Abstract
Micro/nanoplastics (M/NPs) have become prevalent in aquatic environments due to their widespread applications. Likewise, ubiquitous ecological macromolecules can adsorb onto M/NPs to form an “eco-corona”, which significantly alters their environmental behaviors including aggregation dynamics, adsorption/desorption, and bioavailability. Therefore, it is necessary to analyze the role of eco-corona in assessing the environmental risks of M/NPs. This review systematically summarizes the formation mechanisms of eco-corona and evaluates its regulatory effects on the stability and ecotoxicity of M/NPs. Compared with other ecological macromolecules (e.g., natural organic matter and extracellular polymeric substances), humic acid (HA) tightly binds to M/NPs through electrostatic and hydrophobic interactions, significantly affecting their hetero-aggregation behavior and colloidal stability. In terms of bioavailability, the various functional groups on the HA surface can regulate the surface charge and hydrophobicity of M/NPs, thereby affecting their bioaccumulation and “Trojan horse” effect. Notably, the HA corona alleviates M/NPs-induced growth inhibition and oxidative stress. Genotoxicity assessment further showed that HA corona can regulate the expression of genes related to oxidative stress response and detoxification pathways. Future studies should focus on the synergistic effects between eco-corona and co-existing pollutants in complex aquatic environments to elucidate the long-term ecological risks associated with eco-corona formation.
1. Introduction
Plastic pollution, as a global environmental issue, has attracted significant attention from scholars both domestically and internationally in recent years. By 2022, global plastic production has surpassed 400 million tons, of which China accounts for 32% [1]. A significant amount of plastic waste enters aquatic environments and gradually decomposes into medium-sized plastics (5–40 mm), microplastics (MPs, 1–5000 µm), and nanoplastics (NPs, 1–100 nm) [2]. Among these, micro/nanoplastics (M/NPs) have been widely detected in aquatic environments such as rivers, oceans, lakes, and reservoirs [3,4,5], and are referred to as “aquatic PM2.5”. According to statistics, M/NPs have been detected in 100% of turtles, 59% of whales, 36% of seals, and 40% of seabirds [6]. Every year, up to 100,000 sea mammals and one million seabirds die due to plastic pollution [7]. Meanwhile, M/NPs could induce various stress effects such as behavioral changes [8], immunotoxicity [9], and reproductive defects [10], posing potential threats to the aquatic ecosystem and human health.
However, current research on the biological effects of M/NPs was mainly focused on the causal relationship between the pristine particles and biological responses, while systematic studies on their interfacial effects in real aquatic environments are rare. In fact, due to their high specific surface area, hydrophobicity, and stability, M/NPs can quickly interact with ecological macromolecules (e.g., humic acid (HA), fulvic acid (FA), extracellular polymeric substances (EPS), and proteins), eventually forming an organic shell of varying thickness on the particle surface, known as the “eco-corona” [11,12]. The formation of eco-corona significantly altered the surface properties and hetero-aggregation of M/NPs. Since M/NPs can similarly be regarded as colloidal particles, the hetero-aggregation stability is a key factor in evaluating the environmental behavior of M/NPs [13,14,15]. For example, the HA corona increased the electrostatic repulsion between polyethylene glycol terephthalate (PET) NPs by providing more negative charges and produced steric hindrance to limit the hetero-aggregation of PET NPs [16].
Meanwhile, eco-corona has been shown to alter the biological effects of M/NPs. Most studies reported that eco-corona reduced the ecological risk of M/NPs by inhibiting cell adhesion, reducing oxidative stress, and alleviating the expression of related genes [17]. However, some studies have found that eco-corona could exacerbate the negative effects of M/NPs, including prolonging their residence time in the intestine and inducing more severe oxidative stress [18,19]. Moreover, the eco-corona could act as a “Trojan horse” for certain functionalized biomolecules. As a result, M/NPs could be internalized by cells and enter the biological tissues of the gastrointestinal tract more easily [20]. Therefore, it is very important to systematically elucidate the biological effects of eco-corona.
Based on these facts, this review investigated the mechanisms by which the eco-corona affects the biological effects of M/NPs, mainly using natural organic matter (NOM) and EPS as the typical ecological macromolecules. First, the physicochemical properties of M/NPs were described. Then, the interactions between M/NPs and different ecological macromolecules were summarized systematically. The impact of eco-corona formation on the hetero-aggregation and stability of M/NPs was clarified. Finally, the effects of eco-corona formation on the bioavailability of M/NPs were analyzed. The results provided a scientific basis for the comprehensive evaluation of the ecological risks of M/NPs in natural aquatic environments.
2. Physicochemical Properties of Typical Natural Organic Macromolecules
2.1. Natural Organic Matter
As the typical ecological macromolecules, NOM and EPS are the main components of the eco-corona in aquatic environments. NOM is a complex organic mixture in surface water or groundwater, which is from biological activities or external sources by the hydrological cycle. Thus, NOM is easily influenced by conditions of the climate and geological and topographical features. In natural water environments, the concentration of NOM varies from 0.5 mg·L−1 in seawater and groundwater to over 30 mg·L−1 in wetlands [21]. As shown in Figure 1, NOM was mainly composed of humic substances (e.g., HA, FA, humin) and non-humic components (such as carbohydrates, amino acids, and proteins) [22,23]. Notably, certain biopolymers like sodium alginate (SA)—a polysaccharide extracted from brown algae—were also classified as NOM [24]. Based on the particle size difference, NOM could be categorized into particulate organic matter and dissolved organic matter (DOM) [21]. Among them, HA is an important component of NOM with a concentration ranging from 1 to 15 mg·L−1 in freshwater [25,26]. Due to the presence of phenolic and carboxyl groups, HA contains both hydrophobic and hydrophilic properties [27]. Thus, HA could interact with organic pollutants, heavy metals, and M/NPs. Compared with HA, FA has a larger specific surface area and higher oxygen content, containing various reactive functional groups such as carboxylic acid, phenolic hydroxyl, hydroxyl, quinone, and amine. Due to the proton dissociation of these functional groups, FA can interact with metal ions and organic compounds through mechanisms such as hydrogen bonding and ion pairing [28]. SA is an anionic polysaccharide rich in hydroxyl and carboxyl groups. It is highly water-soluble and exhibits strong adsorption affinity for heavy metal ions and organic pollutants [29]. In addition, the research related to humin in the aquatic environments was limited due to the property of insoluble in water.
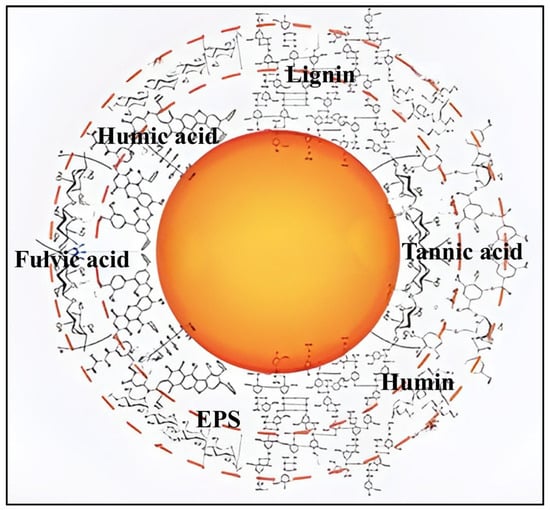
Figure 1.
Main components and structure of eco-corona.
2.2. Extracellular Polymeric Substances
As another type of ecological macromolecules, EPS are mainly derived from the biological secretions of microorganisms (e.g., algae and bacteria) and invertebrates (e.g., Daphnia magna, D. magna) [30,31] (Figure 1). Generally, EPS could be divided into soluble EPS and bound EPS. Soluble EPS are dissolved in the liquid media such as mucus, soluble macromolecules, and colloids, which are loosely bound to the outer cell wall or completely dissolved in the solution. The bound EPS typically exhibited a bilayer structure, with the inner layer being tightly bound and the outer layer loosely bound, performing a more compact structure [32]. In terms of molecular composition, the EPS primarily consists of lipids, proteins, polysaccharides, DNA, and other natural polymers. These components could form highly hydrated, gel-like, three-dimensional matrices through van der Waals forces, hydrogen bonds, and electrostatic forces [33]. The composition and structure of EPS determined the unique physicochemical properties. The three-dimensional structure provided a high specific surface area, which enabled EPS to interact with compounds through adsorption. Due to the large number of negatively charged functional groups (e.g., amino, phosphate, carboxyl, and hydroxyl groups), EPS could adsorb cationic compounds via ion exchange [34]. Additionally, EPS contained various hydrophobic or hydrophilic functional groups (e.g., carbonyl, carbon–carbon or carbon–hydrogen bonds, carboxyl, and hydroxyl groups), given the hydrophobic or hydrophilic properties [35,36]. The hydrophilic–hydrophobic property aided in the accumulation and dissolution of environmental pollutants and facilitated microbial adhesion and pollutant attachment. Hence, the microbial colonization and pollutant migration were affected [32].
The natural aquatic environment contains numerous exogenous organic substances capable of acting as dispersants through adsorption onto M/NPs. Among these, bovine serum albumin (BSA) is a highly hydrophobic spherical macromolecule and is often used as a model protein [37]. Sodium dodecyl sulfate (SDS) is an anionic surfactant with high amphiphilicity and adsorption capacity [38]. In addition, cells or organisms release some small molecule metabolites during normal metabolism, such as Oryzias melastigma (O. melastigma) secretions (OMS) [39] and microcystin (MC), a metabolite of cyanobacteria [40,41].
3. Formation Mechanism of the Eco-Corona
Currently, various M/NPs have been used to investigate their interactions with diverse ecological macromolecules [42,43,44,45,46]. As shown in Table 1, the interaction modes of the eco-corona formation mainly included surface electrostatic interactions, hydrophobic interactions, van der Waals forces, ligand exchange interactions, hydrogen bonding, and other high-energy adsorption chemical bonds. Among them, electrostatic and hydrophobic interactions are the two most common mechanisms.

Table 1.
Formation mechanisms of eco-corona on M/NPs with distinct surface functionalization.
3.1. Electrostatic and Hydrophobic Interactions
The electrostatic interactions between the charged M/NPs and biomolecules were driven by the electrostatic force [63,64]. For example, the HA exposes negatively charged carboxyl groups, which can electrostatically attract positively charged polystyrene (PS) NPs-NH2 to form an eco-corona while repelling negatively charged PS NPs-COOH [65]. Similarly, negatively charged EPS and SA have a more significant modifying effect on positively charged M/NPs than on negatively charged ones. The hydrophobic interactions referred to the tendency of non-polar molecules to aggregate and move away from the aqueous phase. The pristine NPs primarily form eco-coronas with biomolecules through the hydrophobic interactions. For example, high-protein EPS formed eco-corona with raw PS NPs through hydrophobic adhesion on marine phytoplankton [66]. Meanwhile, Feng et al. [65] reported that the neutral PS NPs adsorbed the aromatic rings of HA through hydrophobic interactions to form an eco-corona.
