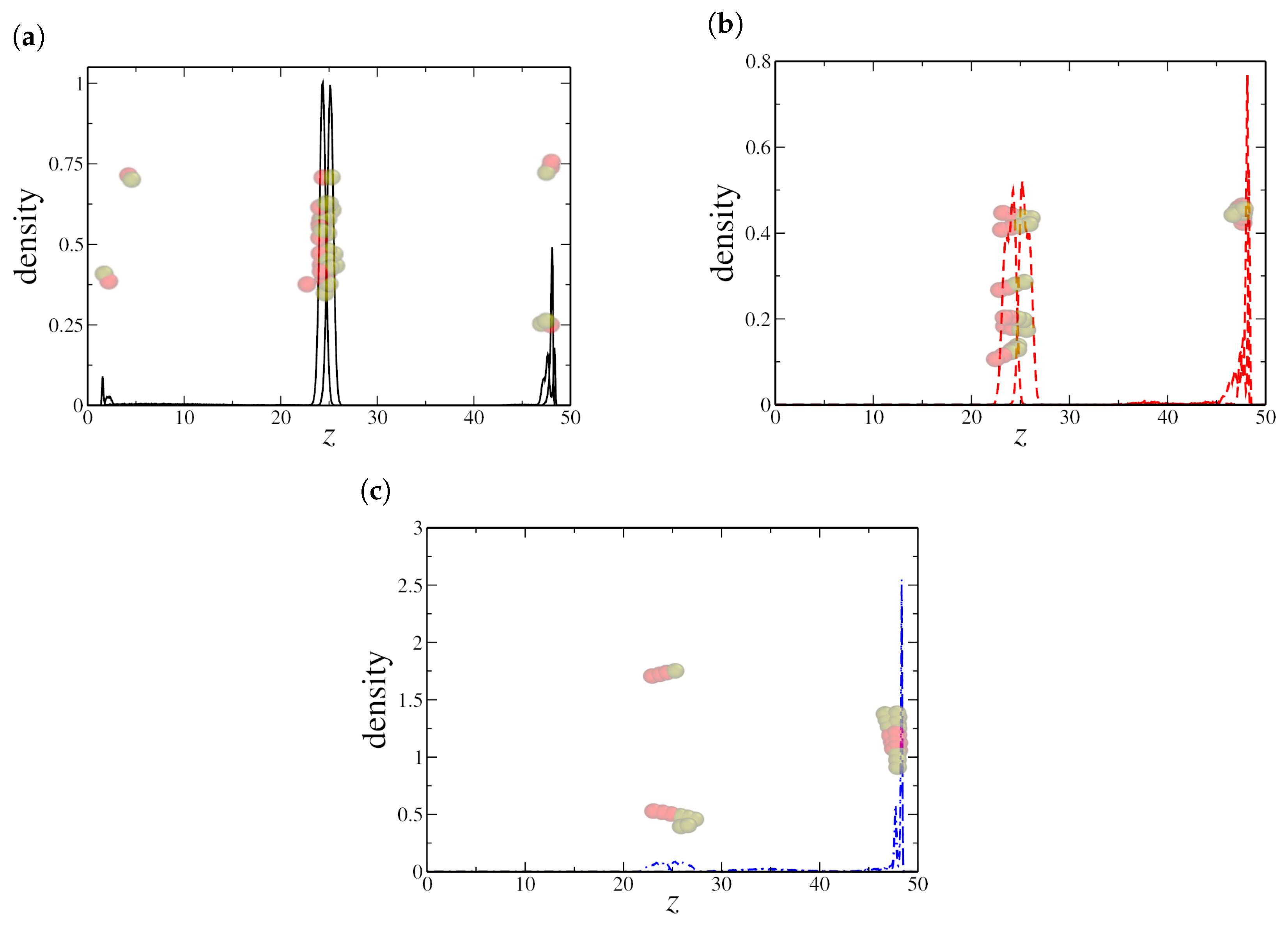
Interestingly, the two NR sizes that exhibited surfactant-like behavior also displayed a discontinuity in surface tension. By analyzing the density distribution at these critical points, specifically at for dimeric NRs and for quadrimeric NRs, we observe in Figure 4a,b that both cases exhibit a well-defined cluster within the oil region. Examination of the corresponding snapshots reveals that these clusters take the form of cylindrical micelles, a characteristic structure commonly observed in surfactant systems.

Figure 4.
Density profile along the z-axis at critical nanorod densities where discontinuities in surface tension were observed: (a) dimeric NRs at and (b) quadrimeric NRs at . (c,d) show the corresponding oil and water density profiles, indicating the formation of nanorod aggregates in the bulk oil region. Here, the oil–water interfaces are located at the peaks in the NRs density profile, near and .
