Hydrotalcite Supported on Polycaprolactone:Poly(methyl methacrylate) Fiber Membranes for Chlorogenic Acid Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrotalcite Modified with CTAB (LDH-CTAB)
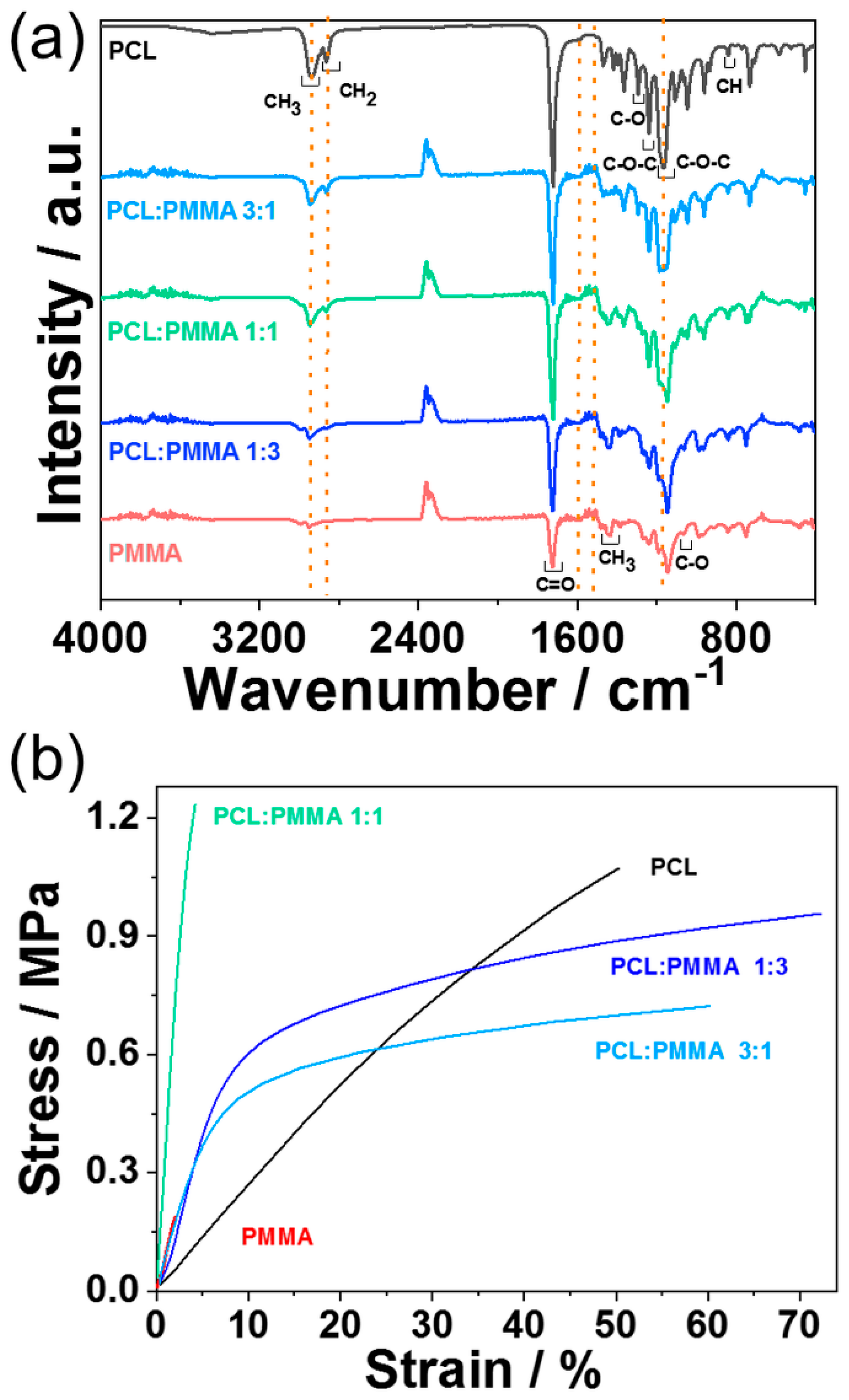
2.3. PCL:PMMA Fibers
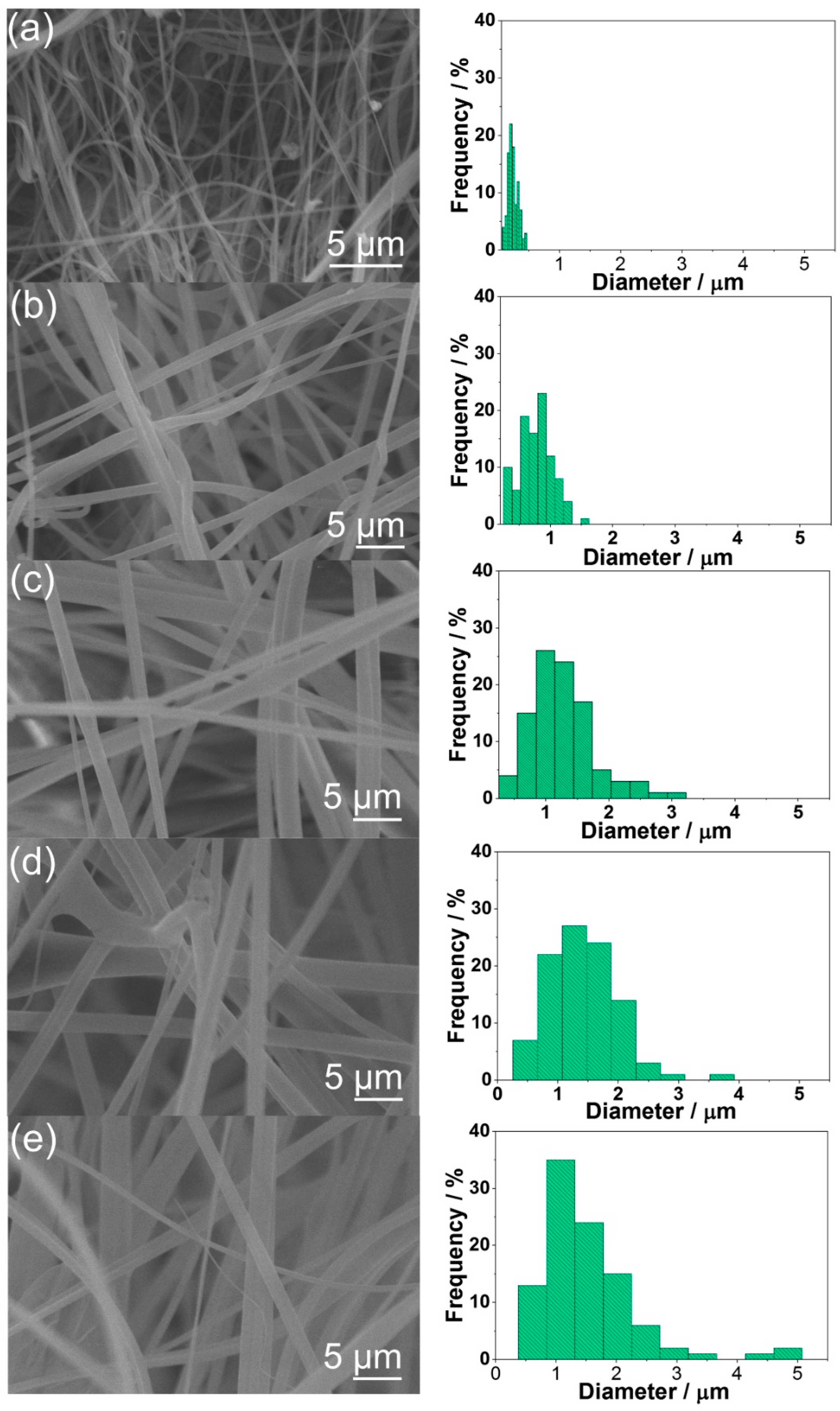
2.4. PCL:PMMA:LDH-CTAB (PPLDH) Fibers
2.5. Material Characterizations
2.6. Chlorogenic Acid (CGA) Removal Assays
2.6.1. LDH Loose Particles
2.6.2. Filter Membranes
3. Results and Discussion
3.1. LDH Characterization
3.2. PCL:PPMA Fibers
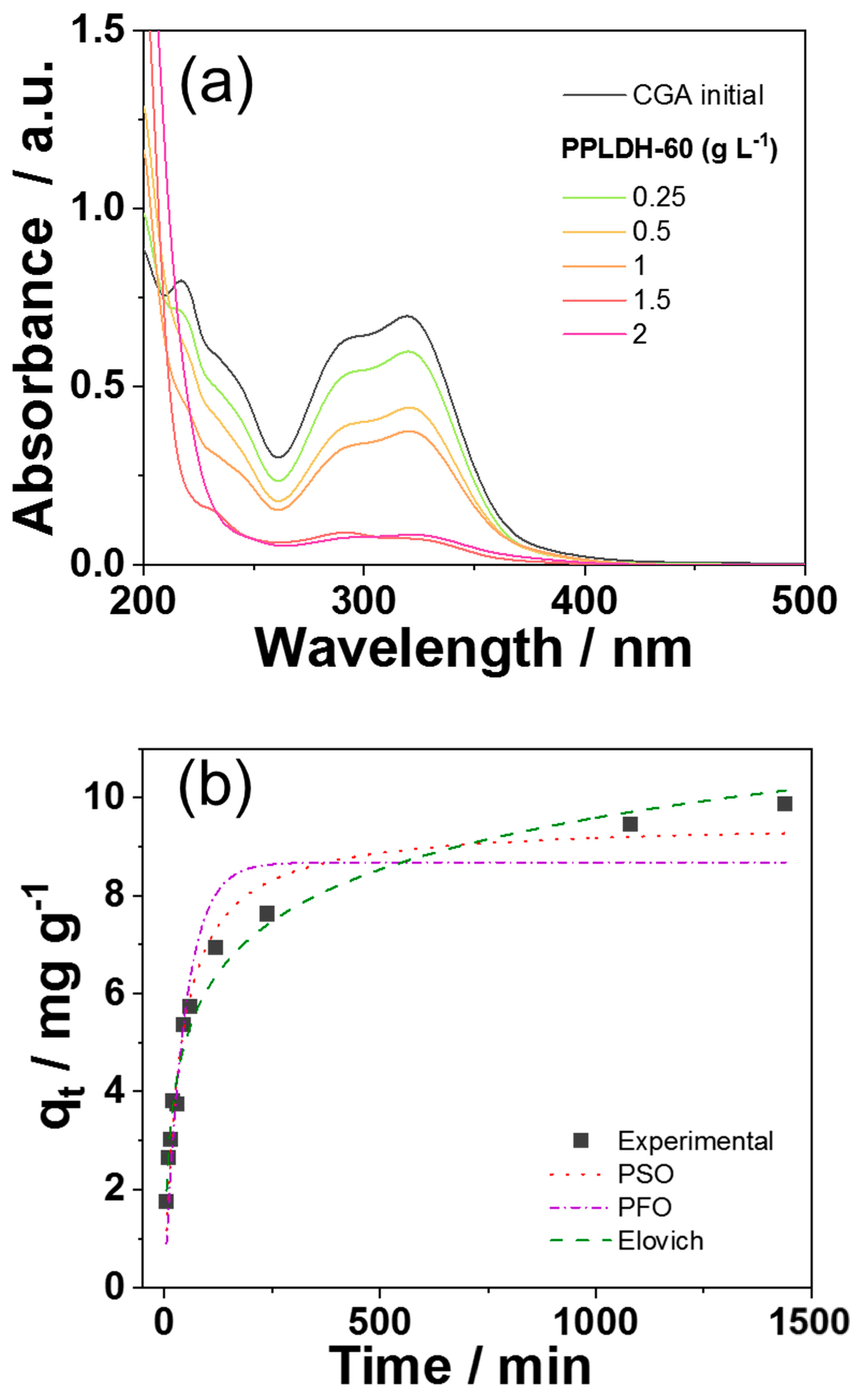
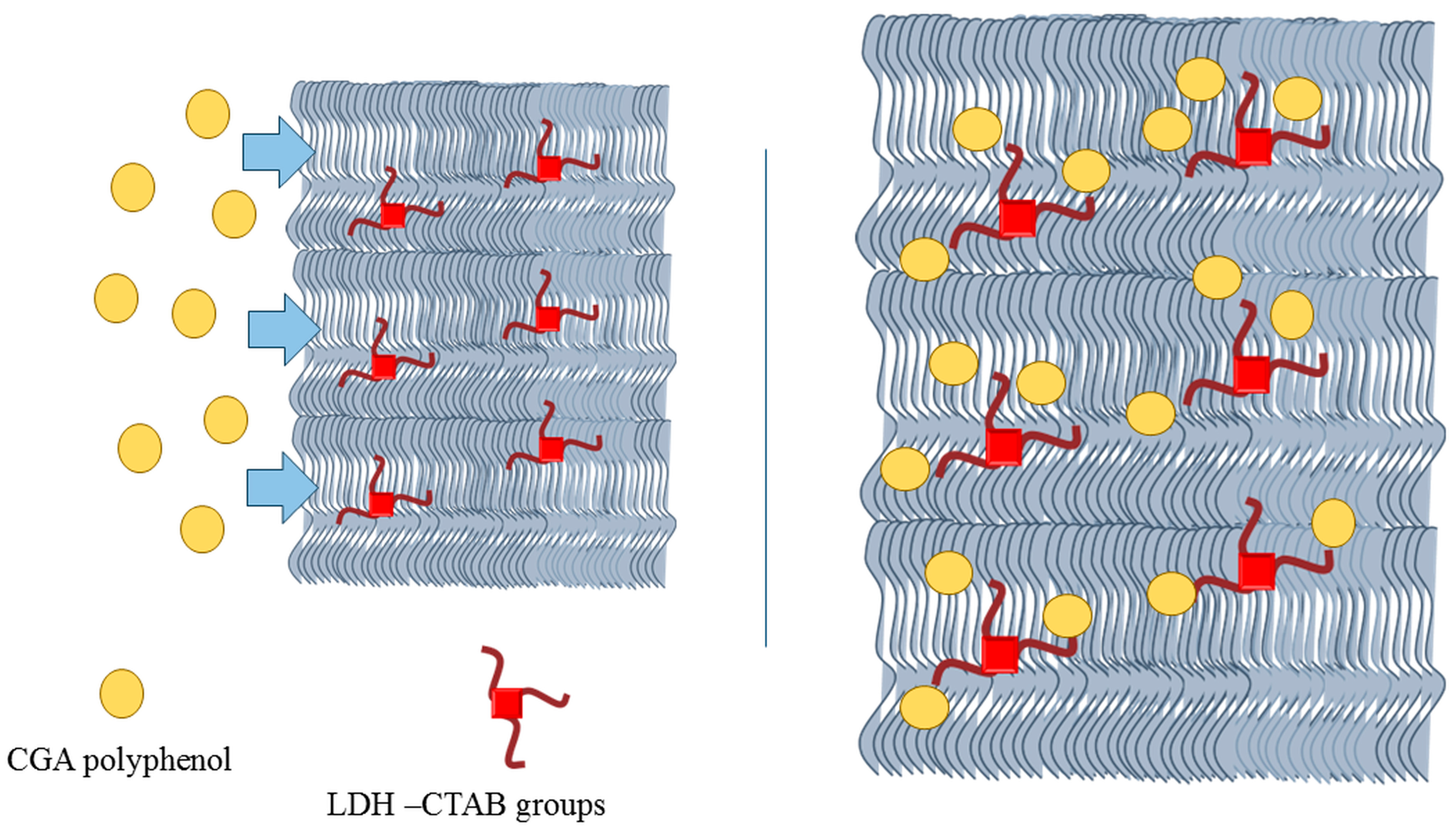
3.3. PPLDH Adsorbing Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kundu, A.; Vadassery, J. Chlorogenic acid-mediated chemical defence of plants against insect herbivores. Plant Biol. 2019, 21, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Sas, O.G.; Castro, M.; Domínguez, Á.; González, B. Removing phenolic pollutants using Deep Eutectic Solvents. Sep. Purif. Technol. 2019, 227, 115703. [Google Scholar] [CrossRef]
- Tarahi, M.; Gharagozlou, M.; Niakousari, M.; Hedayati, S. Protein–Chlorogenic Acid Interactions: Mechanisms, Characteristics, and Potential Food Applications. Antioxidants 2024, 13, 777. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Nematollahzadeh, A.; Shojaei, A.; Karimi, M. Chemically modified organic/inorganic nanoporous composite particles for the adsorption of reactive black 5 from aqueous solution. React. Funct. Polym. 2015, 86, 7–15. [Google Scholar] [CrossRef]
- Paris, E.C.; Malafatti, J.O.D.; Sciena, C.R.; Junior, L.F.N.; Zenatti, A.; Escote, M.T.; Moreira, A.J.; Freschi, G.P.G. Nb2O5 nanoparticles decorated with magnetic ferrites for wastewater photocatalytic remediation. Environ. Sci. Pollut. Res. 2021, 28, 23731–23741. [Google Scholar] [CrossRef]
- Meirelles, M.R.; Malafatti, J.O.D.; Escote, M.T.; Pinto, A.H.; Paris, E.C. Magnetic Adsorbent Based on Faujasite Zeolite Decorated with Magnesium Ferrite Nanoparticles for Metal Ion Removal. Magnetochemistry 2023, 9, 136–149. [Google Scholar] [CrossRef]
- Mascarenhas, B.C.; Tavares, F.A.; Paris, E.C. Functionalized faujasite zeolite immobilized on poly(lactic acid) composite fibers to remove dyes from aqueous media. J. Appl. Polym. Sci. 2020, 137, 48561. [Google Scholar] [CrossRef]
- Nascimento, A.C.A.; Ruellas, T.M.O.; Malafatti, J.O.D.; Paris, E.C. Enhanced adsorption of chlorogenic acid polyphenol by layered double hydroxide modified with sodium dodecyl sulfate. J. Phys. Conf. Ser. 2023, 2516, 012001. [Google Scholar] [CrossRef]
- Pagalan, E.; Sebron, M.; Gomez, S.; Salva, S.J.; Ampusta, R.; Macarayo, A.J.; Joyno, C.; Ido, A.; Arazo, R. Activated carbon from spent coffee grounds as an adsorbent for treatment of water contaminated by aniline yellow dye. Ind. Crops Prod. 2020, 145, 111953. [Google Scholar] [CrossRef]