3.2. Hydrogen Bonding and van der Waals Forces
As for weaker intermolecular forces, hydrogen bonding commonly occurs between molecules containing functional groups like hydroxyl groups. Van der Waals forces are the attractive forces caused by polarization fluctuations between atoms, while π–π interactions occur as weak interactions between molecules containing aromatic rings. For example, oxygen-containing groups (e.g., -OH, C=O) on aged polyethylene (PE) and polypropylene (PP) MPs bound EPS polysaccharides via hydrogen bonding [67,68]. Meanwhile, PS MPs interacted with EPS through π–π interactions between their benzene rings and EPS aromatic structures, with N/O groups serving as key binding sites [11]. Degradable polylactic acid (PLA) MPs also utilized hydrogen bonding for EPS adsorption, while non-degradable PS MPs additionally formed π–π complexes with FA [69]. Van der Waals forces contributed to interactions in Scenedesmus obliquus EPS-PS NPs systems, alongside electrostatic/hydrophobic effects [47]. Meanwhile, the ligand exchange was referred to as the replacement or binding between biomolecules and active sites on the particle surface to form a more stable structure. Ligand exchange occurred when low-binding-energy citrate coatings on Ag NPs were replaced by NOM during eco-corona formation [70].
4. Effects of Eco-Corona Formation on the Hetero-Aggregation Stability of M/NPs
4.1. Applications and Limitations of DLVO and XDLVO Theories
When M/NPs interact with natural organic macromolecules in water to form an eco-corona, their functionalization, surface charge, and hydrophobicity undergo significant changes (Figure 2). These alterations affect the hetero-aggregation and stability of M/NPs. The Derjaguin–Landau–Verwey–Overbeek (DLVO) theory, a colloidal dispersion stability theory, is widely used to evaluate the hetero-aggregation stability of colloidal particles. However, since it only incorporates electrostatic repulsion and van der Waals attraction generated by counter ions in the electric double layer, predictions may exhibit substantial deviations. Consequently, the extended DLVO (XDLVO) theory, which accounts for additional potential forces, can more accurately predict hetero-aggregation stability between colloidal particles [71]. As shown in Table 2, the presence of an eco-corona can influence M/NP hetero-aggregation behaviors and stability through electrostatic interactions, steric hindrance, or molecular bridging.
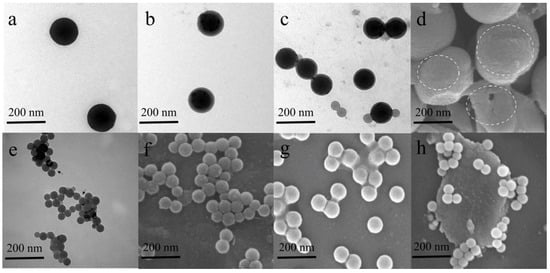
Figure 2.
TEM characterization of PS NPs with distinct surface functionalization and eco-corona formation mediated by ecological macromolecules: (a) pristine of PS NPs, (b) pristine of PS NPs-NH2, (c) pristine of PS NPs-COOH, (d) aged plain PS NPs, (e) PS NPs coated with EPS corona, (f) PS NPs coated with HA corona, (g) PS NPs coated with FA corona, (h) PS NPs coated with NOM corona [51,72,73,74,75].

Table 2.
Regulatory mechanisms of eco-corona on hetero-aggregation stability of M/NPs.
4.2. Regulatory Mechanisms of Eco-Corona on Hetero-Aggregation of M/NPs
For the electrostatic interactions, the zeta (ζ) potential was a key parameter determining the electrostatic repulsion between particles [77]. This interaction could be easily influenced by various factors (e.g., particle characteristics, pH conditions, and environmental media) [78]. Thereby, the stability of M/NPs hetero-aggregation was affected. For example, EPS could inhibit the hetero-aggregation of PS MPs by reducing the ζ potential of PS MPs to increase the electrostatic repulsion. However, PS NPs were shown a higher affinity for tryptophan, tyrosine, and humic acid-like substances in the EPS compared to MPs due to the larger specific surface area. As a result, the hetero-aggregation of NPs was promoted by the reduction in electrostatic repulsion [84]. Meanwhile, the environment-related pH values and the presence of electrolytes could affect the charge states of M/NPs, significantly affecting the hetero-aggregation of plastic particles. Generally, higher pH values increased the electrostatic repulsion between NPs, thereby inhibiting hetero-aggregation. In saline media, the adsorption of NOM on NPs reduced hetero-aggregation and sedimentation, exhibiting good stabilization effects [60]. For instance, Liu et al. [37] found that HA and EPS enhanced the stability of PS NPs as the pH increased in a salt solution containing NaCl and CaCl2. Kong et al. [89] found that HA enhanced the stability of PS NPs in NaCl solutions but reduced the stability in CaCl2 solutions. This might be due to the stronger charge-shielding effect of multivalent cations on the surface of NPs, which enhanced their hetero-aggregation. However, the experimental conditions mainly involved NaCl and CaCl2 solutions, which may not fully simulate the more complex ionic composition, and the interaction of particulate matter in natural water bodies.
Comparatively, the effect of steric hindrance was due to the occupation of space on the surface of M/NPs by the eco-corona. As a result, the direct contacts between particles were prevented to reduce the hetero-aggregation of M/NPs. For instance, Chen et al. [90] found that the additional steric hindrance improved the stability of PS NPs in the presence of HA corona. The adsorption of EPS reduced the hetero-aggregation rates of PS NPs due to the enhanced steric hindrance [91]. Additionally, the aggregates formed by SA adsorbing and bridging PSL NPs-COOH and PS NPs were stable due to the steric hindrance [79,92]. In aged NPs, Li et al. [48] reported that the presence of NOM reduced the hetero-aggregation of ozone-aged PS NPs, leading to enhanced stability by steric hindrance. As PS NPs aged, the steric hindrance caused by HA weakened, thereby reducing the inhibition effect of HA on the hetero-aggregation of PS NPs [93]. Additionally, Wu et al. [94] reported that the HA corona stabilized three types of negatively charged PS NPs (PS, PS-COOH, n-PS-NH2) through steric hindrance under high salinity conditions.
The molecular bridging effects were the connection of ecological macromolecules with M/NPs through the multiple binding sites to promote the formation of aggregates. For example, HA could bind to the hydroxyl groups of oxidized PET NPs through its carboxyl groups, and the hetero-aggregation of colloidal particles was promoted [95]. In addition, the presence of cations altered the charge of particles through the electrostatic interactions in electrolyte solutions, and divalent cations (primarily Ca2+) can also enhance the hetero-aggregation of M/NPs through molecular bridging. For NOM, high concentrations of NOM and HA could strengthen the hetero-aggregation of PS NPs in CaCl2 solutions through the bridging effects [83,96]. Liu et al. [37] found that SA enhanced the hetero-aggregation of PS NPs in CaCl2 solutions through the increased molecular bridging effects. Most studies on the bridging mechanism of Ca2+ to EPS have focused on the evaluation of the hetero-aggregation of metal nanoparticles [80,85,97], with relatively little research on M/NPs. Xiong et al. [98] investigated the effects of EPS on the hetero-aggregation of PS MPs in the presence of Ca2+ and found that the EPS corona enhanced the hetero-aggregation with PS MPs through molecular bridging of Ca2+. In fact, the hetero-aggregation and stability of M/NPs in the presence of eco-corona were affected by the combined effects of the above-mentioned effects. Ruan et al. [81] and Li et al. [86] found that the promotion or inhibition of the hetero-aggregation of PS NPs depended on the balance between the steric hindrance and bridging effects in the existence of NOM corona. Likewise, the hetero-aggregation behaviors of 100 nm PS NPs in HA and FA were also determined by the surface charge heterogeneity of PS NPs, steric hindrance, and bridging effects [99]. Studies on other biomolecules (FA, EPS) also obtained the same conclusion, indicating that this phenomenon is widely universal [71,100]. In addition, Sun et al. [101] found that hydration layer repulsion greatly influenced the hetero-aggregation of M/NPs. For HA corona, the steric hindrance was the main mechanism for the enhanced stability of NPs, and hydration layer exclusion also played a certain role.
5. Effects of Eco-Corona on the Bioavailability of M/NPs
The biological effects of M/NPs would be responded to because of the presence of eco-corona due to the alterations of interfacial properties. Thus, as shown in Figure 3, the roles of eco-corona on the bioavailability of M/NPs were summarized from bioaccumulation, biological effects, and molecular mechanisms, respectively.
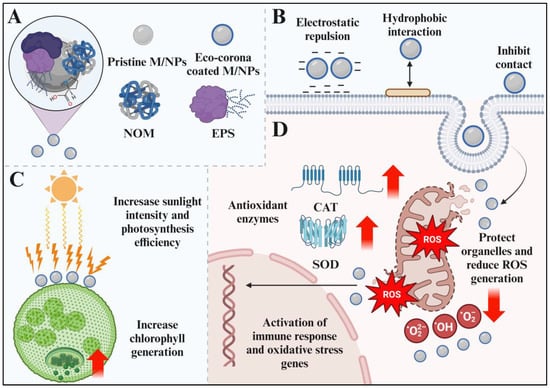
Figure 3.
The formation of eco-corona and the toxicity of M/NPs coated with eco-corona to aquatic organisms. (A) Formation of eco-corona. (B) Effects the adhesion of M/NPs to the cell surface. (C) Protecting algae from damage by M/NPs. (D) Effects on M/NPs-induced cytotoxicity and gene expression. Created using Biorender online tool (www.biorender.com).
5.1. Bioaccumulation
Generally, receptor-mediated active endocytosis is the primary pathway for cells to uptake the M/NPs in aquatic environments [89,102]. Before that, cell adhesion is the key step in the endocytosis process [103]. Recent studies have revealed that M/NPs could adhere to the lipid bilayer of the cell membrane through receptor–ligand interactions, electrostatic interactions, and hydrophobic interactions. Moreover, M/NPs with high hydrophobicity and positive charges are more likely to adhere to the cell surface and penetrate the lipid bilayer [104,105]. However, the formation of eco-corona modified the surface characteristics of M/NPs, thereby affecting the uptake, accumulation, and bioavailability of aquatic organisms (Figure 4).