In contrast, hexameric NRs exhibit a different aggregation behavior. This difference suggests that longer nanorods experience distinct packing constraints, likely due to steric hindrance and enhanced interparticle interactions. In fact, hexameric NRs show spherical micelles at very low concentrations. This shape generally forms when the surfactant-like agents have a larger hydrophobic region relative to their hydrophilic counterpart, leading to a more isotropic arrangement in the bulk phase. The absence of cylindrical micelles for hexameric NRs may indicate that their shape prevents efficient alignment into elongated aggregates, leading to a different mode of self-assembly. Furthermore, the formation of spherical micelles rather than cylindrical ones may contribute to the lack of a discontinuity in surface tension, as the transition in aggregation behavior does not significantly affect the interfacial properties in the same manner as observed for shorter nanorods.
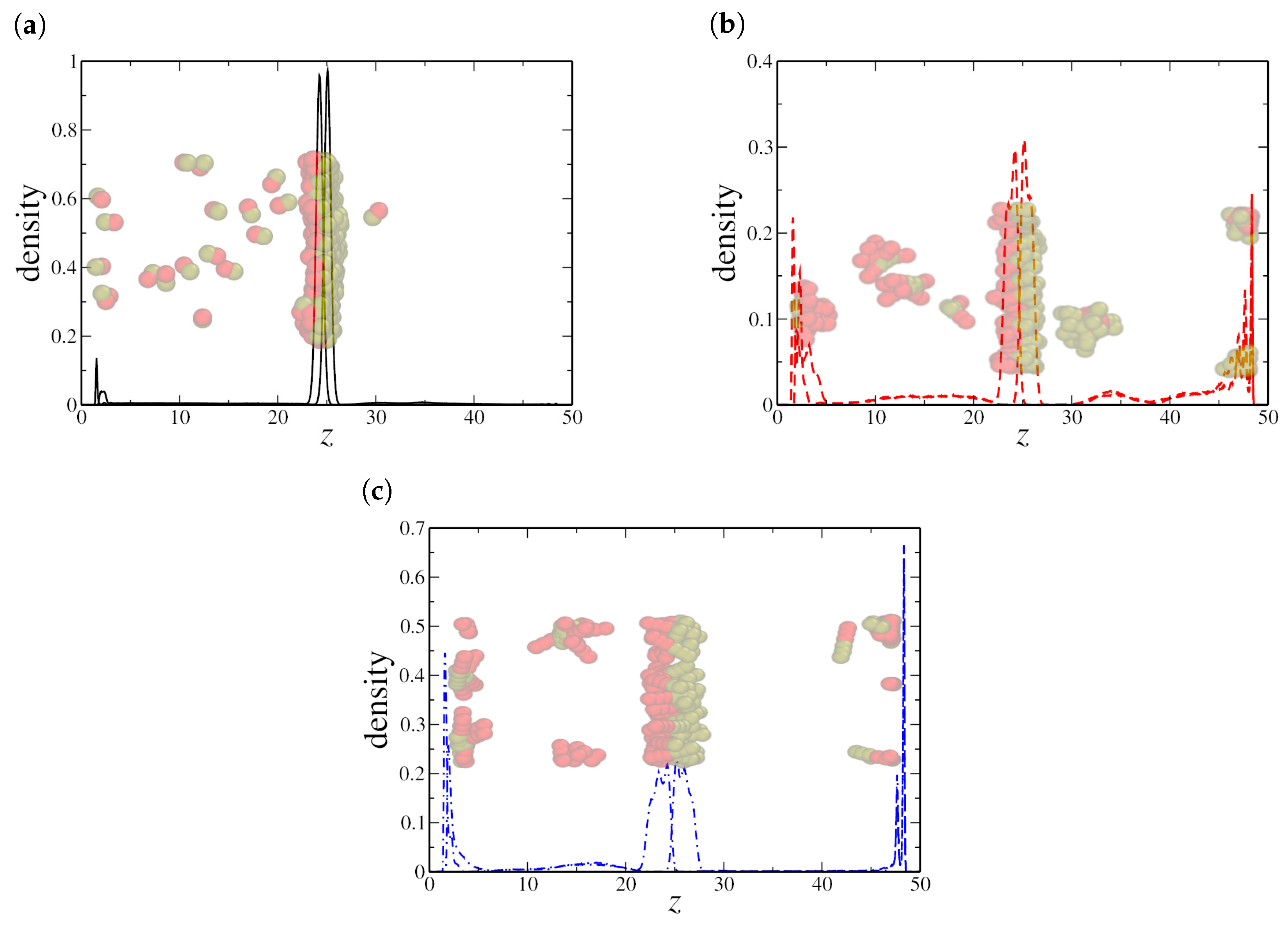
At higher nanorod densities, specifically at shown in Figure 5a–c, the density profiles reveal distinct aggregation behaviors depending on the nanorod length. For dimeric NRs, a well-defined monolayer is observed at the oil–water interface, along with a small fraction of nanorods dispersed in the aqueous phase. This suggests that, while the interface remains saturated with dimeric NRs, some excess nanorods remain solvated in water rather than forming additional aggregates in the oil region. In the case of quadrimeric NRs, we observe both interfacial adsorption and the formation of spherical micelles in the bulk oil and water regions. This behavior aligns with the expected self-assembly of surfactant-like molecules, where excess nanorods, beyond interfacial saturation, transition to micellar structures in the bulk. Interestingly, for hexameric NRs, we observe both interfacial alignment and the presence of spherical micelles in the water region, similar to the quadrimeric case but with a stronger tendency toward micellar formation.