- Paris, E.C.; Malafatti, J.O.D.; Musetti, H.C.; Manzoli, A.; Zenatti, A.; Escote, M.T. Faujasite zeolite decorated with cobalt ferrite nanoparticles for improving removal and reuse in Pb2+ ions adsorption. Chin. J. Chem. Eng. 2020, 28, 1884–1890. [Google Scholar] [CrossRef]
- Malafatti, J.O.D.; Tavares, F.A.; Neves, T.R.; Mascarenhas, B.C.; Quaranta, S.; Paris, E.C. Modified Silica Nanoparticles from Rice Husk Supported on Polylactic Acid as Adsorptive Membranes for Dye Removal. Materials 2023, 16, 2429. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Bakry, B.M.; Adlii, A.; Yakout, S.M.; El-Zaidy, M.A. Facile conversion of kaolinite into clay nanotubes (KNTs) of enhanced adsorption properties for toxic heavy metals (Zn2+, Cd2+, Pb2+, and Cr6+) from water. J. Hazard. Mater. 2019, 374, 296–308. [Google Scholar] [CrossRef]
- Yang, K.; Yan, L.-G.; Yang, Y.-M.; Yu, S.-J.; Shan, R.-R.; Yu, H.-Q.; Zhu, B.-C.; Du, B. Adsorptive removal of phosphate by Mg-Al and Zn-Al layered double hydroxides: Kinetics, isotherms and mechanisms. J. Hazard. Mater. 2014, 124, 36–42. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Ribeiro, C. [Mg-Al]-LDH and [Zn-Al]-LDH as matrices for removal of high loadings of phosphate. Mater. Res. 2018, 21, e20171001–e20171009. [Google Scholar] [CrossRef]
- Crepaldi, E.L.; Valim, J.B. Hidróxidos duplos lamelares: Síntese, estrutura, propriedades e aplicações. Quim. Nova 1998, 21, 300–311. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Silva, R.A.; Lopes, J.A.; Eulálio, D.; dos Santos, R.M.M.; De Aquino, L.A.; Vergütz, L.; Novais, R.F.; Da Costa, L.M.; Pinto, F.G.; et al. Layered double hydroxides: Nanomaterials for applications in agriculture | Hidróxidos duplos lamelares: Nanomateriais para aplicações na agricultura. Rev. Bras. Cienc. Do Solo 2015, 39, 1–13. [Google Scholar] [CrossRef]
- Malafatti, J.O.D.; Ruellas, T.M.D.O.; Meirelles, M.R.; Thomazi, A.C.; Renda, C.G.; Paris, E.C. Nanocarriers of Eu3+ doped silica nanoparticles modified by APTES for luminescent monitoring of cloxacillin. AIMS Mater. Sci. 2021, 8, 760–775. [Google Scholar] [CrossRef]
- Lima, R.C.; Espinosa, J.W.M.; Gurgel, M.F.C.; Paris, E.C.; Leite, E.R.; Joya, M.R.; Pizani, P.S.; Varela, J.A.; Longo, E. Photoluminescence in disordered Sm-doped PbTiO3: Experimental and theoretical approach. J. Appl. Phys. 2006, 100, 034917. [Google Scholar] [CrossRef]
- Moura, A.P.; Cavalcante, L.S.; Sczancoski, J.C.; Stroppa, D.G.; Paris, E.C.; Ramirez, A.J.; Varela, J.A.; Longo, E. Structure and growth mechanism of CuO plates obtained by microwave-hydrothermal without surfactants. Adv. Powder Technol. 2010, 21, 197–202. [Google Scholar] [CrossRef]