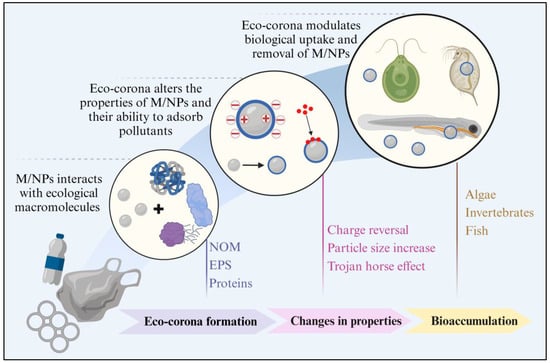
Figure 4.
Effects of eco-corona formation on surface properties and bioaccumulation of M/NPs. Created using Biorender online tool (www.biorender.com).
5.1.1. Algae
For algae, due to the accumulation of numerous carboxyl, hydroxyl and amine functional groups on the surface, the algal cell membrane is mostly negatively charged [106]. The electrostatic repulsion and the prevention of direct contact were the main routes of eco-corona to modify the uptake processes of the charged and uncharged M/NPs, respectively (Figure 3B). For example, HA includes various functional groups, such as ketones, hydroxyls, thiol, methoxyl and phenolic compounds. The adsorption of these functional groups reversed the surface charge of PS NPs-NH2 and increased the electrostatic repulsion between the particles and the negatively charged polysaccharide membrane of Chlorella vulgaris (C. vulgaris), which effectively reduced the bioaccumulation of PS NPs-NH2 [43,107]. Similarly, the negatively charged EPS corona generated significant electrostatic repulsion between PS NPs-NH2 and S. obliquus, resulting in the inhibition of the contact of PS NPs with the cell membrane [108]. Therefore, it is reasonable to speculate that the negatively charged functional groups of NOM and EPS could improve the electrostatic repulsion and then inhibit the internalization of the positively charged M/NPs by algal cells. For uncharged particles, the eco-corona could block the direct contact between M/NPs and algal cells. The EPS released by algae acted as an extracellular protective barrier, preventing direct contact between the PS NPs and Chlorella sp., significantly reducing the bioavailability of PS NPs [109,110]. However, the bioavailability of negatively charged particles was relatively less by the impact of eco-corona due to the pre-existing repulsive effect of the same charge as the cell membrane. For example, the adsorption of negatively charged PS NPs-COOH on Phaeodactylum tricornutum (P. tricornutum) was found to be negligible, while the presence of EPS corona did not change the bioavailability of PS NPs-COOH [74].
5.1.2. Invertebrates
As for aquatic invertebrates, they mainly ingest M/NPs into the intestine through the filter-feeding process [111]. Similarly to algae, invertebrates (e.g., D. magna) can secrete EPS in the form of polysaccharides or proteins in response to environmental stimuli or metabolism. The adsorption of these EPS on the M/NPs surface caused an increase in particle size, making them more readily ingested by D. magna [31]. Upon ingestion, the presence of the EPS corona made plastic particles remain in the intestine longer and more difficult to eliminate. After 6 h of exposure, 20% of PS NPs coated with EPS corona remained in the intestine of D. magna, while only 15% of the original PS NPs remained [31]. It is worth noting that the secretion of EPS was essentially a defense mechanism of invertebrates, but the bioaccumulation of M/NPs was unexpectedly enhanced in this process. Different from endogenous EPS, Wu et al. [112] found that the NOM corona around PS NPs and PS NPs-NH2 cut off the contact between NPs and the intestinal tissue of D. magna. At the same time, HA enhanced the hydrophilicity of the plastic particles, ultimately preventing NPs from passing through the intestinal epithelial cells and entering the biological tissue. This difference suggests that the biotic/abiotic properties of eco-corona determine the direction of its regulation on the accumulation of M/NPs. The formed eco-corona gave M/NPs a new biological identity, thereby affecting the response at the cellular level. Compared with the exogenous environmental substance NOM, as a biological metabolite, the EPS corona had biological similarities with cells, making M/NPs more likely to accumulate in invertebrates [113].
5.1.3. Fish
With regard to fish, M/NPs were able to be absorbed on gill filaments through filtration or accumulated in the intestinal lumen through oral administration [114]. The formation of eco-corona significantly changed the biosorption and clearance behavior of M/NPs, and this effect was tissue-specific: accumulation was inhibited in the gills, while accumulation was enhanced in the intestine. For example, Zheng et al. [115] used Oreochromis niloticus (O. niloticus) as the test organism to evaluate the effects of DOM on the bioavailability of PA MPs. The results indicated that the HA corona on the surface of PA MPs inhibited the accumulation of PA MPs by hindering direct contact with gill tissues and increasing electrostatic repulsion [116,117]. However, the formed eco-corona reduced the ability of fish to clear M/NPs in the intestine. The formation of the BSA corona increased the apparent viscosity of PS MPs and promoted the bioaccumulation of PS MPs in the intestine of Danio rerio (D. rerio) [118]. Once the accumulation of MPs in the oral cavity exceeds a tolerance threshold, and then the fish could expel the MPs by coughing [119]. HA corona reduced the coughing behavior in fish, lowered the clearance rate of MPs, and further increased the number of MPs swallowed into the gut [115].
Meanwhile, surface aging of M/NPs (such as photooxidation and NOM-driven aging) aggravates the interactions with biological tissues and affects their bioaccumulation by changing physicochemical properties (such as roughness and oxygen-containing functional groups). For example, the small-sized PA MPs could accelerate their excretion from the intestine of D. rerio by activating the activity of efflux pump proteins [120]. The presence of NOM changed the particle size and edge properties of PA MPs by driving the photoaging process, significantly increasing the accumulation of MPs in the intestine [18,121]. The interaction between PA MPs and the intestinal villi was intensified due to the rough surface and cracks [122]. Aged PS MPs with serrated edges retain longer in the intestine of D. rerio larvae than spherical MPs [123]. Additionally, NOM aging incorporated numerous oxygen-containing functional groups on MPs surface, promoting the binding of large molecular MPs to the intestinal mucus gel layer through hydrogen bonding, making it difficult for D. rerio to excrete MPs directly [124]. Intestinal tissue damage promoted the diffusion of M/NPs through epithelial cells into the circulatory system or diffusion between adjacent cells at tight junctions, ultimately crossing biological barriers and depositing in the liver and brain [18,115,125].
Additionally, NPs could transfer heavy metals and other harmful chemicals into organisms and various environments through the “Trojan horse” effect, resulting in greater toxic effects than MPs. The adsorption and release of pollutants by NPs depended on the competition between pollutants, organisms, and the environment, in which ecological macromolecules could interfere with the “Trojan horse” effect of NPs by competing for binding sites and enhancing electrostatic repulsion. For example, PS NPs increased the absorption and accumulation of phenanthrene in the gills and liver of Oncorhynchus mykiss, but the addition of NOM decreased phenanthrene absorption by 70%. This reduction was attributed to NOM competing with phenanthrene for adsorption sites on the surface of PS NPs, weakening the “Trojan horse” effect of PS NPs by lowering their transport of phenanthrene [126]. Monikh et al. [127] investigated the “Trojan horse” effect of nanoplastic debris and found that NPs fragments increased the bioavailability of Ag+ by altering the uptake pathway and promoting its transport to the organs of D. magna. The presence of NOM prevented Ag+ from binding to toxic action sites, reducing the bioavailability of Ag+. However, some studies suggested that NOM could increase the accumulation of heavy metals by changing the surface properties of NPs. For instance, Qiao et al. [128] found that NOM promoted the adsorption of Cu2+ onto PS MPs, increasing the accumulation of Cu2+ in the liver and intestine of D. rerio. This was attributed to the aromatic structure of NOM interacting with MP to form a highly conjugated co-polymer, which increased the electron density and made it easier for MPs to adsorb heavy metal ions [129]. Additionally, the Cu2+ concentration (50 µg/L) used in the experiment may exceed typical levels found in most natural water bodies. Such high-concentration exposure could amplify toxic effects, potentially limiting the applicability of the experimental findings to real-world environmental conditions.
5.2. Biological Effects
Studies have shown that M/NPs could damage the cell structure and cause multilevel biological effects. Eco-corona significantly altered the original toxicity of M/NPs through multiple routes such as physical barrier effect, biomolecular regulation, and oxidative stress intervention. Relevant studies about the impact of eco-corona formation on the biological effects of diverse M/NPs are shown in Table 3.

Table 3.
Toxicological alternations induced by the formation of eco-corona.
5.2.1. Growth Inhibition and Oxidative Stress
Firstly, the formation of eco-corona effectively alleviated the inhibition of algal photosynthesis and growth caused by M/NPs (Figure 3C). Its protective mechanism was mainly reflected in (1) blocking the direct contact between M/NPs and cell membrane and (2) activating the compensatory stress response of the organism. For example, PS NPs-NH2 covering the surface of C. vulgaris reduced the efficiency of photosynthesis, disrupted cellular material exchange, and even led to cell apoptosis. The presence of HA corona upregulated the contents of chlorophyll a and b and effectively alleviated the toxicity of PS NPs-NH2 by enhancing the self-regulation ability of C. vulgaris [132]. Additionally, HDPE MPs acted as a barrier to block the minimum light intensity required by Chlamydomonas reinhardtii, leading the cells to produce EPS and increase cell density to relieve cell stress [133]. Low concentrations of PS NPs could directly damage the cell membrane of Microcystis aeruginosa and inhibit photosynthesis. However, increased concentrations of PS NPs induced the release of MC from algal cells. The MC corona reduced the accumulation of PS NPs and alleviated growth inhibition, ultimately allowing algae to recover quickly after the inhibition period [134,135].
Secondly, at the cellular level, M/NPs could stimulate cells to generate excessive ROS by reacting with oxygen and damaging cell structures [136]. Excessive accumulation of ROS in cells could reduce membrane fluidity, disrupt metabolism and gene expression, destroy cell structure, and ultimately induce cell apoptosis [137,138]. The presence of eco-corona could alleviate oxidative damage by reducing the probability of contact between M/NPs and cell structures [139] and directly exerting free radical scavenging function [140]. For example, both the BSA and HA corona significantly downregulated the level of ROS induced by PS NPs, thereby alleviating apoptosis of D. rerio [55]. Notably, the EPS corona could not only reduce the transmembrane input of ROS by consuming extracellular oxidation reaction sites, but also directly remove free radicals through active groups such as phenolic hydroxyl groups, ultimately reducing the toxicity of PS NPs to P. tricornutum [74] and Chlorella sp. [141].