Figure 5.
Density profile along the z-axis for (a) dimeric, (b) quadrimeric, and (c) hexameric nanorods at a high density (). Snapshots from the final frame show nanorod distributions, where (a,b) exhibit both interfacial adsorption and micelle formation in the bulk, while (c) reveals stronger bulk aggregation and interfacial alignment. Here, the oil–water interfaces are located at the peaks in the NRs density profile, near and , respectively.
These results reinforce the trends observed at lower densities and provide further insights into the role of nanorod length in determining their interfacial and bulk phase behavior. The presence of a stable interfacial monolayer for all nanorod sizes confirms their surface activity. However, the difference in bulk aggregation modes—dimeric NRs remaining partially solvated in water, quadrimeric NRs forming spherical micelles, and hexameric NRs showing a dominant micellar phase—highlights how steric effects and hydrophobic interactions dictate their self-assembly. The fact that hexameric NRs do not form cylindrical micelles, even at higher densities, suggests that their aspect ratio favors isotropic aggregation rather than elongated structures. This further explains why the surface tension remains constant in their case, as the system transitions smoothly from interfacial adsorption to bulk self-assembly without a sharp discontinuity. These observations confirm that nanorod length plays a crucial role in determining both the interfacial and bulk phase organization, directly impacting the interfacial tension behavior observed in the simulations.
3.2. Nanoconfined Oil–Water
To further explore the effect of spatial constraints on the interfacial properties of Janus nanorods, we analyzed a nanoconfined system. Unlike the fully periodic system, where nanorods could freely distribute at the interface or within the bulk phases, confinement imposes additional constraints on their organization. The presence of two distinct liquid–solid interfaces further influences their behavior, potentially altering their efficiency in modulating the liquid–liquid interface.
Our results indicate that, under nanoconfinement, all nanorod sizes—dimeric, quadrimeric, and hexameric—led to an increase in interfacial tension, as shown in Figure 6a. This contrasts sharply with the unconfined case, where shorter nanorods (dimers and quadrimers) effectively reduced interfacial tension. The most pronounced increase was observed for hexameric nanorods, exhibiting behavior similar to that found in the fully periodic system. These findings suggest that, under confinement, even the shorter nanorods experience steric restrictions that hinder their optimal adsorption at the interface, thereby reducing their surfactant-like efficiency. In contrast, hexameric nanorods remain largely unaffected by confinement, as their interfacial behavior is already limited by steric hindrance in the bulk system. Figure 6b shows that the nematic order parameter S at the oil–water interface under confinement follows a trend similar to the unconfined case: dimeric nanorods exhibit the highest degree of alignment, and S decreases progressively with increasing nanorod length. This confirms that orientational ordering at the liquid–liquid interface is more favorable for shorter nanorods. However, at the liquid–solid interfaces, the trend is reversed—Figure 6c reveals that hexameric nanorods display a significantly higher degree of nematic ordering. This suggests that, under strong confinement near solid walls, longer nanorods preferentially align due to geometric constraints, leading to enhanced ordering despite their lower interfacial activity.

Figure 6.
(a) Relative interfacial tension as a function of nanorod density in the confined system. The values are normalized by the surface tension of the system in the absence of nanorods (). (b) Nematic order parameter for nanorods at the oil–water interface at the center of the box, , and (c) for nanorods at the liquid–solid interfaces, or .
At very low nanorod densities (), dimeric NRs induce a slight reduction in interfacial tension. This behavior can be attributed to the high density of nanorods adsorbed at the oil–water interface and only a small fraction present at the solid–liquid interfaces, while very few remain in the bulk phases, as indicated by the density profile in Figure 7a. The strong interfacial localization at low concentrations suggests that the system is below the critical aggregation threshold, allowing nanorods to efficiently reduce surface tension. However, for quadrimeric and hexameric nanorods, a higher density is observed at the oil–solid interface compared to the oil–water interface. This difference suggests that longer nanorods preferentially adsorb onto the solid surfaces rather than stabilizing the liquid–liquid interface.
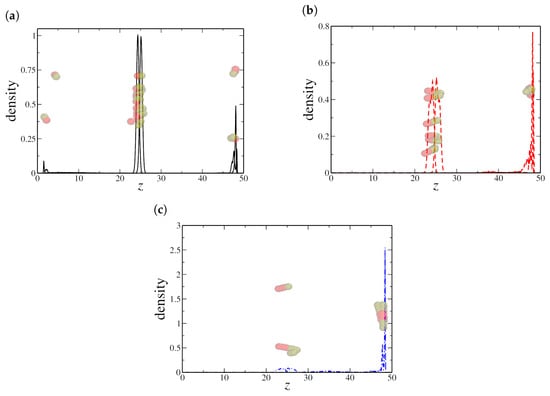
Figure 7.