- Leite, E.R.; Paris, E.C.; Longo, E. Direct Amorphous-to-Cubic Perovskite Phase Transformation for Lead Titanate. J. Am. Ceram. Soc. 2000, 41, 1539–1541. [Google Scholar] [CrossRef]
- Paris, E.C.; Espinosa, J.W.M.; de Lazaro, S.; Lima, R.C.; Joya, M.R.; Pizani, P.S.; Leite, E.R.; Souza, A.G.; Varela, J.A.; Longo, E. Er3+ as marker for order-disorder determination in the PbTiO3 system. Chem. Phys. 2007, 335, 7–14. [Google Scholar] [CrossRef]
- Manzoli, A.; Shimizu, F.M.; Mercante, L.A.; Paris, E.C.; Oliveira, O.N.; Correa, D.S.; Mattoso, L.H.C. Layer-by-layer fabrication of AgCl-PANI hybrid nanocomposite films for electronic tongues. Phys. Chem. Chem. Phys. 2014, 16, 24275–24281. [Google Scholar] [CrossRef]
- Ruellas, T.M.O.; Peçanha, L.O.O.; Domingos, G.H.S.; Sciena, C.R.; Malafatti, J.O.D.; Paris, E.C.; Maestrelli, S.C.; Giraldi, T.R. Zinc oxide pieces obtained by pressing and slip casting: Physical, structural and photocatalytic properties. Environ. Technol. 2019, 42, 1861–1873. [Google Scholar] [CrossRef]
- de Jesus, E.T.; Moreira, A.J.; Sá, M.C.; Freschi, G.P.G.; Joya, M.R.; Li, M.S.; Paris, E.C. Potential of Nb2O5 nanofibers in photocatalytic degradation of organic pollutants. Environ. Sci. Pollut. Res. 2021, 28, 69401–69415. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Behrens, A.M.; Sandler, A.D.; Kofinas, P. A Review of the Fundamental Principles and Applications of Solution Blow Spinning. ACS Appl. Mater. Interfaces 2016, 8, 34951–34963. [Google Scholar] [CrossRef]
- Dadol, G.C.; Kilic, A.; Tijing, L.D.; Lim, K.J.A.; Cabatingan, L.K.; Tan, N.P.B.; Stojanovska, E.; Polat, Y. Solution blow spinning (SBS) and SBS-spun nanofibers: Materials, methods, and applications. Mater. Today Commun. 2020, 25, 101656. [Google Scholar] [CrossRef]
- Kenry; Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Tomecka, E.; Wojasinski, M.; Jastrzebska, E.; Chudy, M.; Ciach, T.; Brzozka, Z. Poly(L-lactic acid) and polyurethane nanofibers fabricated by solution blow spinning as potential substrates for cardiac cell culture. Mater. Sci. Eng. C 2017, 75, 305–316. [Google Scholar] [CrossRef]
- Bonan, R.F.; Bonan, P.R.F.; Batista, A.U.D.; Perez, D.E.C.; Castellano, L.R.C.; Oliveira, J.E.; Medeiros, E.S. Poly(lactic acid)/poly(vinyl pyrrolidone) membranes produced by solution blow spinning: Structure, thermal, spectroscopic, and microbial barrier properties. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Mercante, L.A.; Facure, M.H.M.; Locilento, D.A.; Sanfelice, R.C.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Solution blow spun PMMA nanofibers wrapped with reduced graphene oxide as an efficient dye adsorbent. New J. Chem. 2017, 41, 9087–9094. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Li, P.; Jia, L.; Wang, F.; Liu, Y.; Wang, H.; Yu, L.; Li, B. One-Step, Large-Scale Blow Spinning to Fabricate Ultralight, Fibrous Sorbents with Ultrahigh Oil Adsorption Capacity. ACS Appl. Mater. Interfaces 2021, 13, 6631–6641. [Google Scholar] [CrossRef]