5.2.2. Photoaging M/NPs
When M/NPs were exposed to ultraviolet (UV) radiation in sunlight, their surface properties and toxicological effects changed significantly [130]. UV radiation broke M/NPs into fragments with more oxygen functional groups, higher roughness, and larger specific surface area [142]. Ecological macromolecules in aquatic environments could accelerate the photoaging of M/NPs by affecting the penetration depth of ultraviolet light and participating in free radical reactions. During the NOM-driven photoaging process, hydroxyl radicals formed on the surface increased the oxidative potential of M/NPs, which stimulated D. rerio intestinal cells to produce more ROS, leading to more severe oxidative stress and intestinal damage [51]. Compared to the pristine PA MPs, NOM-driven photoaged PA MPs unbalanced the redox status of the D. rerio intestine, leading to the shedding of intestinal epithelial cells and thinning of the intestinal wall [18]. Notably, the rough surface and newly added oxygen-containing functional groups produced by the photoaging process significantly enhanced the adsorption capacity of M/NPs for coexisting pollutants. Taking PVC MPs as an example, the aged PVC MP has more adsorption sites for pollutants, significantly enhancing the toxicity of tetrabromobisphenol A to S. obliquus [143]. However, Giri and Mukherjee [47] found that the EPS-driven aging process alleviated the toxicity of PS NPs to S. obliquus. This was due to EPS increasing the particle size by promoting the hetero-aggregation of PS NPs, thereby preventing cellular uptake and reducing ROS production.
In fact, the impact of eco-corona on the biological effects of M/NPs depended on the characteristics of M/NPs and the composition of eco-corona. Among these properties, particle sizes were an important factor with regard to the characteristics of M/NPs due to the differences in toxic mechanisms [144]. Larger MPs, for example, could obstruct light transmission and impair photosynthesis in S. obliquus, whereas smaller MPs tended to adhere to cell membranes, compromising their structural integrity. The presence of HA corona mitigated the toxicity of small MPs by preventing their adhesion to algal surfaces but did not counteract the light-blocking effects of larger MPs [19]. Similarly, the OMS corona has been found to alleviate immune and energy metabolism suppression caused by 5 μm PS MPs, but it exacerbated the immunotoxicity of 50 nm PS NPs. This heightened toxicity of smaller NPs is attributed to their increased ability to penetrate the chorion and disrupt inflammatory responses and immune enzyme activity [39]. Furthermore, the eco-corona formed by different ecological macromolecules affected the biological effects of M/NPs in distinct ways. For instance, the BSA corona aggravated oxidative stress and heart rate abnormalities in D. rerio embryos exposed to PET NPs, exhibiting a stronger toxicity-enhancing effect than the SDS corona [145]. The greater dispersibility and smaller size of BSA-PET NPs facilitated their penetration through embryonic membranes, leading to more pronounced damage to cell membranes and mitochondria compared to SDS-PET NPs.
5.3. Molecular Mechanism
After entering cells, M/NPs interfered with gene transcription and induced the expression of specific genes by excessive ROS production or crossing the nuclear membrane to damage DNA [146,147,148]. Eco-corona activates the detoxification and antioxidant defense systems in organisms by regulating the expression of oxidative stress-related genes (Figure 3D). Glutathione-S-transferase (GST) [149], glutathione peroxidase (GPx) [150], and catalase (CAT) [151] are key antioxidant enzymes that resist oxidative stress and are considered biomarkers for oxidative stress assessment. Heat shock protein (HSP70) and P-glycoprotein (P-GP) are responsible for exporting various harmful compounds out of cells and are the most common stress-induced chaperone [152,153]. The NOM and HA corona alleviated the upregulation of detoxification and oxidative stress-related genes (GST, CAT, HSP70, and P-GP) induced by PS NPs, significantly reducing the oxidative damage caused by M/NPs to D. magna [61,154]. Notably, the FA corona with a more negative charge exacerbated the upregulation of gene expression and effectively alleviated the toxicity of PS NPs by regulating the activities of antioxidant enzymes such as GPx, CAT, and SOD [154,155]. Similarly, melanocortin 2 receptor (MC2R) [156] and interleukin 10 (IL10) [157] have anti-inflammatory effects and are crucial in stress response and immune regulation. Short-term exposure to PS NPs induced anti-inflammatory and oxidative stress responses in Dicentrarchus labrax. PS NPs coated with NOM corona showed synergistic anti-inflammatory properties by activating anti-inflammatory factors such as MC2R and IL10, restoring the levels of inflammatory markers to normal [158].
Although eco-corona generally exhibited toxicity mitigation effects, some studies have shown that they might enhance the genotoxicity of M/NPs by exacerbating oxidative stress. NRF2 and its inhibitor KEAP1 are key components of the Nrf2-Keap1-ARE signaling pathway and are essential to the antioxidant system [159,160]. Luo et al. [118] found that the BSA corona induced excessive ROS production by increasing the accumulation of MPs, further aggravating the inhibition of NRF2 and KEAP1 gene expression in the intestine of D. rerio, and seriously damaging the protective function of the antioxidant system. UDP-glucuronosyltransferase (ugt) is a microsomal glycosyltransferase that transforms lipophilic molecules (e.g., bilirubin, steroids, and hormones) into water-soluble excretory metabolites [161]. Similarly, HA corona significantly inhibited the expression of ugt genes (ugt1a1, ugt2a4, etc.) and GST genes (gsta.2, gsto2, etc.) of D. rerio embryos by increasing the particle size of NPs to allow them to adsorb more pollutants, causing more severe oxidative damage and metabolic disorders [18].
6. Perspectives
Ecological macromolecules have been demonstrated to be essential in modulating the stability and biological effects of M/NPs. Current research has made significant progress in understanding the ecological impacts of eco-corona on aquatic organisms such as algae, invertebrates, and fish. However, given the intricacies of environmental factors and the characteristics of ecological macromolecules, further studies are necessary to elucidate the formation mechanism of eco-corona and its subsequent toxic effects in diverse aquatic environments. Future research should prioritize the following aspects:
- (1)
- The properties of the environmental medium significantly determine the aggregation state, stability, and bioavailability of contaminants in aquatic environments. For instance, higher ionic strength could reduce the electrostatic repulsion of TiO2 NPs and increase particle aggregation [162]. Higher temperatures not only facilitate the formation of EPS corona on Ag NPs surfaces but also significantly enhance the structural stability of the EPS-Ag NPs composite system [163]. So far, only the effects of salinity, pH, and different electrolytes on the formation of eco-corona on the surface of M/NPs have been studied, while the role of other influencing factors (such as temperature, conductivity, ion valence, etc.) deserves further investigation.
- (2)
- In aquatic environments, the interaction between M/NPs and co-pollutants could influence the uptake and accumulation of plastics or contaminants in aquatic organisms. Current studies have demonstrated that the formation of eco-corona altered the adsorption and desorption behavior of M/NPs toward heavy metal ions [164]. However, little is known about the effects of eco-corona on the interaction of M/NPs with other types of pollutants (e.g., metal nanoparticles, persistent organic pollutants, pharmaceutical pollutants), and further investigation of potential interfacial reactions is needed.
- (3)
- When M/NPs coated with eco-corona migrate from the external water environment into organisms or cells, the affinity of ecological macromolecules to the M/NPs may change with the changes due to the environment [165]. If biomolecules such as lipids, proteins, and nucleic acids within the organism exhibit a higher affinity for M/NPs, these biomolecules can be exchanged with the adsorbed components on the eco-corona [166]. However, it remains unclear which specific biomolecules would cover or replace the ecological macromolecules adsorbed on the surface of M/NPs, thereby forming a new eco-corona. The consequences of biomolecule replacement and its interaction process are also unclear. Therefore, further research is needed on the fate and transformation of the corona in cells or organisms, which is crucial for evaluating the biological effects of M/NPs.
- (4)
- Currently, knowledge of the biological effects and mechanisms of eco-corona is relatively limited. Studies on the biological effects of M/NPs by eco-corona have mainly centered on membrane adhesion, cellular uptake, growth inhibition, and oxidative stress. However, the impact of eco-corona on genotoxicity and reproductive toxicity has only recently started to be investigated. Therefore, beyond specific toxic endpoints such as DNA damage and apoptosis, the mechanisms of intracellular responses, including enzyme inactivation, cell differentiation, epigenetic modifications, and gene mutations, should be thoroughly explored.
7. Conclusions
This review elucidates the critical role of ecological macromolecules in modulating the environmental behaviors and biological impacts of M/NPs. In aquatic environments, ecological macromolecules are adsorbed on the surface of M/NPs through electrostatic interactions, hydrophobic interactions, hydrogen bonding, ligand exchange, and van der Waals forces to form an eco-corona. Eco-corona alters the hetero-aggregation and stability of M/NPs through mechanisms such as electrostatic interactions, steric hindrance, and molecular bridging. Regarding bioavailability, the feeding behavior and body size of aquatic organisms across different trophic levels shape the effects of eco-corona on the bioaccumulation and toxicity of M/NPs. The formation of the eco-corona reduces the uptake of M/NPs by algae and fish, but makes M/NPs more difficult to be removed from the intestines of fish and invertebrates. Notably, eco-corona competitively binds to coexisting pollutants through electrostatic repulsion and site competition, thereby weakening the “Trojan horse” effect of M/NPs. Meanwhile, eco-corona alters the various toxic effects of M/NPs, including growth inhibition, oxidative stress, and immunosuppression. Genotoxicity assessments reveal that regulating gene expression levels is an important way for eco-corona to change the biological effects of M/NPs. Future research should focus on comprehensively exploring the mechanisms and ecological risks of eco-corona and coexisting pollutants, and provide a scientific basis for pollution mitigation strategies.
Author Contributions
H.Y.: Investigation, Methodology, Validation, Resources, Visualization, Supervision, Project Administration, Funding Acquisition, and Writing—Reviewing and Editing. L.K. and Z.C.: Conceptualization, Investigation, Formal Analysis, Data Curation, Writing—Original Draft, and Writing—Review and Editing. H.X. and Q.Y.: Writing—Review and Editing and Supervision. J.W. Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (42307464) and the Natural Science Foundation of Jiangsu Province, China (KZ20221144).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Plastics Europe. Plastics—The Fast Facts. 2023. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 23 January 2025).