Density profile along the z-axis for (a) dimeric, (b) quadrimeric, and (c) hexameric nanorods at a low density () in the confined system. Snapshots illustrate the nanorod distribution, highlighting strong interfacial adsorption for (a,b), while (c) shows increased aggregation at the solid–fluid interface. Here, the oil–water interface is located at the central peak in the NRs density profile, near , while the solid–fluid interfaces are located in and , respectively.
This competition between adsorption at the liquid–liquid interface and the solid–fluid interfaces plays a crucial role in determining the interfacial behavior of Janus nanorods. In Figure 8, we present the NR density profile for an intermediate concentration, . As shown in Figure 8b,c, larger clusters tend to aggregate at the solid surface, reinforcing this competition and inhibiting the surfactant-like behavior. The preference for solid–fluid adsorption over liquid–liquid adsorption reduces the number of nanorods available to stabilize the oil–water interface, thereby increasing interfacial tension.
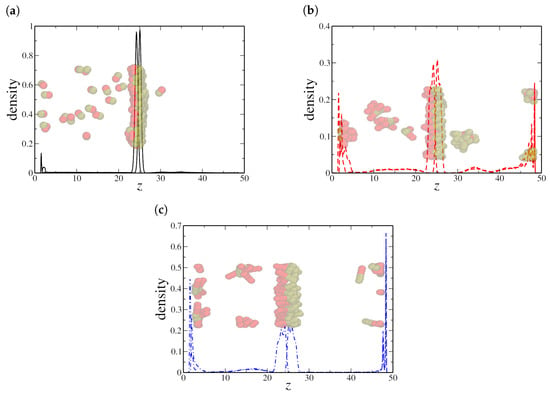
Figure 8.
Density profile along the z-axis for (a) dimeric, (b) quadrimeric, and (c) hexameric nanorods at an intermediate density () in the confined system. Snapshots show nanorod aggregation, with (b,c) displaying a preference for adsorption at the solid–fluid interface rather than the oil–water interface. Here, the oil–water interface is located at the central peak in the NRs density profile, near , while the solid–fluid interfaces are located in and , respectively.
For dimeric NRs, Figure 8a reveals a significant presence of free dimers in the aqueous phase, indicating a transition from predominant interfacial adsorption to partial dispersion in the bulk. This shift suggests that, as nanorod concentration increases, the oil–water interface becomes saturated, leading to excess nanorods migrating into the bulk phase rather than forming additional interfacial layers. As a result, bulk aggregation becomes more prominent, diminishing the efficiency of nanorods in lowering surface tension and potentially altering the overall stability of the interface. This trend aligns with previous observations, reinforcing the idea that nanoconfinement and steric interactions significantly impact the self-assembly and interfacial properties of Janus nanorods.
Finally, the high-density scenario indicates the formation of large aggregates in the water and oil regions for all NR sizes, as shown in Figure 9 for . Even dimeric NRs cluster into a single large aggregate in the oil region (see Figure 9a), while most of the NRs are free in the aqueous region. Clusters with distinct sizes are observed for quadrimeric (Figure 9b) and hexameric (Figure 9c) NRs. In addition to the bulk aggregation, we also observe a significant adsorption of hexameric nanorods at the solid–liquid interface, suggesting that confinement strongly influences the spatial distribution of longer nanorods. The preferential adsorption of hexameric NRs onto solid surfaces rather than at the oil–water interface further distinguishes their behavior from that of shorter nanorods, reinforcing the competition between interfacial adsorption and bulk aggregation.
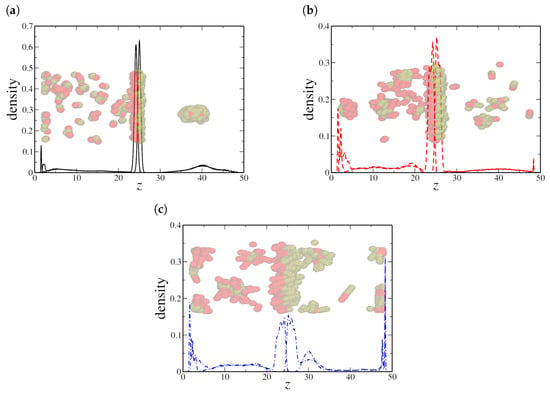
Figure 9.
Density profile along the z-axis for (a) dimeric, (b) quadrimeric, and (c) hexameric nanorods at a high density () in the confined system. Snapshots reveal bulk aggregation for all cases, with hexameric nanorods exhibiting a strong preference for solid–liquid adsorption. Here, the oil–water interface is located at the central peak in the NRs’ density profile, near , while the solid–fluid interfaces are located in and , respectively.