- Xu, X.; Yue, Y.; Cai, D.; Song, J.; Han, C.; Liu, Z.; Wang, D.; Xiao, J.; Wu, H. Aqueous Solution Blow Spinning of Seawater-Stable Polyamidoxime Nanofibers from Water-Soluble Precursor for Uranium Extraction from Seawater. Small Methods 2020, 4, 1–10. [Google Scholar] [CrossRef]
- da Costa Farias, R.M.; Mota, M.F.; Severo, L.L.; de Medeiros, E.S.; Klamczynski, A.P.; de Jesús Avena-Bustillos, R.; de Lima Santana, L.N.; de Araújo Neves, G.; Glenn, G.M.; Menezes, R.R. Green synthesis of porous N-Carbon/Silica nanofibers by solution blow spinning and evaluation of their efficiency in dye adsorption. J. Mater. Res. Technol. 2020, 9, 3038–3046. [Google Scholar] [CrossRef]
- Jia, J.; Lee, L.; Ang, B.C.; Andriyana, A.; Shariful, I.; Amalina, M.A. Fabrication of PMMA / zeolite nanofibrous membrane through electrospinning and its adsorption behavior. Appl. Polym. Sci. 2017, 134, 1–13. [Google Scholar] [CrossRef]
- Oriero, D.A.; Gyan, I.O.; Bolshaw, B.W.; Cheng, I.F.; Aston, D.E. Electrospun biocatalytic hybrid silica—PVA-tyrosinase fi ber mats for electrochemical detection of phenols. Microchem. J. 2015, 118, 166–175. [Google Scholar] [CrossRef]
- Romeo, V.; Gorrasi, G.; Vittoria, V.; Chronakis, I.S. Encapsulation and exfoliation of inorganic lamellar fillers into polycaprolactone by electrospinning. Biomacromolecules 2007, 8, 3147–3152. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Pinto, J.; Nanni, G.; Fragouli, D.; Athanassiou, A. Enhanced oil removal from water in oil stable emulsions using electrospun nanocomposite fiber mats. RSC Adv. 2018, 8, 7641–7650. [Google Scholar] [CrossRef]
- Li, R.; Li, Z.; Yang, R.; Yin, X.; Lv, J.; Zhu, L.; Yang, R. Polycaprolactone/poly(L-lactic acid) composite micro/nanofibrous membrane prepared through solution blow spinning for oil adsorption. Mater. Chem. Phys. 2020, 241, 122338. [Google Scholar] [CrossRef]
- Catauro, M.; Angelo, A.D.; Viola, V.; Cimmino, G.; Pacifico, S. Chlorogenic Acid Hybrids by Sol—Gel Route. Molecules 2023, 28, 3486–3499. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, C.; Wang, P.; Zhang, Y.; Zhang, H. Electrospun chitosan/polycaprolactone nanofibers containing chlorogenic acid-loaded halloysite nanotube for active food packaging. Carbohydr. Polym. 2020, 247, 116711. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Xue, J.; Zhang, H.; Wang, F.; Liu, J. Mechanistic understanding of the effect of zein–chlorogenic acid interaction on the properties of electrospun nanofiber films. Food Chem. X 2022, 16, 100454. [Google Scholar] [CrossRef]