- Gaylarde, C.C.; Neto, J.A.B.; da Fonseca, E.M. Nanoplastics in aquatic systems-are they more hazardous than microplastics? Environ. Pollut. 2021, 272, 115950. [Google Scholar] [CrossRef]
- Singh, N.; Abdullah, M.M.; Ma, X.; Sharma, V.K. Microplastics and nanoplastics in the soil-plant nexus: Sources, uptake, and toxicity. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1613–1642. [Google Scholar] [CrossRef]
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassl, B.; Rowenczyk, L.; Tufenkji, N.; Feng, S.; Wiesner, M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 2021, 16, 501–507. [Google Scholar] [CrossRef]
- Cai, Z.; Li, M.; Zhu, Z.; Wang, X.; Huang, Y.; Li, T.; Gong, H.; Yan, M. Biological degradation of plastics and microplastics: A recent perspective on associated mechanisms and influencing factors. Microorganisms 2023, 11, 1661. [Google Scholar] [CrossRef]
- Jaiswal, S.; Sharma, B.; Shukla, P. Integrated approaches in microbial degradation of plastics. Environ. Technol. Innov. 2020, 17, 100567. [Google Scholar] [CrossRef]
- Susanti, N.K.Y.; Mardiastuti, A.; Wardiatno, Y. Microplastics and the impact of plastic on wildlife: A literature review. IOP Conf. Ser. Earth Environ. Sci. 2020, 528, 12013. [Google Scholar] [CrossRef]
- Liu, Z.; Malinowski, C.R.; Sepúlveda, M.S. Emerging trends in nanoparticle toxicity and the significance of using Daphnia as a model organism. Chemosphere 2022, 291, 132941. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Pérez, E.; Jiang, Q.; Chen, Q.; Jiao, Y.; Huang, Y.; Yang, Y.; Zhao, Y. Polystyrene nanoplastic induces oxidative stress, immune defense, and glycometabolism change in Daphnia pulex: Application of transcriptome profiling in risk assessment of nanoplastics. J. Hazard. Mater. 2021, 402, 123778. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chou, P.I.; Liu, J.; Zhu, Y.; Jun, Y.S. Oxidative roles of polystyrene-based nanoplastics in inducing manganese oxide formation under light illumination. ACS Nano 2022, 16, 20238–20250. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, B.; Zohora, F.; Bakya, J.; Ahmed, S.; Ahmed, S.F.; Mofijur, M.; Chowdhury, A.; Almomani, F. Microplastics as carriers of toxic pollutants: Source, transport, and toxicological effects. Environ. Pollut. 2024, 343, 123190. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.; Zeng, H.; Yang, X.; Hou, X.; Lu, X. Preferential adsorption of medium molecular weight proteins in extracellular polymeric substance alleviates toxicity of small-sized microplastics to Skeletonema costatum. J. Hazard. Mater. 2024, 476, 135034. [Google Scholar] [CrossRef]
- Bradford, S.A.; Sasidharan, S.; Kim, H.; Gomez-Flores, A.; Li, T.; Shen, C. Colloid interaction energies for surfaces with steric effects and incompressible and/or compressible roughness. Langmuir 2023, 37, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Flores, A.; Bradford, S.A.; Hong, G.; Kim, H. Statistical analysis, machine learning modeling, and text analytics of aggregation attachment efficiency: Mono and binary particle systems. J Hazard Mater. 2023, 454, 131482. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Flores, A.; Bradford, S.A.; Cai, L.; Urík, M.; Kim, H. Prediction of attachment efficiency using machine learning on a comprehensive database and its validation. Water Res. 2023, 229, 119429. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Cai, W.; Xia, J.; Sheng, L.; Wang, W.; Liu, H. Aggregation kinetics of fragmental PET nanoplastics in aqueous environment: Complex roles of electrolytes, pH and humic acid. Environ. Pollut. 2021, 268, 115828. [Google Scholar] [CrossRef]
- Liu, S.B.; Zhang, X.R.; Zeng, K.; He, C.T.; Huang, Y.C.; Xin, G.R.; Huang, X.C. Insights into eco-corona formation and its role in the biological effects of nanomaterials from a molecular mechanisms perspective. Sci. Total Environ. 2023, 858, 159867. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, M.; Zhao, J.; Cao, Z.; Zou, W.; Zhou, Q. Photoaging enhanced the adverse effects of polyamide microplastics on the growth, intestinal health, and lipid absorption in developing zebrafish. Environ. Int. 2022, 158, 106922. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, R.; You, J.; Muir, D.C.G.; Zeng, E.Y. Microplastic impacts on microalgae growth: Effects of size and humic acid. Environ. Sci. Technol. 2020, 54, 1782–1789. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Narayana, V.K.B.; Gross, W.; Mohanraj, J.; Thelakkat, M.; Greiner, A.; Schmalz, H.; Kress, H.; Laforsch, C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020, 6, eabd1211. [Google Scholar] [CrossRef]
- Feng, H.; Liang, Y.N.; Hu, X. Natural organic matter (NOM), an underexplored resource for environmental conservation and remediation. Mater. Today Sustain. 2022, 19, 100159. [Google Scholar] [CrossRef]
- Junaid, M.; Wang, J. Interaction of micro(nano)plastics with extracellular and intracellular biomolecules in the freshwater environment. Crit. Rev. Environ. Sci. Technol. 2021, 52, 4241–4265. [Google Scholar] [CrossRef]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles–formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef] [PubMed]
- Hashino, M.; Katagiri, T.; Kubota, N.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Effect of membrane surface morphology on membrane fouling with sodium alginate. J. Membr. Sci. 2011, 366, 258–265. [Google Scholar] [CrossRef]
- Sharma, A.; Anthal, R. Humic substances in aquatic ecosystems: A review. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 18462–18470. [Google Scholar]
- Glover, C.N.; Pane, E.F.; Wood, C.M. Humic substances influence sodium metabolism in the freshwater crustacean Daphnia magna. Physiol. Biochem. Zool. 2015, 78, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front Agronom. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Zhao, T.; Fang, M.; Tang, Z.; Zhao, X.; Xie, F.; Wu, F.; Giesy, J.P. Effects of fulvic acid on aggregation, sedimentation, and adsorption of Fe3O4 magnetic nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 21463–21474. [Google Scholar] [CrossRef]
- Siddiqui, V.U.; Ilyas, R.A.; Sapuan, S.M.; Hamid, N.H.A.; Khoo, P.S.; Chowdhury, A.; Atikah, M.S.N.; Rani, M.S.A.; Asyraf, M.R.M. Alginate-based materials as adsorbent for sustainable water treatment. Int. J. Biol. Macromol. 2025, 298, 139946. [Google Scholar] [CrossRef]
- Siddharth, T.; Sridhar, P.; Vinila, V.; Tyagi, R.D. Environmental applications of microbial extracellular polymeric substance (EPS): A review. J. Environ. Manag. 2021, 287, 112307. [Google Scholar] [CrossRef]
- Nasser, F.; Lynch, I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J. Proteome. 2016, 137, 45–51. [Google Scholar] [CrossRef]
- Ye, T.; Yang, A.; Wang, Y.; Song, N.; Wang, P.; Xu, H. Changes of the physicochemical properties of extracellular polymeric substances (EPS) from Microcystis aeruginosa in response to microplastics. Environ. Pollut. 2022, 315, 120354. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshanee, M.; Das, S. Bacterial extracellular polymeric substances: Biosynthesis and interaction with environmental pollutants. Chemosphere 2023, 332, 138876. [Google Scholar]
- Tang, W.; Wu, M.; Lou, W.; Yang, C. Role of extracellular polymeric substances and enhanced performance for biological removal of carbonaceous organic matters and ammonia from wastewater with high salinity and low nutrient concentrations. Bioresour. Technol. 2021, 326, 124764. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Liu, L.; Shen, Y.; Yan, P.; Chen, Y.; Guo, J.; Fang, F. Understanding the mechanism in aggregation ability between aerobic and anammox granular sludge from the perspective of exopolysaccharides. J. Water Process Eng. 2020, 38, 101629. [Google Scholar] [CrossRef]
- Hong, P.N.; Taing, C.; Phan, P.T.; Honda, R. Polarity-molecular weight profile of extracellular polymeric substances in a membrane bioreactor: Comparison between bulk sludge and cake layers. J. Water Environ. Technol. 2018, 16, 40–53. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Zhou, J.; Tang, J.; Yang, C.; Chen, C.; Huang, W.; Dang, Z. Influence of environmental and biological macromolecules on aggregation kinetics of nanoplastics in aquatic systems. Water Res. 2020, 186, 116316. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.M.; Faggio, C.; Laudicella, V.A.; Sanfilippo, M.; Trischitta, F.; Santulli, A. Effect of sodium dodecyl sulfate (SDS) on stress response in the Mediterranean mussel (Mytilus galloprovincialis): Regulatory volume decrease (Rvd) and modulation of biochemical markers related to oxidative stress. Aquat. Toxicol. 2014, 157, 94–100. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Y.; Xu, Y.; Liu, B.; Wang, C.; Feng, J.; Li, M.; Yin, H.; Sun, L.; Li, P.; et al. Mechanisms of eco-corona effects on micro(nano)plastics in marine medaka: Insights into translocation, immunity, and energy metabolism. J. Hazard. Mater. 2024, 480, 136236. [Google Scholar] [CrossRef]
- Schmidt, J.R.; Wilhelm, S.W.; Boyer, G.L. The Fate of Microcystins in the Environment and challenges for monitoring. Toxins. 2014, 6, 3354. [Google Scholar] [CrossRef] [PubMed]
- Chetwynd, A.J.; Zhang, W.; Thorn, J.A.; Lynch, I.; Ramautar, R. The nanomaterial metabolite corona determined using a quantitative metabolomics approach: A pilot study. Small 2020, 16, 2000295. [Google Scholar] [CrossRef]
- Schefer, R.B.; Armanious, A.; Mitrano, D.M. Eco-corona formation on plastics: Adsorption of dissolved organic matter to pristine and photochemically weathered polymer surfaces. Environ. Sci. Technol. 2023, 57, 14707–14716. [Google Scholar] [CrossRef] [PubMed]
- Khoshnamvand, M.; You, D.; Xie, Y.; Feng, Y.; Sultan, M.; Wei, X.; Li, J.; Fu, A.; Pei, D.S. Presence of humic acid in the environment holds promise as a potential mitigating factor for the joint toxicity of polystyrene nanoplastics and herbicide atrazine to Chlorella vulgaris: 96-H acute toxicity. Chemosphere 2024, 357, 142061. [Google Scholar] [CrossRef]