This aggregation behavior results from the interplay between steric effects, nanorod–nanorod interactions, and surface affinity under confinement. At lower concentrations, nanorods primarily localize at the oil–water interface, effectively reducing interfacial tension. However, as the concentration increases, the interface becomes saturated, and excess nanorods either migrate into the bulk phases—where they form aggregates—or adsorb onto the solid surfaces, as observed for the hexameric NRs. The preference of longer nanorods for solid–liquid adsorption suggests that their larger aspect ratio reduces their efficiency in stabilizing the oil–water interface, shifting their preferred adsorption sites.
It highlights the complex balance between interfacial competition, bulk aggregation, and solid surface interactions in confined systems. The Janus NRs’ surfactant-like efficiency is significantly altered under nanoconfinement, particularly at high concentrations, where steric constraints and surface interactions dominate their self-assembly.
4. Conclusions
In this work, we investigated the interfacial behavior of Janus nanorods (NRs) in water–oil separation under two distinct conditions: (i) a thin oil film surrounded by water under fully periodic boundary conditions and (ii) a nanoconfined system where the fluid mixture was constrained between two purely repulsive plates. We used dissipative particle dynamics (DPD) simulations to analyze the effect of nanorod length and confinement on interfacial tension, self-assembly and adsorption at the oil–water interface.
Our results demonstrate that, in the bulk system, shorter nanorods (dimers and quadrimers) exhibit a surfactant-like behavior, effectively reducing interfacial tension by adsorbing at the oil–water interface. However, as the nanorod length increases, steric constraints and interparticle interactions become more significant, limiting their efficiency as interfacial stabilizers. In particular, hexameric nanorods tend to form bulk aggregates rather than distributing uniformly at the interface, leading to a saturation effect that prevents further reduction in surface tension. Additionally, we observed a transition in aggregation behavior at specific nanorod densities, where dimeric and quadrimeric nanorods formed cylindrical micelles in the oil phase, while hexameric nanorods preferentially assembled into spherical micelles.
Under nanoconfinement, the behavior of nanorods was markedly different. In contrast to the bulk system, where dimers and quadrimers reduced interfacial tension, confinement led to an overall increase in surface tension for all nanorod sizes. This effect is attributed to steric hindrances imposed by the solid boundaries, which restrict nanorod mobility and adsorption at the liquid–liquid interface. Moreover, we found that longer nanorods, particularly hexameric ones, exhibited a strong preference for adsorption at the solid–liquid interface rather than the oil–water interface, further diminishing their ability to act as surfactants. This competition between interfacial adsorption and solid–fluid interaction plays a crucial role in defining the self-assembly and interfacial properties of nanorods under confinement.
Our simulations provide a framework for designing functionalized nanorods for enhanced oil recovery, wastewater treatment, and membrane filtration. By tuning nanorod length and surface properties, adsorption efficiency and interfacial stability can be optimized, improving separation performance. The preference of longer nanorods for solid–liquid adsorption under confinement suggests applications in engineered membranes, highlighting the need to consider steric constraints and aggregation in material design. These insights pave the way for new emulsifiers and next-generation amphiphilic nanoparticles with improved selectivity and efficiency in oil–water separation.
Future studies could explore the impact of additional factors, such as the wettability of the confining walls, polydispersity in nanorod size, and external fields (e.g., shear or electric fields) on nanorod self-assembly. Furthermore, extending the simulations to include different types of amphiphilic nanoparticles could provide a broader understanding of how particle shape and chemistry influence interfacial behavior in confined and unconfined systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17081128/s1, Script to run the simulations.
Author Contributions
Conceptualization, J.R.B. and C.F.d.M.J.; methodology, J.R.B. and C.F.d.M.J.; software, A.P.d.S. and J.R.B.; validation, A.P.d.S.; formal analysis, A.P.d.S. and J.R.B.; investigation, J.R.B.; resources, J.R.B. and C.F.d.M.J.; data curation, J.R.B.; writing—original draft preparation, J.R.B. and A.P.d.S.; writing—review and editing, C.F.d.M.J.; supervision, J.R.B. and C.F.d.M.J.; project administration, J.R.B. and C.F.d.M.J.; funding acquisition, J.R.B. and C.F.d.M.J. All authors have read and agreed to the published version of the manuscript.
Funding
Without public funding, this research would be impossible. JRB thanks the National Council for Scientific and Technological Development (CNPq), under grant numbers 405479/2023-9, 441728/2023-5, and 304958/2022-0. CFM thanks the National Institute of Science and Technology (INCT) on Carbon Nanomaterials (INCT/Nanocarbon), and National Institute of Science and Technology (INCT) of Nanomaterials for Life (INCT/NanoLife). APS acknowledges support from the Coordination for the Improvement of Higher Education Personnel Brasil (CAPES), Financing Code 001.