- Yu, D.; Duan, Z.; Wang, A.; Li, L.; Guo, H.; Deng, B.; Li, D.; Li, H.; Liu, Q. Structure and properties of chlorogenic acid-loaded polylactide fiber prepared by melt spinning. Int. J. Biol. Macromol. 2024, 265, 130810. [Google Scholar] [CrossRef]
- Ejiohuo, O.; Folami, S.O.; Edi, D.; Isaac, J. Polyphenol encapsulated nanofibers in wound healing and drug delivery. Eur. J. Med. Chem. Rep. 2024, 12, 100184. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, R.; Zhang, X.; Yin, X.; Tian, H.; Zhu, L. Preparation, oil adsorption behavior, and mechanism of micro/nanofibrous polycaprolactone membrane prepared through solution blow spinning. Polym. Adv. Technol. 2020, 31, 686–698. [Google Scholar] [CrossRef]
- Song, J.; Li, Z.; Wu, H. Blowspinning: A New Choice for Nanofibers. ACS Appl. Mater. Interfaces 2020, 12, 33447–33464. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Lima-Corrêa, R.A.B.; Castro, C.S.; Damasceno, A.S.; Assaf, J.M. The enhanced activity of base metal modified MgAl mixed oxides from sol-gel hydrotalcite for ethylic transesterification. Renew. Energy 2020, 146, 1984–1990. [Google Scholar] [CrossRef]
- Omdeo, K.G.; Ajay, V.R.; Krishnan, K.; Abitha, V.K.; Nikesh, S.; Sabu, T.; Satyendra, M. Surface modification of synthesized Layered Double Hydroxide [LDH] for methylene blue dye removal in textile industry via photocatalytic activity under visible light. J. Nano Res. 2017, 46, 135–147. [Google Scholar] [CrossRef]
- Zhou, L.; Slaný, M.; Bai, B.; Du, W.; Qu, C.; Zhang, J.; Tang, Y. Enhanced Removal of Sulfonated Lignite from Oil Wastewater with Multidimensional MgAl-LDH Nanoparticles. Nanomaterials 2021, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Rani, A. Adsorption of resorcinol from aqueous solution onto CTAB/NaOH/flyash composites: Equilibrium, kinetics and thermodynamics. J. Environ. Chem. Eng. 2017, 5, 526–538. [Google Scholar] [CrossRef]
- Ceylan, Z.; Mustafaoglu, D.; Malkoc, E. Adsorption of phenol by MMT-CTAB and WPT-CTAB: Equilibrium, kinetic, and thermodynamic study. Part. Sci. Technol. 2018, 36, 716–726. [Google Scholar] [CrossRef]
- Hernández-Padilla, E.S.; Zárate-Guzmán, A.I.; González-Ortega, O.; Padilla-Ortega, E.; Gómez-Durán, A.; Delgado-Sánchez, P.; Aguilar-Aguilar, A.; Cortés, F.B.; Ocampo-Pérez, R. Elucidation of adsorption mechanisms and mass transfer controlling resistances during single and binary adsorption of caffeic and chlorogenic acids. Environ. Sci. Pollut. Res. 2022, 29, 26297–26311. [Google Scholar] [CrossRef]