- Zhang, J.; Zhan, S.; Zhong, L.B.; Wang, X.; Qiu, Z.; Zheng, Y.M. Adsorption of typical natural organic matter on microplastics in aqueous solution: Kinetics, isotherm, influence factors and mechanism. J. Hazard Mater. 2023, 443, 130130. [Google Scholar] [CrossRef]
- Pedroza, R.H.P.; David, C.; Lodeiro, P.; Rey-Castro, C. Interactions of humic acid with pristine poly (lactic acid) microplastics in aqueous solution. Sci. Total Environ. 2024, 908, 168366. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Baimanov, D.; Yuan, H.; Xie, H.; Yu, S.; Zhang, Z.; Yang, J.; Zhao, F.; You, Y.; Guan, Y.; et al. Protein corona-directed cellular recognition and uptake of polyethylene nanoplastics by macrophages. Environ. Sci. Technol. 2024, 58, 14158–14168. [Google Scholar] [CrossRef]
- Giri, S.; Mukherjee, A. Ageing with algal EPS reduces the toxic effects of polystyrene nanoplastics in freshwater microalgae Scenedesmus obliquus. J. Environ. Chem. Eng. 2021, 9, 105978. [Google Scholar] [CrossRef]
- Li, X.; Ji, S.; He, E.; Peijnenburg, W.J.; Cao, X.; Zhao, L.; Xu, X.; Zhang, P.; Qiu, H. UV/ozone induced physicochemical transformations of polystyrene nanoparticles and their aggregation tendency and kinetics with natural organic matter in aqueous systems. J. Hazard Mater. 2022, 433, 128790. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q.; Shan, W.; Hao, Z.; Li, P.; Liu, J. Heteroaggregation of different surface-modified polystyrene nanoparticles with model natural colloids. Sci. Total Environ. 2021, 784, 147190. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; He, Q.; Wu, Z.; Ma, J.; Huangfu, X. Influence of dissolved black carbon on the aggregation and deposition of polystyrene nanoplastics: Comparison with dissolved humic acid. Water Res. 2021, 196, 117054. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Cheng, F.; Zhang, T.; Li, C.; Qu, J.; Chen, J.; Peijnenburg, W.J.G.M. Dissolved organic matter enhanced the aggregation and oxidation of nanoplastics under simulated sunlight irradiation in water. Environ. Sci. Technol. 2022, 56, 3085–3095. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Aquatic behavior and toxicity of polystyrene nanoplastic particles with different functional groups: Complex roles of pH, dissolved organic carbon and divalent cations. Chemosphere 2019, 228, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Ortiz, D.; Nieto-Sandoval, J.; de Pedro, Z.M.; Casas, J.A. Adsorption of micropollutants onto realistic microplastics: Role of microplastic nature, size, age, and NOM fouling. Chemosphere 2021, 283, 131085. [Google Scholar] [CrossRef]
- Ding, L.; Luo, Y.; Yu, X.; Ouyang, Z.; Liu, P.; Guo, X. Insight into interactions of polystyrene microplastics with different types and compositions of dissolved organic matter. Sci. Total Environ. 2022, 824, 153883. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Junaid, M.; Wang, C.; Wang, J. Eco-corona enhanced the interactive effects of nanoplastics and 6:2 chlorinated polyfluorinated ether sulfonate in zebrafish embryos. Sci. Total Environ. 2024, 953, 176223. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhao, J.; Li, L.; Wang, Y.; Dai, X.; Yu, F.; Ma, J. Interfacial interaction between micro/nanoplastics and typical PPCPs and nanoplastics removal via electrosorption from an aqueous solution. Water Res. 2020, 184, 116100. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Z.; Wei, W.; Chen, J.; Ni, B.J. Toxicity of micro/nanoplastics in the environment: Roles of plastisphere and eco-corona. Soil Environ. Health 2023, 1, 100002. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, J.; Zheng, L.; Zhu, W.; Xue, X.; Yu, Y.; Deng, Y.; Wang, H. Adsorption behaviors and mechanisms of humic acid on virgin and aging microplastics. J. Mol. Liq. 2022, 363, 119819. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Gong, Z.; Wang, H.; Huang, H.; Shi, Y.; Zhao, X.; Gao, S. Humic acid and fulvic acid hinder long-term weathering of microplastics in lake water. Environ. Sci. Technol. 2021, 55, 15810–15820. [Google Scholar] [CrossRef]
- Fadare, O.O.; Wan, B.; Guo, L.H.; Xin, Y.; Qin, W.; Yang, Y. Humic acid alleviates the toxicity of polystyrene nanoplastic particles to Daphnia magna. Environ. Sci. J. Integr. Environ. Res. Nano 2019, 6, 1466–1477. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, J.; Xia, J.; Wu, J.; Zeng, Y.; Miao, L.; Lv, B. Transport of polystyrene nanoplastics in porous media: Combined effects of two co-existing substances. Sci. Total Environ. 2023, 897, 165275. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yue, T.; Liu, L.; Zhang, B.; Feng, H.; Li, S.; Liu, X.; Dai, Y.; Zhao, J. Molecular assembly of extracellular polymeric substances regulating aggregation of differently charged nanoplastics and subsequent interactions with bacterial membrane. J. Hazard. Mater. 2023, 457, 131825. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Ma, X.; Guo, B.; Zhang, K. Environmental factors-mediated behavior of microplastics and nanoplastics in water: A review. Chemosphere 2021, 271, 129597. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Liu, Y.; Xu, Y.; Li, S.; Liu, X.; Dai, Y.; Zhao, J.; Yue, T. Benzo[a]pyrene and heavy metal ion adsorption on nanoplastics regulated by humic acid: Cooperation/competition mechanisms revealed by molecular dynamics simulations. J. Hazard Mater. 2022, 424, 127431. [Google Scholar] [CrossRef]
- Shiu, R.F.; Vazquez, C.I.; Chiang, C.Y.; Chiu, M.H.; Chen, C.S.; Ni, C.W.; Gong, G.C.; Quigg, A.; Santschi, P.H.; Chin, W.C. Nano- and microplastics trigger secretion of protein-rich extracellular polymeric substances from phytoplankton. Sci. Total Environ. 2020, 748, 141469. [Google Scholar] [CrossRef]
- Zafar, R.; Arshad, Z.; Choi, N.E.; Li, X.; Hur, J. Unravelling the complex adsorption behavior of extracellular polymeric substances onto pristine and UV-aged microplastics using two-dimensional correlation spectroscopy. Chem. Eng. J. 2023, 470, 144031. [Google Scholar] [CrossRef]
- Xu, S.Y.; Wang, C.Q.; Zhu, P.F.; Zhang, D.Y.; Pan, X.L. Temporospatial nano-heterogeneity of self-assembly of extracellular polymeric substances on microplastics and water environmental implications. J. Hazard. Mater. 2022, 440, 129773. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Xia, S.; Zhao, J. Cu(II) adsorption on Poly(Lactic Acid) microplastics: Significance of microbial colonization and degradation. Chem. Eng. J. 2022, 429, 132306. [Google Scholar] [CrossRef]
- Yang, C.W.; Yuan, L.; Zhou, H.Z.; Zhang, X.; Sheng, G.P. Coating ligand-mediated dynamic formation of natural organic matter (NOM) corona on engineered nanoparticles in natural environments. Environ. Sci. Nano 2021, 8, 1029–1041. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Q.; Yang, Y.; Hou, L.; Zheng, W.; Wu, Z.; Wang, Z. Revealing the contradiction between DLVO/XDLVO theory and membrane fouling propensity for oil-in-water emulsion separation. J Hazard Mater. 2024, 466, 133594. [Google Scholar] [CrossRef]
- Giri, S.; Mukherjee, A. Eco-corona reduces the phytotoxic effects of polystyrene nanoplastics in Allium cepa: Emphasizing the role of ROS. Environ. Exp. Bot. 2022, 198, 104850. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Z.; Bai, H.; Xue, M.; Tang, J. Photochemically induced aging of polystyrene nanoplastics and its impact on norfloxacin adsorption behavior. Sci. Total Environ. 2024, 930, 172511. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Gabellieri, E.; Cioni, P.; Paccagnini, E.; Faleri, C.; Lupetti, P.; Corsi, I.; Morelli, E. Interplay between extracellular polymeric substances (EPS) from a marine diatom and model nanoplastic through eco-corona formation. Sci. Total Environ. 2020, 725, 138457. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, R.; Liu, Q.; Ouyang, G. Impact of different modes of adsorption of natural organic matter on the environmental fate of nanoplastics. Chemosphere 2021, 263, 127967. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, J.; Xia, J.; Wu, J.; You, G.; Miao, L. The long-term release and particle fracture behaviors of nanoplastics retained in porous media: Effects of surfactants, natural organic matters, antibiotics, and bacteria. Sci. Total Environ. 2024, 925, 171563. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Maa, J.P.Y.; Shen, X.; Liang, J.; Yu, L.; Ge, L.; Wang, G. Effects of organic matter on interaction forces between polystyrene microplastics: An experimental study. Sci. Total Environ. 2022, 844, 157186. [Google Scholar] [CrossRef]
- Wang, X.; Bolan, N.; Tsang, D.C.W.; Sarkar, B.; Bradney, L.; Li, Y. A review of microplastics aggregation in aquatic environment: Influence factors, analytical methods, and environmental implications. J. Hazard. Mater. 2021, 402, 123496. [Google Scholar] [CrossRef]
- Pradel, A.; Ferreres, S.; Veclin, C.; El Hadri, H.; Gautier, M.; Grassl, B.; Gigault, J. Stabilization of fragmental polystyrene nanoplastic by natural organic matter: Insight into mechanisms. ACS EST Water 2021, 1, 1198–1208. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, K.F.; Lin, K.Y.A.; Chen, J.K.; Jiang, X.Y.; Lin, C.H. The nephrotoxic potential of polystyrene microplastics at realistic environmental concentrations. J. Hazard. Mater. 2022, 427, 127871. [Google Scholar] [CrossRef]
- Ruan, J.; Yang, J.; Wang, X.; Liang, C.; Li, L.; Zeng, Y.; Wang, J.; Li, Y.; Huang, W.; Chen, C. Heteroaggregation kinetics of oppositely charged nanoplastics in aquatic environments: Effects of particle ratio, solution chemistry, and interaction sequence. J Hazard Mater. 2024, 475, 134857. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; van der Hoek, J.P.; Liu, G.; Lompe, K.M. Natural organic matter stabilizes pristine nanoplastics but destabilizes photochemical weathered nanoplastics in monovalent electrolyte solutions. Environ. Sci. Technol. 2025, 59, 1822–1834. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shen, M.; Li, S.; Fu, Y.; Zhang, D.; Liu, H.; Liu, J. Aggregation kinetics of different surface-modified polystyrene nanoparticles in monovalent and divalent electrolytes. Environ. Pollut. 2019, 255, 113302. [Google Scholar] [CrossRef]