Data Availability Statement
The inputs for espresso-3.2.0 that support the findings of this study are available as Supporting Material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Programme, United Nations World Water Development. The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; United Nations Educational, Scientific and Cultural Organization: London, UK, 2023. [Google Scholar]
- Méndez, C.; Simpson, N.P.; Johnson, F.X.; Birt, A. Climate Change 2023: Synthesis Report (Full Volume) Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2023. [Google Scholar]
- Zhao, Y.; Chang, C.; Ji, H.; Li, Z. Challenges of petroleum wastewater treatment and development trends of advanced treatment technologies: A review. J. Environ. Chem. Eng. 2024, 12, 113767. [Google Scholar] [CrossRef]
- Adetunji1, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, J.; Li, S.; Ge, M.; Teng, L.; Chen, Z.; Lai, Y. Advanced Materials with Special Wettability toward Intelligent Oily Wastewater Remediation. ACS Appl. Mater. Interfaces 2021, 13, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.W.; Lau, E.V.; Poh, P.E. A comprehensive guide of remediation technologies for oil contaminated soil — Present works and future directions. Mar. Pollut. Bull. 2016, 109, 14–45. [Google Scholar] [CrossRef]
- Truskewycz, A.; Gundry, T.D.; Khudur, L.S.; Kolobaric, A.; Taha, M.; Aburto-Medina, A.; Ball, A.S.; Shahsavari, E. Petroleum Hydrocarbon Contamination in Terrestrial Ecosystems—Fate and Microbial Responses. Molecules 2019, 24, 3400. [Google Scholar] [CrossRef] [PubMed]
- Adipah, S. Introduction of Petroleum Hydrocarbons Contaminants and its Human Effects. J. Environ. Sci. Public Health 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Han, M.; Zhang, J.; Chu, W.; Chen, J.; Zhou, G. Research Progress and Prospects of Marine Oily Wastewater Treatment: A Review. Water 2019, 11, 2517. [Google Scholar] [CrossRef]
- Labud, V.; Garcia, C.; Hernandez, T. Effect of hydrocarbon pollution on the microbial properties of a sandy and a clay soil. Chemosphere 2007, 66, 1863–1871. [Google Scholar] [CrossRef]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent improvements in oily wastewater treatment: Progress, challenges, and future opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef]
- Laffon, B.; Pássaro, E.; Valdiglesias, V. Effects of exposure to oil spills on human health: Updated review. J. Toxicol. Environ. Heal. Part B 2016, 19, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef]
- Akizuki, Y.; Bressers, A.; Couse, J.; Healy, C.; Mackey, P.; Martin, D.; Messing, J.; Thomson, J.; Canu, J.; Rosa, L.F.; et al. Oil 2023: Analysis and Forecast to 2028; International Energy Agency—OECD Publishing: Paris, France, 2023. [Google Scholar]
- OILS & FATS International. Vegetable Oil Production in 2023/24 Expected to Be Up on Previous Year; OILS & FATS International: Surrey, UK, 2023. [Google Scholar]
- Arellano, P.; Tansey, K.; Balzter, H.; Boyd, D.S. Detecting the effects of hydrocarbon pollution in the Amazon forest using hyperspectral satellite images. Environ. Pollut. 2015, 205, 225–239. [Google Scholar] [CrossRef]
- Zahri, K.N.M.; Zulkharnain, A.; Sabri, S.; Gomez-Fuentes, C.; Ahmad, S.A. Research Trends of Biodegradation of Cooking Oil in Antarctica from 2001 to 2021: A Bibliometric Analysis Based on the Scopus Database. Int. J. Environ. Res. Public Health 2021, 18, 2050. [Google Scholar] [CrossRef]
- Stark, J.S. Effects of lubricant oil and diesel on macrofaunal communities in marine sediments: A five year field experiment in Antarctica. Environ. Pollut. 2022, 311, 119885. [Google Scholar] [CrossRef]
- Foo, W.H.; Koay, S.S.N.; Chia, S.R.; Chia, W.Y.; Tang, D.Y.Y.; Nomanbhay, S.; Chew, K.W. Recent advances in the conversion of waste cooking oil into value-added products: A review. Fuel 2022, 324, 124539. [Google Scholar] [CrossRef]
- Foo, W.H.; Chia, W.Y.; Tang, D.Y.Y.; Koay, S.S.N.; Lim, S.S.; Chew, K.W. The conundrum of waste cooking oil: Transforming hazard into energy. J. Hazard. Mater. 2021, 417, 126129. [Google Scholar] [CrossRef] [PubMed]
- Tamothran, A.M.; Bhubalan, K.; Anuar, S.T.; Curtis, J.M. The degradation and toxicity of commercially traded vegetable oils following spills in aquatic environment. Environ. Res. 2022, 214, 113985. [Google Scholar] [CrossRef]