- Xu, X.; Song, K.; Guo, J.; Liu, S.; Zhou, X.; He, J. Adsorption behavior of amino functionalized MCM-41 on chlorogenic acid from Eucommia ulmoides leaves. J. Porous Mater. 2023, 30, 71–81. [Google Scholar] [CrossRef]
- Xu, D.; Chen, L.; Liu, X.; Wang, J.; Jiao, S.; Yao, X.; Lin, R. Preparation of porous chitosan aerogels for efficient adsorption and sustained release of chlorogenic acid via enhanced water permeability. Eur. Polym. J. 2024, 207, 112821. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Jin, P.; Jiang, M.; Zhu, G.; Duan, Y.; Yang, Z.; Qiu, H. Preparation of mesoporous silica materials functionalized with various amino-ligands and investigation of adsorption performances on aromatic acids. Chem. Eng. J. 2020, 379, 122405. [Google Scholar] [CrossRef]
- Son, S.R.; Linh, N.T.B.; Yang, H.M.; Lee, B.T. In vitro and in vivo evaluation of electrospun PCL/PMMA fibrous scaffolds for bone regeneration. Sci. Technol. Adv. Mater. 2013, 14, 015009. [Google Scholar] [CrossRef]
- Kruse, M.; Walter, P.; Bauer, B.; Rütten, S.; Schaefer, K.; Plange, N.; Gries, T.; Jockenhoevel, S.; Fuest, M. Electro-spun Membranes as Scaffolds for Human Corneal Endothelial Cells. Curr. Eye Res. 2018, 43, 1–11. [Google Scholar] [CrossRef]
- Simões, M.C.R.; Cragg, S.M.; Barbu, E.; De Sousa, F.B. The potential of electrospun poly(methyl methacrylate)/polycaprolactone core–sheath fibers for drug delivery applications. J. Mater. Sci. 2019, 54, 5712–5725. [Google Scholar] [CrossRef]
- Philip, P.; Tomlal Jose, E.; Chacko, J.K.; Philip, K.C.; Thomas, P.C. Preparation and characterisation of surface roughened PMMA electrospun nanofibers from PEO—PMMA polymer blend nanofibers. Polym. Test. 2019, 74, 257–265. [Google Scholar] [CrossRef]
- Munj, H.R.; Tomasko, D.L. Polycaprolactone-polymethyl methacrylate electrospun blends for biomedical applications. Polym. Sci. Ser. A 2017, 59, 695–707. [Google Scholar] [CrossRef]
- Pirzada, A.M.; Ali, I.; Mallah, N.B.; Maitlo, G. Composite electrospun membranes based on PET-PAN modified with LDH-hybrid as promising adsorbent for pollutants removal from wastewater. Glob. Nest J. 2024, 26, 05650. [Google Scholar] [CrossRef]
- Lv, W.; Mei, Q.; Du, M.; Xiao, J.; Ye, W.; Zheng, Q. Interaction between Poly (vinyl alcohol) and Layered Double Hydroxide (LDH) Particles with Di ff erent Topological Shape and Their Application in Electrospinning. J. Phys. Chem. C 2016, 120, 14435–14443. [Google Scholar] [CrossRef]
- Mushtaq, M.; Wasim, M.; Naeem, M.A.; Khan, M.R.; Yue, S.; Saba, H.; Hussain, T.; Siddiqui, M.Q.; Farooq, A.; Wei, Q. Composite of PLA Nanofiber and Hexadecyl Trimethyl-Ammonium Chloride-Modified Montmorillonite Clay: Fabrication and Morphology. Coatings 2020, 10, 484. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Sharif, N.; Jafari, S.M. Loading of phenolic compounds into electrospun nanofibers and electrosprayed nanoparticles. Trends Food Sci. Technol. 2020, 95, 59–74. [Google Scholar] [CrossRef]