- Huang, S.H.; Zhang, B.; Cui, F.Y.; He, Y.K.; Shi, J.Y.; Yang, X.Y.; Lens, P.N.L.; Shi, W.X. Mechanisms underlying the detrimental impact of micro(nano)plastics on the stability of aerobic granular sludge: Interactions between micro(nano)plastics and extracellular polymeric substances. J. Hazard. Mater. 2024, 478, 135512. [Google Scholar] [CrossRef]
- Huang, R.; Han, Z.; Ma, C.; Liu, H.; Huangfu, X. Stability and mobility of zinc oxide nanoparticles in aquatic environment: Influence of extracellular polymeric substances from cyanobacteria and microalgae. J. Environ. Chem. Eng. 2023, 11, 109069. [Google Scholar] [CrossRef]
- Li, L.; Luo, D.; Luo, S.; Yue, J.; Li, X.; Chen, L.; Chen, X.; Wen, B.; Luo, X.; Li, Y.; et al. Heteroaggregation, disaggregation, and migration of nanoplastics with nanosized activated carbon in aquatic environments: Effects of particle property, water chemistry, and hydrodynamic condition. Water Res. 2024, 266, 122399. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiao, R.; Yu, J.; Wang, D. Combined effects of particle size and humic acid corona on the aggregation kinetics of nanoplastics in aquatic environments. Sci. Total. Environ. 2023, 901, 165987. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, J.; Xia, J.; Zeng, Y.; Miao, L. Influence of natural organic matters on fate of polystyrene nanoplastics in porous media. Sci. Total Environ. 2023, 893, 64504. [Google Scholar] [CrossRef]
- Kong, Y.; Li, X.; Tao, M.; Cao, X.; Wang, Z.; Xing, B. Cation-π mechanism promotes the adsorption of humic acid on polystyrene nanoplastics to differently affect their aggregation: Evidence from experimental characterization and DFT calculation. J. Hazard. Mater. 2023, 459, 132071. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.; Li, H.; Yin, Y.; Song, W.; Guo, H.; Huang, T.; Xing, B. Molecular-level insight into the behavior of metal cations and organic matter during the aggregation of polystyrene nanoplastics. J Hazard Mater. 2024, 473, 134665. [Google Scholar] [CrossRef]
- Mao, Y.; Li, H.; Huangfu, X.; Liu, Y.; He, Q. Nanoplastics display strong stability in aqueous environments: Insights from aggregation behaviour and theoretical calculations. Environ. Pollut. 2020, 258, 113760. [Google Scholar] [CrossRef]
- Guckeisen, T.; Orghici, R.; Rathgeber, S. Correlative effects on nanoplastic aggregation in model extracellular biofilm substances investigated with fluorescence correlation spectroscopy. Polymers 2024, 16, 2170. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; Li, X.; Wang, X.; van der Hoek, J.P.; Liu, G. Combined effects of photoaging and natural organic matter on the colloidal stability of nanoplastics in aquatic environments. Water Res. 2022, 226, 119313. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, R.; Lin, W.; Ouyang, G. Effect of salinity and humic acid on the aggregation and toxicity of polystyrene nanoplastics with different functional groups and charges. Environ. Pollut. 2019, 245, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Han, Y.; Zhang, Y.; Li, J.; Sun, B.; Geng, Z.; Wang, Z.; Xing, B. Exposure order to photoaging and humic acids significantly modifies the aggregation and transformation of nanoplastics in aqueous solutions. Environ. Sci. Technol. 2023, 57, 6520–6529. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Chen, C.; Huang, Z.; Gu, J.; Zhang, Z.; Cai, T.; Peng, J.; Huang, W.; Dang, Z.; Yang, C. Influence of macromolecules and electrolytes on heteroaggregation kinetics of polystyrene nanoplastics and goethite nanoparticles in aquatic environments. J. Hazard. Mater. 2024, 477, 135257. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Du, J.; Pu, L.; Chen, S. Effects of chlorella extracellular polymeric substances on the aggregation and stability of TiO2 nanoparticles as electrolytes. Desalination Water Treat. 2021, 209, 334–341. [Google Scholar] [CrossRef]
- Xiong, S.; Cao, X.; Eggleston, I.; Chi, Y.; Li, A.; Liu, X.; Zhao, J.; Xing, B. Role of extracellular polymeric substances in the aggregation and biological response of micro(nano)plastics with different functional groups and sizes. J. Hazard. Mater. 2023, 446, 130713. [Google Scholar] [CrossRef]
- Cai, L.; Hu, L.; Shi, H.; Ye, J.; Zhang, Y.; Kim, H. Effects of inorganic ions and natural organic matter on the aggregation of nanoplastics. Chemosphere 2018, 197, 142–151. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, H.; Zhao, J.; Luo, X.; Wang, Z.; Xing, B. Photodegradation elevated the toxicity of polystyrene microplastics to grouper (Epinephelus moara) through disrupting hepatic lipid homeostasis. Environ. Sci. Technol. 2020, 54, 6202–6212. [Google Scholar] [CrossRef]
- Sun, H.; Jiao, R.; Gao, Y.; Wang, D. Insight into the aggregation kinetics of nanoplastics in aquatic environments: Combined effects of protein corona and particle size. Sep. Purif. Technol. 2025, 362, 131899. [Google Scholar] [CrossRef]
- Beddoes, C.M.; Case, C.P.; Briscoe, W.H. Understanding nanoparticle cellular entry: A physicochemical perspective. Adv. Colloid Interface Sci. 2015, 218, 48–68. [Google Scholar] [CrossRef]
- Kihara, S.; Ashenden, A.; Kaur, M.; Glasson, J.; Ghosh, S.; van der Heijden, N.; Brooks, A.E.S.; Mata, J.P.; Holt, S.; Domigan, L.J.; et al. Cellular interactions with polystyrene nanoplastics-The role of particle size and protein corona. Biointerphases 2021, 16, 041001. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Nakamura, H.; Ohsaki, S.; Watano, S. Direct translocation of a negatively charged nanoparticle across a negatively charged model cell membrane. Phys. Chem. Chem. Phys. 2021, 23, 10591–10599. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.; Wei, W. Simulation of nanoparticles interacting with a cell membrane: Probing the structural basis and potential biomedical application. NPG Asia Mater. 2021, 13, 52. [Google Scholar] [CrossRef]
- Li, N.; Wang, P.; Wang, S.; Wang, C.; Zhou, H.; Kapur, S.; Zhang, J.; Song, Y. Electrostatic charges on microalgae surface: Mechanism and applications. J. Environ. Chem. Eng. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Hanachi, P.; Khoshnamvand, M.; Walker, T.R.; Hamidian, A.H. Nano-sized polystyrene plastics toxicity to microalgae Chlorella vulgaris: Toxicity mitigation using humic acid. Aquat. Toxicol. 2022, 245, 106123. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Christudoss, A.C.; Chandrasekaran, N.; Peijnenburg, W.J.G.M.; Mukherjee, A. The role of algal EPS in reducing the combined toxicity of BPA and polystyrene nanoparticles to the freshwater algae Scenedesmus obliquus. Plant Physiol. Biochem. 2023, 197, 107664. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Meng, L.; Zeb, A.; Mo, F.; Wang, J.; Shi, R. The interfacial interaction between Dechlorane plus (DP) and polystyrene nanoplastics (PSNPs): An overlooked influence factor for the algal toxicity of PSNPs. Sci. Total Environ. 2023, 905, 167129. [Google Scholar] [CrossRef]
- Debroy, A.; Saravanan, J.S.; Joyce Nirmala, M.; Pulimi, M.; Mukherjee, D.A. Algal EPS modifies the toxicity potential of the mixture of polystyrene nanoplastics (PSNPs) and flame retardant, triphenyl phosphate in freshwater microalgae Chlorella sp. Chemosphere 2024, 366, 143471. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Gao, R.Y.; Wang, Z.J.; Shao, Q.Q.; Hu, Y.W.; Jia, H.B.; Liu, X.J.; Dong, F.Q.; Fu, L.M.; Zhang, J.P. Daphnia magna uptake and excretion of luminescence-labelled polystyrene nanoparticle as visualized by high sensitivity real-time optical imaging. Chemosphere 2023, 326, 138341. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, N.; Lu, L.; Li, N.; Hu, T.; Guo, J.; Zhang, J.; Zeng, Z.; Liang, J. Aggregation behavior of polystyrene nanoplastics: Role of surface functional groups and protein and electrolyte variation. Chemosphere. 2024, 350, 140998. [Google Scholar] [CrossRef]
- Canesi, L.; Balbi, T.; Fabbri, R.; Salis, A.; Damonte, G.; Volland, M.; Blasco, J. Biomolecular coronas in invertebrate species: Implications in the environmental impact of nanoparticles. NanoImpact 2017, 8, 89–98. [Google Scholar] [CrossRef]
- Ding, J.; Huang, Y.; Liu, S.; Zhang, S.; Zou, H.; Wang, Z.; Geng, J. Toxicological effects of nano-and micro-polystyrene plastics on red tilapia: Are larger plastic particles more harmless? J. Hazard. Mater. 2020, 396, 122693. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ding, J.; Xu, H.; Tian, W.; Xu, J.; Zou, H.; Zhu, W. Influences of molecular weight fractionated humic acids on polyamide 66 microplastic stability and toxicity in red tilapia (Oreochromis niloticus). Front. Mar. Sci. 2022, 9, 1060582. [Google Scholar] [CrossRef]
- Lei, C.; Sun, Y.; Tsang, D.C.W.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhao, J.; Xing, B. Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter. Environ. Sci. Nano 2016, 3, 240–255. [Google Scholar] [CrossRef]
- Luo, H.; Du, Q.; Zhong, Z.; Xu, Y.; Peng, J. Protein-coated microplastics corona complex: An underestimated risk of microplastics. Sci. Total Environ. 2022, 851, 157948. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Yan, B.; Yue, T. Surface properties of nanoparticles dictate their toxicity by regulating adsorption of humic acid molecules. ACS Sustain. Chem. Eng. 2021, 9, 13705–13716. [Google Scholar] [CrossRef]
- Zou, W.; Xia, M.L.; Jiang, K.; Cao, Z.; Zhang, X.; Hu, X. Photo-oxidative degradation mitigated the developmental toxicity of polyamide microplastics to zebrafish larvae by modulating macrophage-triggered proinflammatory responses and apoptosis. Environ. Sci. Technol. 2020, 54, 13888–13898. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; van der Hoek, J.P.; Liu, G.; Lompe, K.M. Photo-oxidation of micro- and nanoplastics: Physical, chemical, and biological effects in environments. Environ. Sci. Technol. 2024, 58, 991–1009. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Wu, A.; Tang, Z.; Niu, L.; Wu, F.; Wang, F.; Zhao, T.; Fu, Z. Aggregation and stability of sulfate-modified polystyrene nanoplastics in synthetic and natural waters. Environ. Pollut. 2020, 268, 114240. [Google Scholar] [CrossRef]