- Fingas, M. Vegetable Oil Spills. In Handbook of Oil Spill Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; Chapter 4; pp. 79–91. [Google Scholar] [CrossRef]
- Feng, Y.; Hou, J.; Yang, Y.; Wang, S.; Wang, D.; Cheng, T.; You, Z. Morphology of MoS2 nanosheets and its influence on water/oil interfacial tension: A molecular dynamics study. Fuel 2022, 312, 122938. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Manshad, A.K.; Mohammadi, A.H. Effects of dissolved binary ionic compounds and different densities of brine on interfacial tension (IFT), wettability alteration, and contact angle in smart water and carbonated smart water injection processes in carbonate oil reservoirs. J. Mol. Liq. 2018, 254, 83–92. [Google Scholar] [CrossRef]
- Moradi, S.; Isari, A.A.; Bachari, Z.; Mahmoodi, H. Combination of a new natural surfactant and smart water injection for enhanced oil recovery in carbonate rock: Synergic impacts of active ions and natural surfactant concentration. J. Pet. Sci. Eng. 2019, 176, 1–10. [Google Scholar] [CrossRef]
- Mehrabianfar, P.; Bahraminejad, H.; Manshad, A.K. An introductory investigation of a polymeric surfactant from a new natural source in chemical enhanced oil recovery (CEOR). J. Pet. Sci. Eng. 2021, 198, 108172. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Khaksar Manshad, A.; Mohammadi, A.H. Effects of MgO, γ-Al2O3, and TiO2 Nanoparticles at Low Concentrations on Interfacial Tension (IFT), Rock Wettability, and Oil Recovery by Spontaneous Imbibition in the Process of Smart Nanofluid Injection into Carbonate Reservoirs. ACS Omega 2022, 7, 22161–22172. [Google Scholar] [CrossRef]
- Horstmann, R.; Hecht, L.; Kloth, S.; Vogel, M. Structural and Dynamical Properties of Liquids in Confinements: A Review of Molecular Dynamics Simulation Studies. Langmuir 2022, 38, 6506–6522. [Google Scholar] [CrossRef] [PubMed]
- Krott, L.B.; Gavazzoni, C.; Bordin, J.R. Anomalous diffusion and diffusion anomaly in confined Janus dumbbells. J. Chem. Phys. 2016, 145, 244906. [Google Scholar] [CrossRef] [PubMed]
- Bordin, J.R.; Krott, L.B. Confinement effects on the properties of Janus dimers. Phys. Chem. Chem. Phys. 2016, 18, 28740–28746. [Google Scholar] [CrossRef] [PubMed]
- Bordin, J.R.; Krott, L.B. How Competitive Interactions Affect the Self-Assembly of Confined Janus Dumbbells. J. Phys. Chem. B 2017, 121, 4308–4317. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Huynh, T.T. Sorbent-based devices for the removal of spilled oil from water: A review. Environ. Sci. Pollut. Res. 2021, 28, 28876–28910. [Google Scholar] [CrossRef]
- Lusweti, E.; Kanda, E.K.; Obando, J.; Makokha, M. Effects of oil exploration on surface water quality—A review. Water Pract. Technol. 2022, 17, 2171–2185. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Kalla, S. Use of membrane distillation for oily wastewater treatment—A review. J. Environ. Chem. Eng. 2021, 9, 104641. [Google Scholar] [CrossRef]
- Leão, M.B.; Bordin, J.R.; de Matos, C.F. Specific Surface Area Versus Adsorptive Capacity: An Application View of 3D Graphene-Based Materials for the Removal of Emerging Water Pollutants. Water Air Soil Pollut. 2023, 234, 136. [Google Scholar] [CrossRef]
- Bordin, J.R.; Ferreira de Matos Jauris, C.; Côrtes, P.R.B.; Araújo, W.S.; Moreira, L.S.; Pereira dos Santos, A.; Bitencourt Leão, M.; Moraes, E.E.; Piotrowski, M.J.; Köhler, M.H. Computational condensed matter science contributions to addressing water emerging contaminant pollution: A comprehensive review. J. Phys. Condens. Matter 2025, 37, 113004. [Google Scholar] [CrossRef]
- Gong, J.; Xiang, B.; Sun, Y.; Li, J. Janus smart materials with asymmetrical wettability for on-demand oil/water separation: A comprehensive review. J. Mater. Chem. A 2023, 11, 25093–25114. [Google Scholar] [CrossRef]
- Paiva, F.L.; Secchi, A.R.; Calado, V.; Maia, J.; Khani, S. Slip and momentum transfer mechanisms mediated by Janus rods at polymer interfaces. Soft Matter 2020, 16, 6662–6672. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.T.; Gates, I.D.; Natale, G. Dynamics of Brownian Janus rods at a liquid–liquid interface. Phys. Fluids 2022, 34, 012117. [Google Scholar] [CrossRef]