- Cortés, J.C.; Navarro-Quiles, A.; Santonja, F.J.; Sferle, S.M. Statistical analysis of randomized pseudo-first/second order kinetic models. Application to study the adsorption on cadmium ions onto tree fern. Chemom. Intell. Lab. Syst. 2023, 240, 104910. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- de Oliveira, C.; Renda, C.G.; Moreira, A.J.; Pereira, O.A.P.; Pereira, E.C.; Freschi, G.P.G.; Bertholdo, R. Evaluation of a graphitic porous carbon modified with iron oxides for atrazine environmental remediation in water by adsorption. Environ. Res. 2023, 219, 115054. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, L.; Lv, J. Effects of expandable graphite and modified ammonium polyphosphate on the flame-retardant and mechanical properties of wood flour-polypropylene composites. Polym. Polym. Compos. 2013, 21, 449–456. [Google Scholar] [CrossRef]
- dos Santos, R.M.M.; Gonçalves, R.G.L.; Constantino, V.R.L.; Santilli, C.V.; Borges, P.D.; Tronto, J.; Pinto, F.G. Adsorption of Acid Yellow 42 dye on calcined layered double hydroxide: Effect of time, concentration, pH and temperature. Appl. Clay Sci. 2017, 140, 132–139. [Google Scholar] [CrossRef]
- Zubair, M.; Jarrah, N.; Manzar, M.S.; Al-Harthi, M.; Daud, M.; Mu’azu, N.D.; Haladu, S.A. Adsorption of eriochrome black T from aqueous phase on MgAl-, CoAl- and NiFe- calcined layered double hydroxides: Kinetic, equilibrium and thermodynamic studies. J. Mol. Liq. 2017, 230, 344–352. [Google Scholar] [CrossRef]
- Bharali, D.; Deka, R.C. Adsorptive removal of congo red from aqueous solution by sonochemically synthesized NiAl layered double hydroxide. J. Environ. Chem. Eng. 2017, 5, 2056–2067. [Google Scholar] [CrossRef]
- Zhu, Z.; Ouyang, S.; Li, P.; Shan, L.; Ma, R.; Zhang, P. Persistent organic pollutants removal via hierarchical flower-like layered double hydroxide: Adsorption behaviors and mechanism investigation. Appl. Clay Sci. 2020, 188, 105500. [Google Scholar] [CrossRef]
- Yan, L.; Yin, Y.; Lv, P.; Zhang, Z.; Wang, J.; Long, F. Synthesis and Application of Novel 3D Magnetic Chlorogenic Acid Imprinted Polymers Based on a Graphene-Carbon Nanotube Composite. J. Agric. Food Chem. 2016, 64, 3091–3100. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Pan, J.; Van Der Bruggen, B.; Shen, J. A novel chitosan base molecularly imprinted membrane for selective separation of chlorogenic acid. Sep. Purif. Technol. 2016, 164, 70–80. [Google Scholar] [CrossRef]
- Li, Z.; Al-Wraikat, M.; Hao, C.; Liu, Y. Comparison of Non-Covalent and Covalent Interactions between Lactoferrin and Chlorogenic Acid. Foods 2024, 13, 1245. [Google Scholar] [CrossRef]