- Triebskorn, R.; Braunbeck, T.; Grummt, T.; Hanslik, L.; Huppertsberg, S.; Jekel, M.; Knepper, T.P.; Krais, S.; Müller, Y.K.; Pittroff, M.; et al. Relevance of nano- and microplastics for freshwater ecosystems: A critical review. Trends Analyt. Chem. 2019, 110, 375–392. [Google Scholar] [CrossRef]
- Merrill, G.B.; Hermabessiere, L.; Rochman, C.M.; Nowacek, D.P. Microplastics in marine mammal blubber, melon, & other tissues: Evidence of translocation. Environ. Pollut. 2023, 335, 122252. [Google Scholar] [PubMed]
- Zhang, Y.; Goss, G.G. The “Trojan Horse” effect of nanoplastics: Potentiation of polycyclic aromatic hydrocarbon uptake in rainbow trout and the mitigating effects of natural organic matter. Environ. Sci. Nano 2021, 8, 3685–3698. [Google Scholar] [CrossRef]
- Monikh, F.A.; Vijver, M.G.; Guo, Z.; Zhang, P.; Darbha, G.K.; Peijnenburg, W.J. Metal sorption onto nanoscale plastic debris and trojan horse effects in Daphnia magna: Role of dissolved organic matter. Water Res. 2020, 186, 116410. [Google Scholar] [CrossRef]
- Qiao, R.; Lu, K.; Deng, Y.; Ren, H.; Zhang, Y. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci. Total Environ. 2019, 682, 128–137. [Google Scholar] [CrossRef]
- Chen, W.; Sandoval, H.; Kubiak, J.Z.; Li, X.C.; Ghobrial, R.M.; Kloc, M. The phenotype of peritoneal mouse macrophages depends on the mitochondria and ATP/ADP homeostasis. Cell Immunol. 2018, 324, 1–7. [Google Scholar] [CrossRef]
- Mao, R.; Lang, M.; Yu, X.; Wu, R.; Yang, X.; Guo, X. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater. 2020, 393, 122515. [Google Scholar] [CrossRef]
- Natarajan, L.; Omer, S.; Jetly, N.; Jenifer, M.A.; Chandrasekaran, N.; Suraishkumar, G.K.; Mukherjee, A. Eco-Corona formation lessens the toxic effects of polystyrene nanoplastics towards marine microalgae Chlorella sp. Environ. Res. 2020, 188, 109842. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; You, D.; Xie, Y.; Feng, Y.; Sultan, M.; Pei, D.S.; Fu, A. Alleviating binary toxicity of polystyrene nanoplastics and atrazine to Chlorella vulgaris through humic acid interaction: Long-term toxicity using environmentally relevant concentrations. Chemosphere 2024, 358, 142111. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, K.; Kashian, D.R. Extracellular polymeric substances in green alga facilitate microplastic deposition. Chemosphere 2022, 286, 131814. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yuan, Y.; Li, Y.; Liu, X.; Wang, X.; Fan, Z. Polystyrene nanoplastics affect growth and microcystin production of Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2021, 28, 13394–13403. [Google Scholar] [CrossRef]
- Abomohra, A.; Hanelt, D. Recent advances in micro-/nanoplastic (MNPs) removal by microalgae and possible integrated routes of energy recovery. Microorganisms 2022, 10, 2400. [Google Scholar] [CrossRef]
- Liu, T.; Hou, B.; Wang, Z.; Yang, Y. Polystyrene microplastics induce mitochondrial damage in mouse GC-2 cells. Ecotoxicol. Environ. Saf. 2022, 237, 113520. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cong, J.Y.; Wu, J.; Chen, Y.Z.; Fan, H.Y.; Wang, X.L.; Duan, Z.H.; Wang, L. Nanoplastic-protein corona interactions and their biological effects: A review of recent advances and trends. Trends Analyt. Chem. 2023, 166, 117206. [Google Scholar] [CrossRef]
- He, Y.; Peng, G.; Jiang, Y.; Zhao, M.; Wang, X.; Chen, M.; Lin, S. Environmental hazard potential of nano-photocatalysts determined by nano-bio interactions and exposure conditions. Small 2020, 16, 1907690. [Google Scholar] [CrossRef]
- Natarajan, L.; Annie Jenifer, M.; Peijnenburg, W.J.G.M.; Mukherjee, A. Algal extracellular polymeric substances (algal-EPS) for mitigating the combined toxic effects of polystyrene nanoplastics and nano-TiO2 in Chlorella sp. Nanotoxicology 2023, 17, 143–156. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, T.; Qu, G.; Jia, H.; Zhu, L. Probing the aging processes and mechanisms of microplastic under simulated multiple actions generated by discharge plasma. J. Hazard. Mater. 2020, 398, 122956. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Zhang, L.; Xu, M.; Gao, L.; Zhao, B. The interaction of micro/nano plastics and the environment: Effects of ecological corona on the toxicity to aquatic organisms. Ecotoxicol. Environ. Saf. 2022, 243, 113997. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, C.; Wang, Y.; Fu, L.; Man, M.; Chen, L. Realistic polyethylene terephthalate nanoplastics and the size-and surface coating-dependent toxicological impacts on zebrafish embryos. Environ. Sci. J. Integr. Environ. Res. Nano 2020, 7, 2313–2324. [Google Scholar] [CrossRef]
- Barabadi, H.; Najafi, M.; Samadian, H.; Azarnezhad, A.; Vahidi, H.; Mahjoub, M.A.; Koohiyan, M.; Ahmadi, A. A systematic review of the genotoxicity and antigenotoxicity of biologically synthesized metallic nanomaterials: Are green nanoparticles safe enough for clinical marketing? Medicina 2019, 55, 439. [Google Scholar] [CrossRef]
- Khan, A.R.; Ulhassan, Z.; Li, G.; Lou, J.; Iqbal, B.; Salam, A.; Azhar, W.; Batool, S.; Zhao, T.; Li, K.; et al. Micro/ nanoplastics: Critical review of their impacts on plants, interactions with other contaminants (antibiotics, heavy metals, and polycyclic aromatic hydrocarbons), and management strategies. Sci. Total Environ. 2024, 912, 169420. [Google Scholar] [CrossRef]
- Shukla, R.K.; Badiye, A.; Vajpayee, K.; Kapoor, N. Genotoxic potential of nanoparticles: Structural and functional modifications in DNA. Front. Genet. 2021, 12, 728250. [Google Scholar] [CrossRef]
- Gunderson, M.P.; Nguyen, B.T.; Reyes, J.C.C.; Holden, L.L.; French, J.M.; Smith, B.D.; Lineberger, C. Response of phase I and II detoxification enzymes, glutathione, metallothionein and acetylcholine esterase to mercury and dimethoate in signal crayfish (Pacifastacus leniusculus). Chemosphere 2018, 208, 749–756. [Google Scholar] [CrossRef]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef]
- Zámocký, M.; Koller, F. Understanding the structure and function of catalases: Clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol. 1999, 72, 19–66. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Ceckova, M.; Micuda, S.; Pavek, P. Expression and function of pglycoprotein in normal tissues: Effect on pharmacokinetics. Methods Mol. Biol. 2010, 596, 199–222. [Google Scholar] [PubMed]
- Fadare, O.O.; Wan, B.; Liu, K.; Yang, Y.; Zhao, L.; Guo, L.H. Eco-corona vs protein corona: Effects of humic substances on corona formation and nanoplastic particle toxicity in Daphnia magna. Environ. Sci. Technol. 2020, 54, 8001–8009. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Ghosh, S. Therapeutic potential of fulvic acid in chronic inflammatory diseases and diabetes. J. Diabetes Res. 2018, 2018, 5391014. [Google Scholar] [CrossRef]
- Faught, E.; Schaaf, M.J.M. Molecular mechanisms of the stress-induced regulation of the inflammatory response in fish. Gen. Comp. Endocrinol. 2024, 345, 114387. [Google Scholar] [CrossRef]
- Khan, A.R.; Wallbom, N.; Sundh, H.; Sandblom, E.; Tort, L.; Jönsson, E. Sea water acclimation of rainbow trout (Oncorhynchus mykiss) modulates the mucosal transcript immune response induced by Vibrio anguillarum and Aeromonas salmonicida vaccine, and prevents further transcription of stress-immune genes in response to acutes. Fish Shellfish Immunol. 2024, 152, 109733. [Google Scholar]
- Brandts, I.; Balasch, J.C.; Gonçalves, A.P.; Martins, M.A.; Pereira, M.L.; Tvarijonaviciute, A.; Teles, M.; Oliveira, M. Immuno-modulatory effects of nanoplastics and humic acids in the European seabass (Dicentrarchus labrax). J. Hazard Mater. 2021, 414, 125562. [Google Scholar] [CrossRef]
- Fuse, Y.; Endo, Y.; Araoi, S.; Daitoku, H.; Suzuki, H.; Kato, M.; Kobayashi, M. The possible repositioning of an oral anti-arthritic drug, auranofin, for Nrf2-activating therapy: The demonstration of Nrf2-dependent anti-oxidative action using a zebrafish model. Free Radic. Biol. Med. 2018, 115, 405–411. [Google Scholar] [CrossRef]
- Raghunath, A.; Nagarajan, R.; Sundarraj, K.; Panneerselvam, L.; Perumal, E. Genomewide identification and analysis of Nrf2 binding sites—Antioxidant response elements in zebrafish. Toxicol. Appl. Pharmacol. 2018, 360, 236–248. [Google Scholar] [CrossRef]
- Zhou, J.; Miners, J.O. Enzyme kinetics of uridine diphosphate glucuronosyltransferases (UGTs). Methods Mol Biol. 2014, 1113, 203–228. [Google Scholar]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid. Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Gasco, R.; Worms, I.A.M.; Kantarciyan, A.; Slaveykova, V.I. Diatom-derived extracellular polymeric substances form eco-corona and enhance stability of silver nanoparticles. Environ. Sci. Nano 2024, 11, 4138–4150. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, Q.; Jiang, J.; Dalu, T.; Kadushkin, A.; Singh, J.; Fakhrullin, R.; Wang, F.; Cai, X.; Li, R. Coronas of micro/nano plastics: A key determinant in their risk assessments. Part Fibre Toxicol. 2022, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Nasser, F.; Constantinou, J.; Lynch, I. Nanomaterials in the environment acquire an “eco-corona” impacting their toxicity to Daphnia magna—A call for updating toxicity testing policies. Proteomics 2020, 20, e1800412. [Google Scholar] [CrossRef]
- Soliman, M.G.; Martinez-Serra, A.; Antonello, G.; Dobricic, M.; Wilkins, T.; Serchi, T.; Fenoglio, I.; Monopoli, M.P. Understanding the role of biomolecular coronas in human exposure to nanomaterials. Environ. Sci. Nano 2024, 11, 4421–4448. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).