- Naidu, K.C.B.; Kumar, N.S.; Banerjee, P.; Reddy, B.V.S. A review on the origin of nanofibers/nanorods structures and applications. J. Mater. Sci. Mater. Med. 2021, 32, 68. [Google Scholar] [CrossRef]
- Rafael Bordin, J. Distinct self-assembly aggregation patters of nanorods with decorated ends: A simple model study. Fluid Phase Equilibria 2019, 499, 112251. [Google Scholar] [CrossRef]
- Farajpour, A.; Ghayesh, M.H.; Farokhi, H. A review on the mechanics of nanostructures. Int. J. Eng. Sci. 2018, 133, 231–263. [Google Scholar] [CrossRef]
- Gupta, D.; Varghese, B.S.; Meera Suresh, C.P.; Gupta, T.K. Nanoarchitectonics: Functional nanomaterials and nanostructures—A review. J. Nanoparticle Res. 2022, 24, 196. [Google Scholar] [CrossRef]
- Bai, L.; Wang, X.; Sun, X.; Li, J.; Huang, L.; Sun, H.; Gao, X. Enhanced superhydrophobicity of electrospun carbon nanofiber membranes by hydrothermal growth of ZnO nanorods for oil–water separation. Arab. J. Chem. 2023, 16, 104523. [Google Scholar] [CrossRef]
- Yan, F.; Tong, L.; Qin, H.; Guo, W.; Liu, J.; Xie, W.; Gao, P.; Xiao, H. Preparation of superhydrophilic-underwater superoleophobic silica nanorods modified fiber membrane for efficient oil-in-water emulsion separation. Ceram. Int. 2023, 49, 23317–23325. [Google Scholar] [CrossRef]
- Yin, X.; Yu, S.; Wang, L.; Wang, J.; Wang, B.; Li, H.; Chen, Z. Dual-functional underliquid superhydrophobic and superoleophobic stainless steel mesh decorated with Ni3S2 nanorods for continuous oil/water separation. Surf. Coatings Technol. 2022, 434, 128177. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, L.; Jia, N.; Wang, R.; Liu, L.; Gao, C. Polyphenol-metal manipulated nanohybridization of CNT membranes with FeOOH nanorods for high-flux, antifouling and self-cleaning oil/water separation. J. Membr. Sci. 2020, 600, 117857. [Google Scholar] [CrossRef]
- Yin, X.; Yu, S.; Wang, L.; Li, H.; Xiong, W. Design and preparation of superhydrophobic NiS nanorods on Ni mesh for oil-water separation. Sep. Purif. Technol. 2020, 234, 116126. [Google Scholar] [CrossRef]
- Ahmed, F.U.; Upadhaya, D.; Dhar Purkayastha, D.; Krishna, M.G. Stable hydrophilic and underwater superoleophobic ZnO nanorod decorated nanofibrous membrane and its application in wastewater treatment. J. Membr. Sci. 2022, 659, 120803. [Google Scholar] [CrossRef]
- He, H.; Li, Z.; Ouyang, L.; Liang, Y.; Yuan, S. Hierarchical WO3@Cu(OH)2 nanorod arrays grown on copper mesh with superwetting and self-cleaning properties for high-performance oil/water separation. J. Alloy. Compd. 2021, 855, 157421. [Google Scholar] [CrossRef]
- Español, P.; Warren, P.B. Perspective: Dissipative particle dynamics. J. Chem. Phys. 2017, 146, 150901. [Google Scholar] [CrossRef]
- Hoogerbrugge, P.J.; Koelman, J.M.V.A. Simulating Microscopic Hydrodynamic Phenomena with Dissipative Particle Dynamics. Europhys. Lett. 1992, 19, 155. [Google Scholar] [CrossRef]
- Español, P.; Warren, P. Statistical Mechanics of Dissipative Particle Dynamics. Europhys. Lett. 1995, 30, 191. [Google Scholar] [CrossRef]
- Limbach, H.; Arnold, A.; Mann, B.; Holm, C. ESPResSo—An extensible simulation package for research on soft matter systems. Comput. Phys. Commun. 2006, 174, 704–727. [Google Scholar] [CrossRef]
- Arnold, A.; Lenz, O.; Kesselheim, S.; Weeber, R.; Fahrenberger, F.; Roehm, D.; Košovan, P.; Holm, C. ESPResSo 3.1: Molecular Dynamics Software for Coarse-Grained Models. In Proceedings of the Meshfree Methods for Partial Differential Equations VI; Griebel, M., Schweitzer, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–23. [Google Scholar]
- Moeendarbary, E.; Ng, T.; Zangeneh, M. Dissipative particle dynamics: Introduction, methodology and complex fluid applications—A review. Int. J. Appl. Mech. 2009, 1, 737–763. [Google Scholar] [CrossRef]
- Khedr, A.; Striolo, A. DPD Parameters Estimation for Simultaneously Simulating Water–Oil Interfaces and Aqueous Nonionic Surfactants. J. Chem. Theory Comput. 2018, 14, 6460–6471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, X.; Liang, C.; Wang, Z.; Jia, Y. Dissipative particle dynamics to study asphaltenes and surfactants interactions at the oil–water interface. J. Mol. Liq. 2023, 381, 121802. [Google Scholar] [CrossRef]
- Parsons, J.D. Nematic ordering in a system of rods. Phys. Rev. A 1979, 19, 1225–1230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).