- Simanaviciute, D.; Klimaviciute, R.; Rutkaite, R. Equilibrium adsorption of caffeic, chlorogenic and rosmarinic acids on cationic cross-linked starch with quaternary ammonium groups. Int. J. Biol. Macromol. 2017, 95, 788–795. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in Adsorption. Part XI. A System. J. Chem. Soc. 1960, 846, 3973–3993. [Google Scholar]
- Nayak, A.K.; Pal, A. Enhanced adsorption of gentian violet dye from water using lignocellulosic agricultural waste modified with di- And tri-carboxylic acids: Artificial intelligence modeling, practical comprehension, mechanistic and regeneration analyses. J. Environ. Chem. Eng. 2021, 9, 105578. [Google Scholar] [CrossRef]
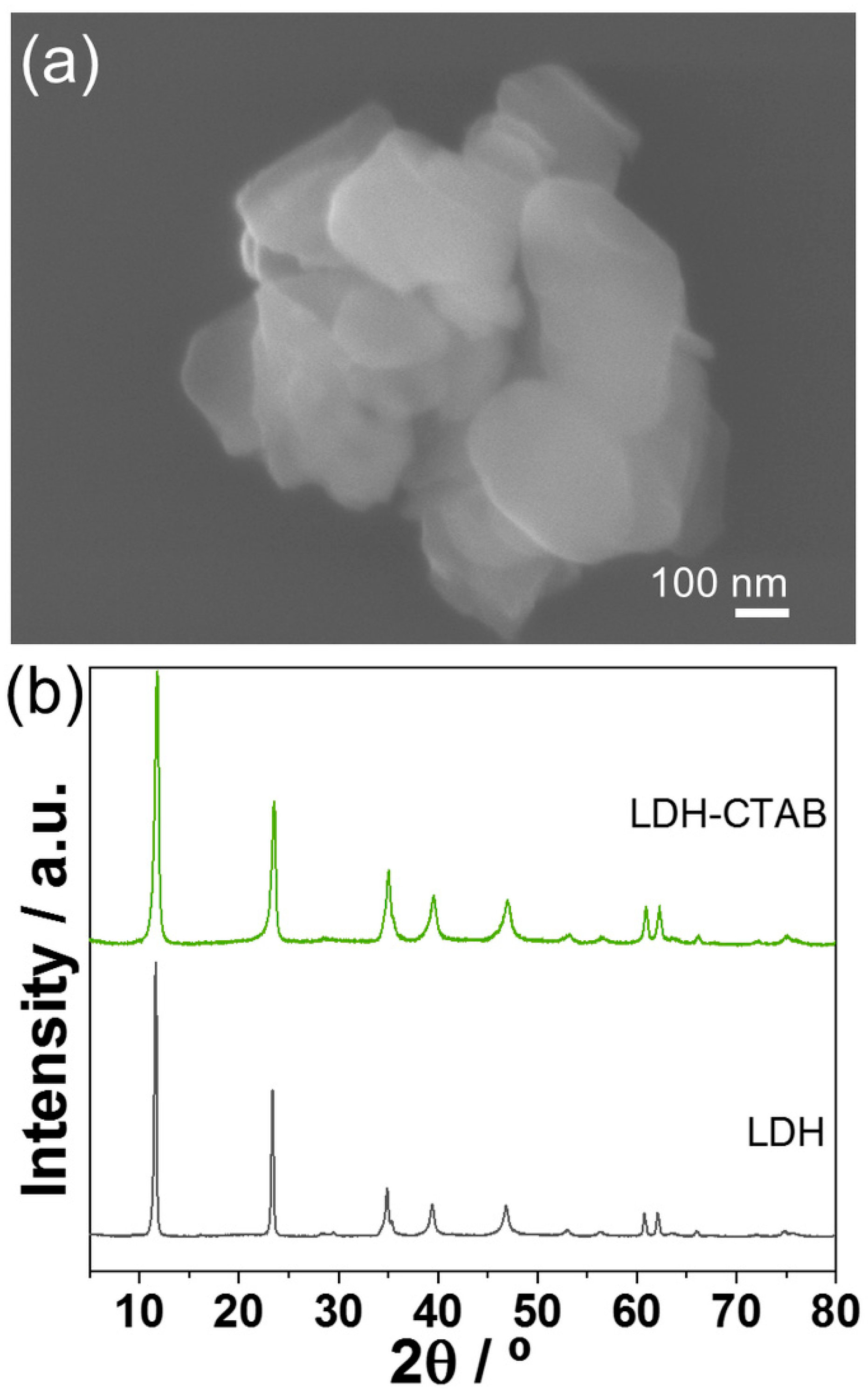






| Sample | d (Å) | c (Å) | a (Å) |
|---|---|---|---|
| LDH | 7.6 | 22.8 | 1.5 |
| LDH-CTAB | 7.5 | 22.5 | 1.5 |
| PFO Model | PSO Model | Elovich Model | |||
|---|---|---|---|---|---|
| qe1 (mg g−1) | 8.67 | qe2 (mg g−1) | 9.50 | α (mg g−1 min−1) | 0.804 |
| k1 (min−1) | 0.022 | K2 (g mg−1 min−1) | 0.003 | β (mg g−1) | 0.653 |
| R2 | 0.892 | R2 | 0.967 | R2 | 0.979 |
| X2 | 0.234 | X2 | 0.014 | X2 | 1.17 |
| Δq (%) | 14.6 | Δq(%) | 3.74 | Δq(%) | 30.7 |
| Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|
| qmax/mg g−1 | kL/L mg−1 | R2 | n | k/mg1−1/n L1/n g−1 | R2 |
| 9.7 | 1.3 | 0.9067 | 10 | 15.6 | 0.0934 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, A.C.d.A.; Malafatti, J.O.D.; Silva, M.L.L.S.e.; Moreira, A.J.; Thomazi, A.C.; Quaranta, S.; Paris, E.C. Hydrotalcite Supported on Polycaprolactone:Poly(methyl methacrylate) Fiber Membranes for Chlorogenic Acid Removal. Water 2025, 17, 931. https://doi.org/10.3390/w17070931
Nascimento ACdA, Malafatti JOD, Silva MLLSe, Moreira AJ, Thomazi AC, Quaranta S, Paris EC. Hydrotalcite Supported on Polycaprolactone:Poly(methyl methacrylate) Fiber Membranes for Chlorogenic Acid Removal. Water. 2025; 17(7):931. https://doi.org/10.3390/w17070931
Chicago/Turabian StyleNascimento, Andressa Cristina de Almeida, João Otávio Donizette Malafatti, Maria Luiza Lopes Sierra e Silva, Ailton José Moreira, Adriana Coatrini Thomazi, Simone Quaranta, and Elaine Cristina Paris. 2025. "Hydrotalcite Supported on Polycaprolactone:Poly(methyl methacrylate) Fiber Membranes for Chlorogenic Acid Removal" Water 17, no. 7: 931. https://doi.org/10.3390/w17070931
APA StyleNascimento, A. C. d. A., Malafatti, J. O. D., Silva, M. L. L. S. e., Moreira, A. J., Thomazi, A. C., Quaranta, S., & Paris, E. C. (2025). Hydrotalcite Supported on Polycaprolactone:Poly(methyl methacrylate) Fiber Membranes for Chlorogenic Acid Removal. Water, 17(7), 931. https://doi.org/10.3390/w17070931










